Documente Academic
Documente Profesional
Documente Cultură
Process Mineralogy and Application in Mineral Processing and Extractive Metallurgy (Joe Zhou) PDF
Încărcat de
Aldo Pablo0 evaluări0% au considerat acest document util (0 voturi)
940 vizualizări13 paginiExtraction of most metals and minerals is largely driven by mineralogical factors. Process mineralogy helps address such issues related to ore processing and metal extraction. Case studies covering gold, silver and base metals ores are provided.
Descriere originală:
Titlu original
Process Mineralogy and Application in Mineral Processing and Extractive Metallurgy (Joe Zhou).pdf
Drepturi de autor
© © All Rights Reserved
Formate disponibile
PDF, TXT sau citiți online pe Scribd
Partajați acest document
Partajați sau inserați document
Vi se pare util acest document?
Este necorespunzător acest conținut?
Raportați acest documentExtraction of most metals and minerals is largely driven by mineralogical factors. Process mineralogy helps address such issues related to ore processing and metal extraction. Case studies covering gold, silver and base metals ores are provided.
Drepturi de autor:
© All Rights Reserved
Formate disponibile
Descărcați ca PDF, TXT sau citiți online pe Scribd
0 evaluări0% au considerat acest document util (0 voturi)
940 vizualizări13 paginiProcess Mineralogy and Application in Mineral Processing and Extractive Metallurgy (Joe Zhou) PDF
Încărcat de
Aldo PabloExtraction of most metals and minerals is largely driven by mineralogical factors. Process mineralogy helps address such issues related to ore processing and metal extraction. Case studies covering gold, silver and base metals ores are provided.
Drepturi de autor:
© All Rights Reserved
Formate disponibile
Descărcați ca PDF, TXT sau citiți online pe Scribd
Sunteți pe pagina 1din 13
1
PROCESS MIERALOGY AD APPLICATIO I MIERAL
PROCESSIG AD EXTRACTIVE METALLURGY
Joe Zhou
Joe Zhou Mineralogy Ltd., Canada
Joe.zhou.mineralogy@gmail.com
ABSTRACT
The ultimate goal of a mineral processing project is to recover as much of the target
mineral(s) as possible from the ore being treated to achieve the best economics.
Selection of the mineral processing techniques and development of the flowsheet are
the most important steps in achieving this goal. However, the recoveries of valuable
minerals may be not satisfactory due to the complexity of the ore although the mineral
processing plant is well designed and built. In fact, extraction of most metals and
minerals is largely driven by mineralogical factors that often cause low recoveries,
low grade, high reagent consumption and other processing issues. Mineralogical
factors affecting ore processing and metal extraction may include mineral type,
metal/element deportment, grain size, liberation and association, surface chemistry,
and concentration and liberation characteristics of minerals detrimental to processing
(e.g., cyanide- and oxygen-consuming minerals, organic carbon, clays, etc.). Process
mineralogy helps address such issues related to ore processing and metal extraction. It
is widely used as a predictive and trouble-shooting tool in mineral processing and
extractive metallurgy, and provides useful information on process selection, flowsheet
development, recovery improvement and reagent consumption optimization.
This paper presents an overview of process mineralogy and focuses on practical
processing problems encountered in ore processing. It also provides a comparison of
commonly used mineralogical techniques and methods for selecting the right tool for
the problem. Case studies covering gold, silver and base metals ores are provided
from a variety of processing options such as gravity separation, flotation, cyanidation
and pre-oxidation.
Keywords: process mineralogy, mineral processing, extractive metallurgy, prediction,
trouble-shooting
ITRODUCTIO
The minerals industry employs various processes to extract valuable metals and
minerals from different ores. Commonly used processes include crushing, grinding,
gravity concentration, flotation, leaching, magnetic and electrostatic separation, and
refining. Fine grinding and oxidative pretreatment are also commonly used in
processing of refractory ores. These processes can be used separately for different
ores, but more often they are employed collectively in many processing projects.
Presented at the First International Metallurgical Meeting Peru 2012, October 26
th
,
2012, Lima, Peru
2
Process selection is the most important step in a mineral processing project. Generally
speaking, the factors affecting process selection of a project include geological,
mineralogical, metallurgical, environmental, geographical, economical and political
situations (Marsden and House, 2006). Among these factors, mineralogical and
metallurgical factors have a direct impact on process selection as they determine the
response of the ore to mineral processing techniques. In many metallic ores, the
recoveries of metals and minerals of interest are often driven by mineralogical factors
such as grain size, liberation, surface chemistry, association with other minerals,
coating and rimming, presence of cyanicides, oxygen consumers, preg-robbers and
clay minerals, and locking in other minerals as trace substitution. Among these
mineralogical factors, liberation, grain size and association (locking in other minerals
as fine-grained inclusions and/or trace substitution) are the most common factors
affecting gold ore processing and metal extraction. Processing of PGE ores faces the
same challenges as gold. Fine grain size and locking of PGM in sulphide and non-
sulphide minerals often cause PGM losses. Presence of talc in some PGE ores (such
as the Merensky reef and Stillwater Complex) can cause significant processing
difficulties (Cole, 2002). In the UG-2 ore located in the Bushveld Complex, the PGM
are finely disseminated with the average grain size being about 10 microns, so that
grain size, liberation and association tend to dictate mineral floatability (Nel et al,
2004). The presence of PGE in pentlandite and other sulphide minerals as solid
solution is another issue that causes processing difficulties. Compared to gold and
PGE, silver mineralogy is more complex. In Pb-Zn-Ag ore, silver often occurs as
inclusions in and attachment to primary lead and zinc minerals (commonly galena and
sphalerite) and can be lost with these minerals during flotation. In silver ore
containing significant amounts of pyrargyrite, proustite and stephanite, silver recovery
by cyanidation can be very low because these minerals dissolve slowly in cyanide
solution. Silver can also occur in other minerals as submicroscopic silver as well,
making silver extraction more difficult (Zhou, 2010). Base metal ores are mainly
processed by flotation. In processing of base metal ores, high content of pyrite, fine
grain size of galena, intergrowth of chalcopyrite with sphalerite and presence of clay
minerals can cause serious processing problems. Surface chemistry may affect
extraction of base metals as well. In some base metal ores containing precious metals,
precious metal-bearing minerals (such as electrum and kstelite) may misreport to
zinc concentrate rather than copper concentrate because of the surface coating (Zhou
et al., 2005). As high-grade nickel sulphide ores are being depleted and processing
laterite ores continue to pose challenges, the future of nickel extraction lies in low-
grade ultramafic ores. The main challenge in processing low-grade ultramafic ores is
the presence of MgO-containing silicate minerals. The impact of MgO content on Ni
recovery is very pronounced and high MgO requires the effective rejection of MgO
containing minerals. A study conducted on fibrous minerals in ultramafic nickel
sulphide ores indicated the significant depressing effect of MgO on pentlandite
flotation. As the impact of fibrous minerals on slurry viscosity, grinding, and flotation
becomes understood, nickel recovery will increase, making the mining and low-grade
ultramafic deposits viable (Xu et al, 2012). In iron ore processing, elevated sulphur
content of the concentrate is a common issue in some operations. To be able to reduce
the sulphur content, the sulphide mineralogy including mineral speciation, grain size,
liberation and association with iron minerals must be well understood and determined.
