Documente Academic
Documente Profesional
Documente Cultură
Nuclear Structure
Încărcat de
api-237070241Titlu original
Drepturi de autor
Formate disponibile
Partajați acest document
Partajați sau inserați document
Vi se pare util acest document?
Este necorespunzător acest conținut?
Raportați acest documentDrepturi de autor:
Formate disponibile
Nuclear Structure
Încărcat de
api-237070241Drepturi de autor:
Formate disponibile
Nuclear Structure
All matter is composed of atoms that are in turn composed of a heavier, central,
positively charged core surrounded by a less massive negatively charged cloud of
electrons. The nucleus is composed of protons and neutrons which have about the
same mass and are known as nucleons.
The number of protons in a nucleus tells you which element the atom is. Atoms of a
given element always have the same number of protons. Three numbers describe the
composition of a nucleus:
Atomic Number (Z) the number of protons in the nucleus
Neutron Number (N) the number of neutrons in the nucleus
Atomic Mass Number (A) the number of nucleons in the nucleus
An element or atom is described using the notation
A
Z
where
Z is the atomic number
A is the atomic mass number
X is the generic symbol for the element
Four fundamental interactions occur between the particles in an atom: gravitation,
electromagnetism, the strong nuclear force, and the weak nuclear force.
Gravitation is by far the weakest of the four interactions. Its effects are only
significant when it acts between massive objects and it plays almost no role at the
atomic level.
Electromagnetism acts between electrically charges particles, either attracting or
repelling the particles on which it acts. The magnetic and electrostatic forces produce
the electromagnetic interaction. It is electromagnetism that binds negatively charged
electrons to the positively charged protons in the nucleus to form atoms.
The strong nuclear force is a powerful attractive force that is much stronger than the
electrostatic force. This force is strong enough to overwhelm the repulsive
electromagnetic force and bind the nucleons together to form the nucleus. The strong
nuclear force has a very short range, approximately one femtometre, which is less
than the diameter of a nucleus. At greater distances, the strong force is practically
unobservable.
The weak nuclear force is responsible for processes in which one particle changes
into another type of particle.
Although neutrons have no charge, they actually help to hold the nucleus together.
Neutrons add to the strong nuclear force without adding to the repulsive electrostatic
force of the positively charged protons. Most atoms have at least as many neutrons as
protons. In general, the more protons there are in the nucleus, the higher the
proportion of neutrons that are needed to hold the nucleus together.
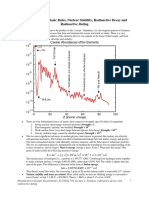
When the number of neutrons is plotted against the number of protons for various
isotopes, a pattern for nuclear stability is observed. Stable nuclei tend to have equal
numbers of protons and neutrons for the first 20 elements. Larger stable elements
contain more neutrons than protons to counteract the proton-proton repulsion. Above
Z = 82, no number of neutrons can produce the force required to form a stable nucleus
and they will decay.
