Documente Academic
Documente Profesional
Documente Cultură
ST Gregorys As Level Physics Handbook Completed 2015
Încărcat de
api-239434818Titlu original
Drepturi de autor
Formate disponibile
Partajați acest document
Partajați sau inserați document
Vi se pare util acest document?
Este necorespunzător acest conținut?
Raportați acest documentDrepturi de autor:
Formate disponibile
ST Gregorys As Level Physics Handbook Completed 2015
Încărcat de
api-239434818Drepturi de autor:
Formate disponibile
A Level Physics
Student Handbook
A level Physics Handbook 2012 St Gregorys Catholic College, Bath
- 1 -
1 ST GREGORYS CATHOLIC COLLEGE PHYSICS DEPARTMENT ................................................................................................... 2
1.1 WHAT IS PHYSICS? ................................................................................................................................................................. 2
1.2 WHY STUDY PHYSICS?............................................................................................................................................................. 2
2 PHYSICS AT ST GREGORYS CATHOLIC COLLEGE ..................................................................................................................... 3
2.1 AIMS .................................................................................................................................................................................. 3
2.2 EXPECTATIONS ...................................................................................................................................................................... 3
2.2.1 Attendance .............................................................................................................................................................. 3
2.2.2 Completing homework ............................................................................................................................................. 4
2.2.3 Keeping records of your work ................................................................................................................................... 4
2.2.4 Reviewing work ........................................................................................................................................................ 5
2.2.5 Contacting your teachers ......................................................................................................................................... 5
2.3 ASSESSMENT ........................................................................................................................................................................ 6
2.3.1 Homework ............................................................................................................................................................... 6
2.3.2 Tests ........................................................................................................................................................................ 6
2.3.3 Monitoring and increasing your progress .................................................................................................................. 6
2.3.4 External assessment ................................................................................................................................................. 6
3 RESOURCES ........................................................................................................................................................................... 7
3.1 TEXTBOOKS .......................................................................................................................................................................... 7
3.2 REVISION GUIDES .................................................................................................................................................................. 7
3.3 PHYSICS REVIEW .................................................................................................................................................................... 7
3.4 WEBSITES ............................................................................................................................................................................ 8
3.5 INSTITUTE OF PHYSICS MEMBERSHIP ........................................................................................................................................... 8
3.6 PHYSICS VLE ........................................................................................................................................................................ 8
3.7 BOOKS ................................................................................................................................................................................ 9
4 THE COURSE ........................................................................................................................................................................ 11
4.1 THE AS COURSE AT A GLANCE ................................................................................................................................................. 11
4.2 THE A2 COURSE AT A GLANCE ................................................................................................................................................. 12
4.3 THE AS PHYSICS SPECIFICATION ............................................................................................................................................... 13
4.4 THE A2 PHYSICS SPECIFICATION ............................................................................................................................................... 21
4.5 GENERAL SKILLS .................................................................................................................................................................. 30
4.5.1 Physics terms, definitions and units ........................................................................................................................ 31
5 PRACTICAL ASSESSMENT ..................................................................................................................................................... 46
5.1 G483 (AS) & G486 (A2) ..................................................................................................................................................... 46
5.2 DEFINITIONS ....................................................................................................................................................................... 47
5.3 MEASURING INSTRUMENTS .................................................................................................................................................... 49
5.4 EXPERIMENTAL TECHNIQUES & ADVICE FOR SPECIFIC APPARATUS ..................................................................................................... 50
5.5 CALCULATING UNCERTAINTIES ................................................................................................................................................. 52
5.5.1 Estimation of uncertainty using the spread of repeat readings. ............................................................................... 52
5.5.2 Estimation of uncertainty from a single reading ...................................................................................................... 52
5.5.3 Determining the uncertainties in derived quantities. ............................................................................................... 53
5.6 SIGNIFICANT FIGURES ............................................................................................................................................................ 55
5.6.1 Raw data ............................................................................................................................................................... 55
5.6.2 Processing raw data ............................................................................................................................................... 55
5.7 GRAPHS ............................................................................................................................................................................ 58
5.8 ADVICE TO AS CANDIDATES IN PRACTICAL PHYSICS ........................................................................................................................ 59
6 SKILLS .................................................................................................................................................................................. 61
6.1 SETTING OUT YOUR CALCULATIONS ........................................................................................................................................... 61
6.2 REARRANGING EQUATIONS ..................................................................................................................................................... 62
6.3 POWERS OF TEN AND STANDARD FORM ..................................................................................................................................... 64
7 SI UNITS .............................................................................................................................................................................. 67
8 SYMBOLS ............................................................................................................................................................................ 68
9 DATA BOOKLET ................................................................................................................................................................... 70
A level Physics Handbook 2012 St Gregorys Catholic College, Bath
- 2 -
1 St Gregorys Catholic College Physics Department
We are delighted that you have chosen to study A Level physics and we hope that this course
will give you a greater understanding of how and why Physics is so important in the modern
world.
Please take time to read through this handbook which covers our expectations and
assessment information.
1.1 What is Physics?
Physics is the study of matter and its motion through time and space, including the related
concepts of force and energy. Physics concerns everything from the smallest building blocks
of matter created in the big bang to the ultimate fate of the whole universe.
Physics is not set in stone and fixed for all time. Like other sciences it is constantly evolving as
new theories, discoveries and ways of thinking gradually take the place of previous
knowledge and understanding. You can be part of this new age of discovery.
Isaac Newton describes the fun and excitement of studying Physics:
I do not know what I may appear to the world, but to myself I seem to have been only like a
boy playing on the sea-shore, and diverting myself in now and then finding a smoother pebble
or a prettier shell than ordinary, whilst the great ocean of truth lay all undiscovered before
me.
You will study things that you never knew before, and learn about relatively new discoveries
that have changed our ways of thinking.
1.2 Why study Physics?
Physics is not only interesting, it is also highly marketable. Of all the subjects listed for entry
on to a degree, physics came second only to maths in the number of times it was listed as
essential in a recent report by twenty of the leading UK universities. Not only is physics a
preferred subject for university, it is also the first step towards careers in not just engineering
and science, but also finance, law, architecture and journalism.
With an A level in Physics you have proved that you possess a wide range of key skills, exactly
what employers and universities are looking for. A level Physics covers a wide range of
transferable skills from the use of IT in data-logging experiments; to the numerical skills
that are the bedrock of the subject, essential in problem-solving and in practical work; to skill
in written expression needed to write explanations.
A level Physics Handbook 2012 St Gregorys Catholic College, Bath
- 3 -
2 Physics at St Gregorys Catholic College
2.1 Aims
The course has been designed to:
- provide seamless progression from the Key Stage 4 programme of study and enable
students to sustain and develop an enjoyment of, and interest in, physics and its
applications
- develop an understanding of the link between theory and experiment and foster the
development of skills in the design and execution of experiments
- develop essential knowledge and understanding in physics and, where appropriate, the
applications of physics with an appreciation of their significance and the skills needed for
the use of these in new and changing situations
- demonstrate the importance of physics as a human endeavour that interacts with social,
philosophical, economic and industrial matters
- be a suitable preparation for higher educational courses in physics and related courses.
You will have two physics teachers who each teach through the series of OCR A modules,
examined in the Summer term.
Our approach begins with the consideration of an application that draws on many different
areas of physics, and then moves on to the laws, theories and models of physics underlying
this application.
2.2 Expectations
Studying Physics at A level requires commitment. You will have at least 9 hours of Physics per
fortnight and most of you will need to put in at least as much time again outside of lessons if
you are to achieve your best.
2.2.1 Attendance
It is important that you attend all lessons. If you miss any lessons it is your responsibility to
catch up the class work and the homework by the next lesson. At times people may (for
various reasons) be timetabled into additional twilight lessons on Wednesdays. These are
part of your timetable and are not negotiable.
A level Physics Handbook 2012 St Gregorys Catholic College, Bath
- 4 -
2.2.2 Completing homework
Your teachers set your homework for you to practice using what you have been learning and
so that you and they can assess your progress. This enables your teachers to give you the
best support going through the course.
It is a basic expectation that homework will be handed in on time. Teachers have a constant
cycle of setting and marking work and cannot be expected to mark work just when you feel
like doing it.
If you experience difficulties in completing homework it is essential that you contact your
teacher before the deadline.
2.2.3 Keeping records of your work
We ask you to be organised, and keep everything in a place where you can easily find your
work and notes easily. Since we live in the 21st century, not everything is now done on
paper. There will be times when you are required to do work on paper (still very common!),
and times when an electronic activity is the only option/requirement.
So, in most cases, you will need a hybrid system of organising your work..
Paper-based storage system
This is a traditional way of storing notes/information. Very heavily dependent on your
organisational skills, and still required in many aspects of your studies at A-level, such as
practical work, and individual note-taking
Have two files for the subject:
You should have a ring binder to store all your current notes. This is convenient since the
specification breaks down into well-defined sections. After the completion of each section,
transfer the notes into the second ring binder. Label the dividers with the different sections
of the physics specification.
Electronic Storage system
You can use a laptop/desktop to store the notes/work you produce in class and resources
that you want to take from the VLE.
1. Make a folder such as Physics AS,
2. Make subfolders for each Unit you study (Unit 1, Unit2, etc.)
3. Make any further subfolders in the Unit folder for different sections
(Good enough to eat, Probing the heart of matter, etc.)
Using this method you do not have to do any transfer any notes, since they will be
automatically in the right folder.
Essential: Backup your work on a weekly basis. This can be done by using a USB stick, or
external hard drive. In this way, you will always have a backup set of notes if your laptop
develops a problem, or goes missing.
A level Physics Handbook 2012 St Gregorys Catholic College, Bath
- 5 -
2.2.4 Reviewing work
From the very first lesson, you will need to make sure that you understand the work that you
have done. Passive reading is not a constructive way of consolidating knowledge and
understanding.
Highlight the main key points
If you have any questions, annotate your notes and ask a teacher.
Practice calculations
Remember that the questions set by your teacher are a minimum if you need more
practice, do it!
Ask for assistance
Your teachers can and will assist you with any areas of work that you need assistance with,
but you need to let them know! Contact them by email or speak to them in person, either at
the end of a lesson, or via the Physics room in G2.
Definitions and equations
We suggest that you build up a list of definitions (could be done as a spreadsheet so that you
can sort by module or alphabetically) and an equations page that you can use for reference
throughout the course and while revising.
2.2.5 Contacting your teachers
Should you be experiencing any difficulties with anything physics related, please speak to us.
If you cannot see us in school, your teachers can be contacted by email and at normal times
you should get a response within a couple of days.
MR BURN (Room G2)
burns@st-gregorys.bathnes.sch.uk
MR COOPER (Room S5)
cooperm@st-gregorys.bathnes.sch.uk
A level Physics Handbook 2012 St Gregorys Catholic College, Bath
- 6 -
2.3 Assessment
2.3.1 Homework
You will be set regular homework by your teachers. Some of this you will be expected to
mark yourself and some they will collect in for marking.
2.3.2 Tests
To enable you and us to assess your suitability for the course, you will be given a test in class
in the middle of October in year 12 and 13. If you are working hard and making good
progress, this should be no problem.
In year 12 you will have mock exams just to make sure that you are on track.
2.3.3 Monitoring and increasing your progress
Above all, we want you to enjoy and succeed at Physics. Even if at times you do not seem to
be working towards this goal, your teachers will to everything that they can to get you there.
You will be told your minimum target grade early on in the year. It is likely that this will be
considerably lower than you are aiming for because the calculation takes a lot of data into
account, rather than focussing on your physics ability. Your teachers will discuss this with you
and agree a challenge target grade which you will work towards.
If at any stage your teachers think that you could be doing better, they will put things into
place to support you in this. In the first instance this might be a suggestion that you come
and see them outside lessons to go through something that you are struggling with, or to
come to the twilight lesson for a period until you are up to speed. If your teachers have more
serious concerns they may start you on the sixth form staging procedure.
2.3.4 External assessment
You will sit two external exams each summer (there are no January exams in Physics); one
for each module. Your coursework (see chapter 5) will also be submitted in the summer term.
A level Physics Handbook 2012 St Gregorys Catholic College, Bath
- 7 -
3 Resources
3.1 Textbooks
You will have to purchase the course physics textbook which covers the whole of the AS and
A2 level OCR physics course.
Physics 1 for OCR with CD-ROM
By David Sang & Gurinder Chadha
Published by Cambridge OCR Advanced Sciences
ISBN-13: 978-0521724555
Physics 2 for OCR with CD-ROM
By David Sang & Gurinder Chadha
Published by Cambridge OCR Advanced Sciences
ISBN-13: 978-0521738309
It is essential that you bring your book with you every lesson as your teachers will require you
to use them during class time.
3.2 Revision Guides
A revision guide is an invaluable companion to the course. We strongly recommend the OCR
specific revision guide which you can buy yourself of through the school.
3.3 Physics review
To aid you the A level physics course, Philip Allen Publishers offer a magazine called Physics
Review which provides specially written articles for A level students and grade boosting
advice from examiners.
Each issue features:
- Specially written articles by leading scientists on how physics works, helping students
apply theory to the real world
- Grade-boosting advice from examiners who know that they need to do to get those top
grades
- Additional online support each issue features tailored resources to revise, research and
improve understanding of key topics
To find out more go to www.philipallan.co.uk/physicsreview
A level Physics Handbook 2012 St Gregorys Catholic College, Bath
- 8 -
3.4 Websites
There are some fantastic resources on the internet that can help you throughout the A level course:
- S-Cool
Great revision website. Interactive activities.
http://www.s-cool.co.uk/a-level/physics
- SchoolPhysics
Excellent animations and revision resources
http://www.schoolphysics.co.uk
- Java Applets (Animations)
Brilliant interactive resources for a wide range of
topics
http://www.walter-fendt.de/ph14e/
- Spark Science
Excellent website for the OCR A Physics course.
http://www.science-spark.co.uk/
- Phet Interactive Simulations
Fun, interactive, research-based simulations of
physical phenomena from the PhET project at
the University of Colorado.
https://phet.colorado.edu/en/simulations/catego
ry/physics
- Hyperphysics
Lots of straightforward, linked up notes that go
beyond the course
http://hyperphysics.phy-
astr.gsu.edu/hbase/hframe.html
- Institute of Physics
Many resources and links for supporting classwork,
revision, careers and general interest.
http://www.physics.org
- Teaching Advanced Physics
This website contains detailed ideas and
resources for teaching and learning A Level
physics.
http://tap.iop.org/
- OCR A Level Physics
Download all important documents from the
official OCR website including course specifications
and summaries.
http://www.ocr.org.uk/qualifications/as-a-level-
gce-physics-a-h158-h558/
3.5 Institute of Physics membership
You can join the Institute of Physics for free as a student. You will receive:
- Regular updates on whats new in physics
- Exam and university guidance
- Information about careers from physics
- The chance to interact with other young physicists
By joining the IOP not only will you become part of the UKs largest physics community, but
you will also get full access to Physics World online and physicsworld.com. You will also
receive regular updates on upcoming science TV programmes, events, competitions and lots
of other exclusive 16-19 member offers.
Join here: http://members.iop.org/16-19.asp
3.6 Physics VLE
The Physics VLE website contains a number of resources. These can be accessed in school and at home (via
the school science website). Your teachers will sometimes ask you to download work from here so it is
important that you know the password.
A level Physics Handbook 2012 St Gregorys Catholic College, Bath
- 9 -
3.7 Books
If you want to read a book about physics but not specifically the A level course, you may find
these of interest:
Very accessible, a great hook book if youre not yet totally convinced by Physics:
Quantum: A guide for the perplexed Al-Khalili
Highly recommend that for any student keen on doing a Physics degree:
The Quantum Story: A history in 40 moments Baggott
Prof. Brian Coxs favourite book:
Cosmos Sagan
With Feynmans writing style any of his books is an enjoyable read:
Six Easy Pieces: Fundamentals of Physics Explained Feynman
QED - The Strange Theory of Light and Matter Feynman
Six Easy Pieces - The Fundamentals of Physics Explained Feynman
Six Not-So-Easy Pieces - Einstein's Relativity, Symmetry and Space-Time Feynman
'Surely You're Joking, Mr Feynman!' - Adventures of a Curious Character as told
to Ralph Leighton
Feynman
The Meaning of It All Feynman
What Do You Care What Other People Think Feynman
The Character of Physical Law Feynman
Space and cosmology
The Edge of Physics: Dispatches from the Frontiers of Cosmology Ananthaswamy
Cosmology: A Very Short Introduction (Very Short Introductions) Coles
The Eerie Silence: Are We Alone in the Universe? Davies
The Fabric of the Cosmos: Space, Time and the Texture of Reality Greene
Birth of Time - How We Measured the Age of the Universe Gribbin
Stardust Gribbin
Big Bang: The Most Important Scientific Discovery of All Time and Why You Need
to Know About It
Singh
Bang! The Complete History of the Universe May, Moore and Lintott
A Brief History of the Universe McEvoy
Particle physics
Particle Physics: A Very Short Introduction Close
Cosmic Onion Close
Antimatter Close
Time
Time, Space and Things Ridley
Introducing Time Callender
A Brief History of Time Hawking
Black Holes Wormholes and Time Machines Al Khalili
Quantum physics
Quantum Theory Cannot Hurt You: A Guide to the Universe Chown
Decoding Reality: The Universe as Quantum Information Vedra
In Search of Schrodinger's Cat - Quantum Physics and Reality Gribbin
Quantum Theory: A Very Short Introduction Polkinghorne
Quantum Physics: A Beginner's Guide Ras
Introducing Quantum Theory McEvoy
A level Physics Handbook 2012 St Gregorys Catholic College, Bath
- 10 -
String theory
Superstrings A Theory of Everything Davies
The Elegant Universe: Superstrings, Hidden Dimensions and the Quest for the
Ultimate Theory
Greene
The Little Book of String Theory Gubser
Science and religion
The Mind of God: Science and the Search for Ultimate Meaning Davies
God Particle - If the Universe is the Answer, What is the Question? Lederman
Certainty
Science and Certainty O'Kirk
In Search of Schrodinger's Cat Gribbin
The Drunkard's Walk: How Randomness Rules Our Lives Mlodinow
Einstein
Relativity: A Very Short Introduction Stannard
Einstein for Beginners Schwarts and McGuinness
Why Does E=mc2? Cox
E = mc2 - A Biography of the World's Most Famous Equation Bodanis
Einsteins Universe the Laypersons Guide Calder
Applications of physics
The Making of the Atomic Bomb Rhodes
The Quantum Frontier: The Large Hadron Collider Lincoln
General interest
Impossibility - The Limits of Science and the Science of Limits Barrow
The Goldilocks Enigma: Why is the Universe Just Right for Life? Davies
Guide to Science Edey
Chaos Gleick
The New Science of Strong Materials Gordon
Five Equations that Changed the World Guillen
Middle World - The Restless Heart of Matter and Life Hawking
Physics of the Impossible Kaku
Physics of Star Trek Krauss
Fear of Physics - A Guide for the Perplexed Krauss
Pioneers In Science Physics Lafferty
Physics for Future Presidents Muller
The Road to Reality: A Complete Guide to the Laws of the Universe Penrose
A level Physics Handbook 2012 St Gregorys Catholic College, Bath
- 11 -
4 The course
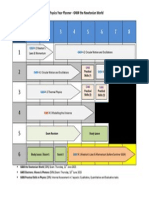
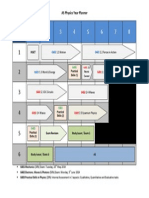
4.1 The AS course at a glance
A level Physics Handbook 2012 St Gregorys Catholic College, Bath
- 12 -
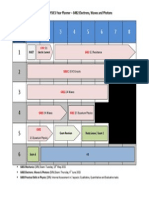
4.2 The A2 course at a glance
A level Physics Handbook 2012 St Gregorys Catholic College, Bath
- 13 -
4.3 The AS Physics specification
AS Unit G481: Mechanics
This unit consists of three teaching modules:
Module 1: Motion
1.1.1 Physical quantities and units
1.1.2 Scalars and vectors
1.1.3 Kinematics
1.1.4 Linear motion
Module 2: Forces in action
1.2.1 Force
1.2.2 Nonlinear motion
1.2.3 Equilibrium
1.2.4 Car safety
Module 3: Work and energy
1.3.1 Work and conservation of energy
1.3.2 Kinetic and potential energies
1.3.3 Power
1.3.4 Behaviour of springs and materials
AS Unit G482: Electrons, Waves and Photons
This unit consists of five teaching modules:
Module 1: Electric current
2.1.1 Electric current
Module 2: Resistance
2.2.1 Circuit symbols
2.2.2 E.m.f. and p.d.
2.2.3 Resistance
2.2.4 Resistivity
2.2.5 Power
Module 3: DC circuits
2.3.1 Series and parallel circuits
2.3.2 Practical circuits
Module 4: Waves
2.4.1 Wave motion
2.4.2 Electromagnetic waves
2.4.3 Interference
2.4.4 Stationary waves
Module 5: Quantum physics
2.5.1 Energy of a photon
2.5.2 The photoelectric effect
2.5.3 Wave-particle duality
2.5.4 Energy levels in atoms
A level Physics Handbook 2012 St Gregorys Catholic College, Bath
- 14 -
AS Unit G481: Mechanics
1.1.1 Physical quantities and unit
(a) explain that some physical quantities consist of a numerical magnitude and a unit;
(b) use correctly the named units listed in this specification as appropriate;
(c) use correctly the following prefixes and their symbols to indicate decimal sub-multiples or multiples of
units: pico (p), nano (n), micro (), milli (m), centi (c), kilo (k), mega (M), giga (G), tera (T);
(d) Make suitable estimates of physical quantities included within this specification.