This paper introduces the scope of process mineralogy and mineralogical factors that
may affect mineral processing and extractive metallurgy of various ores, and
discusses the application of process mineralogy through case studies.
3
OBJECTIVES AD ROLES OF PROCESS MIERALOGY
Process mineralogy is an inter-discipline in the fields of mineralogy and metallurgy. It
uses the theories, principles, methods and tools of mineralogy to study all
mineralogical characteristics of an ore and potential problems related to mineral
processing and extractive metallurgy with prediction and trouble-shooting being two
major objectives and roles. Process mineralogy provides useful information on
process selection, flowsheet development, recovery improvement and reagent
consumption optimization. The information acquired from a process mineralogical
study can be used as a guide for a metallurgical testwork program for process design
or optimization. The scope of a process mineralogy program may include but not
limited to the following:
Prediction:
1. Response of a new ore to various processes and most likely processing options
2. Estimated recovery of valuable minerals and grade of concentrate
3. Potential mineralogical factors affecting ore processing and metal extraction
Trouble-shooting:
1. Deportment of valuable minerals in tailings and deleterious elements in
concentrates
2. Cause for valuable losses and opportunity for recovery improvement
3. Cause for high reagent consumption and opportunity for reagent optimization
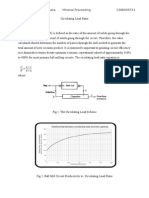
Figure 1 is a good example of using mineralogy as a predictive tool. It illustrates some
common forms and carriers of gold in different gold ores and indicates the impact of
gold deportment on extractive metallurgy. The large gold grain in Figure 1(1) is
coarse (approximately 600 x 1000 m) and completely liberated, and can be
recovered by gravity, flotation or cyanidation. High gold recovery (>90%) is expected,
and can generally be achieved when gold occurs as coarse grains like this one.
However, gold leaching by cyanidation is a slow, diffusion-controlled reaction, and
large gold particles can take many days to fully dissolve. Therefore, gold can be lost
to tailings owing to insufficient residence time during cyanide leaching. To avoid gold
losses, it is better recover the coarse gold by gravity prior to cyanide leaching. The
gold grains in Figure 1(2) are medium-grained and entirely locked in pyrite, and are
not recoverable by direct cyanidation. These gold grains can only be recovered by fine
grinding followed by cyanidation, with or without flotation, or by pre-oxidation of the
pyrite followed by cyanidation. Gold grains in Figure 1(3) are extremely fine, and
locked in arsenopyrite. This type of gold may be recovered by ultra-fine grinding
followed by cyanidation of the sulphide concentrate, although this is in fact seldom
beneficial, as the gold grains are usually finer than the finest practical grind size that
can be achieved in commercial mills. Therefore, the only alternative is to oxidize the
arsenopyrite to liberate the gold, and then leach with cyanide. Gold in Figure 1(4)
occurs as sub-microscopic gold in disseminated pyrite, making the gold extremely
refractory. Pre-concentration of the gold in these types of ores by gravity or flotation
is usually inefficient, and therefore, to recover gold in this type of ore, pre-oxidation
processing (autoclaving, roasting and biological oxidation) of the whole ore may be
required to liberate the gold and achieve high recovery efficiency. Based on the gold
deportment shown in Figure 1 and other results, the mineralogist is able to balance the
various types of gold in an ore and to comment on most likely processing options.
4
Figure 1: Gold deportment and impact on gold extractive metallurgy
(1) Liberated coarse gold grain (Au, 6001000m); (2) gold grains (inside red circles) locked
in pyrite (Py); (3) fine-grained gold particles (inside red circles) locked in arsenopyrite (Apy);
(4) submicroscopic gold locked in fine-grained gold-bearing pyrite (yellowish white grains)
that is disseminated in quartz (Q) (Zhou and Fleming, 2007)
Gravity concentration and flotation processes are very effective at recovering large
and fully liberated gold grains, and are used in many gold plants. Gravity
concentration is also an environmentally friendly process because it doesnt use
chemicals, and should be considered when an ore contains certain amount of liberated
gold. However, liberated gold can be lost from a gravity circuit if the grain size is
small or if the gold grain is attached to a low density mineral. The majority of gravity
equipment can only recover gold grains greater than 50m, and some equipment is
effective at sizes down to about 10m (Marsden and House, 2006). A GRG (gravity-
recoverable gold) study led by the late professor Andre Laplante proved that most
gold particles recovered in commercial gravity recovery plants are coarser than 37 m.
This is because gravity circuits are always installed in mills, and the flow to the
gravity circuit is regulated by installing cyclones, with only the cyclone underflow
reporting to the gravity equipment. GRG finer than 37m tends to report to the
cyclone overflow much more readily than its coarser counterpart, and therefore by-
passes the gravity equipment (Laplante and Stauton, 2005). In a typical gold
deportment study, the mineralogist will determine the size distribution of all observed
gold grains and the size distribution of liberated gold grains can be used in assisting
the equipment selection and for predicting the gold recovery by various processes.
Figure 2 shows the size distribution of liberated gold in a copper-gold ore. Gold
balance indicated that liberated gold in this ore accounts for approx. 65% of the head
assay with a size range from <0.5m to >100m. This part of gold can be recovered
by cyanidation, and also by flotation at slightly lower recovery. The recovery by
gravity will be much lower than cyanidation and flotation (approx. 33%). Flotation is
a common process that is used in many base metals and precious metals ore
processing plants. It is very effective at recovering both liberated valuable and
sulphide minerals over 10m. In flotation of gold ores, liberated gold grains less than
3
Au
1
2 4
More free-milling More refractory
Liberated,
coarse-grained
Locked,
medium-grained
Locked,
fine-grained
Locked,
submicroscopic
Gravity,
Flotation,
Cyanidation
Fine grinding
Cyanidation
Flotation &
Preoxidation
Flotation,
Fine grinding
Preoxidation &
Cyanidation
Pre-oxidation
& Cyanidation
Flotation
Py
Q Apy
5
10m can also be recovered by flotation, but the efficiency is much lower. In addition
to unliberated gold, gold losses to gravity and flotation tailings include very fine
liberated gold grains as well as coarse liberated gold grains whose surface is coated or
rimmed by non-floatable material (e.g., iron oxides or hydroxides, silicates) and
hydrophobic gold grains whose surface is coated with fine hydrocarbon or sulphurous
material (Zhou, Jago and Martin, 2004; Marsden and House, 2006).
