Documente Academic
Documente Profesional
Documente Cultură
Common Ions Charges Chart
Încărcat de
api-233736029Titlu original
Drepturi de autor
Formate disponibile
Partajați acest document
Partajați sau inserați document
Vi se pare util acest document?
Este necorespunzător acest conținut?
Raportați acest documentDrepturi de autor:
Formate disponibile
Common Ions Charges Chart
Încărcat de
api-233736029Drepturi de autor:
Formate disponibile
Common Ions and Their Charges
Monatomic
Cations
Name
Monatomic
Anions
Name
H
+
hydrogen F
-
fluoride
Li
+
lithium Cl
-
chloride
Na
+
sodium Br
-
bromide
K
+
potassium I
-
iodide
Rb
+
rubidium O
2-
oxide
Cs
+
cesium S
2-
sulfide
Be
2+
beryllium Se
2-
selenide
Mg
2+
magnesium Te
2-
telluride
Ca
2+
calcium N
3-
nitride
Sr
2+
strontium P
3-
phosphide
Ba
2+
barium As
3-
arsenide
Al
3+
aluminum
Ga
3+
gallium
In
3+
indium
Ag
+
silver (memorize)
Zn
2+
zinc (memorize)
Monatomic
Cations
(multiple
oxidation
state)
Name
(Roman numeral
gives the positive
charge!)
Polyatomic
Ions
To
memorize
Name
Fe
3+
iron(III) NH
4
+
ammonium
Fe
2+
iron(II) NO
2
-
nitrite
Cu
2+
copper(II) NO
3
-
nitrate
Cu
+
copper(I) SO
3
2-
sulfite
Cr
3+
chromium(III) SO
4
2-
sulfate
Ni
2+
nickel(II) OH
-
hydroxide
Pb
4+
lead(IV) PO
4
3-
phosphate
Pb
2+
lead(II) CO
3
2-
carbonate
Hg
2+
mercury(II) ClO
3
-
chlorate
C
2
H
3
O
2
-
acetate
Fe
3+
Fe
2+
iron (III)
iron (II)
26
atomic
number
ion
charge
ion
name
symbol
(IUPAC)
KEY
58 59 60 61 62 63 64 65 66 67 68 69 70 71
90 91 92 93 94 95 96 97 98 99 100 101 102 103
Ce
3+
cerium
Pr
3+
praseodymium
Nd
3+
neodymium
Pm
3+
promethium
Sm
3+
samarium(III)
samarium(II)
Eu
3+
Eu
2+
europium(III)
europium(II)
Th
4+
thorium
Pa
5+
Pa
4+
protactinium(V)
protactinium(IV)
U
6+
U
4+
uranium(VI)
uranium(IV)
Gd
3+
gadolinium
Tb
3+
terbium
Dy
3+
dysprosium
Ho
3+
holmium
Er
3+
erbium
Tm
3+
thulium
Yb
3+
Yb
3+
ytterbium(III)
ytterbium(II)
Sm
2+
Lu
3+
lutetium
Np
5+
Pu
4+
Pu
6+
plutonium(IV)
Am
3+
Am
4+
americium(IV)
americium(III)
neptunium
Bk
3+
Bk
4+
berkelium(IV)
berkelium(III) Cm
3+
curium
Cf
3+
californium
Es
3+
einsteinium
Fm
3+
fermium
Md
2+
Md
3+
mendelevium(II)
mendelevium(III)
Lr
3+
lawrencium
No
2+
No
3+
nobelium(II)
nobelium(III) plutonium(VI)
PERIODIC TABLE OF IONS acetate
arsenate
arsenite
benzoate
borate
bromate
carbonate
chlorate
chloride
chlorite
chromate
cyanate
cyanide
dichromate
CH3COO
AsO4
3
AsO3
3
C6H5COO
BO3
3
BrO3
CO3
2
ClO3
Cl
ClO2
CrO4
2
CNO
CN
Cr2O7
2
oxalate
perchlorate
periodate
permanganate
peroxide
phosphate
pyrophosphate
sulfate
sulfite
thiocyanate
thiosulfate
ammonium
hydronium
C2O4
2
ClO4
IO4
MnO4
O2
2
PO4
3
P2O7
4
SO4
2
SO3
2
SCN
S2O3
2
NH4
+
H3O
+
POSITIVEPOLYATOMIC IONS
TABLE OF POLYATOMIC IONS
H2PO4
HCO3
HC2O4
HSO4
HS
HSO3
OH
ClO
IO3
HPO4
2
NO3
NO2
SiO4
4
hydrogen carbonate
hydrogen oxalate
hydrogen sulfate
hydrogen sulfide
hydrogen sulfite
hydroxide
hypochlorite
iodate
nitrate
nitrite
orthosilicate
monohydrogen phosphate
dihydrogen phosphate
1 2
3 4 5 6 7 8 9 10
13 14 15 16 17 18 11 12
19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36
Ti
4+
titanium(IV)
Ti
3+
titanium(III)
