Documente Academic
Documente Profesional
Documente Cultură
Expt. 1 PH and Buffer Systems
Încărcat de
Dizzle Jalian Dela Cruz0 evaluări0% au considerat acest document util (0 voturi)
82 vizualizări1 paginăThis document outlines procedures for two experiments on buffer systems and pH. The first examines how buffer capacity is affected by the concentration of the buffer and the ratio of the conjugate base to weak acid. Various buffer solutions will be prepared at different pH levels and concentrations, then sodium hydroxide or hydrochloric acid will be added to test the buffering effect. The second experiment involves titrating an amino acid sample with potassium hydroxide while measuring pH to determine the pKa values and draw the structures at each pKa.
Descriere originală:
pH and Buffer Systems
Drepturi de autor
© © All Rights Reserved
Formate disponibile
PDF, TXT sau citiți online pe Scribd
Partajați acest document
Partajați sau inserați document
Vi se pare util acest document?
Este necorespunzător acest conținut?
Raportați acest documentThis document outlines procedures for two experiments on buffer systems and pH. The first examines how buffer capacity is affected by the concentration of the buffer and the ratio of the conjugate base to weak acid. Various buffer solutions will be prepared at different pH levels and concentrations, then sodium hydroxide or hydrochloric acid will be added to test the buffering effect. The second experiment involves titrating an amino acid sample with potassium hydroxide while measuring pH to determine the pKa values and draw the structures at each pKa.
Drepturi de autor:
© All Rights Reserved
Formate disponibile
Descărcați ca PDF, TXT sau citiți online pe Scribd
0 evaluări0% au considerat acest document util (0 voturi)
82 vizualizări1 paginăExpt. 1 PH and Buffer Systems
Încărcat de
Dizzle Jalian Dela CruzThis document outlines procedures for two experiments on buffer systems and pH. The first examines how buffer capacity is affected by the concentration of the buffer and the ratio of the conjugate base to weak acid. Various buffer solutions will be prepared at different pH levels and concentrations, then sodium hydroxide or hydrochloric acid will be added to test the buffering effect. The second experiment involves titrating an amino acid sample with potassium hydroxide while measuring pH to determine the pKa values and draw the structures at each pKa.
Drepturi de autor:
© All Rights Reserved
Formate disponibile
Descărcați ca PDF, TXT sau citiți online pe Scribd
Sunteți pe pagina 1din 1
EXERCISE 1: pH and Buffer Systems
Procedures:
A. Factors affecting Buffer capacity
1. Effect of concentration of the buffer
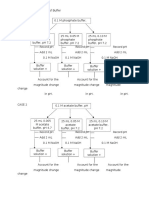
Using 0.1 acetate buffer, pH 4.7, prepare 25 mL each of the buffer solutions with the following
concentrations at pH 4.7:
i.
0.005 M
ii.
0.05 M
iii.
0.10 M
Record the pH of the buffer solutions. Add 2 mL of 0.1 M NaOH to each 25 mL buffer
samples. Record the pH of each buffer solution after the addition of alkali. Account for the
magnitude of the change in pH.
2. Effect on the ration of the Conjugate Base to the Weak acid
From the Henderson-Hasselbalch equation, calculate the ratio of acetic acid and acetate
required to produce buffer solution with:
i.
pH 3.7
ii.
pH 4.7
iii.
pH 5.7
Using 0.1 M stock solutions of the weak acid and conjugate base, make up 25 mL of the buffer
solution. Measure and record the pH of the buffer solution. Divide into two equal parts. Add
2 mL of 0.1 N NaOH to one part and 2 mL 0.1 N HCl on the other. Record the pH of each buffer
solution after addition of alkali or acid. Account for the magnitude of the pH shift in each with
reference to the direction of pH shift.
B. Titration of an Amino Acid
In this part of the experiment you will titrate an amino acid sample which will be assigned to you
by your instructor.
Pipette 10 mL of the amino acid sample into 25 mL or 50 mL beaker. Adjust pH to 1.5 using 1 N
HCl. Use a burette to add 0.1 M KOH in approximately 1.0 mL increments until about pH 12 is reached
(record the accurate volume of each increment
Stir well and measure the pH after each addition. Construct a titration curve by plotting pH against
the volume of KOH added. Label your graph properly. From your graph, determine the pKas of your
sample. Draw the structure of your amino acid at each pKa value.