Figure 2: Prediction of gold recovery by common processes
In addition to being a predictive tool in process flowsheet development, process
mineralogy also plays an important role in process optimization and plant operation,
and is often used as a trouble-shooting tool by operating mines. Due to the complexity
of the ore, the recoveries of valuable minerals may be not satisfactory although the
processing flowsheet is well developed and implemented. In other words, minerals of
interest can be lost to the tailings for mineralogical reasons. When recovery and
concentrate grade drop or fluctuate, or acceptable recovery is achieved only with the
use of significantly more reagents or more complex pretreatment processes, it usually
indicates the change of feed mineralogy. Once this happens, a detailed mineralogical
study program should be considered to find out what has caused the processing
issue(s) and how to improve the plant performance. The information acquired from
such a mineralogy program can be used as a guide for process optimization.
Figure 3 shows the distribution of gold in a cyanidation tail. The sample, assaying
8.3g/t Au and 92g/t Ag, originates from a bulk flotation concentrate subjected to
regrinding to approximately 33 microns, followed by gravity concentration and 24
hour cyanide leaching of the gravity tailing. The leach residue was filtered and
washed and then dried at low temperature for a complete gold and silver deportment
study. The results showed that liberated gold in the sample was effectively recovered
by gravity and subsequent cyanide leaching. The gold left in leach residue is all
locked in other minerals as micron-size inclusions (0.5-13m) and submicroscopic
gold with a total of 74% in pyrite, 7.5% in arsenopyrite and 18.5% in non-sulfide
0
5
10
15
20
25
30
0-10 10-20 20-40 40-60 60-80 80-100 100-120
D
i
s
t
r
i
b
u
t
i
o
n
(
%
)
Grain Size (m)
Size Distribution of Liberated Gold
Flotation recoverable gold
Cyanide recoverable gold
Gravity recoverable gold
6
minerals. Visible gold inclusions (approx. 40%) locked in other minerals are not
recoverable at current grinding fineness unless the sample is ground much finer. To
extract submicroscopic gold (approx. 60%) locked in sulphide minerals, oxidative
pretreatment is required to create a porous structure through which the cyanide leach
solution can penetrate to reach the minute gold particles. The study also showed that
the silver lost in the leach residue occurs mainly as liberated freibergite, acanthite and
stephanite (up to 85m) with minor amounts of native silver and other silver minerals.
It is understandable that those coarse and liberated silver minerals were not recovered
by gravity due to their lower specific gravity, but why they did not dissolve during
cyanide leaching? Mineralogical studies suggested that the concentration of cyanide
in leach solution was too low for silver leaching although all liberated gold was
dissolved to completion. The slow-leaching kinetics of silver minerals is considered to
be another main cause for silver loss. Based on the mineralogical findings, the process
was optimized by incorporating an oxidative pretreatment for gold extraction. High
cyanide concentration and extended leach process has substantially improved the
silver recovery.
Figure 3: Distribution of gold in a cyanidation tail
MIERALOGICAL FACTORS AFFECTIG MIERAL PROCESSIG
There are a number of mineralogical factors that may affect mineral processing and
extractive metallurgy of various ores. Some common factors are listed in Table 1.
Among these factors, liberation, grain size and association are the most important
ones. The impact of these mineralogical factors on mineral processing and extractive
metallurgy is discussed below. More information can be found in the reference (Zhou,
Jago and Martin, 2004; Zhou and Fleming, 2007; Zhou, 2010; Zhou, 2011).
1. Liberation
Most valuable minerals, if not liberated, will not be recovered by conventional
processes, or it can be recovered only at low recovery and low concentrate grade. This
makes liberation extremely important in many (if not all) mineral processing projects.
Prior to the recovery process, valuable minerals need to be liberated from non-
valuable minerals with those they are associated. Poor liberation will result in low
recovery and grade for most valuable minerals. In the case of precious and base metal
7
ores (such as Au, Ag, PGM, Cu, Pb, Zn and Ni), encapsulation in sulphide and
silicate minerals is a common and major cause for valuable mineral losses. In the UG-
2 ore, 25% of PGM were locked in silicates and finer grinding was required in a
secondary milling step to increase the PGM recoveries (Nel et al., 2004). In gold
mining industry, as the free-milling gold ores are being depleted and more refractory
ores are discovered and processed, liberation becomes even more important in such a
project. In addition to physical liberation (such as fine grinding and ultrafine grinding),
several chemical liberation processes (such as pressure oxidation) have been
developed and implemented. In processing of refractory gold ores, particularly the
Carlin-type gold ores in Nevada, the sulphide host minerals must first be oxidized, to
create a porous structure through which the cyanide leach solution can penetrate to
reach the minute gold particles. This oxidation is traditionally achieved by roasting,
autoclaving or bacterial oxidation.
Table 1: Common mineralogical factors
# Mineralogical Factors Associated Ores & Processes
1 Liberation/locking Various ores & processes
2 Grain size Various ores & processes
3 Association Various ores & processes
4 Coating & rimming Mainly flotation, also gravity and leaching
5 Surface chemistry Mainly flotation, also gravity and leaching
6 Cyanicides & oxygen consumers Mainly cyanide leaching
7 Preg-robbing Cyanide leaching of gold and silver ores
8 Refractoriness
Mainly cyanide leaching, also gravity and
flotation of precious metals ores
9 Slow-dissolving kinetics Cyanide leaching of gold and silver ores
10
Deleterious minerals/toxic
elements (As, Hg, asbestos)
Mainly leaching and flotation of base and
precious metals ores, but can also affect other
ore processing
11
Gangue mineralogy (clays,
sulfide gangue and more)
Flotation and leaching of base and precious
metals ores, flotation and magnetic separation
of iron ores, tailings disposal and more
2. Grain Size
Grain size is one of the most important mineralogical factors that affect processing of
many ores. For example, gold is mainly recovered from ores using gravity, flotation,
cyanidation or a combination of these processes. Gold grain size can be a significant
factor driving the efficiency of gold recovery processes. As discussed above, liberated
gold can be recovered by gravity concentration but it can be lost from a gravity circuit
if the grain size is fine. The majority of gravity equipment can only effectively
recover gold grains greater than 50m. Fine gold, which is too small to be recovered
by the installed equipment (Marsden and House, 2006):
<500m: sluices
<200m: jigs
<50 to 100m: spirals
<50m: shaking tables
<20m: centrifugal concentrators (and down to about 10m in some cases)
8
As mentioned above, flotation is a common process that is used in many gold ore
processing plants. It is very effective at recovering both liberated gold (which tends to
be naturally floatable), and gold-bearing sulphide minerals. Mineralogical
examinations conducted on metallurgical products have shown that gold grains over
10m can be effectively recovered by flotation. Gold grains less than 10m can also
be recovered by flotation, but the efficiency is much lower. Gold losses to flotation
tailings include very fine gold grains as well as coarse gold grains whose surface is
coated or rimmed by non-floatable material (e.g., iron oxides or hydroxides, silicates)
(Zhou, Jago and Martin, 2004; Marsden and House, 2006).