S-ar putea să vă placă și
- Mhf4u Culminating Project Sept 2014Document4 paginiMhf4u Culminating Project Sept 2014api-237070241100% (1)
- Mcr3u Formula SheetDocument1 paginăMcr3u Formula Sheetapi-237070241Încă nu există evaluări
- Method of Statement-RTDocument7 paginiMethod of Statement-RTbuddhikasat50% (2)
- Nuclear Stability - Nature Seeks Lowest EnergyDocument7 paginiNuclear Stability - Nature Seeks Lowest EnergyWaqas SaeedÎncă nu există evaluări
- Atomic Theory & Structure: Oakland Schools Chemistry Resource UnitDocument52 paginiAtomic Theory & Structure: Oakland Schools Chemistry Resource UnithendraprimaÎncă nu există evaluări
- What Is Radioactivity?Document27 paginiWhat Is Radioactivity?Davies MasumbaÎncă nu există evaluări
- The Mighty NucleusDocument3 paginiThe Mighty NucleusSenio, Lexis Jan A.Încă nu există evaluări
- Atomic Nucleus and Nuclear EnergyDocument41 paginiAtomic Nucleus and Nuclear EnergyArundhati KulkarniÎncă nu există evaluări
- Elementary Particles and Their InteractionsDocument22 paginiElementary Particles and Their InteractionsCecilia PicayoÎncă nu există evaluări
- KairiDocument2 paginiKairiyannaungsoe890Încă nu există evaluări
- Victoria Catholic School: Submitted By: Jessica V. MarceloDocument4 paginiVictoria Catholic School: Submitted By: Jessica V. MarceloEric CastilloÎncă nu există evaluări
- Physical Science Reference GuideDocument27 paginiPhysical Science Reference GuideCarlos MasikaÎncă nu există evaluări
- Atomic Number, Mass and CompositionDocument8 paginiAtomic Number, Mass and CompositionManasvi KhandelwalÎncă nu există evaluări
- SEMICONDUCTOR BASICS EXPLAINEDDocument94 paginiSEMICONDUCTOR BASICS EXPLAINEDArindam SenÎncă nu există evaluări
- Nuclear Medicine Physics (Essentials)Document3 paginiNuclear Medicine Physics (Essentials)joseÎncă nu există evaluări
- Atom and The Location of Its Major ComponentsDocument11 paginiAtom and The Location of Its Major ComponentsAldenramosÎncă nu există evaluări
- Nuclear Phy Lecture1Document11 paginiNuclear Phy Lecture1foyshall foraejeeÎncă nu există evaluări
- Modern Physics: Structure of Atoms - Structure of An AtomDocument4 paginiModern Physics: Structure of Atoms - Structure of An AtomAbhay VishwakarmaÎncă nu există evaluări
- PHY-2251 (B) Physics - IV Nuclear Physics ChapterDocument20 paginiPHY-2251 (B) Physics - IV Nuclear Physics Chapterjoy bakshiÎncă nu există evaluări
- NeutronDocument3 paginiNeutronDaliboyina VaishnavÎncă nu există evaluări
- Helium Atom: Jump To Navigationjump To SearchDocument2 paginiHelium Atom: Jump To Navigationjump To SearchWYNÎncă nu există evaluări
- The Structure of The AtomDocument2 paginiThe Structure of The Atomapi-666588628Încă nu există evaluări
- Nuclear Forces and Energy Levels ExplainedDocument3 paginiNuclear Forces and Energy Levels Explainedbilalkhan3567Încă nu există evaluări
- New Harmonize 1Document10 paginiNew Harmonize 1najeebmuhammedbello222Încă nu există evaluări
- Nuclei IDocument20 paginiNuclei IShoaibÎncă nu există evaluări
- Modern Physics Atom and Its StructureDocument13 paginiModern Physics Atom and Its StructureRAHUL CHOUDHARYÎncă nu există evaluări
- Atomic StructureDocument19 paginiAtomic StructureElmarÎncă nu există evaluări
- Nuclear ModelDocument11 paginiNuclear Modelzeamayf.biasÎncă nu există evaluări
- Nuclear PhysicsDocument20 paginiNuclear PhysicsShubh GuptaÎncă nu există evaluări