S-ar putea să vă placă și
- The Chemistry of Dairy Products - A Chemical Analysis of Milk, Cream and ButterDe la EverandThe Chemistry of Dairy Products - A Chemical Analysis of Milk, Cream and ButterÎncă nu există evaluări
- Advanced Pharmaceutical analysisDe la EverandAdvanced Pharmaceutical analysisEvaluare: 4.5 din 5 stele4.5/5 (2)
- PH and BuffersDocument4 paginiPH and BuffersNirmalya Chatterjee100% (1)
- Chem 131A: PH and Buffers ExperimentDocument4 paginiChem 131A: PH and Buffers ExperimentLinh NguyễnÎncă nu există evaluări
- Chem - Lab Report 3Document11 paginiChem - Lab Report 3mahzebÎncă nu există evaluări
- Isoelectric PointDocument24 paginiIsoelectric PointSangeeta RayÎncă nu există evaluări
- Expt 2 PH and Buffer SystemDocument8 paginiExpt 2 PH and Buffer SystemHeart YokingcoÎncă nu există evaluări
- Preparation of Buffer SolutionDocument8 paginiPreparation of Buffer SolutionESTHER WONG TZE YIING -Încă nu există evaluări
- P T B S: Reparation and Esting of Uffer OlutionsDocument4 paginiP T B S: Reparation and Esting of Uffer Olutionsadolfo olmosÎncă nu există evaluări
- Exp 1 Prepare Buffer SolnDocument4 paginiExp 1 Prepare Buffer SolnLaila FaeizahÎncă nu există evaluări
- Buffer Preparation and PH Measurement Using The Electrometric Method and Colorimetric MethodDocument2 paginiBuffer Preparation and PH Measurement Using The Electrometric Method and Colorimetric MethodArndrei CunananÎncă nu există evaluări
- Regine T. Diaz, Lordjel Kin M. Eleda: Figure 1. PH MeterDocument2 paginiRegine T. Diaz, Lordjel Kin M. Eleda: Figure 1. PH MeterArndrei CunananÎncă nu există evaluări
- SadasdsadsadDocument2 paginiSadasdsadsadArndrei CunananÎncă nu există evaluări
- Name: Castil, Joyce B. Date Submitted: January 15, 2021 Lab Schedule: TTH, 8:00 - 11:00 RatingDocument31 paginiName: Castil, Joyce B. Date Submitted: January 15, 2021 Lab Schedule: TTH, 8:00 - 11:00 RatingJoyce Castil (Joyceee)Încă nu există evaluări
- Labexercise 2Document7 paginiLabexercise 2Ma Catherine MalanogÎncă nu există evaluări
- Acid Base TitrationDocument12 paginiAcid Base TitrationMsfaeza HanafiÎncă nu există evaluări
- Part B BuffersDocument2 paginiPart B BuffersAltaf Hussain KhanÎncă nu există evaluări
- Plugin bl302 PkavalueDocument3 paginiPlugin bl302 PkavalueSurya KumarÎncă nu există evaluări
- BufferDocument6 paginiBufferGladys CastilloÎncă nu există evaluări
- Buffer SolutionsV3 PDFDocument4 paginiBuffer SolutionsV3 PDFKarunakarÎncă nu există evaluări
- GA7 Potentio Titr Rev7 99Document9 paginiGA7 Potentio Titr Rev7 99Jerome SadudaquilÎncă nu există evaluări
- Lab 6 Using PH ElectrodeDocument14 paginiLab 6 Using PH ElectrodeMina VoÎncă nu există evaluări
- Wis Ap Chem Lab 17 Buffer Solutions ArcherDocument7 paginiWis Ap Chem Lab 17 Buffer Solutions Archerapi-201479236Încă nu există evaluări
- Experiment 1 Preparation of Buffer Solution: Biochemical EngineeringDocument3 paginiExperiment 1 Preparation of Buffer Solution: Biochemical EngineeringMohamedZakariaAbdullahÎncă nu există evaluări
- Written Report Expt.8Document5 paginiWritten Report Expt.8Nicole NatanauanÎncă nu există evaluări
- Chem 160.1 Ex2 BufferDocument8 paginiChem 160.1 Ex2 BufferAsi JenÎncă nu există evaluări
- Mixture of Carbonate BicarbonateDocument9 paginiMixture of Carbonate BicarbonateIan Justine SanchezÎncă nu există evaluări
- Potentiometric Titration CurvesDocument5 paginiPotentiometric Titration CurvesDavid GrahamÎncă nu există evaluări
- Acid Base TitrationDocument5 paginiAcid Base TitrationFernando NainggolanÎncă nu există evaluări
- Lab Report 11Document3 paginiLab Report 11PaulÎncă nu există evaluări
- Quis Kimia Analitik Dasar Departement of Chemical Education Rombel 001. Sunday: 10.00 - 12.30 Please Your Answer This Any ProblemsDocument1 paginăQuis Kimia Analitik Dasar Departement of Chemical Education Rombel 001. Sunday: 10.00 - 12.30 Please Your Answer This Any ProblemsriaayumaharaniÎncă nu există evaluări
- Lab Experiment 3 - PH TitrationDocument1 paginăLab Experiment 3 - PH Titrationfive shadowsÎncă nu există evaluări