Cyanidation is the most efficient and widely applied process for extracting gold from
various ores, and high recoveries (>80%) are usually expected and commonly
achieved. The gold left in the leaching tailings is often encapsulated in sulphide, oxide
and/or silicate minerals that are impervious to the cyanide leach solution. However,
liberated gold can also be lost to cyanidation tailings. This generally occurs when
some of the gold in the ore is too big or too slow-leaching to dissolve to completion in
the time allocated to the leaching process. Studies have indicated that a spherical gold
particle with a diameter of 44 m will take approximately 13 hours to dissolve under
normal cyanide leach conditions, while particles with a diameter of 150 m will take
48 hours or more (Fleming, 1992). Generally, when an ore contains large liberated
gold particles, the flowsheet will incorporate a gravity circuit to recover these
particles, so it is not necessary to design for excessively long leach times. An average
gold plant would have a 24 hours leach residence time (Fleming, 1998). Deschenes
and his team at CANMET have made a breakthrough in extracting gold and silver in
high-grade Au-Ag ores (Deschnes et al, 2009; Rajala and Deschnes, 2009;
Deschnes and Fulton, 2011).
In the processing of base metal ores, grain size also has a significant impact. Galena,
sphalerite and chalcopyrite are often associated with each other. Galena is often fine-
grained and sphalerite is relatively coarse. Chalcopyrite often occurs as inclusions in
sphalerite (so-called chalcopyrite disease) in addition to being discrete particles. To
recover these minerals of interest, the Cu-Pb-Zn ore often needs to be ground to such
a degree that these minerals are liberated from other minerals and also separated from
each other. Both over-grinding and under-grinding may cause processing issues. To
maximize the recovery of valuable minerals, grain size distribution of each mineral
needs to be determined through a mineralogy program.
3. Association
Association is also an important mineralogical factor that may impact ore processing.
In processing of copper-gold ores (such as porphyry Cu-Au ores), precious metals
(Au and Ag) are often recovered into copper concentrate by flotation with or without
gravity concentration. When gold is intimately associated with copper minerals, it will
report to copper concentrate. However, if gold is associated with pyrite, iron oxide
and silicate minerals, the recovery of gold might be lower. In some plants, a separate
sulfide flotation circuit may be required to produce an Au-bearing sulfide concentrate.
Gold with silicate is a common association in many ores. When gold is finely
disseminated in silicates, the extraction can be challenging because the ore has to be
ground finer to make the gold accessible to the cyanide solution. It has been found
that a substantial amount of gold (up to 50%) in some ores can occur as tiny
9
Category Technique Technique Application MDL
Qualitative/Semi-Quant OM Optical Microscopy
Mineral ID & qualitative/semi-quant
mineral analysis of bulk samples
High (%)
ADIS Automated Digital Image System High (%)
XRD X-ray Diffracton High (%)
SEM Scanning Electron Microscopy
Mineral ID & qualitative/semi-quant
elemental analysis of individual particles
High (%)
MLA Mineral Liberation Analyser Low (%)
QEMSCAN
Quantitative Evaluation of Materials
by Scanning Electron Microscopy
Low (%)
EPMA Electron Probe Microanalysis Low (ppm)
PIXE Proton-induced X-ray Emission Low (ppm)
D-SIMS
Dynamic Secondary Ion Mass
Spectrometry
Low (ppm-ppb)
LAM-ICP-MS
Laser Ablation Microprobe
Inductively Coupled Plasma Mass
Spectrometry
Low (ppm-ppb)
Synchrotron Synchrotron Radiation Light Source Low (ppm-ppb)
TOF-LIMS
Time of Flight Laser Ion Mass
Spectrometry
Low (ppm)
TOF-SIMS
Time of Flight Secondary Ion Mass
Spectrometry
Low (ppm)
XPS X-ray Photon Spectrometry Surface analysis of bulk material Low
Surface Analysis
Surface analysis of bulk material &
individual particle
Mineral ID & qualitative/semi-quant
mineral analysis of bulk samples
Semi-Quant
Quantitative
Quantitative mineral analysis of bulk
samples and individual particles
Quantitative
Quantitaive elemental analysis of
individual particles
inclusions (a few microns) in quartz or other minerals, which makes gold extraction
extremely difficult and uneconomic.
Same as the porphyry copper-gold ores, flotation is the principal process for
recovering gold minerals and copper sulphides in iron copper gold (IOCG) ores. Gold
in IOCG ores is mainly associated with copper sulphides and, to a lesser extent, iron
oxide minerals (mainly as inclusions or submicroscopic gold in hematite). Liberated
gold and gold associated with copper sulphides can be recovered by flotation into a
copper concentrate and high gold recovery (over 80%) can be achieved. However,
gold can be lost to flotation tails if it is fine-grained or if it is associated with hematite.
To recover the fine-grained gold particles (often below 20 m) and gold that is
associated (exposed) with hematite or other gangue, cyanide leaching may be
considered. Gold locked in hematite or other gangue as tiny inclusions will not be
recovered unless the ore is ground finer. Submicroscopic gold in hematite is not
recoverable by conventional techniques. Precious metals associated with zinc
minerals (mainly sphalerite) in Au-Ag ore and Cu-Pb-Zn-Au-Ag ore is another
example of unfavourable association (Zhou, 2005).
Table 2 lists a number of techniques commonly used in process mineralogy. These
techniques can be used separately, but a typical mineralogy program often uses a
comprehensive mineralogical and analytical approach including several techniques.
The advantage of using such a comprehensive approach is that a large sample can be
studied to get better statistics and each mineralogical issue will be addressed properly
using specific techniques.
It should be noted that each technique is designed for certain purposes and has its
advantages & limitations. They should be selected wisely and used properly.
Table 2: Techniques commonly used in process mineralogy
10
APPLICATIO OF PROCESS MIERLOGY I MIERAL PROCESSIG
AD EXTRACTIVE METALLURGY
Case 1: Process mineralogy of a refractory sulphide gold ore
Gold in refractory sulphide gold ores is predominantly associated with sulphide
minerals and occurs as both fine-grained gold particles and/or submicroscopic gold.
Microscopic gold (visible gold) usually accounts only for an insignificant portion,
with the majority of gold being contained in sulphides. To extract gold from
refractory ores, the ore needs to be finely ground and pre-oxidation is required.
Sulphide host minerals must first be oxidized to create a porous structure through
which the cyanide leach solution can penetrate to reach the minute gold particles.