V
3+
vanadium(III)
V
5+
vanadium(V)
Cr
3+
Cr
2+
chromium(III)
chromium(II)
Mn
2+
Mn
4+
manganese(II)
manganese(IV)
Fe
3+
Fe
2+
iron (III)
iron (II)
Co
2+
Co
3+
cobalt (II)
cobalt (III)
Ni
2+
Ni
3+
nickel (II)
nickel (III)
Cu
2+
Cu
+
copper (II)
copper (I)
Ga
3+
gallium
Sc
3+
scandium
Y
3+
yttrium
La
3+
lanthanum
Ac
3+
actinium
Zr
4+
zirconium
Hf
4+
hafnium
Nb
5+
Nb
3+
niobium(V)
niobium(III)
37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54
Mo
6+
molybdenum
Rh
3+
rhodium
Ru
3+
Ru
4+
ruthenium(III)
ruthenium(IV)
Pd
2+
Pd
4+
paladium(II)
paladium(IV)
Ag
+
silver
Cd
2+
cadmium
Pt
4+
Pt
2+
platinum(IV)
platinum(II)
55 56 57 72 73 74 75 76 77 78 79 80 81 82 83 84 85 86
Au
3+
Au
+
gold (III)
gold (I)
Hg
2+
Hg
+
mercury (II)
mercury (I)
Tl
+
Tl
3+
thallium(I)
thallium(III)
Pb
2+
Pb
4+
lead (II)
lead (IV)
Bi
3+
Bi
5+
bismuth(III)
bismuth(V)
Sn
4+
Sn
2+
tin (IV)
tin (II)
Sb
3+
Sb
5+
antimony(III)
antimony(V)
Tc
7+
Ta
5+
tantalum
W
6+
tungsten
Re
7+
rhenium
Os
4+
osmium
Ir
4+
iridium
87 88 89
H
+
hydrogen
Li
+
lithium
Be
2+
beryllium
Na
+
sodium
Mg
2+
magnesium
K
+
potassium
Ca
2+
Rb
+
rubidium
Sr
2+
strontium
Cs
+
cesium
Ba
2+
calcium
barium
Fr
+
francium
Ra
2+
radium
B
boron
C
carbon nitride
N
3-
oxide
O
2-
fluoride
F
-
neon
Ne
Al
3+
aluminum
Si
silicon phosphide
P
3-
sulfiide
S
2-
chloride
Cl
-
argon
Ar
helium
He
Zn
2+
zinc
In
3+
indium
Ge
4+
germanium
As
3-
arsenide selenide
Se
2-
bromide
Br
-
krypton
Kr
telluride
Te
2-
Po
2+
polonium(II)
polonium(IV)
Po
4+
iodide
I
-
xenon
Xe
astatide
At
-
radon
Ra
hydride
H
-
1
1
2
3 4 5 6 7 8 9 10 11 12
13 14 15 16
17 18
technetium
S-ar putea să vă placă și
- 1.1 Atomic Structure Multiple ChoiceDocument13 pagini1.1 Atomic Structure Multiple ChoiceAmmaarah PatelÎncă nu există evaluări
- Material WeldingDocument1 paginăMaterial WeldingDebashish ChatterjeeÎncă nu există evaluări
- Ionic Bonding Part 1 EdexcelDocument4 paginiIonic Bonding Part 1 EdexcelKevin The Chemistry Tutor100% (1)
- IGCSE Chemistry Chemical BondsDocument7 paginiIGCSE Chemistry Chemical BondsdanielmahsaÎncă nu există evaluări
- Nandan Dec 2011Document59 paginiNandan Dec 2011Vishvajeet Singh50% (2)
- Elements, Compounds and MixturesDocument4 paginiElements, Compounds and MixturesFatema KhatunÎncă nu există evaluări
- NEET UG Chemistry Chemical Kinetics PDFDocument23 paginiNEET UG Chemistry Chemical Kinetics PDFPritam MandalÎncă nu există evaluări
- Periodicity Chemistry Worksheet: A. Periodic TableDocument9 paginiPeriodicity Chemistry Worksheet: A. Periodic TableRhea FrancisÎncă nu există evaluări
- Chemical Bonding IPEDocument37 paginiChemical Bonding IPEAdiChemAdi100% (1)
- Basic Inorganic Nomenclature FOR IIT-JEE ENTRANCE TEST by S.K.sinha See Chemistry Animations atDocument5 paginiBasic Inorganic Nomenclature FOR IIT-JEE ENTRANCE TEST by S.K.sinha See Chemistry Animations atmyiitchemistry88% (17)
- 10th Periodic Classification MCQDocument4 pagini10th Periodic Classification MCQRanjit SinghÎncă nu există evaluări
- Ionic Equations WorksheetDocument1 paginăIonic Equations Worksheetgimarreyes23Încă nu există evaluări