- For Other Uses, See .: Atom (Disambiguation)Document2 paginiFor Other Uses, See .: Atom (Disambiguation)Hana SpahicÎncă nu există evaluări
- Atomic Nucleus Structure and Binding EnergyDocument10 paginiAtomic Nucleus Structure and Binding EnergyKristiyan GeorgievÎncă nu există evaluări
- Atomic NucleusDocument3 paginiAtomic NucleusMaria Aamer Sabah100% (1)
- Basic Rad Phy1Document99 paginiBasic Rad Phy1Rashmi RajanÎncă nu există evaluări
- Atomic Structure EvolutionDocument2 paginiAtomic Structure EvolutionJessica BeasleyÎncă nu există evaluări
- Lecture-1 - Atomic & Nuclear Physics - FundamentalsDocument17 paginiLecture-1 - Atomic & Nuclear Physics - Fundamentalstrr367267Încă nu există evaluări
- Basic Electricity: By: Adriana MartinezDocument8 paginiBasic Electricity: By: Adriana MartinezSa HaÎncă nu există evaluări
- IG Chem - Nuclear MatterDocument1 paginăIG Chem - Nuclear MatterRyno BredenkampÎncă nu există evaluări
- Atoms and ElementsDocument18 paginiAtoms and ElementsJACK CAMPBELLÎncă nu există evaluări
- What is an atomDocument14 paginiWhat is an atomSwati RoyÎncă nu există evaluări
- Atoms Ni JohannDocument1 paginăAtoms Ni JohannRACHELLE MAE LLAGASÎncă nu există evaluări
- STEMGENPHYSICS2 - HO 5 Atomic and Nuclear PhenomenaDocument12 paginiSTEMGENPHYSICS2 - HO 5 Atomic and Nuclear PhenomenaKyle Cedric MitalÎncă nu există evaluări
- Mithun's Physics Revision Notes (Unit 1)Document6 paginiMithun's Physics Revision Notes (Unit 1)Mithun Dev SabaratnamÎncă nu există evaluări
- AtomDocument12 paginiAtomatgimale.comÎncă nu există evaluări
- Atomic Structure EssayDocument4 paginiAtomic Structure EssayholliebaldwinnÎncă nu există evaluări
- Lec 2 Basic PhysicsDocument26 paginiLec 2 Basic PhysicsHashir AliÎncă nu există evaluări
- Nuclear Stability, Radioactive Decay and DatingDocument8 paginiNuclear Stability, Radioactive Decay and Datingshawnah jamesÎncă nu există evaluări
- Structure and Properties of the Atom ExplainedDocument9 paginiStructure and Properties of the Atom ExplainedenesÎncă nu există evaluări
- Note For EJUDocument18 paginiNote For EJUmr.draungnaingwinÎncă nu există evaluări
- Basic Concepts: Electricity-Ohm's Law - Law of ResistanceDocument8 paginiBasic Concepts: Electricity-Ohm's Law - Law of ResistanceBSMK60Încă nu există evaluări
- Nuclear Chemistry FundamentalsDocument16 paginiNuclear Chemistry FundamentalsManohar MaripeÎncă nu există evaluări
- Atomic StructureDocument28 paginiAtomic StructureJohn Vince Ramos PapÎncă nu există evaluări
- Chapter 13Document13 paginiChapter 13Shyam 07Încă nu există evaluări
- AtomsDocument6 paginiAtomsshreyaÎncă nu există evaluări
- 1. introduction and basic definitionsDocument75 pagini1. introduction and basic definitionsma9858056Încă nu există evaluări
- The Four Fun ForcesDocument2 paginiThe Four Fun ForcesZehnouni AbderezakÎncă nu există evaluări
- Mejarito, Jian Kristin C. BSED Science 3-C Modern PhysicsDocument4 paginiMejarito, Jian Kristin C. BSED Science 3-C Modern PhysicsallisonÎncă nu există evaluări
- Chemistry Assignment 1Document4 paginiChemistry Assignment 1joegabriel901Încă nu există evaluări
- Atoms and Elements: Atomic StructureDocument5 paginiAtoms and Elements: Atomic StructureJohn Rey Siwala EduqueÎncă nu există evaluări
- Particles and Radiation ExplainedDocument42 paginiParticles and Radiation ExplainedDarren Contreras100% (1)