Figure 4 shows the gold-bearing arsenopyrite (4A) and pyrite (4B) in a sulphide gold
ore. The ore is a highly refractory ore with only about 2% of gold being fine-grained
"free gold" (not locked in sulphide grains), making gold extraction difficult because
the gold is mainly locked inside the two main sulphide minerals arsenopyrite (75%
Au) and arsenic-rich pyrite (23% Au). The ore is treated through grinding, flotation,
pressure oxidation and carbon-in-leach circuits. Gold recovery is ~83%.
Figure 4: Gold in a refractory sulphide ore
A: Micron-size gold inclusions in arsenopyrite (Apy); B: Submicroscopic gold-bearing pyrite (Py)
Case 2: Process mineralogy of a Pb-Zn-Ag ore
In Pb-Zn-Ag ores, silver is often associated with sulfides and is recovered by flotation
and concentrate smelting with or without leaching to recover the base metals as well
as the silver values. As discussed above, a number of mineralogical factors, such as
liberation, association, grain size, and rimming and coating, can impact flotation and
leaching of silver minerals. In a Pb-Zn-Ag operation processing high-grade silver ore
(~400g/t Ag), the silver recovery by flotation was approx. 85% for a number of years.
The mill endeavors to recover additional silver to the lead concentrate and minimize
the placement of silver to the zinc concentrate. To explore the opportunity for further
silver recovery improvement, a silver deportment study was initiated to identify the
cause for silver loss and to determine the possible recovery increase. The conclusions
are listed below (Zhou, 2010).
1. Silver occurred mainly as freibergite ((Ag, Cu, Fe)
12
(Sb, As)
4
S
13
), dyscrasite
(Ag
3
Sb), pyrargyrite (Ag
3
SbS
3
) with a moderate to minor amounts of acanthite
(Ag
2
S), native silver and other minerals.
B
51.9ppm Au
Py
11
2. Silver also occurs in galena as inclusions and submicroscopic silver.
3. Lead Tail (Zinc Feed) contained 60 g/t Ag (accounting for ~14% of the head
assay). Approximately 15% of the lost silver was liberated and associated with
galena, and can be recovered in lead circuit without further grinding, which means
the current silver recovery in lead circuit can be increased by 2%.
4. Zinc Tail (i.e. Final Tail) contained 48 g/t Ag (accounting for 12% of the head
assay). Approximately 15% of the lost silver (with a maximum of 20%) was
locked in galena, sphalerite and other sulphide minerals and was recoverable by
flotation. This indicates that 2% of the silver lost in the Final Tail can be
recovered in zinc circuit.
5. Theoretically, a total improvement of 4% recovery can be expected.
6. The main causes for silver loss are unfloated liberated galena and silver minerals
and association of silver with sulphide gangue and non-sulphide gangue.
Figure 5 presents some lost silver minerals in the flotation tail. Liberated galena with
dyscrasite inclusions (Figure 5-1) and minor amounts of liberated silver minerals (5-2)
can be recovered in the lead circuit without finer grinding. The collector chemistry
needs to be reviewed to maximize the recovery of these liberated silver minerals. To
recover silver minerals and galena associated with non-sulphide gangue (5-3) and
sulphide gangue (5-4), the ore needs to be ground finer to liberate the silver minerals.
Composite particles shown in Figure 5-3 and 5-4 can be recovered into lead or zinc
concentrate without finer grinding, but they will lower the concentrate grade.
Figure 5: Silver minerals lost in flotation tail
1: Liberated galena with dyscrasite inclusions (inside red circles); 2: Liberated dyscrasite
grain (Dy); 3: Freibergite (Fr) associated with galena (Gn) and silicate (Si); 4: Dyscrasite
(inside red circle) locked in arsenopyrite (Apy)
3
2
Dy
4
Apy
12
DISCUSSIO
The minerals industry is continuing its growth as the world economy keeps growing.
A number of large projects are being developed and will be commissioned in the next
few years. At the same time, the minerals industry is facing challenges in processing
difficult ores with low grade and complex mineralogy. To help determine the mining
method, extraction process requirements, and in particular, the performance and
optimization of all processes involved, process mineralogy is becoming increasingly
important in a mineral processing project. It includes ore characterization during pre-
feasibility and feasibility study stages and subsequent periodic mineralogical analyses
of feed, intermediate process streams and final products during plant operation. As a
predictive and trouble-shooting tool, process mineralogy helps address all issues and
problems related to mineral processing and extractive metallurgy and provides useful
information on process selection, flowsheet development, recovery improvement and
cost reduction.
ACKOWLEDGEMETS
The author wishes to acknowledge the conference organizers for opportunity to
present this paper at the First International Metallurgical Meeting Peru 2012. Mining
companies that have contributed indirectly to this paper by providing projects and
financial support are also acknowledged.
REFERECES
Cole, S., A review of PGM metallurgy highlighting recent process innovations. In:
Proc 34
th
Annual Meeting of Canadian Mineral Processors. CMP, Ottawa, 2002, pp
445-467.
Deschnes, G., Rajala, J., Guo, H., Fulton, M. and Mortazavi, S., Leaching of Gold
and Silver from Kupol Samples: Part I: Preliminary Investigation. In: Proc 41
st
Annual Meeting of Canadian Mineral Processors, CMP, Ottawa, Canada, 2009, 409-
426.
Deschnes, G. and Fulton, M., The CELP Technology for Leaching Gold and Silver
from High Grdae Ores: Preliminary Investigation for Two New Potential Applications.
In: Proc 43
rd
Annual Meeting of Canadian Mineral Processors, CMP, Ottawa,
Canada, 2011, 467-476.
Fleming, C.A., 1992, Hydrometallurgy of precious metals recovery. Hydrometallurgy
30, pp 127-162. Elsevier Science Publishers B.V., Amsterdam.
Fleming, C.A., 1998. Cyanidation of gold and silver, in Gold Symposium, pp 1-49
(Royal Melbourne Institute of Technology: Melbourne).
Laplante, A.R. and Staunton, W.P. 2005 Gravity recovery of gold An overview of
recent developments. Treatment of Gold Ores, First International Symposium, 44
th
Annual Conference of Metallurgists of CIM, Calgary, Alberta, Canada (edited by G.
Deschenes, D. Hodouin and L. Lorenzen), pp 49-63.
13
Marsden, J. and House, I. 2006. The chemistry of gold extraction, second edition (ed:
E Horwood), pp 651 (The Society for Mining, Metallurgy and Exploration Inc:
Littleton).
Nel, E., Theron, J., Martin, C. and Raabe, H., 2004. PGM ore processing at Impalas
UG-2 concentrator in Rustenburg, South Africa. In: Proceedings 36
th
Annual Meeting
of Canadian Mineral Processors, pp 121-139.
Rajala, J. and Deschnes, G., Extraction of Gold and Silver at the Kupol Mill using
CELP. In: World Gold 2009, The Southern African Institute of Mining and
Metallurgy, Johannesburg, South Africa, 2009, 35-42.
Xu, M., Dai, Z., Dong, J., Ford, F., and Lee, A. W., 2012: Fibrous minerals in
ultramafic nickel sulphide ores. CIM Journal, Vol. 3, No. 1, 2012, 1-8.