- AP Chem CH 7 Practice QuizDocument8 paginiAP Chem CH 7 Practice QuizOmprakash LatiyalÎncă nu există evaluări
- Precipitation ReactionsDocument3 paginiPrecipitation ReactionsborgiamatriceÎncă nu există evaluări
- Boyles LawDocument4 paginiBoyles LawCarespe Mae SartagudaÎncă nu există evaluări
- Acid and Base Worksheet 1-07-08 Ans KeyDocument4 paginiAcid and Base Worksheet 1-07-08 Ans KeyShazia FarheenÎncă nu există evaluări
- Ionic Compound Formula WritingDocument2 paginiIonic Compound Formula WritingJia Ru0% (1)
- 11U CHEMISTRY EXAM REVIEW QUESTIONS June 2010Document9 pagini11U CHEMISTRY EXAM REVIEW QUESTIONS June 2010Maden betoÎncă nu există evaluări
- The Periodic Table of ElementsDocument41 paginiThe Periodic Table of ElementsPawan GoswamiÎncă nu există evaluări
- SL Score ! /30: Practice Exam: Paper 1 Topic 4: BondingDocument7 paginiSL Score ! /30: Practice Exam: Paper 1 Topic 4: Bondingraja_tanukuÎncă nu există evaluări
- Chemical Bonding WS Packet Margie Core 2013Document4 paginiChemical Bonding WS Packet Margie Core 2013Lama DebanaÎncă nu există evaluări
- Alkanes, Alkenes and AlcoholsDocument83 paginiAlkanes, Alkenes and AlcoholsG M Ali KawsarÎncă nu există evaluări
- Notes On Mole ConceptDocument10 paginiNotes On Mole Conceptsufian83% (12)
- Common Ions Charges ChartDocument2 paginiCommon Ions Charges Chartapi-233736029100% (1)
- Class 9 Structure of Atom-MCQDocument1 paginăClass 9 Structure of Atom-MCQbrcrao100% (2)
- Solved Multiple Choice Questions Chemical EquilibriumDocument12 paginiSolved Multiple Choice Questions Chemical EquilibriumApeksha Garg100% (2)
- Periodic Table WorksheetDocument4 paginiPeriodic Table Worksheettony zouÎncă nu există evaluări
- 110 Oxidation NumberDocument3 pagini110 Oxidation NumberTerry100% (1)
- Sec2 Chemistry NotesDocument5 paginiSec2 Chemistry NotesChai Yi チアイÎncă nu există evaluări
- Chemistry Solutes Solvents Solubility Solutions VCBCCTDocument17 paginiChemistry Solutes Solvents Solubility Solutions VCBCCTDIONYSUS100% (1)
- PeriodicityDocument6 paginiPeriodicityHadi AlnaherÎncă nu există evaluări
- Practice Problems On Net Ionic EquationsDocument3 paginiPractice Problems On Net Ionic EquationsZainabÎncă nu există evaluări
- ks3 Metals and Non MetalsDocument3 paginiks3 Metals and Non MetalsTasdidaa Shamsi100% (1)
- Chemistry Metals WorksheetDocument5 paginiChemistry Metals WorksheetRosina KaneÎncă nu există evaluări
- Physical and Chemical Changes NotesDocument2 paginiPhysical and Chemical Changes NotesMidhun Bhuvanesh.B 7AÎncă nu există evaluări
- Latin Names and SymbolsDocument1 paginăLatin Names and SymbolsDiana230598100% (3)
- Electrolysis PDFDocument14 paginiElectrolysis PDFBaryaÎncă nu există evaluări
- 2.11 Counting Atoms Practice WorksheetDocument2 pagini2.11 Counting Atoms Practice WorksheetzahraaÎncă nu există evaluări
- Metals Worksheet 1Document2 paginiMetals Worksheet 1Sharizah Bte Md Amin100% (1)
- Charles Law PDFDocument3 paginiCharles Law PDFIvan BayonaÎncă nu există evaluări
- CH 3 ReviewDocument4 paginiCH 3 ReviewAref DahabrahÎncă nu există evaluări