- AtomsDocument3 paginiAtomsMarlen Rocío Toledo PachecoÎncă nu există evaluări
- Your Journey To The Basics Of Quantum Realm Volume II: Your Journey to The Basics Of Quantum Realm, #2De la EverandYour Journey To The Basics Of Quantum Realm Volume II: Your Journey to The Basics Of Quantum Realm, #2Evaluare: 5 din 5 stele5/5 (1)
- Sph3u Student SyllabusDocument3 paginiSph3u Student Syllabusapi-237070241Încă nu există evaluări
- Sph3u ExamreviewDocument2 paginiSph3u Examreviewapi-237070241Încă nu există evaluări
- Mhf4u Student SyllabusDocument3 paginiMhf4u Student Syllabusapi-237070241Încă nu există evaluări
- Forces and Motion-1Document8 paginiForces and Motion-1api-237070241Încă nu există evaluări
- Phase ChangeDocument1 paginăPhase Changeapi-237070241Încă nu există evaluări
- Resonance and Sound ReviewDocument3 paginiResonance and Sound Reviewapi-237070241Încă nu există evaluări
- RadioactivityDocument3 paginiRadioactivityapi-237070241Încă nu există evaluări
- Newtons Laws of MotionDocument33 paginiNewtons Laws of Motionapi-237070241Încă nu există evaluări
- Review SlidesDocument16 paginiReview Slidesapi-237070241Încă nu există evaluări
- Kinematics Review SolutionsDocument2 paginiKinematics Review Solutionsapi-237070241Încă nu există evaluări
- Cofunction IdentitiesDocument1 paginăCofunction Identitiesapi-237070241Încă nu există evaluări
- Lab Report RubricDocument3 paginiLab Report Rubricapi-237070241Încă nu există evaluări
- 02 Forces and MotionDocument8 pagini02 Forces and Motionapi-237070241Încă nu există evaluări
- Mhf4u Course Calendar Sept 2014-1Document5 paginiMhf4u Course Calendar Sept 2014-1api-237070241Încă nu există evaluări
- Dotcross QuestionsDocument2 paginiDotcross Questionsapi-237070241Încă nu există evaluări
- Kinematics ReviewDocument2 paginiKinematics Reviewapi-237070241Încă nu există evaluări
- Mcv4u Final Exam ReviewDocument2 paginiMcv4u Final Exam Reviewapi-237070241Încă nu există evaluări
- Dotcross AnswersDocument1 paginăDotcross Answersapi-237070241Încă nu există evaluări
- Mcr3u Student SyllabusDocument4 paginiMcr3u Student Syllabusapi-237070241Încă nu există evaluări
- Kinematics ReviewDocument3 paginiKinematics Reviewapi-237070241Încă nu există evaluări
- Standing WavesDocument2 paginiStanding Wavesapi-237070241Încă nu există evaluări
- Standing Waves ReviewDocument2 paginiStanding Waves Reviewapi-237070241Încă nu există evaluări
- Cartesian VectorsDocument6 paginiCartesian Vectorsapi-237070241Încă nu există evaluări
- Rube Goldberg at BronteDocument4 paginiRube Goldberg at Bronteapi-237070241Încă nu există evaluări
- Chapter 6 ReviewDocument1 paginăChapter 6 Reviewapi-237070241Încă nu există evaluări
- 04 - Relative ExtremaDocument4 pagini04 - Relative Extremaapi-237070241Încă nu există evaluări
- Optimzation WorksheetDocument3 paginiOptimzation Worksheetapi-237070241Încă nu există evaluări
- Modern Physics Problem Sheets NewDocument20 paginiModern Physics Problem Sheets NewXyzÎncă nu există evaluări
- Unit 3 - Two Dimensional NMR: Week - 2 - AssignmentDocument4 paginiUnit 3 - Two Dimensional NMR: Week - 2 - AssignmentSaurav PaulÎncă nu există evaluări
- Fundamental Atomic Data: Appendix ADocument70 paginiFundamental Atomic Data: Appendix AHJÎncă nu există evaluări