Zhou, J., Jago, B. and Martin, C., 2004. Establishing the process mineralogy of gold
ores. In: Proceedings 36
th
Annual Meeting of Canadian Mineral Processors, pp 199-
226.
Zhou, J., Martin, C., Blatter, P., Boss, P.-A. and Robitaille, 2005. The process
mineralogy of precious metals in LaRonde flotation products and its effect on process
optimization. In: Proceedings Treatment of Gold Ores, First International Symposium,
44
th
Annual Conference of Metallurgists of CIM, Calgary, (eds: G Deschenes, D
Hodouin and L Lorenzen), pp 93-109.
Zhou, J. and Fleming, C.A., 2007. Gold in tailings mineralogical characterisation
and metallurgical applications. In: Proceedings World Gold 2007. Cairns, (eds: J
Avraamides, G Deschnes and D Tucker), pp. 311-317.
Zhou, J., 2010. Process mineralogy of silver ores and applications in flowsheet design
and plant optimization. In: Proceedings of the 42
nd
Annual Meeting of Canadian
Mineral Processors, CMP, Ottawa, Canada, 2010, 143-161.
Zhou, J., The mineralogy and predictive metallurgy of major types of gold ores. In:
World Gold 2011, Proceedings of the 50
th
Annual Conference of Metallurgists of CIM,
Montreal, QC, Canada, 1-15.
S-ar putea să vă placă și
- Extractive Metallurgy 1: Basic Thermodynamics and KineticsDe la EverandExtractive Metallurgy 1: Basic Thermodynamics and KineticsÎncă nu există evaluări
- P1 (Intro) & P2Document38 paginiP1 (Intro) & P2Khana Rizki MaulanaÎncă nu există evaluări
- Mineral Processing A DasDocument36 paginiMineral Processing A DasYallarling NagureÎncă nu există evaluări
- Alumina to Zirconia: The History of the CSIRO Division of Mineral ChemistryDe la EverandAlumina to Zirconia: The History of the CSIRO Division of Mineral ChemistryEvaluare: 1 din 5 stele1/5 (1)
- Assignment 2 ProcessMineralogyDocument8 paginiAssignment 2 ProcessMineralogyinung84Încă nu există evaluări
- Kinetic Modelling of Gold Leaching and Cyanide Consumption inDocument10 paginiKinetic Modelling of Gold Leaching and Cyanide Consumption inAlejandro ValenzuelaÎncă nu există evaluări
- Extraction and Separation of Manganese and Iron From Ferruginous Manganese Ores A ReviewDocument18 paginiExtraction and Separation of Manganese and Iron From Ferruginous Manganese Ores A Reviewrichard100% (1)
- Particles The Bridge Between Geology and MetallurgyDocument16 paginiParticles The Bridge Between Geology and MetallurgypiMLeonÎncă nu există evaluări
- Mineral Processing Lab ManualDocument11 paginiMineral Processing Lab ManualChimwemwe KaongaÎncă nu există evaluări
- Clays and Clay Minerals: Proceedings of the Fourteenth National Conference, Berkeley, CaliforniaDe la EverandClays and Clay Minerals: Proceedings of the Fourteenth National Conference, Berkeley, CaliforniaS. W. BaileyÎncă nu există evaluări
- Overview of Mineral Processing Methods: August 2015Document14 paginiOverview of Mineral Processing Methods: August 2015Zanele MbathaÎncă nu există evaluări
- Syllabus Mining and Mineral ProcessingDocument9 paginiSyllabus Mining and Mineral ProcessingArief NuzulÎncă nu există evaluări
- Sampling PDFDocument30 paginiSampling PDFNikhil SubarnoÎncă nu există evaluări
- Conceptual Models In Exploration Geochemistry: The Canadian Cordillera And Canadian ShieldDe la EverandConceptual Models In Exploration Geochemistry: The Canadian Cordillera And Canadian ShieldÎncă nu există evaluări
- A New Look at Mineral Maps and The Potential Relationships of PDFDocument4 paginiA New Look at Mineral Maps and The Potential Relationships of PDFDaniel Valdes JamettÎncă nu există evaluări
- New Perspectives on Gold HydrometallurgyDocument10 paginiNew Perspectives on Gold HydrometallurgySteven TremolÎncă nu există evaluări
- 15A Mathematical Model of The Leaching of Gold in Cyanide SolutionsDocument16 pagini15A Mathematical Model of The Leaching of Gold in Cyanide SolutionsuchihaituÎncă nu există evaluări
- Comminution and Liberation of MineralsDocument12 paginiComminution and Liberation of MineralsRuben AltamiranoÎncă nu există evaluări
- Liberation, Separation, ExtractionDocument100 paginiLiberation, Separation, Extractiongaol_bird009Încă nu există evaluări
- Comminution and Sizing InHard Rock Gold MiningDocument6 paginiComminution and Sizing InHard Rock Gold MiningandestaÎncă nu există evaluări
- Mineral Processing MethodsDocument69 paginiMineral Processing MethodsChamal_JaliyaÎncă nu există evaluări
- Dense Medium SeparationDocument28 paginiDense Medium SeparationAdel Niño Liza Iligan100% (1)
- Metals From Ores: An Introduction: CRI SONDocument8 paginiMetals From Ores: An Introduction: CRI SONSaumya Subhra NandiÎncă nu există evaluări
- Metallurgy SummarizedDocument17 paginiMetallurgy SummarizedHeli VentenillaÎncă nu există evaluări
- Dense Medium Separation (DMS)Document10 paginiDense Medium Separation (DMS)Ethar SalamÎncă nu există evaluări
- 3rd International Geometallurgy Conference 2016 Paper Number (PDFDrive)Document22 pagini3rd International Geometallurgy Conference 2016 Paper Number (PDFDrive)W ZuoÎncă nu există evaluări
- Beyond Reconciliation - A Proactive Approach To Mining DataDocument7 paginiBeyond Reconciliation - A Proactive Approach To Mining DataCraigÎncă nu există evaluări
- Hydro Metallurgy of Complex Sulfide Ores by P. Dharma RaoDocument172 paginiHydro Metallurgy of Complex Sulfide Ores by P. Dharma RaoFerudun Akyol100% (1)
- World's Richest Tin Lode Geology and StructureDocument13 paginiWorld's Richest Tin Lode Geology and StructureLeslie JCÎncă nu există evaluări
- Flotation: By: Raheel MemonDocument24 paginiFlotation: By: Raheel MemonJEANCARLOCGÎncă nu există evaluări