- Six Types of Chemical Reaction WorksheetDocument0 paginiSix Types of Chemical Reaction WorksheetMax SaubermanÎncă nu există evaluări
- C) ConductionDocument17 paginiC) ConductionDaniel Happy100% (1)
- Experimental Techniques (TOPIC 2)Document17 paginiExperimental Techniques (TOPIC 2)ChaudhryAbdullahÎncă nu există evaluări
- CH 9 WorksheetsDocument5 paginiCH 9 Worksheetsadaglio001100% (1)
- Metals and Non Metals WorksheetDocument2 paginiMetals and Non Metals WorksheetKamaljeet Singh100% (1)
- Science Notes For Class 10 Chapter 5 Periodic Classification of ElementsDocument4 paginiScience Notes For Class 10 Chapter 5 Periodic Classification of Elementscrazy about readingÎncă nu există evaluări
- Stoichiometry Worksheet 2Document1 paginăStoichiometry Worksheet 2body99Încă nu există evaluări
- Class XI Redox Reactions NotesDocument5 paginiClass XI Redox Reactions NoteseasaÎncă nu există evaluări
- Question Bank of Chapter 1Document4 paginiQuestion Bank of Chapter 1lovika malhotraÎncă nu există evaluări
- Chemistry Valencies and Atomic Nos.Document1 paginăChemistry Valencies and Atomic Nos.kskkingÎncă nu există evaluări
- Elements and Compounds For MYP 3Document18 paginiElements and Compounds For MYP 3Maira ButtÎncă nu există evaluări
- The Particulate Nature of Matter 1 MSDocument7 paginiThe Particulate Nature of Matter 1 MSKHANÎncă nu există evaluări
- Worksheet #3 - Mole ConceptDocument6 paginiWorksheet #3 - Mole Conceptjfkdmfmdf100% (1)
- Oxidation States of Transition MetalsDocument7 paginiOxidation States of Transition MetalsMannevaram AbhinavareddiÎncă nu există evaluări
- Thermo Kin Ws CompleteDocument20 paginiThermo Kin Ws CompleteMohommad YawariÎncă nu există evaluări
- D and F BlockDocument12 paginiD and F BlockJinal VadodariyaÎncă nu există evaluări
- FORM 4 ENERGY CHANGES IN CHEMICAL AND PHYSICAL PROCESSES ANS Teacher - Co - .KeDocument8 paginiFORM 4 ENERGY CHANGES IN CHEMICAL AND PHYSICAL PROCESSES ANS Teacher - Co - .KeCitron AkhalaÎncă nu există evaluări
- CBSE Class 10 Chemistry Worksheet - Chemical Reactions and EquationsDocument4 paginiCBSE Class 10 Chemistry Worksheet - Chemical Reactions and EquationsAsh snowÎncă nu există evaluări
- Questions Periodic TableDocument25 paginiQuestions Periodic TablejuandesabiÎncă nu există evaluări
- Empirical Formulae A: QuestionsDocument5 paginiEmpirical Formulae A: QuestionsAlisha TuliÎncă nu există evaluări
- Chemical Arithmetic and Reactions: ObjectivesDocument24 paginiChemical Arithmetic and Reactions: Objectivesgoputs6386Încă nu există evaluări
- Le Chatelier WorksheetDocument1 paginăLe Chatelier WorksheetRawanఌÎncă nu există evaluări
- IonsDocument1 paginăIonsghazal khanÎncă nu există evaluări
- Ccs Ela Reading Assessment 7th GradeDocument6 paginiCcs Ela Reading Assessment 7th Gradeapi-233736029Încă nu există evaluări
- Daniel F Life Is Like EssayDocument3 paginiDaniel F Life Is Like Essayapi-233736029Încă nu există evaluări
- Bianca R Life Essay Thesis PicDocument2 paginiBianca R Life Essay Thesis Picapi-233736029Încă nu există evaluări
- Aidan H Life Is Like EssayDocument2 paginiAidan H Life Is Like Essayapi-233736029Încă nu există evaluări
