Documente Academic
Documente Profesional
Documente Cultură
Pathophysiology of Myocardial Infarction
Încărcat de
kobe_andre15Descriere originală:
Titlu original
Drepturi de autor
Formate disponibile
Partajați acest document
Partajați sau inserați document
Vi se pare util acest document?
Este necorespunzător acest conținut?
Raportați acest documentDrepturi de autor:
Formate disponibile
Pathophysiology of Myocardial Infarction
Încărcat de
kobe_andre15Drepturi de autor:
Formate disponibile
ACSC41 23/10/03 5:42 PM Page 88
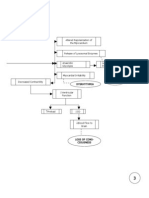
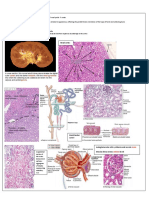
41 Pathophysiology of acute myocardial infarction
Start here Macrophages
and T-lymphocytes Stunned myocardium
Fibrous cap
24 h Mainly dead
Transmural necrosed zone myocytes and
Lipid core Infarct appears pale, most cells dead, neutrophils
neutrophils present—
Plaque rupture, coagulation necrosis
platelet aggregation
Granulation tissue
moves inward and
replaces dead tissue
with scar tissue
2h
Subendocardial necrosed zone 5–7 days
Cellular damage progressing, but
still partially reversible with reperfusion
Thrombus propagates
into and along
coronary artery
Endocardium
Mixture of living
and dead myocytes;
Cross-section of ventricular wall Scar substrate for
served by thrombosed artery thin re-entrant
firm arrhythmias
grey
0h
> 3 months
Finish here
41.1
Infarction is tissue death caused by ischaemia. Acute myo- complete thrombotic occlusion of the infarct-related artery. The
cardial infarction (MI) occurs when localized myocardial incidence of total occlusion fell to 65% 12–24 h after symptom
ischaemia causes the development of a defined region of nec- onset due to spontaneous fibrinolysis. Fresh thrombi on top
rosis. MI is most often caused by rupture of an atherosclerotic of ruptured plaques have also been demonstrated in the infarct-
lesion in a coronary artery. This causes the formation of a throm- related arteries in patients dying of MI.
bus that plugs the artery, stopping it from supplying blood to the
region of the heart that it supplies. Mechanisms and consequences of plaque rupture
Coronary plaques which are prone to rupture are typically small
Role of thrombosis in MI and nonobstructive, with a large lipid-rich core covered by a thin
Pivotal studies by DeWood and colleagues showed that coron- fibrous cap. Activated macrophages and T-lymphocytes localized
ary thrombosis is the critical event resulting in MI. Of patients at the site of plaque rupture are thought to release metalloproteases
presenting within 4 h of symptom onset with ECG evidence and cytokines which weaken the fibrous cap, rendering it liable
of transmural MI, coronary angiography showed that 87% had to tear or erode due to the shear stress exerted by the bloodflow.
88 Pathology and therapeutics
ACSC41 23/10/03 5:42 PM Page 89
Plaque rupture reveals subendothelial collagen, which serves Evolution of the infarct
as a site of platelet adhesion, activation and aggregation. This Both infarcted and unaffected myocardial regions undergo
results in: progressive changes over the hours, days and weeks following
1 The release of substances such as thromboxane A2 (TXA2 ), coronary thrombosis. This process of postinfarct myocardial
fibrinogen, 5-hydroxytryptamine (5-HT), platelet activating fac- evolution leads to the occurrence of characteristic complications
tor and ADP, which further promote platelet aggregation. at predictable times after the initial event (see Chapter 42).
2 Activation of the clotting cascade, leading to fibrin formation Ischaemia causes an immediate loss of contractility in the
and propagation and stabilization of the occlusive thrombus. affected myocardium, a condition termed hypokinesis. Necrosis
The endothelium is often damaged around areas of coronary starts to develop in the subendocardium (which is most prone to
artery disease. The resulting deficit of antithrombotic factors ischaemia; see Chapter 37), about 15–30 min after coronary occlu-
such as thrombomodulin and prostacyclin enhances thrombus sion. The necrotic region grows outward towards the epicardium
formation. In addition, the tendency of several platelet-derived over the next 3–6 h, eventually spanning the entire ventricular
factors (e.g. TXA2, 5-HT) to cause vasoconstriction is increased wall. In some areas (generally at the edges of the infarct) the
in the absence of endothelial-derived relaxing factors. This may myocardium is stunned (reversibly damaged) and will eventually
promote the development of local vasospasm, which worsens recover if bloodflow is restored. Contractility in the remaining
coronary occlusion. viable myocardium increases, a process termed hyperkinesis.