1.1.2 Scalars and vectors
(a) define scalar and vector quantities and give examples;
(b) draw and use a vector triangle to determine the resultant of two coplanar vectors such as
displacement, velocity and force;
(c) calculate the resultant of two perpendicular vectors such as displacement, velocity and force;
(d) resolve a vector such as displacement, velocity and force into two perpendicular components.
1.1.3 Kinematics
(a) define displacement, instantaneous speed, average speed, velocity and acceleration;
(b) select and use the relationships
average speed = distance
time
acceleration = change in velocity
time
to solve problems;
(c) apply graphical methods to represent displacement, speed, velocity and acceleration;
(d) determine velocity from the gradient of a displacement against time graph;
(e) determine displacement from the area under a velocity against time graph;
(f) determine acceleration from the gradient of a velocity against time graph.
1.1.4 Linear Motion
(a) derive the equations of motion for constant acceleration in a straight line from a velocity against time
graph;
(b) Select and use the equations of motion for constant acceleration in a straight line:
(c) apply the equations for constant acceleration in a straight line, including the motion of bodies falling
in the Earths uniform gravitational field without air resistance;
(d) explain how experiments carried out by Galileo overturned Aristotles ideas of motion;
(e) describe an experiment to determine the acceleration of free fall g using a falling body;
(f) apply the equations of constant acceleration to describe and explain the motion of an object due to a
uniform velocity in one direction and a constant acceleration in a perpendicular direction.
A level Physics Handbook 2012 St Gregorys Catholic College, Bath
- 15 -
1.2.1 Force
(a) Solve problems using the relationship:
net force = mass acceleration (F = ma)
appreciating that acceleration and the net
force are always in the same direction;
(b) define the newton;
(c) apply the equations for constant acceleration and F = ma to analyse the motion of objects;
(d) recall that according to the special theory of relativity, F = ma cannot be used for a particle travelling at
very high speeds because its mass increases.
1.2.2 Nonlinear motion
(a) explain that an object travelling in a fluid experiences a resistive or a frictional force known as drag;
(b) state the factors that affect the magnitude of the drag force;
(c) determine the acceleration of an object in the presence of drag;
(d) state that the weight of an object is the gravitational force acting on the object;
(e) select and use the relationship:
weight = mass acceleration of free fall (W = mg);
(f) describe the motion of bodies falling in a uniform gravitational field with drag;
(g) use and explain the term terminal velocity.
1.2.3 Equilibrium
(a) draw and use a triangle of forces to represent the equilibrium of three forces acting at a point in an
object;
(b) state that the centre of gravity of an object is a point where the entire weight of an object appears to act;
(c) describe a simple experiment to determine the centre of gravity of an object;
(d) explain that a couple is a pair of forces that tends to produce rotation only;
(e) define and apply the torque of a couple;
(f) define and apply the moment of force;
(g) explain that both the net force and net moment on an extended object in equilibrium is zero;
(h) apply the principle of moments to solve problems, including the human forearm;
(i) select and use the equation for density:
(j) select and use the equation for pressure
where F is the force normal to the area A.
1.2.4 Car Safety
(a) define thinking distance, braking distance and stopping distance;
(b) analyse and solve problems using the terms thinking distance, braking distance and stopping distance;
(c) describe the factors that affect thinking distance and braking distance;
(d) describe and explain how air bags, seat belts and crumple zones in cars reduce impact forces in accidents;
(e) describe how air bags work, including the triggering mechanism;
(f) describe how the trilateration technique is used in GPS (global positioning system) for cars.
A level Physics Handbook 2012 St Gregorys Catholic College, Bath
- 16 -
1.3.1 Work and Conservation of Energy
(a) define work done by a force;
(b) define the joule;
(c) calculate the work done by a force using
W = Fx and W = Fx cos ;
(d) state the principle of conservation of energy;
(e) describe examples of energy in different forms, its conversion and conservation, and apply the principle
of energy conservation to simple examples;
(f) apply the idea that work done is equal to the transfer of energy to solve problems.
1.3.2 Kinetic and potential energies
(a) select and apply the equation for kinetic energy ;
(b) apply the definition of work done to derive the equation for the change in gravitational potential energy;
(c) select and apply the equation for the change in gravitational potential energy near the Earths surface
E
p
= mgh;
(d) analyse problems where there is an exchange between gravitational potential energy and kinetic
energy;
(e) apply the principle of conservation of energy to determine the speed of an object falling in the Earths
gravitational field.
1.3.3 Power
(a) define power as the rate of work done;
(b) define the watt;
(c) calculate power when solving problems;
(d) state that the efficiency of a device is always less than 100% because of heat losses;
(e) select and apply the relationship for efficiency
(f) interpret and construct Sankey diagrams.
1.3.4 Behaviour of springs and materials
(a) describe how deformation is caused by a force in one dimension and can be tensile or compressive;
(b) describe the behaviour of springs and wires in terms of force, extension, elastic limit, Hookes law and
the force constant (i.e. force per unit extension or compression);
(c) select and apply the equation F = kx, where k is the force constant of the spring or the wire;
(d) determine the area under a force against extension (or compression) graph to find the work done by the
force;
(e) select and use the equations for elastic potential energy:
(f) define and use the terms stress, strain, Young modulus and ultimate tensile strength (breaking stress);
(g) describe an experiment to determine the Young modulus of a metal in the form of a wire;
(h) define the terms elastic deformation and plastic deformation of a material;
(i) describe the shapes of the stress against strain graphs for typical ductile, brittle and polymeric materials.
A level Physics Handbook 2012 St Gregorys Catholic College, Bath
- 17 -
AS Unit G482: Electrons, Waves and Photons
2.1.1 Charge and Current
(a) explain that electric current is a net flow of charged particles;
(b) explain that electric current in a metal is due to the movement of electrons, whereas in an electrolyte
the current is due to the movement of ions;
(c) explain what is meant by conventional current and electron flow;
(d) select and use the equation Q = It;
(e) define the coulomb;
(f) describe how an ammeter may be used to measure the current in a circuit;
(g) recall and use the elementary charge e = 1.6 10
-19
C;
(h) describe Kirchhoffs first law and appreciate that this is a consequence of conservation of charge;
(i) state what is meant by the term mean drift velocity of charge carriers;
(j) select and use the equation I = Anev;
(k) describe the difference between conductors, semiconductors and insulators in terms of the number
density n.
2.2.1 Circuit symbols
(a) recall and use appropriate circuit symbols as set out in SI Units, Signs, Symbols and Abbreviations
(ASE, 1981) and Signs, Symbols and Systematics (ASE, 1995);
(b) interpret and draw circuit diagrams using these symbols.
2.2.2 E.m.f and p.d.
(a) define potential difference (p.d.);
(b) select and use the equation W = VQ;
(c) define the volt;
(d) describe how a voltmeter may be used to determine the p.d. across a component;
(e) define electromotive force (e.m.f.) of a source such as a cell or a power supply;
(f) describe the difference between e.m.f. and p.d. in terms of energy transfer.
2.2.3 Resistance
(a) define resistance;
(b) select and use the equation for resistance ;
(c) define the ohm;
(d) state and use Ohms law;
(e) describe the IV characteristics of a resistor at constant temperature, filament lamp and light-
emitting diode (LED);
(f) describe an experiment to obtain the IV characteristics of a resistor at constant temperature,
filament lamp and light-emitting diode (LED);
(g) describe the uses and benefits of using light-emitting diodes (LEDs).
2.2.4 Resistivity
(a) define resistivity of a material;
(b) select and use the equation ;
(c) describe how the resistivities of metals and semiconductors are affected by temperature;
(d) describe how the resistance of a pure metal wire and of a negative temperature coefficient (NTC)
thermistor is affected by temperature.
A level Physics Handbook 2012 St Gregorys Catholic College, Bath
- 18 -
2.2.5 Power
(a) describe power as the rate of energy transfer;
(b) select and use power equations
(c) explain how a fuse works as a safety device (HSW 6a);
(d) determine the correct fuse for an electrical device;
(e) select and use the equation W = IVt;
(f) define the kilowatt-hour (kW h) as a unit of energy;
(g) calculate energy in kW h and the cost of this energy when solving problems (HSW 6a).
2.3.1 Series and parallel circuits
(a) state Kirchhoffs second law and appreciate that this is a consequence of conservation of energy;
(b) apply Kirchhoffs first and second laws to circuits;
(c) select and use the equation for the total resistance of two or more resistors in series;
(d) select and use the equation for the total resistance of two or more resistors in parallel;
(e) solve circuit problems involving series and parallel circuits with one or more sources of e.m.f.;
(f) explain that all sources of e.m.f. have an internal resistance;
(g) explain the meaning of the term terminal p.d.;
(h) select and use the equations:
e.m.f. = I (R + r), and e.m.f. = V + Ir .
2.3.2 Practical circuits
(a) draw a simple potential divider circuit;
(b) explain how a potential divider circuit can be used to produce a variable p.d.;
(c) select and use the potential divider equation ;
(d) describe how the resistance of a light-dependent resistor (LDR) depends on the intensity of light;
(e) describe and explain the use of thermistors and light-dependent resistors in potential divider circuits;
(f) describe the advantages of using data-loggers to monitor physical changes (HSW 3).
A level Physics Handbook 2012 St Gregorys Catholic College, Bath
- 19 -
2.4.1 Wave motion
(a) describe and distinguish between progressive longitudinal and transverse waves;
(b) define and use the terms displacement, amplitude, wavelength, period, phase difference, frequency
and speed of a wave;
(c) derive from the definitions of speed, frequency and wavelength, the wave equation v = f;
(d) select and use the wave equation v = f;
(e) explain what is meant by reflection, refraction and diffraction of waves such as sound and light.
2.4.2 Electromagnetic waves
(a) state typical values for the wavelengths of the different regions of the electromagnetic spectrum
from radio waves to -rays;
(b) state that all electromagnetic waves travel at the same speed in a vacuum;
(c) describe differences and similarities between different regions of the electromagnetic spectrum;
(d) describe some of the practical uses of electromagnetic waves;
(e) describe the characteristics and dangers of UV-A, UV-B and UV-C radiations and explain the role of
sunscreen (HSW 6a);
(f) explain what is meant by plane polarised waves and understand the polarisation of electromagnetic
waves;
(g) explain that polarisation is a phenomenon associated with transverse waves only;
(h) state that light is partially polarised on reflection;
(i) recall and apply Maluss law for transmitted intensity of light from a polarising filter.
2.4.3 Interference
(a) state and use the principle of superposition of waves;
(b) apply graphical methods to illustrate the principle of superposition;
(c) explain the terms interference, coherence, path difference and phase difference;
(d) state what is meant by constructive interference and destructive interference;
(e) describe experiments that demonstrate two-source interference using sound, light and microwaves;
(f) describe constructive interference and destructive interference in terms of path difference and phase
difference;
(g) use the relationships intensity = power/cross-sectional area intensity amplitude
2
;
(h) describe the Young double-slit experiment and explain how it is a classical confirmation of the wave-
nature of light (HSW 1);
(i) Select and use the equation for electromagnetic waves;
(j) describe an experiment to determine the wavelength of monochromatic light using a laser and a double
slit (HSW 1);
(k) describe the use of a diffraction grating to determine the wavelength of light (the structure and use of a
spectrometer are not required);
(l) select and use the equation dsin = n;
(m) explain the advantages of using multiple slits in an experiment to find the wavelength of light.
2.4.4 Stationary waves
(a) explain the formation of stationary (standing) waves using graphical methods;
(b) describe the similarities and differences between progressive and stationary waves;
(c) define the terms nodes and antinodes;
(d) describe experiments to demonstrate stationary waves using microwaves, stretched strings and air
columns;
(e) determine the standing wave patterns for stretched string and air columns in closed and open pipes;
(f) use the equation:
separation between adjacent nodes (or antinodes) = /2;
(g) define and use the terms fundamental mode of vibration and harmonics;
(h) determine the speed of sound in air from measurements on stationary waves in a pipe closed at one end.
A level Physics Handbook 2012 St Gregorys Catholic College, Bath
- 20 -
2.5.1 Energy of a photon
(a) describe the particulate nature (photon model) of electromagnetic radiation;
(b) state that a photon is a quantum of energy of electromagnetic radiation;
(c) select and use the equations for the energy of a photon:
(d) define and use the electronvolt (eV) as a unit of energy;
(e) use the transfer equation for electrons and other charged particles;
(f) describe an experiment using LEDs to estimate the Planck constant h using the equation (no knowledge
of semiconductor theory is expected).
2.5.2 The photoelectric effect
(a) describe and explain the phenomenon of the photoelectric effect;
(b) explain that the photoelectric effect provides evidence for a particulate nature of electromagnetic radiation
while phenomena such as interference and diffraction provide evidence for a wave nature;
(c) define and use the terms work function and threshold frequency;
(d) state that energy is conserved when a photon interacts with an electron;
(e) select, explain and use Einsteins photoelectric equation ;
(f) explain why the maximum kinetic energy of the electrons is independent of intensity and why the
photoelectric current in a photocell circuit is proportional to intensity of the incident radiation.
2.5.3 Waveparticle duality
(a) explain electron diffraction as evidence for the wave nature of particles like electrons;
(b) explain that electrons travelling through polycrystalline graphite will be diffracted by the atoms and the
spacing between the atoms;
(c) select and apply the de Broglie equation ;
(d) explain that the diffraction of electrons by matter can be used to determine the arrangement of atoms and
the size of nuclei.
2.5.4 Energy levels in atoms
(a) explain how spectral lines are evidence for the existence of discrete energy levels in isolated atoms, ie in
a gas discharge lamp;
(b) describe the origin of emission and absorption line spectra;
(c) use the relationships:
A level Physics Handbook 2012 St Gregorys Catholic College, Bath
- 21 -
4.4 The A2 Physics specification
AS Unit G484: The Newtonian World
This unit consists of three teaching modules:
Module 1: Newtons laws and momentum
4.1.1 Newtons laws of motion
4.1.2 Collisions
Module 2: Circular motion and oscillations
4.2.1 Circular motion
4.2.2 Gravitational Fields
4.2.3 Simple harmonic oscillations
Module 3: Thermal Physics
4.3.1 Solid, liquid and gas
4.3.2 Temperature
4.3.3 Thermal properties of materials
AS Unit G485: Fields, Particles and Frontiers of Physics
This unit consists of five teaching modules:
Module 1: Electric and magnetic fields
5.1.1 Electric fields
5.1.2 Magnetic fields
5.1.3 Electromagnetism
Module 2: Capacitors and exponential decay
5.2.1 Capacitors
Module 3: Nuclear physics
5.3.1 The nuclear atom
5.3.2 Fundamental particles
5.3.3 Radioactivity
5.3.4 Nuclear fission and fusion
Module 4: Medical imaging
5.4.1 X-rays
5.4.2 Diagnostic methods in medicine
5.4.3 Ultrasound
Module 5: Modelling the universe
5.5.1 Structure of the universe
5.5.2 The evolution of the universe
A level Physics Handbook 2012 St Gregorys Catholic College, Bath
- 22 -
AS Unit G484: The Newtonian World
4.1.1 Newtons laws of motion
(a) state and use each of Newton's three laws motion;
(b) define linear momentum as the product of mass and velocity and appreciate the vector nature
of momentum;
(c) define net force on a body as equal to rate of change of its momentum;
(d) select and apply the equation to solve problems;
(e) explain that F = ma is a special case of Newtons second law when mass m remains constant;
(f) define impulse of a force;
(g) recall that the area under a force against time graph is equal to impulse;
(h) recall and use the equation impulse = change in momentum.
4.1.2 Collisions
(a) state the principle of conservation of momentum;
(b) apply the principle of conservation of momentum to solve problems when bodies interact in one
dimension;
(c) define a perfectly elastic collision and an inelastic collision;
(d) explain that whilst the momentum of a system is always conserved in the interaction between bodies,
some change in kinetic energy usually occurs.
4.2.1 Circular motion
(a) define the radian;
(b) convert angles from degrees into radians and vice versa;
(c) explain that a force perpendicular to the velocity of an object will make the object describe a
circular path;
(d) explain what is meant by centripetal acceleration and centripetal force;
(e) select and apply the equations for speed and centripetal acceleration ;
(f) select and apply the equation for centripetal force
4.2.2 Gravitational fields
(a) describe how a mass creates a gravitational field in the space around it;
(b) define gravitational field strength as force per unit mass;
(c) use gravitational field lines to represent a gravitational field;
(d) state Newtons law of gravitation;
(e) select and use the equation for the force between two point or spherical objects;
(f) select and apply the equation for the gravitational field strength of a point mass;
(g) select and use the equation to determine the mass of the Earth or another similar object;
A level Physics Handbook 2012 St Gregorys Catholic College, Bath
- 23 -
(h) explain that close to the Earths surface the gravitational field strength is uniform and
approximately equal to the acceleration of free fall;
(i) analyse circular orbits in an inverse square law field by relating the gravitational force to the centripetal
acceleration it causes;
(j) define and use the period of an object describing a circle;
(k) derive the equation from first principles;
(l) select and apply the equation for planets and satellites (natural and artificial);
(m) select and apply Keplers third law to solve problems;
(n) define geostationary orbit of a satellite and state the uses of such satellites.