- Makanza Flotation (2008)Document23 paginiMakanza Flotation (2008)Richard CookÎncă nu există evaluări
- Rapid Determination of Bond Rod-Mill Work IndexDocument5 paginiRapid Determination of Bond Rod-Mill Work IndexCraig TaylorÎncă nu există evaluări
- Of Ore And: Simulated Processing CoalDocument28 paginiOf Ore And: Simulated Processing CoalJeromeÎncă nu există evaluări
- The Gold Mine of Sakdrissi - Results and Analyses and A Calculation of The Prehistoric Gold-ExploitationDocument25 paginiThe Gold Mine of Sakdrissi - Results and Analyses and A Calculation of The Prehistoric Gold-ExploitationTamta ChanturiaÎncă nu există evaluări
- Classification 1Document23 paginiClassification 1Sachin NaikÎncă nu există evaluări
- MINE292-Lecture10-Gravity Separation-2014 PDFDocument34 paginiMINE292-Lecture10-Gravity Separation-2014 PDFraliaga59Încă nu există evaluări
- Why Electrowinning Hates IronDocument7 paginiWhy Electrowinning Hates IronJOSE MACASSIÎncă nu există evaluări
- Ore Reserve Estimation Method - 911 MetallurgistDocument24 paginiOre Reserve Estimation Method - 911 MetallurgistAvinash BarsaÎncă nu există evaluări
- Introduction to Extractive MetallurgyDocument229 paginiIntroduction to Extractive MetallurgyrunganiÎncă nu există evaluări
- Circulating Load RatioDocument2 paginiCirculating Load RatioNatashaEgieara100% (1)
- CIP/CIL/CIC Adsorption Circuit Process Selection GuideDocument8 paginiCIP/CIL/CIC Adsorption Circuit Process Selection GuideSheila Mae GardonÎncă nu există evaluări
- 10 1 1 90 7867 PDFDocument11 pagini10 1 1 90 7867 PDFAlejandro NavarroÎncă nu există evaluări
- Mineral ProcessingDocument10 paginiMineral ProcessingMahmoud MahmoudmÎncă nu există evaluări
- Copper HydrometallurgyDocument11 paginiCopper HydrometallurgyRonald QuilangÎncă nu există evaluări
- Mineral Resource and Ore Reserve Estimation: Second EditionDocument14 paginiMineral Resource and Ore Reserve Estimation: Second EditionRenzo YaringañoÎncă nu există evaluări
- The role of process mineralogy in overcoming mineralogical barriersDocument25 paginiThe role of process mineralogy in overcoming mineralogical barriersfaouzi rachidÎncă nu există evaluări
- SDM Intro To Flowing Film ConcentratorsDocument7 paginiSDM Intro To Flowing Film Concentratorsdrrcc0761Încă nu există evaluări
- Continuous Vat Leaching Pilot Trials Demonstrate Successful Copper RecoveryDocument9 paginiContinuous Vat Leaching Pilot Trials Demonstrate Successful Copper RecoveryGeorgi SavovÎncă nu există evaluări
- 9 10 Test Methods For Characterising OreDocument130 pagini9 10 Test Methods For Characterising OreWillan Villanueva BolañosÎncă nu există evaluări
- Geometallurgy What Why and HowDocument46 paginiGeometallurgy What Why and HowW ZuoÎncă nu există evaluări
- Savana Mining Pan-American Mineral JigDocument1 paginăSavana Mining Pan-American Mineral JigWyattYeagerÎncă nu există evaluări
- Platreef PDFDocument299 paginiPlatreef PDFpleasure masangoÎncă nu există evaluări
- Geometallurgy Roles and Application in Mining OperationDocument25 paginiGeometallurgy Roles and Application in Mining OperationFahrul Rozzi Usman100% (1)
- Preliminary EvaluationDocument5 paginiPreliminary EvaluationAryan AnandÎncă nu există evaluări
- Preg-Robbing Phenomena in The Cyanidation of Sulphide Gold Ores PDFDocument20 paginiPreg-Robbing Phenomena in The Cyanidation of Sulphide Gold Ores PDFboanerges wino pattyÎncă nu există evaluări
- Queen's University - Mining Engineering - ListDocument3 paginiQueen's University - Mining Engineering - ListNitinkiet103Încă nu există evaluări
- HiH 5 Words and Grammar 3Document1 paginăHiH 5 Words and Grammar 3Aldo PabloÎncă nu există evaluări
- Training Materials Festo DonNTUDocument81 paginiTraining Materials Festo DonNTUAldo PabloÎncă nu există evaluări
- 03 Att A3 Constr Direct Man Power 12mar2012Document1 pagină03 Att A3 Constr Direct Man Power 12mar2012Aldo PabloÎncă nu există evaluări
- Consumo Consumo: P. PrespDocument17 paginiConsumo Consumo: P. PrespAldo PabloÎncă nu există evaluări
- AMT-C With SAP ADMS - 201603Document59 paginiAMT-C With SAP ADMS - 201603Aldo PabloÎncă nu există evaluări
- Morococha Project - Contracting Plan (Abr 08, 2011)Document1 paginăMorococha Project - Contracting Plan (Abr 08, 2011)Aldo PabloÎncă nu există evaluări
- C18 Acert Locomotive Engine: Eu Stage Iiib 563 Bkw/755 BHP at 1900 RPMDocument4 paginiC18 Acert Locomotive Engine: Eu Stage Iiib 563 Bkw/755 BHP at 1900 RPMSuat YamanÎncă nu există evaluări
- Webinar - Why DLCC Is Essential For Cat Dealers - 201508Document29 paginiWebinar - Why DLCC Is Essential For Cat Dealers - 201508Aldo PabloÎncă nu există evaluări
- Lubrication Equipment CatalogDocument60 paginiLubrication Equipment Catalogjairo269Încă nu există evaluări
- 03 Att A3 Constr Direct Man Power 12mar2012Document1 pagină03 Att A3 Constr Direct Man Power 12mar2012Aldo PabloÎncă nu există evaluări
- Morococha Project - Contracting Plan (Abr 08, 2011)Document1 paginăMorococha Project - Contracting Plan (Abr 08, 2011)Aldo PabloÎncă nu există evaluări
- Heating A Volume of Liquid: Parameter To Be Entered Repair of Tank FacesDocument1 paginăHeating A Volume of Liquid: Parameter To Be Entered Repair of Tank FacesAldo PabloÎncă nu există evaluări
- General Lubrication Products: High Pressure Stationary Grease PumpsDocument35 paginiGeneral Lubrication Products: High Pressure Stationary Grease PumpsAldo PabloÎncă nu există evaluări
- Manual Wiring SystemDocument29 paginiManual Wiring SystemAldo PabloÎncă nu există evaluări
- Electronic Buzzer Eks / Eksp: Signalling Device For Dry and Damp RoomsDocument4 paginiElectronic Buzzer Eks / Eksp: Signalling Device For Dry and Damp RoomsAldo PabloÎncă nu există evaluări