- Kelan M Life Essay Thesis PicDocument2 paginiKelan M Life Essay Thesis Picapi-233736029Încă nu există evaluări
- Jessica G Life Is Like EssayDocument2 paginiJessica G Life Is Like Essayapi-233736029Încă nu există evaluări
- 20 Project Presentation GuidelinesDocument4 pagini20 Project Presentation Guidelinesapi-233736029Încă nu există evaluări
- Robotics Unit Sneha TharayilDocument8 paginiRobotics Unit Sneha Tharayilapi-233736029Încă nu există evaluări
- Ccs Ela Reading Assessment 8th Grade-StudentDocument9 paginiCcs Ela Reading Assessment 8th Grade-Studentapi-233736029Încă nu există evaluări
- Bio-Inspired Robot Cob-EDocument8 paginiBio-Inspired Robot Cob-Eapi-233736029Încă nu există evaluări
- Bio-Inspired Robot-CribotDocument6 paginiBio-Inspired Robot-Cribotapi-233736029Încă nu există evaluări
- Youtube Parent Consent FormDocument3 paginiYoutube Parent Consent Formapi-233736029100% (1)
- Classbook Wall Assignment DescriptionDocument2 paginiClassbook Wall Assignment Descriptionapi-233736029Încă nu există evaluări
- Robot Prototype Design Project InstructionsDocument7 paginiRobot Prototype Design Project Instructionsapi-233736029Încă nu există evaluări
- Coastal Landforms Table Graphic OrganizerDocument2 paginiCoastal Landforms Table Graphic Organizerapi-233736029Încă nu există evaluări
- Group Project Partner EvaluationsDocument1 paginăGroup Project Partner Evaluationsapi-233736029Încă nu există evaluări
- Data Analysis WorksheetDocument1 paginăData Analysis Worksheetapi-233736029Încă nu există evaluări
- 6th Grade Final Exam Study GuideDocument8 pagini6th Grade Final Exam Study Guideapi-233736029Încă nu există evaluări
- 7th Science Final Exam Study GuideDocument8 pagini7th Science Final Exam Study Guideapi-233736029Încă nu există evaluări
- Evidences of Evolution Graphic OrganizerDocument1 paginăEvidences of Evolution Graphic Organizerapi-233736029Încă nu există evaluări
- Coastal Landforms Table Graphic OrganizerDocument2 paginiCoastal Landforms Table Graphic Organizerapi-233736029Încă nu există evaluări
- Beach Sediment InvestigationDocument1 paginăBeach Sediment Investigationapi-233736029Încă nu există evaluări
- Shaping The Land Test Study GuideDocument7 paginiShaping The Land Test Study Guideapi-233736029Încă nu există evaluări
- Sle Presentation 6-8 Presentation Rubric CcssDocument2 paginiSle Presentation 6-8 Presentation Rubric Ccssapi-233736029Încă nu există evaluări
- Notes - Ionic BondsDocument15 paginiNotes - Ionic Bondsapi-233736029Încă nu există evaluări
- Covalent BondsDocument13 paginiCovalent Bondsapi-233736029Încă nu există evaluări
- Notes - Chemical EquationsDocument7 paginiNotes - Chemical Equationsapi-233736029Încă nu există evaluări
- Catholic Social Teaching and The Un Declaration of Human RightsDocument2 paginiCatholic Social Teaching and The Un Declaration of Human Rightsapi-233736029Încă nu există evaluări
- Chapter 21 Sec 2-The EyeDocument23 paginiChapter 21 Sec 2-The Eyeapi-233736029Încă nu există evaluări
- Mnemonics For JeeDocument2 paginiMnemonics For JeeAdarsh VargheseÎncă nu există evaluări