Sudden death and acute coronary syndrome onset show a A progression of cellular, histological and gross changes
circadian variation (daily cycle), peaking at around 9 a.m. with develop within the infarct. Although alterations in the gross
a trough at around 11 p.m. Levels of catecholamines peak about appearance of infarcted tissue are not apparent for at least 6 h
an hour after awakening in the morning, resulting in maximal after the onset of cell death, cell biochemistry and ultrastructure
levels of platelet aggregability, vascular tone, heart rate and begin to show abnormalities within 20 min. Cell damage is pro-
blood pressure, which may trigger plaque rupture and thrombosis. gressive, becomingly increasingly irreversible over about 12 h.
Increased physical and mental stress can also cause MI and This period therefore provides a window of opportunity during
sudden death, supporting a role for increases in catecholamines which thrombolysis and reperfusion may salvage some of the
in MI pathophysiology. Furthermore, chronic β-adrenergic infarct (see Chapter 43).
receptor blockade abolishes the circadian rhythm of MI. Between 4 and 12 h after cell death starts, the infarcted
Autopsies of young subjects killed in road accidents often myocardium begins to undergo coagulation necrosis, a pro-
show small plaque ruptures in susceptible arteries, suggesting cess characterized by cell swelling, organelle breakdown and
that plaque rupture does not always have pathological con- protein denaturation. After about 18 h, neutrophils (phagocytic
sequences. The degree of coronary occlusion and myocardial lymphocytes) enter the infarct. Their numbers reach a peak after
damage caused by plaque rupture probably depends on systemic about 5 days, and then decline. After 3– 4 days, granulation tis-
catecholamine levels, as well as local factors such as plaque sue appears at the edges of the infarct zone. This consists of
location and morphology, the depth of plaque rupture, and the macrophages, fibroblasts, which lay down scar tissue, and new
extent to which coronary vasoconstriction occurs. capillaries. The infarcted myocardium is especially soft be-
Severe and prolonged ischaemia produces a region of nec- tween 4 and 7 days, and is therefore maximally prone to ruptur-
rosis spanning the entire thickness of the myocardial wall. Such ing. This event is usually fatal, may occur at any time during the
a transmural infarct usually causes ST segment elevation (i.e. first 2 weeks and is responsible for about 10% of MI mortality.
STEMI, see Chapter 38). Less severe and protracted ischaemia As the granulation tissue migrates inward toward the centre of
can arise when: the infarct over several weeks, the necrotic tissue is engulfed
1 Coronary occlusion is followed by spontaneous reperfusion. and digested by the macrophages. The granulation tissue then
2 The infarct-related artery is not completely occluded. progressively matures, with an increase in connective (scar)
3 Occlusion is complete, but an existing collateral blood supply tissue and loss of capillaries. After 2–3 months, the infarct has
prevents complete ischaemia. healed, leaving a noncontracting region of the ventricular wall
4 The oxygen demand in the affected zone of myocardium is that is thinned, firm and pale grey.
smaller. Infarct expansion, the stretching and thinning of the infarcted
Under these conditions, the necrotic zone may be mainly limited wall, may occur within the first day or so after a MI, especially
to the subendocardium, typically causing non-ST segment eleva- if the infarction is large or transmural, or has an anterior loca-
tion MI. tion. Over the course of several months, there is progressive
The classification of acute MI according to the presence or dilatation, not only of the infarct zone, but also of healthy
absence of ST segment elevation is designed to allow rapid de- myocardium. This process of ventricular remodelling is
cision-making concerning whether thrombolysis should be caused by an increase in end-diastolic wall stress. Infarct expan-
initiated (see Chapter 42). This classification replaces the pre- sion puts patients at a sub-stantial risk for the development of
vious one, based on the presence or absence of Q waves on the congestive heart failure, ventricular arrhythmias, and free wall
ECG, which was less useful for guiding immediate therapy. rupture.