- Exercises With Solutions in Radiation Physics) 1 Radiation Sources and Radioactive DecayDocument27 paginiExercises With Solutions in Radiation Physics) 1 Radiation Sources and Radioactive DecayDavitMartinezÎncă nu există evaluări
- RadioactivitiesDocument31 paginiRadioactivitiesapi-3743896Încă nu există evaluări
- Problemas 1HRMNDocument101 paginiProblemas 1HRMNSarah Jayne BurlinghamÎncă nu există evaluări
- (BP504, Pharmacognosy & Phytochemistry II, Unit 1Document9 pagini(BP504, Pharmacognosy & Phytochemistry II, Unit 1vishu950753Încă nu există evaluări
- Lecture 16 - Nuclear Fission and FussionDocument4 paginiLecture 16 - Nuclear Fission and FussionMuhammadHamzaÎncă nu există evaluări
- Physics Decay LabDocument4 paginiPhysics Decay LabKamal GrahamÎncă nu există evaluări
- 3-Formation of Heavy ElementsDocument29 pagini3-Formation of Heavy ElementsJhester de leonÎncă nu există evaluări
- Nature - Disintegration of Uranium by Neutrons A New Type of Nuclear ReactionDocument2 paginiNature - Disintegration of Uranium by Neutrons A New Type of Nuclear ReactionbelleblackÎncă nu există evaluări
- 7.nuclear Chemistry and Environmental Chemistry ExerciseDocument38 pagini7.nuclear Chemistry and Environmental Chemistry ExerciseYogy YÎncă nu există evaluări
- Nuclear Weapons Captain Shaifful Nizam Bin Abdul KholidDocument63 paginiNuclear Weapons Captain Shaifful Nizam Bin Abdul KholidShaifful NizamÎncă nu există evaluări
- Angelica Pazziuagan, Answer Sheet Module 3 Part 1Document4 paginiAngelica Pazziuagan, Answer Sheet Module 3 Part 1angelica pazziuaganÎncă nu există evaluări
- Impact of Season and Location On The Natural Radioactivity in Marine Macroalgae (Gracilaria Edulis) of Coastal Tamil Nadu, India PDFDocument9 paginiImpact of Season and Location On The Natural Radioactivity in Marine Macroalgae (Gracilaria Edulis) of Coastal Tamil Nadu, India PDFBećo PehlivanovićÎncă nu există evaluări
- TransmutationDocument2 paginiTransmutationZakir Hussain NagthanÎncă nu există evaluări
- Class-8-General Science-Chapter-5 - Inside The AtomDocument8 paginiClass-8-General Science-Chapter-5 - Inside The AtomONE CLICK COMPUTERÎncă nu există evaluări
- POGIL Avg Atomic Mass KEYDocument4 paginiPOGIL Avg Atomic Mass KEYbobÎncă nu există evaluări
- Atomic Structure DPP 2 PDFDocument2 paginiAtomic Structure DPP 2 PDFtan jig0% (1)
- Lab 8 - Nuclear PhysicsDocument9 paginiLab 8 - Nuclear PhysicsRichard HuangÎncă nu există evaluări
- Hydrotector - Screening Tool: FeaturesDocument2 paginiHydrotector - Screening Tool: Featuresabidhussain470Încă nu există evaluări
- Neutron - Wikipedia, The Free EncyclopediaDocument15 paginiNeutron - Wikipedia, The Free EncyclopediaMaxim ŠporkiÎncă nu există evaluări
- Basics in NMRDocument75 paginiBasics in NMRPrashant PandeyÎncă nu există evaluări
- Nuclear Stability NotesDocument13 paginiNuclear Stability NotesHENRY MARTINEZÎncă nu există evaluări
- Nuclear Medicine Physics Part 2Document27 paginiNuclear Medicine Physics Part 2هبلتنى الكورةÎncă nu există evaluări
- Nuclear Pollution: Submitted by Nandhini. GDocument15 paginiNuclear Pollution: Submitted by Nandhini. GNandhini GÎncă nu există evaluări
- Nuclear ProliferationDocument39 paginiNuclear ProliferationDasuni De SilvaÎncă nu există evaluări
- Brit A1 FormDocument2 paginiBrit A1 FormRavishwar NarayanÎncă nu există evaluări
- DPP 1 NucleiDocument3 paginiDPP 1 NucleiSidhu MoosewaalaÎncă nu există evaluări