4.2.3 Simple harmonic oscillations
(a) describe simple examples of free oscillations;
(b) define and use the terms displacement, amplitude, period, frequency, angular frequency
and phase difference;
(c) select and use the equation period = 1/frequency;
(d) define simple harmonic motion;
(e) select and apply the equation a = (2f)
2
x as the defining equation of simple harmonic
motion;
(f) select and use x = Acos(2ft) or x = Asin(2ft) as solutions to the equation a = (2f)
2
x ;
(g) select and apply the equation v
max
= (2f)A for the maximum speed of a simple harmonic
oscillator;
(h) explain that the period of an object with simple harmonic motion is independent of its
amplitude;
(i) describe, with graphical illustrations, the changes in displacement, velocity and
acceleration during simple harmonic motion;
(j) describe and explain the interchange between kinetic and potential energy during simple
harmonic motion;
(k) describe the effects of damping on an oscillatory system;
(l) describe practical examples of forced oscillations and resonance;
(m)describe graphically how the amplitude of a forced oscillation changes with frequency near
to the natural frequency of the system;
(n) describe examples where resonance is useful and other examples where resonance
should be avoided.
4.3.1 Solid, liquid and gas
(a) describe solids, liquids and gases in terms of the spacing, ordering and motion of atoms or
molecules;
(b) describe a simple kinetic model for solids, liquids and gases;
(c) describe an experiment that demonstrates Brownian motion and discuss the evidence for
the movement of molecules provided by such an experiment;
(d) define the term pressure and use the kinetic model to explain the pressure exerted by
gases;
(e) define internal energy as the sum of the random distribution of kinetic and potential
energies associated with the molecules of a system;
(f) explain that the rise in temperature of a body leads to an increase in its internal energy;
(g) explain that a change of state for a substance leads to changes in its internal energy but not its
temperature;
(h) describe using a simple kinetic model for matter the terms melting, boiling and evaporation.
A level Physics Handbook 2012 St Gregorys Catholic College, Bath
- 24 -
4.3.2 Temperature
(a) explain that thermal energy is transferred from a region of higher temperature to a region of lower
temperature;
(b) explain that regions of equal temperature are in thermal equilibrium;
(c) describe how there is an absolute scale of temperature that does not depend on the
property of any particular substance (ie the thermodynamic scale and the concept of absolute
zero);
(d) convert temperatures measured in kelvin to degrees Celsius (or vice versa):
T (K)= (C) + 273.15;
(e) state that absolute zero is the temperature at which a substance has minimum internal
energy.
4.3.3 Thermal properties of materials
(a) define and apply the concept of specific heat capacity;
(b) select and apply the equation E = mc;
(c) describe an electrical experiment to determine the specific heat capacity of a solid or a liquid;
(d) describe what is meant by the terms latent heat of fusion and latent heat of vaporisation.
4.3.4 Ideal gases
(a) state Boyles law;
(b) select and apply
(c) state the basic assumptions of the kinetic theory of gases;
(d) state that one mole of any substance contains 6.02 10
23
particles and that 6.02 10
23
mol
-1
is the
Avogadro constant N
A
;
(e) select and solve problems using the ideal gas equation expressed as
pV = NkT and pV = nRT, where N is the number of atoms and n is the number of moles;
(f) explain that the mean translational kinetic energy of an atom of an ideal gas is directly proportional to
the temperature of the gas in kelvin;
(g) select and apply the equation for the mean translational kinetic energy of atoms.
A level Physics Handbook 2012 St Gregorys Catholic College, Bath
- 25 -
AS Unit G485: Fields, Particles and Frontiers of Physics
5.1.1 Electric fields
(a) state that electric fields are created by electric charges;
(b) define electric field strength as force per unit positive charge;
(c) describe how electric field lines represent an electric field;
(d) select and use Coulombs law in the form ;
(e) select and apply for the electric field strength of a point charge;
(f) select and use for the magnitude of the uniform electric field strength between charged parallel plates;
(g) explain the effect of a uniform electric field on the motion of charged particles;
(h) describe the similarities and differences between the gravitational fields of point masses and the electric
fields of point charges.
5.1.2 Magnetic fields
(a) describe the magnetic field patterns of a long straight current-carrying conductor and a long solenoid;
(b) state and use Flemings left-hand rule to determine the force on current conductor placed at right angles
to a magnetic field;
(c) select and use the equations F = BIL and F = BILsin;
(d) define magnetic flux density and the tesla;
(e) select and use the equation F = BQv for the force on a charged particle travelling at right angles to a
uniform magnetic field;
(f) analyse the circular orbits of charged particles moving in a plane perpendicular to a uniform magnetic
field by relating the magnetic force to the centripetal acceleration it causes;
(g) analyse the motion of charged particles in both electric and magnetic fields;
(h) explain the use of deflection of charged particles in the magnetic and electric fields of a mass
spectrometer (HSW 6a)
5.1.3 Electromagnetism
(a) define magnetic flux;
(b) define the weber.
(c) select and use the equation for magnetic flux ; =BAcos
(d) define magnetic flux linkage;
(e) state and use Faradays law of electromagnetic induction;
(f) state and use Lenzs law;
(g) select and use the equation: induced e.m.f. = rate of change of magnetic flux linkage;
(h) describe the function of a simple ac generator;
(i) describe the function of a simple transformer;
(j) select and use the turns-ratio equation for a transformer;
(k) describe the function of step-up and step-down transformers.
A level Physics Handbook 2012 St Gregorys Catholic College, Bath
- 26 -
5.2.1 Capacitors
(a) define capacitance and the farad;
(b) select and use the equation Q = VC;
(c) state and use the equation for the total capacitance of two or more capacitors in series;
(d) state and use the equation for the total capacitance of two or more capacitors in parallel;
(e) solve circuit problems with capacitors involving series and parallel circuits;
(f) explain that the area under a potential difference against charge graph is equal to energy stored by a
capacitor;
(g) select and use the equations and for a charged capacitor;
(h) sketch graphs that show the variation with time of potential difference, charge and current for a capacitor
discharging through a resistor;
(i) define the time constant of a circuit;
(j) select and use time constant = CR;
(k) analyse the discharge of capacitor using equations of the form
(l) explain exponential decays as having a constant-ratio property;
(m) describe the uses of capacitors for the storage of energy in applications such as flash photography,
lasers used in nuclear fusion and as back-up power supplies for computers (HSW 6a).
5.3.1 The nuclear atom
(a) describe qualitatively the alpha-particle scattering experiment and the evidence this provides for the existence, charge and small size of the nucleus (HSW 1, 4c);
(b) describe the basic atomic structure of the atom and the relative sizes of the atom and the nucleus;
(c) select and use Coulombs law to determine the force of repulsion, and Newtons law of gravitation to determine the force of attraction, between two protons at nuclear
separations and hence the need for a short-range, attractive force between nucleons (HSW 1, 2, 4);
(d) describe how the strong nuclear force between nucleons is attractive and very short-ranged;
(e) estimate the density of nuclear matter;
(f) define proton and nucleon number;
(g) state and use the notation for the representation of nuclides;
(h) define and use the term isotopes;
(i) use nuclear decay equations to represent simple nuclear reactions;
(j) state the quantities conserved in a nuclear decay.
5.3.2 Fundamental particles
(a) explain that since protons and neutrons contain charged constituents called quarks they are, therefore,
not fundamental particles;
(b) describe a simple quark model of hadrons in terms of up, down and strange quarks and their respective
antiquarks, taking into account their charge, baryon number and strangeness;
(c) describe how the quark model may be extended to include the properties of charm, topness and
bottomness;
(d) describe the properties of neutrons and protons in terms of a simple quark model;
(e) describe how there is a weak interaction between quarks and that this is responsible for decay;
(f) state that there are two types of decay;
(g) describe the two types of decay in terms of a simple quark model;
(h) state that (electron) neutrinos and (electron) antineutrinos are produced during
+
and
-
decays,
respectively;
(i) state that a
-
particle is an electron and a
+
particle is a positron;
(j) state that electrons and neutrinos are members of a group of particles known as leptons.
A level Physics Handbook 2012 St Gregorys Catholic College, Bath
- 27 -
5.3.3 Radioactivity
(a) describe the spontaneous and random nature of radioactive decay of unstable nuclei;
(b) describe the nature, penetration and range of -particles, -particles and -rays;
(c) define and use the quantities activity and decay constant;
(d) select and apply the equation for activity A = N;
(e) select and apply the equations A = A
0
e
-t
and N = N
0
e
-t
where A is the activity and N is the number of undecayed nuclei;
(f) define and apply the term half-life;
(g) select and use the equation t
1/2
= 0.693;
(h) compare and contrast decay of radioactive nuclei and decay of charge on a capacitor in a CR circuit
(HSW 5b);
(i) describe the use of radioactive isotopes in smoke alarms (HSW 6a);
(j) describe the technique of radioactive dating (ie carbon-dating).
5.3.4 Nuclear fission and fusion
(a) select and use Einsteins massenergy equation E = mc
2
;
(b) define binding energy and binding energy per nucleon;
(c) use and interpret the binding energy per nucleon against nucleon number graph;
(d) determine the binding energy of nuclei using E = mc
2
and masses of nuclei;
(e) describe the process of induced nuclear fission;
(f) describe and explain the process of nuclear chain reaction;
(g) describe the basic construction of a fission reactor and explain the role of the fuel rods, control rods and
the moderator (HSW 6a and 7c);
(h) describe the use of nuclear fission as an energy source (HSW 4 and 7c);
(i) describe the peaceful and destructive uses of nuclear fission (HSW 4 and 7c);
(j) describe the environmental effects of nuclear waste (HSW 4, 6a and b, 7c);
(k) describe the process of nuclear fusion;
(l) describe the conditions in the core of stars that make fusion possible;
(m) calculate the energy released in simple nuclear reactions.
5.4.1 X-Rays
(a) describe the nature of X-rays;
(b) describe in simple terms how X-rays are produced;
(c) describe how X-rays interact with matter (limited to photoelectric effect, Compton Effect and pair
production);
(d) define intensity as the power per unit cross-sectional area;
(e) select and use the equation to show how the intensity I of a collimated X-ray beam varies with thickness x
of medium;
(f) describe the use of X-rays in imaging internal body structures including the use of image intensifiers and
of contrast media (HSW 3, 4c and 6);
(g) explain how soft tissues like the intestines can be imaged using barium meal;
(h) describe the operation of a computerised axial tomography (CAT) scanner;
(i) describe the advantages of a CAT scan compared with an X-ray image (HSW 4c, 6).
A level Physics Handbook 2012 St Gregorys Catholic College, Bath
- 28 -
5.4.2 Diagnosis methods in medicine
(a) describe the use of medical tracers like technetium-99m to diagnose the function of organs;
(b) describe the main components of a gamma camera;
(c) describe the principles of positron emission tomography (PET);
(d) outline the principles of magnetic resonance, with reference to precession of nuclei, Larmor frequency,
resonance and relaxation times;
(e) describe the main components of an MRI scanner;
(f) outline the use of MRI (magnetic resonance imaging) to obtain diagnostic information about internal
organs (HSW 3, 4c and 6a);
(g) describe the advantages and disadvantages of MRI (HSW 4c & 6a);
(h) describe the need for non-invasive techniques in diagnosis (HSW 6a);
(i) explain what is meant by the Doppler effect;
(j) explain qualitatively how the Doppler effect can be used to determine the speed of blood.
5.4.3 Ultrasound
(a) describe the properties of ultrasound;
(b) describe the piezoelectric effect;
(c) explain how ultrasound transducers emit and receive high-frequency sound;
(d) describe the principles of ultrasound scanning;
(e) describe the difference between A-scan and B-scan;
(f) calculate the acoustic impedance using the equation Z = c;
(g) calculate the fraction of reflected intensity using the equation
(h) describe the importance of impedance matching;
(i) explain why a gel is required for effective ultrasound imaging techniques.
5.5.1 Structure of the universe
(a) describe the principal contents of the universe, including stars, galaxies and radiation;
(b) describe the solar system in terms of the Sun, planets, planetary satellites and comets;
(c) describe the formation of a star, such as our Sun, from interstellar dust and gas;
(d) describe the Suns probable evolution into a red giant and white dwarf;
(e) describe how a star much more massive than our Sun will evolve into a super red giant and then either a neutron star or black hole;
(f) define distances measured in astronomical units (AU), parsecs (pc) and light-years (ly);
(g) state the approximate magnitudes in metres, of the parsec and light-year;
(h) state Olbers paradox;
(i) interpret Olbers paradox to explain why it suggests that the model of an infinite, static universe is incorrect (HSW 7);
(j) select and use the equation
(k) describe and interpret Hubbles redshift observations;
(l) state and interpret Hubbles law (HSW 1 & 2);
(m) convert the Hubble constant H
0
from its conventional units (km s
-1
Mpc
-1
) to SI (s
-1
);
(n) state the cosmological principle;
(o) describe and explain the significance of the 3K microwave background radiation (HSW 1).
A level Physics Handbook 2012 St Gregorys Catholic College, Bath
- 29 -
5.5.2 The evolution of the universe
(a) explain that the standard (hot big bang) model of the universe implies a finite age for the universe (HSW
1, 2, 7);
(b) select and use the expression: age of universe 1/H
0
;
(c) describe qualitatively the evolution of universe 10
-43
s after the big bang to the present;
(d) explain that the universe may be open, flat or closed, depending on its density (HSW 7);
(e) explain that the ultimate fate of the universe depends on its density;
(f) define the term critical density;
(g) select and use the expression for critical density of the universe ;
(h) explain that it is currently believed that the density of the universe is close to, and possibly exactly equal
to, the critical density needed for a flat cosmology (HSW 7).
A level Physics Handbook 2012 St Gregorys Catholic College, Bath
- 30 -
4.5 General Skills
Units
SI units will be used throughout this specification. Knowledge of SI multipliers will be required. A table of the
SI multipliers will be included in each examination paper.
Practical Work
Practical work will play an important role throughout the course. Attention is drawn to the specified content
in each unit and the instructions relating to the practical internal assessments.
Mathematical requirements
The following list of requirements is taken from the GCE AS and A level Criteria for Science Subjects. The
sections in bold type [i.e. use of radians, the exponential and log functions] will not be required at AS level,
because the subject content which requires these concepts is not met in this part of the course.
Computation
recognise and use expressions in decimal and standard form
use ratios, fractions and percentages
use calculators to find and use power, exponential and logarithmicfunctions
use calculators to handle sin x, cos x, tan x when x is expressed indegrees or radians
Handling data
use an appropriate number of significant figures
find arithmetic means
make order of magnitude calculations.
Algebra
understand and use the symbols: =, <, <<, >>, >, , ~
change the subject of an equation
substitute numerical values into algebraic equations using appropriate units for physical quantities
solve simple algebraic equations
Graphs
translate information between graphical, numerical and algebraic forms
plot two variables from experimental or other data
understand that y = mx + c represents a linear relationship
determine the slope and intercept of a linear graph
draw and use the slope of a tangent to a curve as a measure of rate of change
understand the possible physical significance of the area between a curve and the x axis and be able to
calculate it or measure it by counting squares as appropriate
use logarithmic plots to test exponential and power law variations
sketch simple functions including y = k/x, y = kx2, y = k/x2, y = sin x, y = cos x, y = e-x
Geometry and Trigonometry
calculate areas of triangles, circumferences and areas of circles, surface areas and volumes of rectangular
blocks, cylinders and spheres
use Pythagoras' theorem, and the angle sum of a triangle
use sin, cos and tan in physical problems
understand the relationship between degrees and radians and translate from one to the other.
A level Physics Handbook 2012 St Gregorys Catholic College, Bath
- 31 -
4.5.1 Physics terms, definitions and units
Item Definition
Quantity In S.I. a quantity is represented by a number a unit, (e.g. m = 3.0
kg).
Scalar A scalar is a quantity that has magnitude only.
Vector A vector is a quantity that has magnitude and direction.
Force A force on a body is a push or a pull acting on the body from some
external body.
Unit: N
Newtons Third Law If a body A exerts a force on a body B, then B exerts an equal and
opposite force on A.
E F = m a The mass of a body its acceleration is equal to the vector sum of the
forces acting on the body. This vector sum is called the resultant
force.
Resolving a vector
into components in
particular directions
This means finding vectors (the so-called components) in these
directions, which add together vectorially to make the original vector,
and so, together, are equivalent to this vector.
Density of a material
volume
mass
density = Unit: kg m
3
in which mass and volume apply to any sample of the material.
Moment (or torque)
of a force.
The moment (or torque) of a force about a point is defined as the
force the perpendicular distance from the point to the line of action
of the force,
i.e. moment = F d.
Unit: Nm. [N.B. the unit is not J]
The principle of
moments.
For a system to be in equilibrium, anticlockwise moments about a
point = clockwise moments about the same point.
Centre of gravity. The centre of gravity is the single point within a body at which the
entire weight of the body may be considered to act
Displacement The displacement of a point B from a point A is the shortest
distance from A to B, together with the direction. Unit: m.
Mean Speed
Mean speed =
total distance travelled
total time taken
x
t
A
=
A
Unit: ms
-1
.
Instantaneous Speed instantaneous speed = rate of change of distance
Unit: ms
-1
.
Mean Velocity
Mean velocity =
total displacement
total time taken
Unit: ms
-1
.
A level Physics Handbook 2012 St Gregorys Catholic College, Bath
- 32 -
Instantaneous
Velocity
The velocity of a body is the rate of change of displacement.
Unit: ms
-1
Mean Acceleration
Mean Acceleration =
change in velocity
time taken
v
t
A
=
A
Unit: ms
-2
.
Instantaneous
Acceleration
The instantaneous acceleration of a body is its rate of change of
velocity. Unit: ms
-2
Terminal Velocity The terminal velocity is the constant, maximum velocity of an object
when the resistive forces on it are equal and opposite to the
accelerating force (e.g. pull of gravity).
Work Work done by a force is the product of the magnitude of the force and
the distance moved in the direction of the force.( W.D. = Fxcos u )
Unit: J [= Nm]
Hookes Law The tension in a spring or wire is proportional to its extension from
its natural length, provided the extension is not too great.
Spring constant, k The spring constant is the force per unit extension.
Unit: Nm
-1
.
Energy The energy of a body or system is the amount of work it can do.
Unit: J
Principle of
conservation of
energy
Energy cannot be created or destroyed, only transferred from one
form to another. Energy is a scalar.
Potential energy This is energy possessed by virtue of position. (e.g. Gravitational PE
= mgh). Unit: J
Kinetic energy This is energy possessed by an object by virtue of its motion. Unit: J
Power This is the work done per second, or energy transferred per second.
Unit: watt (W) [= Js
-1
].