- Steel ChemistryDocument22 paginiSteel ChemistryAmit Kumar UkeÎncă nu există evaluări
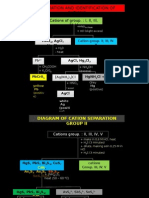
- Separation of CationsDocument9 paginiSeparation of CationsQodri Maulana SipahutarÎncă nu există evaluări
- Is.4843.1968 0 PDFDocument13 paginiIs.4843.1968 0 PDFPravin PatilÎncă nu există evaluări
- Legure Bakra - BronzeDocument25 paginiLegure Bakra - BronzespahicdaniloÎncă nu există evaluări
- SOP Hemodialisa - Parameter Ro Dan AirbersihDocument7 paginiSOP Hemodialisa - Parameter Ro Dan Airbersihjokoblitar100% (3)
- 12 Book Back One Mark With AnswerDocument30 pagini12 Book Back One Mark With AnswerVishnu DasÎncă nu există evaluări
- Equivalent Cross Valve CastDocument1 paginăEquivalent Cross Valve Castg_casalinuovo19812344Încă nu există evaluări
- Comparison List Copper Bronze 2011 1Document7 paginiComparison List Copper Bronze 2011 1NaldoVicenteÎncă nu există evaluări
- Equivalent Cross Valve CastDocument2 paginiEquivalent Cross Valve CastArun JaffersonÎncă nu există evaluări
- Valencias de Los ElementosDocument4 paginiValencias de Los ElementosCarlos Mario Calderón RodríguezÎncă nu există evaluări
- Account Information Sample Information Equipment InformationDocument2 paginiAccount Information Sample Information Equipment Informationdhavit wijayantoÎncă nu există evaluări
- Manual Kapasitas Jalan Indonesia (MKJI)Document11 paginiManual Kapasitas Jalan Indonesia (MKJI)ichwanicovÎncă nu există evaluări
- Material Composition of Different MetalsDocument8 paginiMaterial Composition of Different MetalsAjesh KumarÎncă nu există evaluări
- Element Word SearchDocument2 paginiElement Word Searchpepac4140% (1)
- Bs As - Resol 336-2003 Anexo II Limites AdmisiblesDocument2 paginiBs As - Resol 336-2003 Anexo II Limites AdmisiblesmoncryÎncă nu există evaluări
- Jurnal IonDocument6 paginiJurnal IonAnca OktariaÎncă nu există evaluări
- Sac, Çubuk & Kütükler Shapes, Plates &bars Sac, Çubuk & Kütükler Astm/Asme DIN StasDocument4 paginiSac, Çubuk & Kütükler Shapes, Plates &bars Sac, Çubuk & Kütükler Astm/Asme DIN StaseragornÎncă nu există evaluări
- L80 - Continental AlloysDocument1 paginăL80 - Continental AlloysHans CohnÎncă nu există evaluări
- 연대경제대학원 석사학위논문 학술정보원등록 최종본Document121 pagini연대경제대학원 석사학위논문 학술정보원등록 최종본0514bachÎncă nu există evaluări
- Spesialit Obat Vitamin & Mineral-1Document36 paginiSpesialit Obat Vitamin & Mineral-1yuni panda100% (1)
- (Pet Brojeva I Do Sedam Velikih Slova) : Naputak Županijskom PovjerenstvuDocument9 pagini(Pet Brojeva I Do Sedam Velikih Slova) : Naputak Županijskom PovjerenstvuFranbartÎncă nu există evaluări
- Micronutrient Deficiencies in Blueberries and Their Correction Dr. David Kissel University of Georgia Athens GADocument27 paginiMicronutrient Deficiencies in Blueberries and Their Correction Dr. David Kissel University of Georgia Athens GADavid GluhićÎncă nu există evaluări
- Sengle Ckue: Carbon Nano TobescntDocument4 paginiSengle Ckue: Carbon Nano TobescntSathish100% (1)
- TonisitasDocument6 paginiTonisitasPutri PajarianaÎncă nu există evaluări