- Summary: Phthalate (KHP) Solution Which The Molarity Is Already Known. Expressing The Chemical ReactionDocument15 paginiSummary: Phthalate (KHP) Solution Which The Molarity Is Already Known. Expressing The Chemical ReactionDayledaniel SorvetoÎncă nu există evaluări
- Preparing Buffers and Buffer CapacityDocument7 paginiPreparing Buffers and Buffer CapacityAndreaKatovic100% (1)
- Experimennt 5 - Examination of BuffersDocument7 paginiExperimennt 5 - Examination of BuffersMuhammad Riv'at NalÎncă nu există evaluări
- CHY 47.1 Procedure Factors Affecting Buffers Capacity 1st Sem 2021-2022Document5 paginiCHY 47.1 Procedure Factors Affecting Buffers Capacity 1st Sem 2021-2022Kathryne May JinonÎncă nu există evaluări
- Experiment 1 Preparation of Buffer SolutionsDocument16 paginiExperiment 1 Preparation of Buffer Solutionsmohamad ashaziq89% (56)
- Buffer (Larutan Penyangga)Document6 paginiBuffer (Larutan Penyangga)Budiman ApriyossaÎncă nu există evaluări
- WINSEM2022-23 BBIT206P LO VL2022230503900 Reference Material I 23-12-2022 Experiment 2 Acid-Base Titration PH Meter FADocument4 paginiWINSEM2022-23 BBIT206P LO VL2022230503900 Reference Material I 23-12-2022 Experiment 2 Acid-Base Titration PH Meter FAGravity JaiÎncă nu există evaluări
- Phosphorus Acid TitrationDocument2 paginiPhosphorus Acid TitrationJoséMaríaMoreÎncă nu există evaluări
- SchematicDocument4 paginiSchematicCai PascualÎncă nu există evaluări
- Experiment 2Document8 paginiExperiment 2Alok VermaÎncă nu există evaluări
- Exp2 - A-B Titration - w2013-2Document10 paginiExp2 - A-B Titration - w2013-2lovingbubblesÎncă nu există evaluări
- 1 Theory: Buffers and Buffer CapacityDocument5 pagini1 Theory: Buffers and Buffer Capacitygrim_ripperÎncă nu există evaluări
- Acid-Base Equilibria and TitrationsDocument2 paginiAcid-Base Equilibria and TitrationsraeeamdeeirÎncă nu există evaluări
- Lab 3: Introduction To Acids Base Chemistry Part A Experimental Determination of Acid Dissociation Constant, KaDocument10 paginiLab 3: Introduction To Acids Base Chemistry Part A Experimental Determination of Acid Dissociation Constant, Kaenock yegonÎncă nu există evaluări
- Exp 6 PH Metric TitrationDocument3 paginiExp 6 PH Metric TitrationDeep DaveÎncă nu există evaluări
- Lab Experiment 3 Ka Determination Through PH TitrationDocument4 paginiLab Experiment 3 Ka Determination Through PH TitrationxmusiqaÎncă nu există evaluări
- PH BuffersDocument5 paginiPH BuffersReem NasserÎncă nu există evaluări
- CPB 30103 Biochemical Engineering UniKL MICET Experiment 1: Preparation of Buffer Solution Full Lab ReportDocument10 paginiCPB 30103 Biochemical Engineering UniKL MICET Experiment 1: Preparation of Buffer Solution Full Lab ReportSiti Hajar Mohamed0% (1)
- Activity No. 1 - Preparation of A BufferDocument3 paginiActivity No. 1 - Preparation of A BufferJoshua Abelgas100% (1)
- Acid and Base Titrations Using A PH MeterDocument5 paginiAcid and Base Titrations Using A PH MeterJason Oliver ChanÎncă nu există evaluări
- AP Chemistry - Titration Curves of Strong and Weak Acids and BasesDocument5 paginiAP Chemistry - Titration Curves of Strong and Weak Acids and BasesJonathan Chen100% (2)
- Vdocument - in Buffer Solutions 568b0b28b560dDocument14 paginiVdocument - in Buffer Solutions 568b0b28b560dAmiraÎncă nu există evaluări
- Practical 04 - Estimation of PKa by Half Neutralization MethodDocument10 paginiPractical 04 - Estimation of PKa by Half Neutralization Methodsandi fernandoÎncă nu există evaluări
- Titration Curves of Acid-Base and Determination of PH and Concentration From The Equivalence PointDocument24 paginiTitration Curves of Acid-Base and Determination of PH and Concentration From The Equivalence PointMiguel Angel Roldan MartinÎncă nu există evaluări
- Buffers and Redox Laboratory 6 v2Document12 paginiBuffers and Redox Laboratory 6 v2skyeandoÎncă nu există evaluări
- PH Meters Purdue University Instrument Van Project Acid-Base Titration Using A PH MeterDocument5 paginiPH Meters Purdue University Instrument Van Project Acid-Base Titration Using A PH MeterNatsu PatnaikÎncă nu există evaluări
- Buffer CapacityDocument3 paginiBuffer Capacityharshalgarse100% (1)
- Determination of Concentration of Acetic Acid in VinegarDocument22 paginiDetermination of Concentration of Acetic Acid in VinegarFatin Izzati Hasnan100% (1)