Efficiency of a
system
% efficiency = (useful work (or energy) out / work put in) x 100
Unit: none
Electric current, I. This is the rate of flow of electric charge. I = Q/t. Unit: A
Potential difference
(p.d.), V.
The p.d. between two points is the energy converted from electrical
potential energy to some other form per coulomb of charge flowing
from one point to the other. Unit: volt (V) [= JC
-1
].
Ohms Law. The current flowing through a metal wire at constant temperature is
proportional to the p.d. across it.
Electrical
Resistance, R.
The resistance of a conductor is the p.d. (V) placed across it divided
by the resulting current (I) through it. R = V / I
Unit: ohm (O) [= VA
-1
].
Resistivity, The resistance, R, of a metal wire of length L and cross-sectional area
A is given by R = L / A, in which , the resistivity, is a constant (at
constant temperature) for the material of the wire.
Unit: O m
A level Physics Handbook 2012 St Gregorys Catholic College, Bath
- 33 -
Superconducting
transition
temperature
The temperature at which a material, when cooled, loses all its
electrical resistance, and becomes super-conducting. Some materials
(e.g. copper) never become superconducting however low the
temperature.
The Law of
Conservation of
Charge.
Electric charge cannot be created or destroyed, (though positive and
negative charges can neutralise each other). Charge cannot pile up
anywhere.
e.m.f. The e.m.f. of a source is the energy converted from some other form
(e.g. chemical) to electrical potential energy per coulomb of charge
flowing through the source.
Unit: V.
Progressive wave A pattern of disturbances travelling through a medium and carrying
energy with it, involving the particles of the medium oscillating about
their equilibrium positions.
Transverse wave A transverse wave is one where the particle oscillations are at right
angles to the direction of travel (or propagation) of the wave.
Longitudinal wave A longitudinal wave is one where the particle oscillations are in line
with (parallel to) the direction of travel (of propagation) of the wave.
Polarised wave A polarised wave is a transverse wave in which particle oscillations
occur in only one of the directions at right angles to the direction of
wave propagation.
Wavelength of a
progressive wave
The wavelength of a progressive wave is the minimum distance
(measured along the direction of propagation) between two points on
the wave oscillating in phase.
Frequency of a wave The frequency of a wave is the number of cycles of a wave that pass a
given point in one second,
[or equivalently the number of cycles of oscillation per second
performed by any particle in the medium through which the wave is
passing.]
Velocity of a wave The velocity of a wave is the distance that the wave profile moves per
unit time.
Diffraction Diffraction is the spreading out of waves when they meet
obstacles, such as the edges of a slit. Some of the waves energy
travels into the geometrical shadows of the obstacles.
The principle of
superposition.
The principle of superposition states that if waves from two sources
[or travelling by different routes from the same source] occupy the
same region then the total displacement at any one point is the vector
sum of their individual displacements at that point.
In phase Waves arriving at a point are said to be in phase if they have the same
frequency and are at the same point in their cycles at the same time.
[Wave sources are in phase if the waves have the same frequency and
are at the same point in their cycles at the same time, as they leave
the sources.]
Phase difference Phase difference is the difference in position of 2 points within a
cycle of oscillation. It is measured as a fraction of the cycle or as an
angle, where one whole cycle is 2t or 360]
Coherence Waves or wave sources, which have a constant phase difference
A level Physics Handbook 2012 St Gregorys Catholic College, Bath
- 34 -
between them (and therefore must have the same frequency) are said
to be coherent.
Stationary (or
standing) wave
A stationary wave is a pattern of disturbances in a medium, in which
energy is not propagated. The amplitude of particle oscillations is
zero at equally-spaced nodes, rising to maxima at antinodes, midway
between the nodes.
Snells law At the boundary between any two given materials, the ratio of the
sine of the angle of incidence to the sine of the angle of refraction is a
constant.
Refractive Index For light, Snell's Law may be written:
2 2 1 1
sin sin u u n n =
in which u
1
and u
2
are angles to the normal for light passing between
medium 1 and medium 2; n
1
and n
2
are called the
refractive indices of medium 1 and medium 2 respectively.
The refractive index of a vacuum is fixed by convention as exactly 1.
For air, n = 1.000
Critical angle When light approaches the boundary between two media from the
'slower' medium, the critical angle is the largest angle of incidence for
which refraction can occur. The refracted wave is then travelling at
90 to the normal.
Photoelectric effect When light or ultraviolet radiation of short enough wavelength falls
on a surface, electrons are emitted from the surface.
Work function The work function of a surface is the minimum energy needed to
remove an electron from the surface. Unit: J
Ionisation The removal of one or more electrons from an atom.
Ionisation energy The ionization energy of an atom is the minimum energy needed to
remove an electron from the atom in its ground state. Unit: J
Stimulated emission This is the emission of a photon from an excited atom, triggered by
an incident photon of energy equal to the energy gap between the
excited state and a state of lower energy in the atom. The emitted
photon has the same frequency, phase, direction of travel and
polarisation direction as the incident photon.
Population inversion A population inversion is a situation in which a higher energy state in
an atomic system is more heavily populated than a lower energy state
(i.e. a less excited state or the ground state) of the same system.
Pumping Pumping is feeding energy into the amplifying medium of a laser to
produce a population inversion.
Atomic mass
number, A
[nucleon number]
The atomic mass number of an atom is the number of nucleons
(number of protons + number of neutrons) in its nucleus.
Atomic number, Z
[proton number]
The atomic number of an atom is the number of protons in its
nucleus. [This determines the chemical element which the atom
represents.]
Nuclide A nuclide is a particular variety of nucleus, that is a nucleus with a
particular A and Z.
Isotope Isotopes are atoms with the same number of protons, but different
numbers of neutrons in their nuclei.
A level Physics Handbook 2012 St Gregorys Catholic College, Bath
- 35 -
Lepton The leptons are electrons and electron-neutrinos [and analogous pairs
of particles of the so-called second and third generations].
Hadron Hadrons are particles consisting of quarks or antiquarks bound
together. Only hadrons (and quarks or antiquarks themselves) can
feel the strong force.
Baryon A baryon is a hadron consisting of 3 quarks or 3 antiquarks. The best
known baryons are the nucleons, that is the proton and the neutron.
Meson A meson is a hadron consisting of a quark-antiquark pair.
Black body A black body is a body (or surface) which absorbs all the
electromagnetic radiation that falls upon it. No body is a better
emitter of radiation at any wavelength than a black body at the same
temperature.
Wiens displacement
law
The wavelength of peak emission from a black body is inversely
proportional to the absolute (kelvin) temperature of the body.
max
= W/T [W = Wien Constant = 2.90 x 10
-3
m K]
Absolute or kelvin
temperature
The temperature, T in kelvin (K) is related to the temperature, u, in
celsius (C) by: T /K= u /C + 273.15 .
At 0 K (-273.15C) the energy of particles in a body is the lowest it
can possibly be.
Stefans Law [The
Stefan-Boltzmann
law]
The total electromagnetic radiation energy emitted per unit time by a
black body is given by power = A o T
4
in which A is the
bodys surface area and o is a constant called the Stefan constant. [o
= 5.67 10
-8
W m
-2
K
-4
]
Luminosity of a star The luminosity of a star is the total energy it emits per unit time in the
form of electromagnetic radiation. UNIT: W
[Thus we could have written luminosity instead of power in Stefans
law (above).]
Period T for a point
describing a circle
Time taken for one complete circuit.
Frequency f The number of circuits or cycles per second.
Angular velocity e. For an object describing a circle at uniform speed, the angular
velocity e is equal to the angle u swept out by the radius in time At
divided by t . (e = Au /At) UNIT: [rad] s
-1
Simple harmonic
motion (shm)
Shm occurs when an object moves such that its acceleration is always
directed toward a fixed point and proportional to its distance from the
fixed point. (a = e
2
x)
Simple harmonic
motion (shm)
(Alternative
definition)
The motion of a point whose displacement x changes with time t
according to x = A sin (e t +c), where A, e and c are constants.
[Variations of this kind are said to be sinusoidal.]
Period T for an
oscillating body
The time taken for one complete cycle.
Amplitude A of an
oscillating object
The maximum value of the objects displacement (from its
equilibrium position).
Phase
The phase of an oscillation is the angle (et +c) in the equation x = A
A level Physics Handbook 2012 St Gregorys Catholic College, Bath
- 36 -
sin (e t +c). [c is called the phase constant.]
UNIT: rad
Free oscillations
[Natural oscillations]
Free oscillations occur when an oscillatory system (such as a mass
on a spring, or a pendulum) is displaced and released.
[The frequency of the free oscillations is called the systems natural
frequency.]
Damping Damping is the dying away, due to resistive forces, of the amplitude
of free oscillations.
Critical damping Critical damping is the case when the resistive forces on the system
are just large enough to prevent oscillations occurring at all when the
system is displaced and released.
Forced oscillations These occur when a sinusoidally varying driving force is applied to
an oscillatory system, causing it to oscillate with the frequency of the
applied force.
Resonance If, in forced vibrations, the frequency of the applied force is equal to
the natural frequency of the system (e.g. mass on spring), the
amplitude of the resulting oscillations is large. This is resonance.
Momentum The momentum of an object is its mass multiplied by its velocity.
(p = mv). It is a vector. UNIT: kg m s
-1
Newtons laws of
motion: 1st law
An object continues moving at constant speed in a straight line, or
remains at rest, unless acted upon by a resultant force.
Newtons laws of
motion: 2nd law
The rate of change of momentum of an object is proportional to the
resultant force acting on it, and takes place in the direction of that
force.
Newtons laws of
motion: 3rd law
If a body A exerts a force on a body B, then B exerts an equal and
opposite force on A.
The principle of
conservation of
momentum
The vector sum of the momenta of bodies in a system stays constant
even if forces act between the bodies, provided there is no external
resultant force.
Elastic collision. A collision in which there is no change in total kinetic energy.
Inelastic collision. A collision in which kinetic energy is lost.
Boyles law For a fixed mass of gas at constant temperature [unless its density is
very high], the pressure varies inversely as the volume. (pV = k).
Ideal gas An ideal gas strictly obeys the equation of state
pV = nRT, in which n is the number of moles, T is the kelvin
temperature and R is the molar gas constant. R = 8.31 J mol
-1
K
-1
.
Except at very high densities a real gas approximates well to an ideal
gas.
The mole The mole is the S.I. unit of amount of substance, n. It is the amount
containing as many particles (e.g. molecules) as there are atoms in 12
g of carbon12.
Avogadro constant
N
A
This is the number of particles per mole. (N
A
=6.0210
23
mol
-1
).
Internal energy, U,
of a system
This is the sum of the kinetic and potential energies of the particles of
the system.
A level Physics Handbook 2012 St Gregorys Catholic College, Bath
- 37 -
Heat This is energy flow from a region at higher temperature to a region at
lower temperature, due to the temperature difference. In
thermodynamics we deal with heat going into or out of a system. It
makes no sense to speak of heat in a system.
Work If the system is a gas, in a cylinder fitted with a piston, the gas does
work of amount pAV when it exerts a pressure p and pushes the
piston out a small way, so the gas volume increases by AV. Work,
like heat, is energy in transit from (or to) the system.
First law of
thermodynamics
The increase, AU, in internal energy of a system is
AU = Q W in which Q is the heat entering the system and W is the
work done by the system. Any of the terms in the equation can be
positive or negative, e.g. if 100 J of heat is lost from a system Q =
100 J.
Specific heat
capacity c.
The heat required, per kilogram, per degree Celsius or Kelvin, to
raise the temperature of a substance.
UNIT: J kg
-1
K
-1
or J kg
-1
C
-1
Newtons law of
gravitation.
The gravitational force between two particles is proportional to the
product of their masses, m
1
and m
2
, and inversely proportional to their
separation squared, r
2
. F = G m
1
m
2
/r
2
in which G is the gravitational
constant. G = 6.67 10
-11
N m
2
kg
-2
.
Coulombs Law The electrostatic force, F, between two small bodies is proportional
to the product of their charges, Q
1
and Q
2
, and inversely proportional
to their separation squared, r
2
. F = Q
1
Q
2
/4tc
0
r
2
in which c
0
is the
permittivity of free space. c
0
= 8.85 10
-12
Fm
-1
.
Electric field
strength E.
The force experienced per unit charge by a small positive charge
placed in the field. Unit: V m
-1
or N C
-1
.
Gravitational field
strength g.
The force experienced per unit mass by a mass placed in the field.
Unit: m s
-2
or N kg
-1
.
Electric potential V
E
. Electric potential at a point is the work done per unit charge in
bringing a positive charge from infinity to that point.
Unit: V. [= JC
-1
]
Gravitational
potential V
g
.
Gravitational potential at a point is the work done per unit mass in
bringing a mass from infinity to that point. Unit: Jkg
-1
.
Keplers laws of
planetary motion: 1
Each planet moves in an ellipse with the Sun at one focus.
Keplers laws of
planetary motion: 2
The line joining a planet to the centre of the Sun sweeps out equal
areas in equal times.
Keplers laws of
planetary motion: 3
T
2
, the square of the period of the planets motion, is proportional to
r
3
, in which r is the semi-major axis of its ellipse. [For orbits which
are nearly circular, r may be taken as the mean distance of the planet
from the Sun.]
Dark matter Matter which we cant see, or detect by any sort of radiation, but
whose existence we infer from its gravitational effects.
Radial velocity of a
star [in the context
of Doppler shift]
This is the component of a stars velocity along the line joining it and
an observer on the Earth.
A level Physics Handbook 2012 St Gregorys Catholic College, Bath
- 38 -
Capacitor A capacitor is a pair of conducting plates separated by an insulator. If
a p.d. is placed across the plates, they acquire equal and opposite
charges.
Capacitance, C, of a
capacitor
Capacitance = charge on either plate/p.d. between plates
Unit: F (farad) [= C V
-1
]
Dielectric Insulator between the plates of a capacitor, also serving to make the
capacitance larger than if there were just empty space.
Magnetic field
strength, B
(Magnetic flux
density)
This is a vector quantity. Its direction is that in which the North pole
of a freely-pivoted magnet points. Its magnitude is defined by
l I
F
B =
in which F is the force on a length l of wire carrying a current I,
placed perpendicular to the direction of the field. Unit: T (tesla) [=
NA
-1
m
-1
]
Hall voltage When a magnetic field, B, is applied to conductor carrying a current
I, at right angles to the field direction, a so-called Hall voltage
appears across the specimen, at right angles to the B and I directions.
Ampre A The ampre is that current which, when flowing through two infinite,
thin, parallel wires, one metre apart in vacuum, produces a force
between the wires of exactly 2 10
-7
N per metre of length. Unit: A.
Magnetic flux u. If a single-turn coil of wire encloses an area A, and a magnetic field B
makes an angle u with the normal to the plane of the coil, the
magnetic flux through the coil is given by u = AB cos u.
Unit: weber (Wb) =Tm
2
.
Flux linkage Nu If the above coil consists of N turns, the flux linkage is given by Nu.
Unit: Wb or Wb turn.
Faradays law When the flux linking an electrical circuit is changing, an emf is
induced in the circuit of magnitude equal to the rate of change of flux
linkage.
t
N
E
A
u A
=
) (
[Note: The sign is from Lenzs Law, see below]
Lenzs Law. The direction of any current resulting from an induced emf is such as
to oppose the change in flux linkage that is causing the current.
Root-mean-square
(rms) value
If an alternating voltage is read at regular intervals throughout a
cycle, giving the values V
1
, V
2
V
n
, the rms p.d., V
rms
, is defined as
Vrms = 1/n (V
1
2
+ V
2
2
+ ..V
n
2
)
I
ms
, is defined similarly for current.
[A Steady p.d. of magnitude Vrms (and a steady current
of Irms) would give the same power dissipation in a resistor as the
alternating p.d. and current."]
Alpha (o) radiation Fast moving particles, helium nuclei, ejected from certain radioactive
nuclei.
Beta (|) radiation Electrons with speeds just less than the speed of light, ejected from
certain radioactive nuclei.
Gamma () radiation Photons of high energy (high frequency, short wavelength) ejected
from radioactive nuclei.
A level Physics Handbook 2012 St Gregorys Catholic College, Bath
- 39 -
X
A
Z
notation
X is the chemical symbol of the element, A the mass number (number
of protons plus number of neutrons) and Z the atomic number
(number of protons).
Half life
1
2
T of a
nuclide
The time taken for the number of radioactive nuclei N (or the activity
A) to reduce to one half of the initial value. Unit: s.
Activity A The rate of decay (number of disintegrations per second) of a sample
of radioactive nuclei.
Unit: Becquerel (Bq) = s
-1
.
Decay constant
The constant which appears in the exponential decay law
t
e N N
=
0
and determines the rate of decay (the greater , the more rapid the
rate of decay). is related to half life by = ln2/
1
2
T . Unit: s
-1
Radio-isotopes Isotopes (of an element) have the same atomic number Z but different
mass number A; radio-isotopes are simply isotopes which are
radioactive.
Unified atomic mass
unit u.
The unified atomic mass unit is defined as exactly one twelfth of the
mass of one atom of carbon 12. Thus one atom of C
12
has a mass of
exactly 12u.
(1u = 10
-3
kg / N
A
= 1.66 10
-27
kg)
Electron volt (eV). This is the energy transferred when an electron moves between two
points with a potential difference of 1 volt between them.
1 eV = 1.6 10
-19
J
So for an electron being accelerated it is the K.E. acquired when
accelerated through a pd of 1V.
Binding energy of a
nucleus.
The energy that has to be supplied in order to dissociate a nucleus
into its constituent nucleons. [It is therefore not energy which a
nucleus possesses.] Unit: J [or MeV]
Conservation of
mass-energy
Energy cannot be lost or gained, only transferred from one form to
another. We can measure the energy in a body by multiplying its
mass by c
2
.
Nuclear fission Certain nuclei of large mass number (e.g. Uranium-235) can absorb a
neutron and will then split into (usually) two smaller, beta-
radioactive, nuclei. Two or more neutrons are also released. The
fragments have large kinetic energies. This is nuclear fission.
Chain reaction A chain reaction is repeated events of nuclear fission in a sample of
fissile material, initiated by neutrons released in previous fissions.
Flux leakage Flux leakage is said to occur when not all the magnetic flux produced
by the primary of a trans-former is linked with the secondary (and
vice versa).
Eddy currents Eddy currents consist of charge flowing in closed loops in a
conducting material (rather than being constrained to flow along
wires).
Self-inductance L
of a circuit
This is defined by the equation
Induced emf = -L (I/t)
A level Physics Handbook 2012 St Gregorys Catholic College, Bath
- 40 -
in which AI/At is the rate of change of current through the circuit.
Unit: henry (H) = V s A
-1
Reactance X
L
of an
inductor
When a sinusoidal AC voltage is applied to an inductor, the reactance
is given by X
L
= V
rms
/I
rms
where V
rms
and I
rms
are the rms values of the
voltage across, and the current through, the inductor.
It is equal to eL (or 2tfL) UNIT: O
Reactance X
C
of a
capacitor
When a sinusoidal AC voltage is applied to a capacitor, the reactance
is given by X
C
= V
rms
/I
rms
where V
rms
and I
rms
are the rms values of the
voltage across the capacitor and the current through its connecting
wires.