Pathophysiology of acute myocardial infarction 89
S-ar putea să vă placă și
- Myocardial Infarction Risk Factors and ComplicationsDocument4 paginiMyocardial Infarction Risk Factors and ComplicationsHearty ArriolaÎncă nu există evaluări
- Patho of MIDocument2 paginiPatho of MIInchan Montesines0% (1)
- Myocardial InfarctionDocument6 paginiMyocardial InfarctionMaicie Rose Bautista VallesterosÎncă nu există evaluări
- Myocardial InfarctionDocument45 paginiMyocardial InfarctionGopal SinghÎncă nu există evaluări
- Myocardial Infarction Pathophysiology ExplainedDocument3 paginiMyocardial Infarction Pathophysiology ExplainedAbi Habiling100% (3)
- Pathophysiology Worksheet MIDocument2 paginiPathophysiology Worksheet MIpjbedelÎncă nu există evaluări
- Final Myocardial Infarction Pathophysiology PDFDocument3 paginiFinal Myocardial Infarction Pathophysiology PDFDave JoshuaÎncă nu există evaluări
- Myocardial InfractionDocument16 paginiMyocardial InfractionYAMINIPRIYANÎncă nu există evaluări
- Lung Cancer Case PresentationDocument31 paginiLung Cancer Case Presentationapi-498724117Încă nu există evaluări
- Anatomy & Physiology of the HeartDocument5 paginiAnatomy & Physiology of the HeartLyka Milo AvilaÎncă nu există evaluări
- BUERGER's DiseaseDocument2 paginiBUERGER's DiseasechemnikoÎncă nu există evaluări
- Dilated Cardiomyopathy Case StudyDocument29 paginiDilated Cardiomyopathy Case Studydvalitz100% (2)
- Acute Respiratory Failure Pa Tho PhysiologyDocument4 paginiAcute Respiratory Failure Pa Tho Physiologyroseanne18100% (4)
- Pathophysiology of Angina PectorisDocument1 paginăPathophysiology of Angina PectorisCheryl100% (1)
- Pathophysiology of COPD - The BasicsDocument11 paginiPathophysiology of COPD - The BasicstiaranindyÎncă nu există evaluări
- Mitral StenosisDocument43 paginiMitral StenosisEricka SantosÎncă nu există evaluări
- CABGDocument37 paginiCABGMaria Janet BasalloÎncă nu există evaluări
- Pathophysiology TBIDocument1 paginăPathophysiology TBIChester ManaloÎncă nu există evaluări
- Patho MIDocument2 paginiPatho MIbanyenye25100% (2)
- AnginaDocument12 paginiAnginaHermiie Joii Galang Maglaquii0% (1)
- DCM - Heart Failure Due to Dilated CardiomyopathyDocument13 paginiDCM - Heart Failure Due to Dilated CardiomyopathyPrincysuzine PintoÎncă nu există evaluări
- Myocardial Infarction AssignmentDocument23 paginiMyocardial Infarction AssignmentPums100% (6)
- Care for Thoracic Aortic AneurysmDocument67 paginiCare for Thoracic Aortic AneurysmJonathan DiazÎncă nu există evaluări
- Myocardial InfarctionDocument21 paginiMyocardial Infarctionanon_516349434Încă nu există evaluări
- Human Diseases Case Study 18 ADocument4 paginiHuman Diseases Case Study 18 Aairickann100% (1)
- Acute Myocardial InfarctionDocument32 paginiAcute Myocardial InfarctionListya Normalita100% (1)
- PathophysiologyDocument9 paginiPathophysiologypaul andrew laranjo asuncionÎncă nu există evaluări
- Final Paper Rheumatic Heart DiseaseDocument16 paginiFinal Paper Rheumatic Heart DiseasePrincess TumambingÎncă nu există evaluări
- Pathophysiology of Myocardial Infarction (STEMI)Document2 paginiPathophysiology of Myocardial Infarction (STEMI)michaela100% (3)
- Guillain Barre Syndrome PathophysiologyDocument4 paginiGuillain Barre Syndrome Pathophysiologykathy100% (13)
- Guillain Barre Syndrome (GBS) ImanDocument26 paginiGuillain Barre Syndrome (GBS) ImanTowardsLight100% (5)
- Pathophysiology of Congestive Heart FailureDocument3 paginiPathophysiology of Congestive Heart Failuretinayko100% (1)
- Pathophysiology of Brain Abscess Secondary To Chronic Otitis MediaDocument5 paginiPathophysiology of Brain Abscess Secondary To Chronic Otitis Mediafufulabrador100% (1)
- Case Study - Septic ShockDocument16 paginiCase Study - Septic ShockIrene Mae Villanueva Ariola100% (2)