- Vibrator Draw Config Peru Eliptex - D-53482Document1 paginăVibrator Draw Config Peru Eliptex - D-53482Aldo PabloÎncă nu există evaluări
- Bioleaching vs Pressure Hydrometallurgy for ChalcopyriteDocument33 paginiBioleaching vs Pressure Hydrometallurgy for ChalcopyriteAldo PabloÎncă nu există evaluări
- Grinding FundamentalsDocument49 paginiGrinding Fundamentalsalfonsopescador100% (7)
- 17.10 - NIGEL GRIGG - Silver Concentrate Leaching Presentation 2012 Final PDFDocument42 pagini17.10 - NIGEL GRIGG - Silver Concentrate Leaching Presentation 2012 Final PDFAldo PabloÎncă nu există evaluări
- Cement Mill NotebookDocument32 paginiCement Mill NotebookNael92% (50)
- Capacities and performance of jaw crushersDocument7 paginiCapacities and performance of jaw crushersAldo PabloÎncă nu există evaluări
- Belt Analyst 2007 DocumentationDocument99 paginiBelt Analyst 2007 DocumentationAldo PabloÎncă nu există evaluări
- Media Charge Wear Rod MillsDocument3 paginiMedia Charge Wear Rod MillspolsiemprealdoÎncă nu există evaluări
- 5.5x9m EGL Ball Mill PartsDocument20 pagini5.5x9m EGL Ball Mill PartsAldo PabloÎncă nu există evaluări
- SAGParam OpenEJEMPLO2PROPDocument120 paginiSAGParam OpenEJEMPLO2PROPpolsiemprealdoÎncă nu există evaluări
- Goodyear Conveyor HandbookDocument6 paginiGoodyear Conveyor HandbookAldo PabloÎncă nu există evaluări
- Belt Analyst 2007 DocumentationDocument99 paginiBelt Analyst 2007 DocumentationAldo PabloÎncă nu există evaluări
- HPGRSim Openpractica1Document83 paginiHPGRSim Openpractica1Aldo PabloÎncă nu există evaluări
- Modeling metal extraction and electrowinning processesDocument71 paginiModeling metal extraction and electrowinning processesAldo PabloÎncă nu există evaluări
- Metal Hydride Materials For Solid Hydrogen StorageDocument20 paginiMetal Hydride Materials For Solid Hydrogen StorageJaime Cahuasquí SeguraÎncă nu există evaluări
- Astm A311 - 2015 - ObsoletoDocument4 paginiAstm A311 - 2015 - ObsoletoRicardo Ricardo100% (1)
- CHAPTER 9-Industrial ChemistryDocument17 paginiCHAPTER 9-Industrial ChemistryTooling ganeshÎncă nu există evaluări
- CLASS-10TH - CHAPTER - 3 Metals and Non-MetalsDocument3 paginiCLASS-10TH - CHAPTER - 3 Metals and Non-MetalsTanmay LahaÎncă nu există evaluări
- Database Smelter ESDM UpdateDocument1 paginăDatabase Smelter ESDM Updatebudi1widiawanÎncă nu există evaluări
- Aluminum Analysis ReportGlient: Here is a concise, SEO-optimized title for the document:TITLE IGAS Research Reports Aluminum Alloy Purity and PropertiesDocument3 paginiAluminum Analysis ReportGlient: Here is a concise, SEO-optimized title for the document:TITLE IGAS Research Reports Aluminum Alloy Purity and PropertiessalmanÎncă nu există evaluări
- Chapter9 AnswersDocument5 paginiChapter9 AnswersedytfuyÎncă nu există evaluări
- CHEMISTRY Form 4 CHAPTER 4-The Periodic TableDocument47 paginiCHEMISTRY Form 4 CHAPTER 4-The Periodic TableAngie Kong Su MeiÎncă nu există evaluări
- Coordination Compound and Its ChemistryDocument38 paginiCoordination Compound and Its ChemistryAvinash RaiÎncă nu există evaluări
- Content: C-Stähle Für Die WärmebehandlungDocument18 paginiContent: C-Stähle Für Die WärmebehandlungAsad EjazÎncă nu există evaluări
- Ammonium IronDocument13 paginiAmmonium IronA Vidya SagarÎncă nu există evaluări
- Thermal Conductivity of Metal RodDocument3 paginiThermal Conductivity of Metal RodBhargava S Padmashali100% (1)
- Coeficiente de RozamientoDocument2 paginiCoeficiente de RozamientoThomasÎncă nu există evaluări
- Steel Castings, General Requirements, For Pressure-Containing PartsDocument12 paginiSteel Castings, General Requirements, For Pressure-Containing PartsMarco A. R. JimenesÎncă nu există evaluări
- Qor Colors Pigment Information 090414Document3 paginiQor Colors Pigment Information 090414Pat ShepardÎncă nu există evaluări
- Paint SPECIFICATIONSDocument21 paginiPaint SPECIFICATIONSonur gunes100% (1)
- The Reactions of Adipic AcidDocument3 paginiThe Reactions of Adipic AcidmeimeiliuÎncă nu există evaluări
- Com Su 202 DDocument23 paginiCom Su 202 Dsuriya100% (2)
- Chapter 2 Magnetic Effects of Current XDocument25 paginiChapter 2 Magnetic Effects of Current XPawan Kumar GoyalÎncă nu există evaluări
- Mechanical Behavior of Orthodontic TMA WiresDocument10 paginiMechanical Behavior of Orthodontic TMA WiresMuhammad UzairÎncă nu există evaluări
- Gating System Design For Casting Thin Aluminium Alloy (Al-Si) PlatesDocument10 paginiGating System Design For Casting Thin Aluminium Alloy (Al-Si) PlatesKhin Aung ShweÎncă nu există evaluări
- CMF001 Tutorial 4 Physical ChemistryDocument4 paginiCMF001 Tutorial 4 Physical ChemistrycjcmoneyÎncă nu există evaluări
- Annealing (metallurgy) - Heat treatment softens metalsDocument4 paginiAnnealing (metallurgy) - Heat treatment softens metalsStephen MontelepreÎncă nu există evaluări
- Lloyds Approved Welding Consumables SuppliersDocument16 paginiLloyds Approved Welding Consumables SuppliersadammzjinÎncă nu există evaluări
- Basics in Mineral Processing-IntroductionDocument5 paginiBasics in Mineral Processing-Introductionmakedo33Încă nu există evaluări
- 2016 Specimen Paper 2 PDFDocument18 pagini2016 Specimen Paper 2 PDFPepz SupitchaÎncă nu există evaluări
- Chemistry Worksheet0Document6 paginiChemistry Worksheet0Aliyah RasheedÎncă nu există evaluări
- Examen de Evaluación API 571Document18 paginiExamen de Evaluación API 571berray2007100% (2)
- Cast Iron BrochureDocument12 paginiCast Iron BrochureFlamarion BadaroÎncă nu există evaluări
- Science Paper 1: Stage 9Document16 paginiScience Paper 1: Stage 9Dazai KinnieÎncă nu există evaluări