It is equal to 1/eC (or 1/2tfC). UNIT: O
Phasor This is an imaginary vector rotating at the frequency f of the
alternating current. Its length can represent the r.m.s. (or peak) value
of a currents or a p.d., or the value of a resistance or reactance.
Impedance Z When an AC voltage is applied to a combination of resistance and
capacitance or inductance (or both), the impedance is given by Z
=
V
rms
/I
rms
where V
rms
and I
rms
are the rms values of the voltage across
the combination and the current through it. UNIT: O
Resonance
frequency f
0
of an
LCR circuit
This is the frequency of applied p.d. at which I
rms
is maximum, for a
given V
rms
. For a series LCR circuit it is given by
2f
0
= 1/LC
Q factor of an LCR
circuit
This is defined by
0
2 f L
Q
R
t
= in which f
0
is
the resonance frequency.
The Q factor is a measure of the sharpness of the resonance curve
the larger the Q factor, the sharper the resonance curve.
Corpuscular theory
of light
The theory that light consists of a stream of fast-moving particles
(corpuscles).
Lumiferous ether (or
aether)
Supposed universal medium enabling the propagation of light as
waves, even through space.
Action at a distance The idea that one object (e.g. a charged particle) can exert a force on
another object some distance away, without anything happening in
the space in between.
Magnetic line of
force
Line whose direction at each point along it is the direction of the
magnetic field at that point (i.e. the direction in which the North pole
of a very small compass magnet would point, if placed there).
Vortex ether A supposed space-filling cellular medium whose cells can spin. A
spinning cell is called a vortex (literally a whirlpool). Mechanical
effects in this vortex ether mimic the effects of electric and magnetic
fields, and suggest the existence of electromagnetic waves.
Frame of reference A frame of reference can be pictured as a laboratory with a built-in
grid of rulers, so that x, y and z co-ordinates can be assigned to any
event. There are also closely-spaced synchronised clocks, so that the
event can be assigned a time wherever it occurs.
Inertial frames of
reference
These are frames moving at a constant relative velocity, in which
Newtons laws of motion are obeyed.
A level Physics Handbook 2012 St Gregorys Catholic College, Bath
- 41 -
The Principle of
Relativity
The laws of Physics are the same in all inertial frames of reference.
Proper time interval,
At
This is the time between two events as measured in the frame in
which the events occur at the same place.
Time dilation The time, At, between two events as measured in a frame in which the
events occur at different places is greater than At.
Specifically:
2
2
1
t
v
c
t A
A =
in which v is the velocity of the frame relative to the frame in which
the events occur at the same place.
Crystal Solid in which atoms are arranged in a regular array.
Crystalline solid Solid consisting of a crystal, or of many crystals, usually arranged
randomly. The latter is strictly a polycrystalline solid. Metals are
polycrystalline.
Amorphous solid A truly amorphous solid would have atoms arranged quite randomly.
Examples are rare. In practice we include solids such as glass or brick
in which there is no long range order in the way atoms are arranged,
though there may be ordered clusters of atoms.
Polymeric solid Solid made up of chain-like molecules.
Hookes Law for
rods and wires
The tension in a rod or wire is proportional to its extension from its
natural length, provided the extension is not too great.
Stress Stress is the force per unit cross-sectional area when equal opposing
forces act on a body.
Unit: pascal (Pa) or Nm
-2
.
Strain Strain is defined as the extension per unit length due to an applied
stress. Unit: none
The Young Modulus
Young Modulus
tensile stress
tensile strain
E =
Unless otherwise indicated this is defined for the Hookes Law
region. Unit: Pa or Nm
-2
Ductile material A material which can be drawn out into a wire. This implies that
plastic strain occurs under enough stress.
Elastic strain This is strain that disappears when the stress is removed, that is the
specimen returns to its original size and shape.
Plastic (or inelastic)
strain
This is strain that decreases only slightly when the stress is removed.
In a metal it arises from the sliding of layers of atoms over each other
in the crystals.
Elastic limit This is the point at which deformation ceases to be elastic. For a
specimen it is usually measured by the maximum force, and for a
material, by the maximum stress, before the strain ceases to be
elastic.
Dislocations in
crystals
Certain faults in crystals which (if there are not too many) reduce the
stress needed for planes of atoms to slide. The easiest dislocation to
picture is an edge dislocation: the edge of an intrusive, incomplete
A level Physics Handbook 2012 St Gregorys Catholic College, Bath
- 42 -
plane of atoms.
Grain boundaries The boundaries between crystals (grains) in a polycrystalline
material.
Ductile fracture
(necking)
The characteristic fracture process in a ductile material. Fracture of a
rod or wire is preceded by local thinning, increasing the stress.
Creep This is an increase of strain with time, which may sometimes occur
even if the stress is kept constant.
Fatigue failure If a metal specimen is subjected to many cycles of increasing and
decreasing stress, even if the stress is always below the elastic limit,
the metal is likely to develop cracks and eventually break.
Work-hardening
(cold working)
This is the process of causing inelastic strain in a metal, through at
least one application of stress. It raises the elastic limit, but reduces
the extent of the ductile region between elastic limit and fracture.
Annealing This is the process of heating a metal, in order to alter its mechanical
properties. In the case of copper, it will restore most of the ductility
destroyed by any previous work-hardening.
Quench-hardening This is the heating and rapid cooling of certain steels to produce
changes in the crystal structure. The effects include raising of the
elastic limit.
Brittle material Material with no region of plastic flow, which, under tension, fails by
brittle fracture.
Brittle fracture This is the fracture under tension of brittle materials by means of
crack propagation.
Thermoplastic
polymers
When heated, thermoplastic polymers soften, and at higher
temperatures melt. They solidify on cooling. The process can be
repeated.
Thermosetting
polymers
After one episode of heating and cooling, usually during manufacture
of an object, any further heating of a thermosetting polymer may
result in cracking and/or charring, but not melting.
Elastic hysteresis When a material such as rubber is put under stress and the stress is
then relaxed, the stress-strain graphs for increasing and decreasing
stress do not coincide, but form a loop. This is hysteresis (literally,
lag).
X-ray beam intensity This is the X-ray energy per unit area, per unit time, passing normally
through a surface.
X-ray attenuation This is the decrease in intensity of X-rays as they pass through a
material. The attenuation equation is
I=I
0
e
-x
in which I is the intensity of X-rays having passed through thickness
x of material, and I
0
is the original intensity. is a constant dependent
on the material, called the attenuation constant.
X-ray contrast media These are substances introduced into parts of the body to give greater
contrast between them and surrounding areas. Usually they have high
attenuation constants e.g. barium meals for highlighting stomach
and intestines.
CT scanner or CAT
scanner
A device which collects data on X ray transmission at different angles
through slices of the body, and uses it to produce a 3-dimensional
A level Physics Handbook 2012 St Gregorys Catholic College, Bath
- 43 -
picture. CT stands for computerised tomography. A stands for
axial.
Piezoelectric crystal One of several types of crystal which deform when a voltage is
applied between two of their faces, and which, conversely, produce a
voltage when deformed.
Piezoelectric
transducer
This is a piezoelectric crystal used either to generate ultrasound when
an alternating voltage of ultrasonic frequency is applied, or to sense
ultrasound by producing an alternating voltage in response.
Ultrasonic A scan Scan in which the strength of reflections of an ultrasound pulse from
interfaces in the body is shown by the amplitude of a trace.
Ultrasonic B scan Scan in which the strength of reflection of an ultrasound pulse from
an interfaces in the body is shown by the brightness of a trace. An
array of transducers can produce a two dimensional picture.
Acoustic impedance
Z of a material
The acoustic impedance Z of a material of density , in which the
speed of sound is c, is defined by Z = c . The greater the difference
in Z between two materials the greater the fraction of ultrasound
power reflected at an interface between them.
Coupling medium Gel or oil used to exclude air between the skin and the ultrasound
transducer. It reduces the mismatch in Z, and enables more ultrasound
to enter the body instead of being reflected off the skin.
Doppler effect Change in frequency of waves due to relative motion of wave source,
sensor, medium or reflector.
Precession of spin of
proton
Protons have spin and behave like tiny magnets. In a strong
magnetic field their spins wobble or precess around the magnetic
field direction, rather as a spinning tops axis wobbles around the
vertical. There is a natural frequency of wobble (the Larmor
frequency).
Magnetic resonance
of protons
This is the strong absorption of radio waves of the Larmor frequency
by protons in a magnetic field. It results in the spin flipping direction.
Relaxation time of
spin-flipped protons
This is a characteristic time for spin-flipped protons in hydrogen
atoms in a magnetic field to return to their original spin orientation
when the radio frequency is removed. It depends on, and so tells us
about, the tissue which contains the hydrogen atoms.
Absorbed dose This is the radiation energy (for o, |, or radiation, or for X-rays)
absorbed per kilogram of tissue.
The gray (Gy) This is the unit of absorbed dose.
1 Gy = 1 joule per kilogram
Dose equivalent Dose equivalent is defined as Q absorbed dose, in which Q is a
factor (with no units) which depends on the type of radiation, and
takes account of the degree of biological effect of the radiation.
The sievert (Sv) This is the unit of dose equivalent.
Collimator Device for producing (or selecting) a parallel beam (e.g. of gamma
radiation).
Scintillations These are flashes of light (or u-v radiation) given out by certain
crystals (e.g. sodium iodide) when high energy particles, e.g. gamma
ray photons, strike them.
A level Physics Handbook 2012 St Gregorys Catholic College, Bath
- 44 -
Photomultiplier This is an arrangement of electrodes, with different voltages applied
to them, so that electrons emitted from one electrode by the
photoelectric effect are effectively multiplied in number to make a
much larger current.
Positron emission
tomography (PET)
This is a means of mapping the location in the body of ingested
molecules which have been laced with positron-emitting nuclei. The
positrons annihilate with electrons in adjacent tissue and send out
pairs of gamma photons which are detected outside the body.
Nuclear fusion If light nuclei are brought together with enough kinetic energy they
can join together to make a heavier nucleus. Usually a proton or
neutron is produced as well. The fusion products have more kinetic
energy than the original kinetic energy supplied.
Nuclear fission Certain nuclei of large mass number (e.g. Uranium-235) are fissile:
they can absorb a (slow) neutron and will then split into (usually) two
smaller, beta-radioactive, nuclei. Two or more neutrons are also
released. The fragments have large kinetic energies. This is nuclear
fission.
Enrichment of
uranium
This is the process of increasing the proportion of the fissile U-235 to
the non-fissile U-238 in a sample of uranium.
Breeding
(of Pu-239)
If U-238 is bombarded with fast (high energy) neutrons, the resulting
U-239 decays by beta emission in two steps to produce Pu-239
(plutonium-239). This will undergo fission with slow (thermal)
neutrons. Thus nuclear fuel can been bred from U-238.
Convection This is the transfer of heat by the bulk movement of fluids taking
their random molecular energy with them.
Conduction of heat This is the transfer of heat down a temperature gradient by direct
interactions between particles, without the movement of matter in
bulk.
Thermal conduction
equation and thermal
conductivity, K
The rate of flow of heat, AQ/At through a slice of material of cross-
sectional area A is given by
Q/t = - KA(/x)
in which Au /Ax is the temperature gradient: the temperature
difference across the faces of the slice divided by the thickness of the
slice, and K is a constant for the material called its thermal
conductivity.
The Stefan-
Boltzmann law
[Stefans Law]
The total electromagnetic radiation energy emitted per unit time by a
black body is given by power = A o T
4
in which A is the
bodys surface area and o is a constant called the Stefan constant. [o
= 5.67 10
-8
W m
-2
K
-4
]
Wiens displacement
law
The wavelength of peak emission from a black body is inversely
proportional to the absolute (kelvin) temperature of the body.
max
= W/T [W = Wien Constant = 2.90 x 10
-3
m K]
Solar constant This is the solar radiated energy crossing a plane perpendicular to the
line joining the Earth to the Sun, just outside the Earths atmosphere,
per second per unit area. The mean value is 1.35 kW m
-2
.
Greenhouse effect The glass of a greenhouse transmits the visible radiation from the Sun
(and some of the near ultraviolet and near infrared), but does not
transmit the far-infrared given out by the cool contents of the
A level Physics Handbook 2012 St Gregorys Catholic College, Bath
- 45 -
greenhouse, instead absorbing it and re-radiating back into the
greenhouse. Certain gases in the Earths atmosphere act much like
greenhouse glass. The higher the concentration of these gases the
warmer the Earths surface and atmosphere.
Fuel cell Device which uses chemical energy from fuel to provide electrical
energy directly. In a hydrogen fuel cell hydrogen combines with
oxygen producing electrical energy the electrolysis of water in
reverse.
Heat engine Device which takes a working substance (e.g. a gas) through a cycle
of changes during which it does a net amount of work, equal to the
net heat flowing in.
Carnot cycle An ideal reversible cycle, ABCDA, for a heat engine. AB is
isothermal, heat Q
1
flowing in at temperature T
1
. In BC no heat flows,
but the working substance cools (as it does work). CD is isothermal,
heat Q
2
flowing out at temp. T
2
. [T
1
> T
2
and Q
1
> Q
2
] In DA there is
no heat flow but the temperature of the working substance rises (back
to T
1
) as it has work done on it. The net amount of work done by the
working substance during the cycle is Q
1
- Q
2
.
Efficiency of Carnot
cycle
This is defined as
Useful Energy/Energy input x100% = ((Q1 Q2) / Q1) x 100%
This applies to any heat engine which takes heat in at one
temperature and gives it out at one other. For a Carnot cycle, Q
1
/ Q
2
=
T
1
/ T
2
, so efficiency = [(T
1
- T
2
)/ T
1
]100
Refrigerator This device runs a heat engine cycle in reverse, so that for a net input
of work, heat is taken in at a lower temperature and given out at a
higher temperature, the purpose being to keep cool the source from
which the heat is taken.
Heat pump This is essentially a refrigerator, but with the purpose of supplying
heat at the higher temperature. The amount supplied (per cycle) is
greater than the work input, because of the (low temperature) heat
input.
Coefficient of
performance of
refrigerator
This is defined by
COP = (Heat extracted from cold source / work input) x100%
Coefficient of
performance of
heat pump
This is defined by
COP = (Heat delivered from hot sink / work input) x100%
Note that the different top lines reflect the different purposes of the
heat pump and the refrigerator.
Second Law of
thermodynamics
No process is possible the only result of which is the total conversion
of heat into work.
A level Physics Handbook 2012 St Gregorys Catholic College, Bath
- 46 -
5 Practical assessment
5.1 G483 (AS) & G486 (A2)
This unit develops practical and investigative skills developed within contexts encountered during AS
Physics.
Candidates are required to carry out three tasks:
1. Qualitative task [10 marks]
2. Quantitative task [20 marks]
3. Evaluative task [10 marks]
The qualitative and quantitative tasks will test skills of observation and measurement.
Candidates will carry out these tasks under controlled conditions.
Each task will be internally assessed using a mark scheme provided by OCR.
Candidates may attempt more than one task from each category with the best mark from each category
being used to make up the overall mark.
How Science Works
5a Carry out experimental and investigative activities, including appropriate risk management, in a range of
contexts.
5b Analyse and interpret data to provide evidence, recognising correlations and causal relationships.
5c Evaluate methodology, evidence and data, and resolve conflicting evidence.
The mark schemes supplied by OCR will be based on the following generic criteria.
1. Qualitative task
Candidates carry out a
practical task using
instructions supplied by OCR.
Candidates should be able to:
(a) Demonstrate skilful and safe practical techniques using suitable
qualitative methods.
(b) Make, record and communicate valid observations; organise results
suitably.
2. Quantitative task
Candidates carry out a
practical task using
instructions supplied by OCR.
(a) Demonstrate and describe safe and skilful practical techniques for a
quantitative experiment.
(b) Make, record and communicate reliable measurements with appropriate
precision and accuracy.
(c) Analyse the experimental results.
(d) Interpret and explain the experimental results.
3. Evaluative task
This task will extend the
quantitative task.
Candidates will evaluate the
quality of the data and
procedures. Evaluative tasks
will not require additional data
collection.
(a) Evaluate the results and their impact on the experimental
methodology.
(b) Assess the reliability and accuracy of the experiment by calculating
percentage differences and uncertainties.
(c) Evaluate the methodology with a view to improving experimental
precision and accuracy.
(d) Identify weaknesses in the experimental methodology and
measurements.
(e) Understand and suggest improvements to the experimental
procedures and measurements.
The tasks
Tasks, mark schemes and guidance for teachers and technicians can be downloaded from the OCR
Interchange site.
A level Physics Handbook 2012 St Gregorys Catholic College, Bath
- 47 -
5.2 Definitions
Resolution
This is the smallest change in the quantity being measured by a measuring instrument which
gives a perceptible change in the output.
With a digital instrument this is usually 1 in the least significant figure of the output; e.g. a
digital ammeter which gives an output in mA with two decimal places has a resolution of 0.01
mA. With analogue instruments there is no such clear cut answer. For practical purposes,
WJEC takes the resolution of an analogue instrument to be the interval between the smallest
scale divisions, e.g. a metre rule has a resolution of 1 mm (however we allow full marks for
candidates taking half the smallest scale division or similar).
Uncertainty
Uncertainty in measurements is unavoidable and estimates the range within which the answer
is likely to lie. This is usually expressed as an absolute value, but can be given as a percentage.
The normal way of expressing a measurement x
0
, with its uncertainty, u, is x
0
u. This means
that the true value of the measurement is likely to lie in the range x
0
u to x
0
+ u.
For a single static measurement, the uncertainty in the reading can be taken as the resolution
of the instrument. If this is impractical [as with some dynamic measurements] then candidates
will be given the uncertainty in the question. Alternatively they may be asked to estimate the
uncertainty from several readings.
Note: The term error is used in many textbooks instead of uncertainty. This term implies that
something has gone wrong and is therefore best avoided.
Uncertainties can be split up into two different categories:
- Random uncertainties These occur in any measured quantity. The uncertainty of
each reading cannot be reduced by repeat measurement but the more measurements
which are taken, the closer the mean value of the measurements is likely to be to the
true value of the quantity. Taking repeat readings is therefore a way of reducing the
effect of random uncertainties.
- Systematic uncertainties These can be due to a fault in the equipment, or design of
the experiment e.g. possible zero error such as not taking into account the resistance of
the leads when measuring the resistance of an electrical component or use of a ruler at a
different temperature from the one at which it is calibrated. The effect of these cannot be
reduced by taking repeat readings. If a systematic uncertainty is suspected, it must be
tackled either by a redesign of the experimental technique or theoretical analysis. An
example of this sort of uncertainty, the origin of which remains mysterious, is in the
determination of stellar distances by parallax. The differences between the distances, as
determined by different observatories, often exceeds the standard uncertainties by a
large margin.
A level Physics Handbook 2012 St Gregorys Catholic College, Bath
- 48 -
Percentage uncertainty
This is the absolute uncertainty expressed as a percentage of the best estimate of the true value
of the quantity.
Resolution
This is the smallest quantity to which an instrument can measure
Mistake
This is the misreading of a scale or faulty equipment.