- Infective Endocarditis Case ReportDocument40 paginiInfective Endocarditis Case Reportliu_owen17100% (1)
- Spinal Cord InjuriesDocument51 paginiSpinal Cord Injurieswanglee2000Încă nu există evaluări
- Syndrome of Inappropriate Secretion of Anti Diuretic HormoneDocument39 paginiSyndrome of Inappropriate Secretion of Anti Diuretic Hormonefarmasi_hmÎncă nu există evaluări
- Myocardial Infarction Pathophysiology & Schematic DiagramDocument3 paginiMyocardial Infarction Pathophysiology & Schematic DiagramJessica Peñamora100% (1)
- Right-Sided Heart FailureDocument4 paginiRight-Sided Heart FailureKhalid Mahmud ArifinÎncă nu există evaluări
- Myocardial InfarctionDocument16 paginiMyocardial InfarctionCay Sevilla100% (4)
- Acute Myocardial InfarctionDocument53 paginiAcute Myocardial InfarctionYew Ping Hee100% (1)
- Acute Renal FailureDocument13 paginiAcute Renal FailureGlorianne Palor100% (2)
- Acute Myocardial Infarction - CSDocument49 paginiAcute Myocardial Infarction - CSMASII94% (17)
- The Pathophysiology of Acute Coronary SyndromesDocument6 paginiThe Pathophysiology of Acute Coronary SyndromesTulisan NonaÎncă nu există evaluări
- Cardiovascular Pathology GuideDocument97 paginiCardiovascular Pathology GuideIlga ZanÎncă nu există evaluări
- Cardiovascular Disorders SlidesDocument9 paginiCardiovascular Disorders SlidesCenonina Paola TapiaÎncă nu există evaluări
- 13 - Atherosclerotic Plaque HealingDocument12 pagini13 - Atherosclerotic Plaque HealingAndres Felipe Hurtado BautistaÎncă nu există evaluări
- Ischemic Heart Disease NotesDocument8 paginiIschemic Heart Disease NotesDr. Tarush DhawanÎncă nu există evaluări
- Myocardial InfarctionDocument11 paginiMyocardial Infarctionkinzay batoolÎncă nu există evaluări
- Clinical Cardiology: New FrontiersDocument8 paginiClinical Cardiology: New Frontiersayuhati siregarÎncă nu există evaluări
- 2020 Atherosclerotic Plaque HealingDocument12 pagini2020 Atherosclerotic Plaque HealingErnesto ArellanoÎncă nu există evaluări
- Macroscopic Diagnostic GuideDocument21 paginiMacroscopic Diagnostic Guidekapil pancholiÎncă nu există evaluări
- Renal Block 2Document52 paginiRenal Block 2Maya LaPradeÎncă nu există evaluări
- Atherosclerosis: Yang Haibo MD Department of Cardiology, 1 Affiliated Hospital of ZzuDocument22 paginiAtherosclerosis: Yang Haibo MD Department of Cardiology, 1 Affiliated Hospital of Zzuapi-19916399Încă nu există evaluări
- HemostasisDocument16 paginiHemostasismihikaÎncă nu există evaluări
- Reviews: A Fresh Look at Coronary MicroembolizationDocument16 paginiReviews: A Fresh Look at Coronary MicroembolizationKevin TijerinaÎncă nu există evaluări
- Ischemic Heart DiseaseDocument4 paginiIschemic Heart DiseaseBanana CakeÎncă nu există evaluări
- Asada, Y.A - Thrombus-formation-and-propagation-in-the-onset-of-cardiovascular-eventsReviewOpen-Access - 2018Document12 paginiAsada, Y.A - Thrombus-formation-and-propagation-in-the-onset-of-cardiovascular-eventsReviewOpen-Access - 2018Yarin HernandezÎncă nu există evaluări
- Cvs Lec 1Document43 paginiCvs Lec 1data4fourgÎncă nu există evaluări
- Red vs White Renal Infarcts: Gross and Histological DifferencesDocument3 paginiRed vs White Renal Infarcts: Gross and Histological DifferencesnyarieÎncă nu există evaluări
- Introduction To Forensic Psychology Research and Application 4th Edition Bartol Test BankDocument26 paginiIntroduction To Forensic Psychology Research and Application 4th Edition Bartol Test BankMichelleBrayqwetn100% (57)
- RNTCP National Contact Details Directory Data-FinalDocument174 paginiRNTCP National Contact Details Directory Data-Finalwasimkokni100% (2)
- Lifetime Physical Fitness and Wellness A Personalized Program 14th Edition Hoeger Test BankDocument25 paginiLifetime Physical Fitness and Wellness A Personalized Program 14th Edition Hoeger Test BankStevenHughesreozb100% (54)