Suspect results
These are results that lie well outside the normal range e.g. points well away from a line or
curve of best fit. They often arise from mistakes in measurement. These should be recorded
and reason for discarding noted by the candidate.
For a single static measurement, the uncertainty in the reading can be taken as the resolution
of the instrument. If this is impractical [as with some dynamic measurements] then candidates
will be given the uncertainty in the question. Alternatively they may be asked to estimate the
uncertainty from several readings.
Accuracy
A measurement result is considered to be accurate if it is judged to be close to the true value. It
is a quality denoting the closeness of agreement between a measured value and the true value
of that quantity. Accuracy normally refers to an individual result. It can be influenced by
random and systematic errors. It is also possible to refer to the accuracy of the mean value of a
set of results. If there are no systematic errors, the greater the number of measurements the
more accurate the mean value is, i.e. the closer it is expected to be to the true value. If there is
a systematic error in the readings, e.g. because of a zero error, additional measurements will
not improve the accuracy of the mean.
A level Physics Handbook 2012 St Gregorys Catholic College, Bath
- 49 -
5.3 Measuring instruments
The following is a selection of measuring instruments which provide some guidance as to the
skills you can be expected to demonstrate in unit 3 and 6.
The use of the following in the context of individual experiments:
- micrometers and callipers. These may be analogue or digital. It is intended that candidates
will have experience of the use of these instruments with a discrimination of at least
0.01 mm. A typical use is the determination of the diameter of a wire.
- digital top-loading balances.
- measuring cylinders and burettes. This is largely in the context of volume and density
determination.
- force meters (Newton meters).
- stop watches with a discrimination of 0.01 s. It is also convenient to use stopwatches /
clocks with a discrimination of 1 s.
- rules with a discrimination of 1 mm.
- digital multimeters with voltage, current and resistance ranges. The following (d.c.) ranges
and discriminations illustrative the ones which are likely to be useful:
2 V 0.001 V
20 V 0.01 V
10 A 0.01 A
2 A 0.001 A
2 kO 1 O
200 O 0.01 O
Students should be familiar with the technique of starting readings on a high range to
protect the instrument.
- liquid in glass thermometers. -10 110C will normally suffice, though candidates can be
usefully introduced to the advantages of restricted range thermometers. Where appropriate,
digital temperature probes may be used.
A level Physics Handbook 2012 St Gregorys Catholic College, Bath
- 50 -
5.4 Experimental techniques & advice for specific apparatus
Metre Rule
Take the resolution as 1 mm. This may be unduly pessimistic, especially if care is taken to avoid parallax
errors. It should be remembered that all length measurements using rules actually involve two readings one
at each end both of which are subject to uncertainty. In many cases the uncertainty may be greater than this
due to the difficulty in measuring the required quantity, for example due to parallax or due to the speed needed
to take the reading e.g. rebound of a ball, in which case the precision could be 1 cm. In cases involving
transient readings, it is expected that repeats are taken rather than relying on a guess as to the uncertainty.
Standard Masses
For 20g, 50g, 100g masses the precision can be taken as being as being 1g this is probably more accurate
than the manufacturers [often about 3%]. Alternatively, if known, the manufacturers uncertainty can be used.
Digital meters [ammeters/voltmeters]
The uncertainty can be taken as being the smallest measurable division. Strictly this is often too accurate as
manufacturers will quote as bigger uncertainty. [e.g. 2% + 2 divisions]
Thermometers
Standard -10 C to 110 C take precision as 1C
Digital thermometers uncertainty could be 0.1C. However the actual uncertainty may be greater due to
difficulty in reading a digital scale as an object is being heated or cooled, when the substance is not in thermal
equilibrium with itself let alone with the thermometer..
The period of oscillation of a Pendulum/Spring
The resolution of a stop watch, used for measuring a period, is usually 0.01s. Reaction time would increase the
uncertainty and, although in making measurements on oscillating quantities it is possible to anticipate, the
uncertainty derived from repeat readings is likely to be of the order of 0.1 s. To increase accuracy, often 10 (or
20) oscillations are measured. The absolute error in the period [i.e. time for a single oscillation] is then
1
/
10
(or
1
/
20
respectively) of the absolute error in the time for 10 (20) oscillations
e.g. 20 oscillations: Time = 15.8 0.1 s [0.6%] Period
15.8 0.1
20
= s = 0.790 0.005 s
Note that the percentage uncertainty, p, in the period is the same as that in the overall time.
In this case,
0.1
100% 0.6%
15.8
p = = (1 s.f.)
Digital vernier callipers/micrometer
Precision smallest measurable quantity usually 0.01mm
Measuring cylinder / beakers/ burette
Smallest measurable quantity e.g. 1 cm, but this depends upon the scale of the instrument. In the case of
measuring the volume using the line on a beaker, the estimated uncertainty is likely to be much greater.
A level Physics Handbook 2012 St Gregorys Catholic College, Bath
- 51 -
Note candidates must be careful to avoid parallax when taking these measurements, and should state that all
readings were taken at eye level. They should also measure to the bottom of the meniscus.
A level Physics Handbook 2012 St Gregorys Catholic College, Bath
- 52 -
5.5 Calculating uncertainties
5.5.1 Estimation of uncertainty using the spread of repeat readings.
Suppose the value a quantity x is measured several times and a series of different values obtained:
x
1
, x
2
, x
3
..x
n
. [Normally, in our work, n will be a small number, say 3 or 5].
Unless there is reason to suspect that one of the results is seriously out [i.e. it is anomalous], the best
estimate of the true value of x is the arithmetic mean of the readings:
Mean value
1 2
........
n
x x x
x
n
+ +
=
A reasonable estimate of the uncertainty is the range:
i.e.
max min
2
x x
u
= , where
max
x is the maximum and
min
x the minimum reading of x [ignoring any
anomalous readings]
Example
The following results were obtained for the time it took for an object to roll down a slope.
4.5 s, 4.8 s, 4.6 s, 5.1 s, 5.0 s
The best estimate of the true time is given by the mean which is:
4.5 4.8 4.6 5.1 5.0
4.8s
5
t
+ + + +
= =
The uncertainty, u, is given by:
5.1 4.5
0.3s
2
u
= =
The final answer and uncertainty should be quoted, with units, to the same no. of decimal places and
the uncertainty to 1 sig. fig
i.e. t = 4.8 0.3 s
Note that, even if the initial results had be taken to the nearest 0.01 s, i.e. the resolution of an electronic
stopwatch, the final result would still be given to 0.1 s because the first significant figure in the
uncertainty is in the first place after the decimal point.
The percentage uncertainty, p
0.3
100% 6%
4.8
= = . Again, p is only expressed to 1 s.f.
5.5.2 Estimation of uncertainty from a single reading
Sometimes there may only be a single reading. Sometimes all the readings may be identical. Clearly it
cannot be therefore assumed that there is zero uncertainty in the reading(s).
With analogue instruments, it is not expected that interpolated readings will be taken between divisions
(this is clearly not possible with digital instrument anyway). Hence, the uncertainty cannot be less than
the smallest division of the instrument being used, and is recommended it be taken to be the
smallest division. In some cases, however, it will be larger than this due to other uncertainties such as
reaction time [see later] and manufacturers uncertainties. If other sources of random uncertainty are
present, it is expected that in most cases repeat readings would be taken and the uncertainty estimated
from the spread as above.
A level Physics Handbook 2012 St Gregorys Catholic College, Bath
- 53 -
5.5.3 Determining the uncertainties in derived quantities.
Please note that candidates entered for AS award will be required to combine percentage uncertainties.
Very frequently in Physics, the values of two or more quantities are measured and then these are combined to
determine another quantity; e.g. the density of a material is determined using the equation:
m
V
=
To do this the mass, m, and the volume, V, are first measured. Each has its own estimated uncertainty and
these must be combined to produce an estimated uncertainty in the density. The volume itself may have been
determined by combining several independent quantity determinations [e.g. length, breadth and height for a
rectangular solid or length and diameter for a cylindrical wire].
In most cases, quantities are combined either by multiplying or dividing and this will be considered first.
Multiplying by a constant, squaring (e.g. in
3
4
3
r t ), square rooting or raising to some other power are special
cases of this and will be considered next.
1. Multiplying and dividing:
The percentage uncertainty in a quantity, formed when two or more quantities are combined by either
multiplication or division, is the sum of the uncertainties in the quantities which are combined.
Example
The following results were obtained when measuring the surface area of a glass block with a
30cm rule, resolution 0.1cm
Length = 9.7 0.1 cm
Width = 4.4 0.1cm
Note that these uncertainties are estimates from the resolution of the rule.
This gives the following percentage errors:
Length:
L
0.1
100% 1.0%
9.7
p = =
Width
W
0.1
100% 2.2%
4.4
p = =
So the percentage error in the volume,
V
1.0 2.2 3.2% p = + =
Hence surface area = 9.7 4.4 = 42.68 cm 3.2 %
The absolute error in the surface area is now 3.2% of 42.68 = 1.37 cm
Quoted to 1 sig. fig. the uncertainty becomes 1 cm
The correct result, then, is 43 1cm - Note that surface area is expressed to a number of
significant figures which fits with the estimated uncertainty.
2. Raising to a power (eg x
2
, x
1
, x )
The percentage uncertainty in x
n
is n times the percentage uncertainty in x.
e.g. a period (T) is as being 31 seconds with a percentage uncertainty of 2 %,
So T
2
= 961 4%.
4% 961 = 40 (to 1.s.f)
So the period is expressed as T = 960 40 s.
A level Physics Handbook 2012 St Gregorys Catholic College, Bath
- 54 -
Note: x
1
is the same as
1
/x. So the percentage uncertainty in
1
/x is the same as that in x. Can you see
why we ignore the sign?
Note: the percentage uncertainty in x is half the percentage uncertainty in x.
3. Multiplying by a constant
In this case the percentage uncertainty is unchanged. So the percentage uncertainty in 3x or 0.5x or tx
is the same as that in x.
Example: The following determinations were made in order to find the volume of a piece of
wire:
Diameter: d = 1.22 0.02 mm
Length: l = 9.6 0.1 cm
The percentage uncertainties are: p
d
= 1.6%; p
l
= 1.0%.
Working in consistent units, and applying the equation
2
4
d
V l
t
= , we have:
V = 448.9 mm
3
The percentage uncertainty, p
V
= 1.6 2 + 1.0 = 4.2 % = 4 % (to 1 s.f.)
[Note that t and 4 have no uncertainties.]
So the absolute uncertainty u = 448.9 0.04 = 17.956 = 20 (1 s.f.)
So the volume is expressed as V = 450 20 mm
3
.
4. Adding or subtracting quantities [A2 only]
If 2 quantities are added or subtracted the absolute uncertainty is added. This situation does not arise
very frequently as most equations involve multiplication and division only. The e.m.f. / p.d. equation
for a power supply is an exception.
In all cases, when the final % uncertainty is calculated it can then be converted back to an absolute uncertainty
and quoted 1 sig. figure. The final result and uncertainty should be quoted to the same number of decimal
places
A level Physics Handbook 2012 St Gregorys Catholic College, Bath
- 55 -
5.6 Significant figures
5.6.1 Raw data
In all cases we expect candidates to quote any raw data obtained to a number of significant figures consistent
with the resolution of the instrument used.
For example, the width of a microscope slide could be measured using a metre rule, a pair of vernier callipers
or a micrometer:
The resolution of a metre ruler is 1mm and so the width could be correctly written as 25 1 mm
However, if we use a pair of vernier callipers of resolution 0.1 mm then a (possible) correct value could be
25.2 0.1 mm. Similarly 25.0 0.1 mm would be correct; however writing this as 25 0.1mm would be
incorrect.
Finally, if we use a micrometer of resolution 0.01 mm, then a possible value would be written as 25.21
0.01mm.
Note that it is not the number of significant figures which is the issue here; the place of the least significant
figure should reflect the resolution of the instrument. It is expected that the number of significant figures
increases as an instrument with a greater resolution is used i.e. 2 significant figures for the metre ruler, 3 for
the vernier callipers and 4 for the micrometer [unless it is used for determining a very small dimension, e.g.
the diameter of a human hair]. It is probably worth noting that it is very rare for measured values, using school
laboratory equipment to be quoted to more than 3 or 4 significant figures.
5.6.2 Processing raw data
When processing raw data it is usual for the processed data to be given the same number of significant figures
as the raw data e.g. when determining averages, or square or square root values.
One example is finding the period of oscillation from the timings of 20 oscillations. For a stopwatch of
resolution 0.1 s the time taken for 20 oscillations was:
Time for 20 oscillations
Period
Reading 1 Reading 2 Reading 3 Mean
(s) (s) (s) (s) (s)
20.3 20.4 20.6 20.4 1.02
In this case all raw data and processed data are quoted to 3 significant figures. For a series of measurements,
possibly for pendulums of different lengths, all the readings should be quoted to the resolution of the
stopwatch (and not necessarily the same number of significant figures). E.g. for a pendulum with a shorter
length:
Time for 20 oscillations Period
Reading 1 Reading 2 Reading 3 Mean
(s) (s) (s) (s) (s)
8.7 8.4 8.6 8.6 0.43
The whole row is now quoted to the same number, 2, significant figures. This is different from the first set of
readings. It would be incorrect to give this second set of readings to 3 significant figures as it would imply a
precision that wasnt there with readings of 8.70, 8.40 .... implying an uncertainty of 0.01 s.
It is important to remember that when manipulating a value its uncertainty needs to be manipulated in the
same way, e.g. when dividing a value by 10, the uncertainty needs to be divided by 10 also.
Note that significant figures will only be assessed in the practical modules (PH3 and PH6) and not in any of
the theory modules.
A level Physics Handbook 2012 St Gregorys Catholic College, Bath
- 56 -
Quoting uncertainties
The uncertainty, and also the resolution, should be quoted to a maximum of 2 significant figures, with
generally 1 significant figure being sufficient, especially if the significant figure is greater than 2. E.g. writing
0.15 or 18% is acceptable because both values are less than 2 however it would be advisable to write 5.5
as 6. For marking purposes we will accept 1 or 2 significant figures but no more than 2.
Derived data
When more than one result is being used to determine an overall value by using a formula or equation, the
number of significant figures used is determined by the overall, or absolute, uncertainty. Candidates will be
asked to calculate a percentage uncertainty and from this find the absolute uncertainty. The value quoted
should then be consistent with this value e,g. for a solution of 50.3578 5%, this would give an absolute
uncertainty of 0.251789. This should then be quoted to 1 [or 2] significant figures i.e. 0.3 [or 0.25] so
the final result should be written as 50.4 0.3 [or, just, 50.36 0.25].
How many significant figures / decimal places in derived data?
The result from a calculation should be given to no more significant figures than can be justified. This is
usually straightforward when a value for the estimated uncertainty is known. For example, if a resistance value
is calculated as 359.27 O and the estimated uncertainty is 5 O, the result should be quoted as 359 5 O.
Note again that we normally give the uncertainty to 1 significant figure.
Very often however, no information is given about the uncertainty in the data which are used in the
calculation; e.g. the length of a rectangular strip is 48.3 cm and the width is 8.9 cm. What is its area?
Simple multiplication of the two numbers gives the area as 48.1 8.9 cm
2
= 428.09 cm
2
. The basic safe rule
of thumb is to quote the answer to the smallest number of significant figures in the data. In this case 8.9 cm
has 2 significant figures, so the answer should be given as 430 cm
2
[2 significant figrues]. On this occasion the
rule is a little pessimistic. Suppose we knew that the original data were 0.1 cm then:
Percentage uncertainty in length =
0.1
100
48.1
= 0.2 %
Percentage uncertainty in width =
0.1
100
8.9
= 1.1%
So the total percentage uncertainty is 1.3 %. Now 1.3% of 428.09 cm
2
= 5.6 cm
2
, so if we knew the original
uncertainties wed express the final value for the area as 428 6 cm
2
. For this reason either 2 or 3 significant
figures in the answer is acceptable. We just need to remember, if we quote the answer to 3 significant figures,
that we are less sure of the last significant figure than we were in the data.
The same rule of thumb holds when we are raising to a power, e.g. finding the volume of a sphere of radius
5.35 mm.
Volume =
3
4
3
r t = 641.43 mm
3
[by calculation]
We should express this to 3 significant figures, in line with the data, so volume = 641 mm
3
.
Exercise: Assume that the uncertainty in the radius is 0.01 mm. What is the uncertainty in the volume? Are
3 significant figures justified?
A level Physics Handbook 2012 St Gregorys Catholic College, Bath
- 57 -
Can we ever justify giving more significant figures in the result than in the data?
What is the circumference of a cylinder which has a diameter of 3.5 m? [i.e. 2 significant figures]
Circumference = tD = 10.996 m [by calculation].
If we assume that the original diameter was 0.1 m that gives a percentage uncertainty of ~3%. Using the
calculated figure for circumference with 3% uncertainty gives 0.33 m so it would be reasonable to quote the
circumference as 11.0 m, i.e. 3 significant figures. This happens when the answer has a low first significant
figure [1] but the data had a higher first significant figure [3].
Should we ever give fewer significant figures in the result than in the data?
2 microscope slides [assumed to be identical], placed end to end have a total length of 19.5 cm
[3 significant figures]. What is the length of a single microscope slide?
Again, assume an uncertainty of 0.1 cm in the total length. This represents 0.5%.
By calculation: 0.5 19.5 cm = 9.75 cm and 0.5% of 9.75 cm = 0.05 cm.
This suggests that it would be reasonable to quote the length of a single slide as 9.75 cm but to bear in mind
that the uncertainty in this figure is much more than 1 in the last place. On the other hand, it would not be
unreasonable to quote the length as 9.8 cm.
So, the answer to how many significant figures are justified in derived data is that it is usually safe to give the
same number of significant figures as the least accurate piece of data, but that it is often justified to go up by 1
significant figure in the answer, especially if the first significant figure in the answer is lower than in the data.
Another example: Finding 1/T e.g. when wanting to plot 1/T versus m for a spring oscillating:
T = 1.01 s hence 1/T = 0.99 (3s.f. to 2s.f.)
T = 0.99 s hence 1/T = 1.01 (2s.f. to 3s.f.)
A level Physics Handbook 2012 St Gregorys Catholic College, Bath
- 58 -
5.7 Graphs
[derivation of uncertainties from graphs is only expected in A2]
The following remarks apply to linear graphs:
The points should be plotted with error bars. These should be centred on the plotted point and have a length
equal to y
max
y
min
[for uncertainties in the y values of the points]. If identical results are obtained the
precision of the instrument could be used. If the error bars are too small to plot this should be stated.
If calculating a quantity such as gradient or intercept the steepest line and a least steep line should be drawn
which are consistent with the error bars. It is often convenient to plot the centroid of the points to help this
process. This is the point
( )
, x y , the mean x value against the mean y value. The steepest and least steep lines
should both pass through this point.
.
The maximum and minimum gradients, m
max
and m
min
, [or intercepts, c
max
and c
min
] can now be found and the
results quoted as:
gradient =
max max min min
2 2
m m m m +
intercept =
max max min min
2 2
c c c c +
Scales
Graph should cover more than of the graph paper available and awkward scales [e.g. multiples of 3] should
be avoided. Rotation of the paper through t/4 [90 !] may be employed to give better coverage of the graph
paper.
Semi-log and log-log graphs [A2 only]
Students will be expected to be familiar with plotting these graphs as follows:
Semi-log: to investigate relationships of the form:
x
y ka = .
Taking logs: log log log y k x a = + or ln ln ln y k x a = + [It doesnt matter which]
So a plot of log y against x has a gradient loga and an intercept logk .
Examples: Radioactive or capacitor decay, oscillation damping
Log-log: to investigate relationships of the form:
n
y Ax =
Taking logs: log log log y A n x = + [or the equivalent with natural logs]
So a plot of log y against log xhas a gradient n and an intercept log A.
Examples: Cantilever depression or oscillation period as a function of overhang length, Gallilean
moon periods against orbital radius to test relationship.
Note that Log-log or semi-log graph paper will not be required.
Uncertainties from Log graphs: Candidates will not be expected to include error bars in log plots.
A level Physics Handbook 2012 St Gregorys Catholic College, Bath
- 59 -
5.8 Advice to AS candidates in practical physics
- Before commencing any question read the whole question through completely.
- Where possible, repeat all readings so that you may calculate the best value and its uncertainty. If
repeat readings are not required the question will state so. Record all readings, including repeat
readings, and state the units.
- Express any answers including the gradients and intercepts of graphs to a sensible number of
significant figures together with the units.
- Show all intermediate steps in calculations as credit will be given for a correct approach even if the
final answer is faulty.
- Where the question requires it, estimate the uncertainty and/or the percentage uncertainty in a
measured or calculated quantity and express your result as the quantity its uncertainty [see below].
Graphs
- Include a title; insert clearly on each axis a scale and a label with units..
- Make sure the scales are convenient to use, so that readings may easily be taken from the graph avoid
scales which use factors of 3 and that the plotted points occupy at least half of both the vertical and
horizontal extent of the graph grid.
- First consider carefully whether your plotted points suggest a straight line or a curve. Then either draw
in your best line with the aid of a ruler or your curve by freehand sketch.
- When extracting data from a graph, use the best-fit line rather than the original data.
- When determining the gradient of a graph, show clearly on your graph the readings you use. This is
most conveniently done by drawing a right angled triangle this should be large so that accuracy is
preserved.
A level Physics Handbook 2012 St Gregorys Catholic College, Bath
- 60 -
Uncertainties
1. Expressing uncertainties
Use the form x u, where x is the quantity being measured and u its estimated uncertainty.
2. Estimating uncertainties using the resolution of an instrument.
If a single reading is taken and there is no reason to believe that the uncertainty is greater, take the
uncertainty to be the instrument resolution.
3. Estimating uncertainties using the spread of readings.
Take the best estimate of the quantity you are determining as the mean of your readings, and the
estimated uncertainty to be half the spread in the readings, discounting any suspect readings.
i.e.
max min
2
x x
u
=
4. Percentage uncertainties
The percentage uncertainty, p, is calculated from:
Estimated uncertainty
100%
Mean value
p =
Uncertainties in calculated quantities
1. If your result is calculated by multiplying and/or dividing two or more quantities, each of which has
its own uncertainty, the percentage uncertainty in the result is found by adding the percentage
uncertainties in the quantities from which it is derived.
e.g. If is calculated using
ay
D
= , the percentage uncertainty in is:
a y D
p p p p
= + + .
2. If a quantity is calculated by multiplying by a constant, the percentage uncertainty is unchanged.
3. If a quantity is raised to a power, e.g. x
2
, x
3
or x , the percentage uncertainty is multiplied by the
same power.
Example of 2 and 3: The energy, E, stored in a stretched spring is given by
2
1
2
E kx = . Both k and x have
uncertainties, but
1
2
has no uncertainty.
So 2
E k x
p p p = +
A level Physics Handbook 2012 St Gregorys Catholic College, Bath
- 61 -
6 Skills
6.1 Setting out your calculations
It is really important that you learn to set out your calculations properly. This will help you to avoid making
mistakes as the calculations get harder, and ensure that you get all available marks in the exam. Use the
guide below as a template.
Mr Burn is stopped at traffic lights and then accelerates away for 0.9s before reaching a constant speed. In
this time he travels 4.50m
What is his average acceleration?
1. Write down the information given in the question.
s = 4.5m
u = 0
a = ?
t = 0.90s
2. Write down the formula that you are going to use.
s = ut + at
2
3. Rearrange so that the equation it is in terms of the variable you are calculating.
a = 2(s ut)
t
2
4. Substitute in the numbers and calculate an answer.
a = 2 (4.5 - 0)
0.902
= 11.11111
5. Write the final answer with significant figures to match the question and use the correct units.
a = 11 ms
-2
A level Physics Handbook 2012 St Gregorys Catholic College, Bath
- 62 -
6.2 Rearranging equations
First thing to do is to be confident in yourself you
can probably do all this using numbers or common
sense its just when it comes to putting letters in
that it seems a little strange. Every time well start
with real numbers, and then swap letters in for
real numbers to get general equations.
Well start with equations that have no +s and s
in them.
3 x 2 = 6
Using only numbers, what does 3 equal?
3 = 6
2
Whats happened? Ive taken the 2 from the left
hand side to leave the 3 on its own. Changing
sides, its gone from being on the top line to the
bottom.
The same thing happens if we start with the first
equation and rearrange it to leave the 2 on its
own:
2 = 6
3
We could write this as:
2 = 6 x 1
3
This seems a bit pointless until
Taking the 6 over to the other side, using the same
pattern it was on the top so it goes to the
bottom
2 = 1
6 3
Same thing now using letters:
if ab = c
(which means a x b we dont usually put the x
sign in)
then a = c
b
and if m = n , then m = 1 and 1 = 1
p n p n mp
just keep swapping from top to bottom as things
change from the left side to the right.
Rule 1: In an equation, if we take a letter from
one side to the other it changes from top to
bottom (or vice versa).
Q1 cd = e rearrange to show c =
f
Q2 a = c . i) what does c equal?
b d ii) rearrange to show b =
Q3 y = xw . i) what does x = ?
z ii) what does z = ?
What about other powers?
Lets say youve got
x
2
w = y
z
and you want to find out what x equals.
Treat the x
2
as a thing on its own so you should get
x
2
= zy
w
Ultimately important rule
Rule 2: Whatever you do to one side of an
equation, you do to the other.
So to find x, we have to square root x
2
. This means
we have to square root the right hand side too, so
X =
w
zy
Q4 a = bc
2
what does c = ?
Q5 g = GM what does r = ?
r
2
Q6 V = 4r
3
what does r = ?
3
What about equations with + and -?
The key thing is rule 2 whatever you do to one
side of an equation, you do to the other.
If you had a bowl with 4 apples and 3 bananas in
it, and you only wanted two apples, youd have to
take away the bananas. In algebra, if youve got
4a + 3b and want only as, you have to take the 3b
away.
If 4a + 3b = 7 and we want a = something, we have
to take 3b away from the left hand side (LHS), but
by rule 2 we have to take 3b away from the RHS.
So now 4a = 7 3b
So the effect is what was +3b on one side has
become -3b on the other.
A level Physics Handbook 2012 St Gregorys Catholic College, Bath
- 63 -
Rule 3: in an equation, if we take a letter from
one side to the other, it changes from + to (or
vice versa).
To find a on its own, we have to divide the LHS by
4. Rule 2 same for RHS, so
a = (7-3b)
4
Ive put brackets round the RHS to show were
dividing the whole lot by 4.
Another way of showing this is
A = (7-3b)
Q7 5x 4y = 17 What does x = ?
What does y = ?
Q8 a = 8 4f What does f = ?
Q9 x
2
y
2
= 1 What does x = ?
What does y = ?
Q10 3x + y
2
= 4z What does x = ?
What does y = ?
Now, putting ideas together
Q11 x
2
- 4y = 3z
2
What does x = ?
What does y = ?
Q12 x + 5 = 12 What does x = ?
y
2
What does y = ?
Answers: (If you do get some wrong and cant see
why, ASK!)
1: c = e .
df
2: i) c = ad ii) b = ad
b c
3: i) x = yz ii) z = xw
w y
4:
b
a
5:
g
GM
6: r =
4
3V
3
7: x = 17 + 4y y = 5x 17
5 4
8: f = 8 a
4
9: x =
2
y 1+ y = 1 x
2
10: x = 4z y
2
y =
4
x 3 z 4 -
3
11: y = x
2
15z
3
4
12: y =
12
5 + x
A level Physics Handbook 2012 St Gregorys Catholic College, Bath
- 64 -
6.3 Powers of ten and standard form
a
2
means a x a so
10
2
means 10 x 10 = 100
10
3
means 10 x 10 x 10 = 1000
so the power of ten means how many tens have to
be multiplied together, and it also tells you the
number of 0s after the 1 1000 has a 1 and three
0s, so it is 10
3
.
Q1 Write in full, 10
7
and 10
4
Q2 Write as a power of 10, 100 000
and 100 000 000.
Multiplying
100 x 1000 = 100 000
Doing this in powers of 10 we get:
10
2
x 10
3
= 10
5
What have we done to the powers?
Added them!
Rule 1: When we multiply numbers which are
powers of 10 we simply add the powers.
Q3 What is 10
5
x 10
2
written both as a power of 10
and in longhand?
Dividing
100 000 divided by 100 = 1000 (doesnt it?)
Doing this in powers of 10
10
5
divided by 10
2
= 10
3
What have we done with the powers? Subtracted
them!
Rule 2: When we divide numbers which are
powers of 10 we simply subtract the powers.
Q4 What is 10
6
/10
4
written both as a power of 10
and in longhand?
Fractions
100 = 1 .
1000 10
In powers of 10 we get 10
2
10
3
And if we use our dividing rule then this is 10
2-3
=
10
-1
so 10
-1
must mean 1 . = 1 .
10
1
10
Likewise 100 . = 10
2
. = 10
2-5
= 10
-3
100 000 10
5
And what about. 100.? 100. = 10
2
. = 10
2-2
= 10
0
100 100 10
2
But 100 = 1, so 10
0
= 1
100
In fact its fairly straight forward to show that
anything to the power of nought = 1.
So 1 . = 10
0
. = 10
0-3
= 10
-3
1000 10
3
Rule 3: 10-to-the-minus-something just means 1-
over-10-to-the-something.
Rule 4: 10-to-the-nought equals 1.
Q5 Write these as powers of ten:
1.100 1/100 000 a millionth
Standard form
3000 is just three lots of 1000, so 3000 = 3 x 1000
= 3 x 10
3
and 263 is 2.63 lots of 100, so 263 = 2.63 x 100
= 2.63 x 10
2
To turn something into standard from you need
the number part, between 1 and 9.999 and a
power of 10. The simplest way is to put a decimal
point (dp) in after the first number, and then how
ever many numbers there are after the decimal
point is the power of 10.
e.g. 416 000 in standard from:
Put a dp in after the first number 4.16000
there are five numbers after the dp, so the
number is 4.16 x 10
5
(we dont need to put in all
the spare noughts).
A level Physics Handbook 2012 St Gregorys Catholic College, Bath
- 65 -
Q6 Put these numbers in standard form:
i) 46 ii) 2 130000 iii)10 025
Q7 Write these numbers out in full:
i) 1.38 x 10
3
ii) 9.756 x 10
1
iii)6.0-02 x 10
2
Fractions in standard form
Similar sort of thing. You could say that
0.465 = 4.65 x 0.1 = 4.65 x 10
-1
or 0.000 046 5 = 4.65 x 0.00001 = 4.65 x 10
-5
To turn these into standard form, do the number
part a number between 1 and 9.999 - and then
count back to see how many places after the
original dp the first actual number came thats
the minus power of 10.
e.g. in the example 0.000 0465 and the 4 is the
5
th
number after the dp, so it will be 10
-5
The number part will be 4.65 using the actual
numbers that are there so the standard form
version is 4.65 x 10
-5
Q8 Write these numbers in standard from:
i) 0.002 056 ii) 0.000 0001 iii) 0.000 000 02
Q9 Write these numbers out in full:
i) 1.38 x 10
-2
ii) 6.0200 x 10
-5
iii) 9.9 x 10
-4
Multiplying in standard form
You simply multiply the numbers bits together,
and then multiply the powers of 10 bits.
e.g. 467 x 1302
First turn the numbers into standard form:
4.67 x 10
2
x 1.302 x 10
3
= 4.67 x 1.302 x 10
2+3
= 6.08034 x 10
5
You may ask why bother putting it in standard
from when you could do that calculations on your
calculator? Well, for that example, I suppose, it
wasnt necessary, but when we come to a
calculation like finding the energy in a lump of
light, well have calculations like:
E = 6.6 x 10
-34
x 6.0 x 10
14
Doing that in anything other than standard form
would fill the page up with zeros. Lets do this one
anyway:
E = 6.6 x 10
-34
x 6.0 x 10
14
= 6.6 x 6.0 x 10
-34+14
= 39.6 x 10
-20
= 3.96 x 10
1
x 10
20
= 3.96 x 10
1-20
= 3.96 x 10
-19
Ive shown every single step on the way there, but
if youre in the right mode on your calculator, it
should do it for you.
N.B. In the third line down, as were multiplying
we just add the powers as usual, even if one of the
powers is a minus number -34 + 14 = -20
Q10 Calculate the answers to:
2.23 x 10
7
x 3.75 x 10
12
3.14 x 10
20
x 5.7 x 10
-9
9.6 x 10
3
x 1.6 x 10
-19
Squaring etc. numbers in standard form
Remember that a
2
= a x a
So (10
3
)
2
= 10
3
x 10
3
= 10
3+3
= 10
6
= 10
3x2
e.g. (1.3 x 10
4
)
3
= (1.3)
3
x 10
4x3
= 2.2 x 10
12
Dividing in standard from
As with multiplying, you do the numbers bits
together, and you do the powers of ten bits,
remembering the rule for dividing powers of 10
you just subtract the powers.
e.g. 4.6 x 10
7
= 4.6 x 10
7-3
= 2.3 x 10
4
2.0 x 10
3
2.0
Another example if theres a minus power on the
bottom the same rule applies well just be
taking away a minus, and as we all recall, minus a
minus is a plus so
8.0 x 10
3
= 8.0 x 10
3--19
= 5.0 x 10
3+19
1.6 x 10
-19
1.6
= 5.0 x 10
22
A level Physics Handbook 2012 St Gregorys Catholic College, Bath
- 66 -
Q11 Thy these:
8.0 x 10
16
divided by 5.0 x 10
7
6.4 x 10
5
divided by 4.0 x 10
12
6.0 x 10
3
divided by 2.0 x 10
-15
4.5 x 10
-6
divided by 3.0 x 10
-9
Sums with lots of bits in
are just a combination of all the bits weve
looked at so far. Basically, put everything in
standard from, and then do all the numbers bits
together, and all the powers of 10 together. e.g
Calculate:
20 x 467 x 1.6 x 10
-19
5.0 x 10
3
x 3.0 x 10
8
= 2 x 3.14 x 4.67 x 10
2
x 1.6 x 10
-19
5.0 x 10
3
x 3.0 x 10
8
= 2 x 3.14 x 4.67 x 1.6 x 10
2-19-3-8
5.0 x 3.0
= 3.128.. x 10
-28
Answers: (If you do get some wrong and cant see
why, ASK!)
1: 10
7
= 10 000 000 10
4
= 10 000
2: 100 000 = 10
5
1 000 000 000 = 10
9
3: 10
7
10 000 000
4: 10
2
100
5: 10
-2
10
-5
10
-6
6: i) 4.6 x 10
1
ii) 2.13 x 10
6
iii) 1.0025 x 10
4
7: i) 1380 ii) 97.56 iii) 600.2
8: i) 2.056 x10
-3
ii) 1 x 10
7
iii) 2 x 10
-8
9: i) 0.0138 ii) 0.000 0602 iii) 0.000 99
10: i) 8.36 x 10
19
ii) 1.8 x 10
12
iii) 1.5 x 10
-15
N.B. We have the same number of decimal places
in the answer as in the question.
11: i) 1.6 x 10
9
ii) 1.6 x 10
-7
iii) 3.0 x 10
18
iv) 1.5 x 10
+3
12: i) 2.7 x 10
-12
ii) 3.6 x 10
22
Q12 Last questions:
i) 0.5 x 4.0 x 10
3
x 1.6 x 10
-19
9.9 x 10
7
x 1.2 x 10
-12
ii) 6.7 x 10
-11
x 2.0 x 10
30
x 6.0 x 10
24
(1.5 x 10
11
)
2
(This actually calculates the gravitational force
between the Earth & sun!)
A level Physics Handbook 2012 St Gregorys Catholic College, Bath
- 67 -
7 SI Units
The international system of units (SI) is founded on seven SI base units for seven base quantities assumed to
be mutually independent:
Definitions of the SI base units
Quantity Unit Definition
Unit of length
metre
m
The meter is the length of the path travelled by light in vacuum during a time
interval of 1/299 792 458 of a second.
Unit of mass
kilogram
kg
The kilogram is the unit of mass; it is equal to the mass of the international
prototype of the kilogram.
Unit of time
second
s
The second is the duration of 9 192 631 770 periods of the radiation
corresponding to the transition between the two hyperfine levels of the
ground state of the cesium 133 atom.
Unit of electric
current
ampere
A
The ampere is that constant current which, if maintained in two straight
parallel conductors of infinite length, of negligible circular cross-section, and
placed 1 meter apart in vacuum, would produce between these conductors a
force equal to 2 x 10-7 newton per meter of length.
Unit of
thermodynamic
temperature
Kelvin
K
The kelvin, unit of thermodynamic temperature, is the fraction 1/273.16 of
the thermodynamic temperature of the triple point of water.
Unit of amount of
substance
mole
mol
1. The mole is the amount of substance of a system which contains as many
elementary entities as there are atoms in 0.012 kilogram of carbon 12; its
symbol is "mol."
2. When the mole is used, the elementary entities must be specified and may
be atoms, molecules, ions, electrons, other particles, or specified groups of
such particles.
Unit of luminous
intensity
candela
cd
The candela is the luminous intensity, in a given direction, of a source that
emits monochromatic radiation of frequency 540 x 1012 hertz and that has a
radiant intensity in that direction of 1/683 watt per steradian.
The rest of the SI units are defined in terms of the seven base quantities via a system of quantity equations.
The SI derived units for these derived quantities are obtained from these equations and the seven SI base
units. For convenience, some of the quantities have been given special names and symbols. Examples of such
SI derived units are given in the table below.