- StockKamis30Juli'20 PBF. SMSDocument24 paginiStockKamis30Juli'20 PBF. SMSAiko Cheryl SalsabilaÎncă nu există evaluări
- LAB Profile Cheat SheetDocument1 paginăLAB Profile Cheat SheetAndrea BertocchiÎncă nu există evaluări
- BP2016 - Vol.01Document1.334 paginiBP2016 - Vol.01thu dat80% (5)
- Velez College Nursing Students Gain Valuable Clinical ExperienceDocument6 paginiVelez College Nursing Students Gain Valuable Clinical ExperienceAngela Mae Letaban RegisÎncă nu există evaluări
- AOSpine Thoracolumbar Classification System - Poster PDFDocument1 paginăAOSpine Thoracolumbar Classification System - Poster PDFmhmiguelÎncă nu există evaluări
- Lesson 5 Activity RizalDocument3 paginiLesson 5 Activity RizalRachel ValenzuelaÎncă nu există evaluări
- User Instruction Manual: Blood Glucose Monitoring SystemDocument140 paginiUser Instruction Manual: Blood Glucose Monitoring Systemcinthia olivaÎncă nu există evaluări
- Banner HealthcareDocument6 paginiBanner HealthcareValÎncă nu există evaluări
- DirtyUSMLE RocksDocument412 paginiDirtyUSMLE RocksDavid S. Chou90% (30)
- Multiple Role Conflict Faced by WomenDocument14 paginiMultiple Role Conflict Faced by WomenmnhassenmnhÎncă nu există evaluări
- Case Study - Ectopic PregnancyDocument10 paginiCase Study - Ectopic Pregnancykristine keen buanÎncă nu există evaluări
- IM - UTI Harrison's Personal NotesDocument5 paginiIM - UTI Harrison's Personal NotesstoragejoannamsvÎncă nu există evaluări
- Theoretical Framework for MCGP4101 GRADUATION PROJECTDocument13 paginiTheoretical Framework for MCGP4101 GRADUATION PROJECTRayan Al-ShibliÎncă nu există evaluări
- A4 Quantitative Study of Acceptability of Coconut CHPTR 1&2Document16 paginiA4 Quantitative Study of Acceptability of Coconut CHPTR 1&2Adiyan100% (2)
- Cook Poultry and Game DishesDocument7 paginiCook Poultry and Game DishesJohn Michael ItableÎncă nu există evaluări
- Machine Design I: Material Selection in Hip ImplantsDocument8 paginiMachine Design I: Material Selection in Hip ImplantsrihabÎncă nu există evaluări
- New Patient Medical Form: in One SentenceDocument1 paginăNew Patient Medical Form: in One SentenceAzra BarliÎncă nu există evaluări
- DiagnosticsDocument38 paginiDiagnosticssweetyxocolatÎncă nu există evaluări
- Department of Education: Republic of The Philippines Region I La Union Schools Division Office Bacnotan, La UnionDocument1 paginăDepartment of Education: Republic of The Philippines Region I La Union Schools Division Office Bacnotan, La UnionChristineÎncă nu există evaluări
- Editoragrfeat,+4.+Nomor+1+Maret+2016+ 3+Lilies+OKDocument7 paginiEditoragrfeat,+4.+Nomor+1+Maret+2016+ 3+Lilies+OKFarabi MustajirÎncă nu există evaluări
- Success MindsetDocument32 paginiSuccess MindsetHIREN LATHIYAÎncă nu există evaluări
- G1e121020 - Ghery Arrahman - Bahasa InggrisDocument4 paginiG1e121020 - Ghery Arrahman - Bahasa InggrisGhery Arrahman100% (1)
- Understanding Facebook Use Across GenerationsDocument5 paginiUnderstanding Facebook Use Across GenerationsOscar Iván Negrete RodríguezÎncă nu există evaluări
- West Bengal COVID-19 Bulletin Updates Case CountDocument7 paginiWest Bengal COVID-19 Bulletin Updates Case Countmail_smaity4519Încă nu există evaluări
- MIT CSAIL Human Computer Interaction For User Experience Design Online Short CourseDocument10 paginiMIT CSAIL Human Computer Interaction For User Experience Design Online Short CoursePadma PGÎncă nu există evaluări
- RRL StemQuintessences 3Document9 paginiRRL StemQuintessences 3Sean BrionesÎncă nu există evaluări
- Filipino Millennials Stand Out in Views on Social Media, Adulthood and Social ResponsibilityDocument36 paginiFilipino Millennials Stand Out in Views on Social Media, Adulthood and Social ResponsibilityJames De Los ReyesÎncă nu există evaluări