Quantity Unit Symbol
In terms of SI
units
In terms of SI base
units
area m
2
A - m
2
volume m
3
V - m
3
velocity ms
-1
v - ms
-1
acceleration ms
-2
a - ms
-2
frequency hertz Hz - s
-1
force newton N - mkgs
-2
pressure, stress pascal Pa Nm
-2
m
-1
kgs
-2
energy, work, quantity of heat joule J Nm m
2
kgs
-2
power, radiant flux watt W Js
-2
m
2
kgs
-3
electric charge, quantity of electricity coulomb C - sA
electric potential difference volt V JC
-1
m
2
kgs
-4
A
capacitance farad F CV
-1
m
-2
kg
-1
s
4
A
2
electric resistance ohm VA
-1
m
2
kgs
-3
A
-2
magnetic flux weber Wb Vs m
2
kgs
-2
A
-1
magnetic flux density tesla T Wbm
-2
kgs
-2
A
-1
Celsius temperature degree Celsius C - K
activity (of a radionuclide) becquerel Bq - s
-1
dose equivalent
(d)
sievert Sv Jkg
-1
m
2
s
-2
A level Physics Handbook 2012 St Gregorys Catholic College, Bath
- 68 -
8 Symbols
Alphabet
Greek letters &
names
Equation Abbreviation for a Physical
Quantity
Unit symbol
a A o A alpha
A = area
A = nucleon number (atomic mass)
a = acceleration
o = Wein constant
o = alpha particle
b B | B beta
B = magnetic flux density B = bell (sound intensity)
| = beta particle Bq = becquerel (activity)
c C _ X chi
C = capacitance
o
C = degree Celsius
(temperature)
c = speed of light
c = specific heat capacity
d D o A delta
d = diameter D = dioptre (power of a lens)
d = distance dB = decibel (sound intensity)
D = distance from screen to fringe pattern
D = absorbed dose
A = change in
o = little change in
e E c E epsilon
e = charge on an electron eV = electron volt (energy)
E = energy
E
k
= kinetic energy
E = electric field strength
E = Young's Modulus
c = emf
c = tensile strain
c
o
= permittivity of free space
f F | u phi
F = force F = farad (capacitance)
f = frequency
f = focal length
f
e
= focal length of eyepiece lens
f
o
= focal length of objective lens
u = flux
| = work function
g G I gamma
g = gravitational field strength Gy = gray (absorbed dose)
g = acceleration due to gravity
G = gravitational constant
G = conductance
= gamma ray
h H q H eta
h = height H = henry (inductance)
h = Planck constant Hz = hertz (frequency)
H = dose equivalent
H = hubble constant
q = coefficient of viscosity
I I I I iota
I = current Care! Always 'top' and 'tail' I so
that it can be differentiated from
1 or l
I
0
= peak current
I = sound intensity
I = moment of inertia
A level Physics Handbook 2012 St Gregorys Catholic College, Bath
- 69 -
Alphabet
Greek letters &
names
Equation Abbreviation for a Physical
Quantity
Unit symbol
j J 0 theta
J = current density J = joule (energy)
J = moment of inertia
0 = angle
k K k K kappa
k = Boltzmann constant K = kelvin (absolute temperature)
k = spring constant kg = kilogram (mass)
l L A lamda
l = length l = litre ( = 1000cm
3
) (volume)
l = specific latent heat
= wavelength
= decay constant
L = self inductance
L = angular momentum
ln = natural log of
log = log base 10 of
m M M mu
m = mass m = metre (length)
M = magnification m
2
= metre squared (area)
= permeability m
3
= metre cubed (volume)
= coefficient of friction
n N v N nu
N = number N = newton (force)
N
O
= original number
N
A
= Avogadro constant
N = number of turns of wire
n = number of moles
n= order of diffraction
n = number of charge carriers per unit
volume
n = refractive index
o O o O omicron NOT used for anything. it would too easily be confused with the number zero
p P t H pi
P = power Pa = pascal (pressure)
p = pressure
p = momentum
t = 3.14
q Q
Q = charge
Q = heat energy
Q = quality factor
r R P rho
r = radius
R = resistance
R = molar gas constant
R = reacrion force
= density
= resistivity
rad = radian
s S o E sigma
s = displacement s = second (time)
s = slit width Sv = sievert (dose equivalent)
o = conductivity S = siemens (conductance)
o = tensile stress
o = Stefan constant
E = sum of
A level Physics Handbook 2012 St Gregorys Catholic College, Bath
- 70 -
Alphabet
Greek letters &
names
Equation Abbreviation for a Physical
Quantity
Unit symbol
t T t T tau
t = time T = tesla (magnetic flux density)
T = temperature
T = period of a waveform
T
1/2
= half life
T
E
= effective half life
T
B
= bilogical half life
T
P
= physical half life
u U u Y upsilon
u = initial velocity u = atomic mass unit (mass at
atomic levels) u = image distance
U = U-value
U = internal heat of a system
v V
v = velocity V = volt (electric potential)
v = final velocity
v = image distance
V = volume
V = potential difference
V
0
= peak voltage
w W e O omega
W = work done O = ohm (electrical resistance)
e = angular velocity W = watt (power)
w = width of a fringe Wb =weber (magnetic flux)
x X zeta
x = width
X = reactance
y Y + xi y = height
z Z , Z zeta
z = depth
Z = proton number (atomic number)
9 Data Booklet
http://www.ocr.org.uk/Images/70733-datasheet-data-formulae-and-relationships-specimen.pdf
S-ar putea să vă placă și
- Maths IGCSE Quick RevisionDocument6 paginiMaths IGCSE Quick RevisionMahmoudsamir El Shazly100% (1)
- Physics Unit 6 Source BookDocument50 paginiPhysics Unit 6 Source BookÅzmâñ Khäñ100% (1)
- Physics Unit 1 MechanicsDocument49 paginiPhysics Unit 1 Mechanicsamir hamza100% (1)
- Revision Checklist For O-Level Physics 5054: WWW - Revision-Notes - Co.ccDocument24 paginiRevision Checklist For O-Level Physics 5054: WWW - Revision-Notes - Co.ccLion Jude Tissera0% (1)
- Complete Chemistry Revision Guide IGCSEDocument290 paginiComplete Chemistry Revision Guide IGCSEsakibsultan_308Încă nu există evaluări
- PCM Mht-Cet 2020 Pyqs PDFDocument315 paginiPCM Mht-Cet 2020 Pyqs PDFSairaj BadheÎncă nu există evaluări
- Traumatic Brain InjuryDocument32 paginiTraumatic Brain InjuryNayan Maharjan100% (1)
- Practical Tips For A-LEVELS PHYSICSDocument3 paginiPractical Tips For A-LEVELS PHYSICSforum4physics91% (11)
- Brassier - Alien Theory - The Decline of Materialism in The Name of MatterDocument241 paginiBrassier - Alien Theory - The Decline of Materialism in The Name of MatterHansÎncă nu există evaluări
- Edexcel S1 Revision SheetsDocument9 paginiEdexcel S1 Revision SheetsBooksÎncă nu există evaluări
- A Level PhysicsDocument157 paginiA Level Physicsvinod_kumarranÎncă nu există evaluări
- Nutrition Cs Ps enDocument76 paginiNutrition Cs Ps enaradhya bhatiyaÎncă nu există evaluări
- Aqa A Level Chemistry Cheatsheet 3Document24 paginiAqa A Level Chemistry Cheatsheet 3David AdigboÎncă nu există evaluări
- Ar Ranesis@deped Gov PHDocument18 paginiAr Ranesis@deped Gov PHNelzen Garay100% (1)
- As Physics Practical NotesDocument6 paginiAs Physics Practical NotesUltramix100% (1)
- Gce A and As Level Physics Topical Past Papers 1976 To 2003 PDFDocument388 paginiGce A and As Level Physics Topical Past Papers 1976 To 2003 PDFWaleed Bin Khalid100% (1)
- Sudan Chlorination Potocols Dece 17Document58 paginiSudan Chlorination Potocols Dece 17ali abdalkaderÎncă nu există evaluări
- Edexce PHYSICS Revision Notes PDFDocument17 paginiEdexce PHYSICS Revision Notes PDFRyantyler13100% (1)
- As A 2 Physics Practical HandbookDocument48 paginiAs A 2 Physics Practical Handbookmk45a0% (1)
- Cambridge A Level Physics - DefinitionsDocument16 paginiCambridge A Level Physics - DefinitionsK HoÎncă nu există evaluări
- IB Notes: SL Physics Definitions + AstrophysicsDocument7 paginiIB Notes: SL Physics Definitions + AstrophysicsNabsÎncă nu există evaluări
- .Ukimages171726 Specification Accredited A Level Gce Physics A h556.PDF 27Document96 pagini.Ukimages171726 Specification Accredited A Level Gce Physics A h556.PDF 27Ali AÎncă nu există evaluări
- 401985Document82 pagini401985Julio Cesar Garcia ReyesÎncă nu există evaluări
- Chapter 14 OscillationsDocument54 paginiChapter 14 OscillationsPathmanathan Nadeson100% (1)
- AS-AL SOW 9702 v3 1Document187 paginiAS-AL SOW 9702 v3 1Subarna LamsalÎncă nu există evaluări
- EdexcelASPhysics RevisionGuide9781846905957 Pg26to41Document16 paginiEdexcelASPhysics RevisionGuide9781846905957 Pg26to41Ai Ling Lee100% (1)
- IAS Physics SB1 Practs CP1 Student SheetDocument3 paginiIAS Physics SB1 Practs CP1 Student Sheethussain azizÎncă nu există evaluări
- IAL A2 Physics Note (Specification)Document71 paginiIAL A2 Physics Note (Specification)SajitRahman100% (2)
- Physics Unit 4Document13 paginiPhysics Unit 4ua785100% (1)
- Cambridge International As and A Level Physics Revision Guide Cambridge Education Cambridge Uni SamplesDocument5 paginiCambridge International As and A Level Physics Revision Guide Cambridge Education Cambridge Uni SamplesNalaka Abeysekera50% (6)
- h2 Physics DefinitionsDocument7 paginih2 Physics DefinitionsSyed Osama HussainÎncă nu există evaluări
- S1 Edexcel Revision PackDocument9 paginiS1 Edexcel Revision PackJFGHANSAHÎncă nu există evaluări
- Physics Question BanksDocument32 paginiPhysics Question Banksthuthao2007Încă nu există evaluări
- Aqa Physics 2 Revision NotesDocument66 paginiAqa Physics 2 Revision NotesAsif UllahÎncă nu există evaluări
- Questions by Topics S1Document36 paginiQuestions by Topics S1bookdoudahÎncă nu există evaluări
- Electrolysis RevisionDocument15 paginiElectrolysis RevisionPunitha PanchaÎncă nu există evaluări
- As Physics Unit 2 Summary EdexcelDocument1 paginăAs Physics Unit 2 Summary EdexcelMohamed Al SharkawyÎncă nu există evaluări
- Physics P5 in 5 MinutesDocument2 paginiPhysics P5 in 5 Minutesyaseer786Încă nu există evaluări
- A Level Futher Mathematics PDFDocument21 paginiA Level Futher Mathematics PDFNkengafac Armstrong menjuaÎncă nu există evaluări
- Cambridge Learner Guide For As and A Level PhysicsDocument4 paginiCambridge Learner Guide For As and A Level PhysicsVarshLokÎncă nu există evaluări
- Student Unit Guide - Unit 4 Physics On The MoveDocument48 paginiStudent Unit Guide - Unit 4 Physics On The MoveYoke Hock Seow100% (10)
- Edexcel Unit 4 - June 2015 (IAL) Model AnswersDocument18 paginiEdexcel Unit 4 - June 2015 (IAL) Model Answersjosekada100% (1)
- IGCSE Mathematics Model Paper - 2Document9 paginiIGCSE Mathematics Model Paper - 2Kothakonda Praveen KumarÎncă nu există evaluări
- Bissap PDFDocument177 paginiBissap PDFdafemsta_05Încă nu există evaluări
- Golder ReportDocument91 paginiGolder ReportMark Joseph AbelleraÎncă nu există evaluări
- Physiology Practical Manual 2013 For Medical StudentsDocument172 paginiPhysiology Practical Manual 2013 For Medical StudentsAdi MaghiarÎncă nu există evaluări
- O'Level Physics NotesDocument96 paginiO'Level Physics NotesjanifarchowdhuryÎncă nu există evaluări
- Industrial Placement ReportDocument28 paginiIndustrial Placement ReportBadhanÎncă nu există evaluări
- Reptiles. Fijian Crested Iguana 2007DRDocument69 paginiReptiles. Fijian Crested Iguana 2007DRarhitankÎncă nu există evaluări
- EIA Final ReportDocument62 paginiEIA Final ReportAquaquam Tube100% (1)
- Insight IAS OGP Geography @CivilServicesPDFDocument240 paginiInsight IAS OGP Geography @CivilServicesPDFPavansaikumar DasariÎncă nu există evaluări
- Postharvest Treatment of Horticultural CropsDocument9 paginiPostharvest Treatment of Horticultural CropsolaÎncă nu există evaluări
- Resources, Conservation and Recycling: Life Cycle Assessments of Biodegradable, Commercial Biopolymers - A Critical ReviewDocument13 paginiResources, Conservation and Recycling: Life Cycle Assessments of Biodegradable, Commercial Biopolymers - A Critical ReviewSandeep MeenaÎncă nu există evaluări
- Good International Petroleum Industry Practices (2016) by DG of Hydrocarbon, IndiaDocument453 paginiGood International Petroleum Industry Practices (2016) by DG of Hydrocarbon, IndiaRavikumar mahadevÎncă nu există evaluări
- Grigg, Tessa - Final PHD ThesisDocument307 paginiGrigg, Tessa - Final PHD ThesisrabiyaÎncă nu există evaluări
- Data Literacy Course Notes 365 Data ScienceDocument99 paginiData Literacy Course Notes 365 Data ScienceLaís MaranhãoÎncă nu există evaluări
- Student's Guide: English For Academic PurposesDocument28 paginiStudent's Guide: English For Academic PurposesDorj BatÎncă nu există evaluări
- Deflection, Stress Distribution and Failure Modes of Castellated Steel BeamsDocument305 paginiDeflection, Stress Distribution and Failure Modes of Castellated Steel BeamsAdnan NajemÎncă nu există evaluări
- 2015 A2 Physics Time Plan Final Part2Document1 pagină2015 A2 Physics Time Plan Final Part2api-239434818Încă nu există evaluări
- ST Gregs Physics Ocr A - Stud Gde As Final 2015Document16 paginiST Gregs Physics Ocr A - Stud Gde As Final 2015api-239434818Încă nu există evaluări
- 2015 A2 Physics Time Plan Final Part 1Document1 pagină2015 A2 Physics Time Plan Final Part 1api-239434818Încă nu există evaluări
- 2015 As Physics Time Plan Final Part 2Document1 pagină2015 As Physics Time Plan Final Part 2api-239434818Încă nu există evaluări
- 2015 As Physics Time Plan Final Part 1Document1 pagină2015 As Physics Time Plan Final Part 1api-239434818Încă nu există evaluări
- Yearly Plan 2013-14Document1 paginăYearly Plan 2013-14api-239434818Încă nu există evaluări
- As Physics Time Plan FinalDocument1 paginăAs Physics Time Plan Finalapi-239434818Încă nu există evaluări
- Determine Specific Air Capacity Using Air Conditioning UnitDocument13 paginiDetermine Specific Air Capacity Using Air Conditioning UnitTan Xing YanÎncă nu există evaluări
- Physics Investigatory ProjectDocument16 paginiPhysics Investigatory Project9 To 5 GAMERÎncă nu există evaluări
- AMS1117 SeriesDocument8 paginiAMS1117 SeriesMauricio Raul RotmanÎncă nu există evaluări
- Panel PETROTECH Modificado - SAL 14Document1 paginăPanel PETROTECH Modificado - SAL 14Alvaro Reimar Ferrufino MartinezÎncă nu există evaluări
- Current Electricity and Thermodynamics 1665320998Document5 paginiCurrent Electricity and Thermodynamics 1665320998Akshay PandeyÎncă nu există evaluări
- Mirpur University of Science and Technology (Must), Mirpur Mirpur Institute of TechnologyDocument10 paginiMirpur University of Science and Technology (Must), Mirpur Mirpur Institute of TechnologyYounas AhmadÎncă nu există evaluări
- Physics: Chapter: MagnetismDocument17 paginiPhysics: Chapter: MagnetismkushalÎncă nu există evaluări
- Sysh1800 Plus 003Document1 paginăSysh1800 Plus 003Kresen NaickerÎncă nu există evaluări
- Potentiometer: Potentiometer Is An Instrument For Measuring The Potential (Or Voltage) in A Circuit TapsDocument41 paginiPotentiometer: Potentiometer Is An Instrument For Measuring The Potential (Or Voltage) in A Circuit TapsKusmara MarmarÎncă nu există evaluări
- Isa-Plan // Precision Resistors: SMV-PW // Size 4723Document3 paginiIsa-Plan // Precision Resistors: SMV-PW // Size 4723AliÎncă nu există evaluări
- 0708PH PDocument37 pagini0708PH PRana HazemÎncă nu există evaluări
- Relative Motion in One Dimension 1D and 2DDocument16 paginiRelative Motion in One Dimension 1D and 2DChristine RomanillosÎncă nu există evaluări
- Power Quality Enhancement Using Custom Power DevicesDocument1 paginăPower Quality Enhancement Using Custom Power DevicesankitÎncă nu există evaluări
- Medições BateriaDocument4 paginiMedições BateriaFrancisco José da Costa GomesÎncă nu există evaluări
- Example Problem 2: Calculate Time To Complete A Full CircleDocument12 paginiExample Problem 2: Calculate Time To Complete A Full CircleJhela MhorsÎncă nu există evaluări
- Tutorial 1Document4 paginiTutorial 1Kevin TanÎncă nu există evaluări
- TR2432Document3 paginiTR2432CearEo AraGonÎncă nu există evaluări
- How To Use A Megger Insulation TesterDocument3 paginiHow To Use A Megger Insulation TesterjayamolmvÎncă nu există evaluări
- Quantumgravityfromatomicresonance PDFDocument16 paginiQuantumgravityfromatomicresonance PDFGherghe BogdanÎncă nu există evaluări
- Q1.An Electrical Circuit Is Shown in The Figure Below.: ST Thomas More Catholic SchoolDocument54 paginiQ1.An Electrical Circuit Is Shown in The Figure Below.: ST Thomas More Catholic SchooljsÎncă nu există evaluări
- HIPOTDocument5 paginiHIPOTHARIHARANÎncă nu există evaluări
- Basic Electrical Circuits NewDocument40 paginiBasic Electrical Circuits NewSafnas KariapperÎncă nu există evaluări
- Research Paper Revolving Gate Electricity GenerationDocument4 paginiResearch Paper Revolving Gate Electricity GenerationRaja ManeÎncă nu există evaluări
- Electric CircuitsDocument1 paginăElectric CircuitsthinkiitÎncă nu există evaluări
- Mild Hybrid ComponentsDocument13 paginiMild Hybrid ComponentsRoberto ScillieriÎncă nu există evaluări
- Kinematic Viscosity (Astm D-445) : Lube Expert Prepared By: Approved By: Page-1of1Document1 paginăKinematic Viscosity (Astm D-445) : Lube Expert Prepared By: Approved By: Page-1of1ShunmuganathanÎncă nu există evaluări
- 17 Lecture 11-4: 17.1 Chapter 7 Lagrange's Equations (Con)Document9 pagini17 Lecture 11-4: 17.1 Chapter 7 Lagrange's Equations (Con)Ty WilliamsÎncă nu există evaluări
- Electrical Basics Worksheet: Body Electrical Definitions and Rules DefinitionsDocument18 paginiElectrical Basics Worksheet: Body Electrical Definitions and Rules DefinitionsLong HàÎncă nu există evaluări