Documente Academic
Documente Profesional
Documente Cultură
Gouty Arthritis
Încărcat de
virnzrobzDescriere originală:
Drepturi de autor
Formate disponibile
Partajați acest document
Partajați sau inserați document
Vi se pare util acest document?
Este necorespunzător acest conținut?
Raportați acest documentDrepturi de autor:
Formate disponibile
Gouty Arthritis
Încărcat de
virnzrobzDrepturi de autor:
Formate disponibile
INTRODUCTION
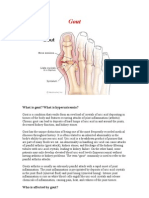
Gout refers to disease that occurs in response to the presence of crystals of monosodium
urate (MSU) in joints, bones, and soft tissues. Both acute arthritis and chronic arthropathy
(tophaceous gout) are considered under the rubric of gout.
The mechanisms of crystal deposition, crystal-induced inflammation, and destructive lesions
of joints and bones associated with macroscopic collections of MSU (tophi) will be reviewed
here. The clinical features, diagnosis, and treatment of acute gout, prevention of recurrent
gout, asymptomatic hyperuricemia, and associated renal diseases are discussed elsewhere.
(See "Clinical manifestations and diagnosis of gout" and"Treatment of acute
gout" and "Prevention of recurrent gout" and "Asymptomatic hyperuricemia" and "Uric acid
nephrolithiasis" and "Uric acid renal diseases".)
HYPERURICEMIA AND GOUT
Hyperuricemia can be caused by underexcretion or overproduction of uric acid, and/or
overconsumption of purine-rich foods that are metabolized to urate. A detailed discussion of
the causes of hyperuricemia and of the normal mechanisms of urate handling are presented
separately. (See "Asymptomatic hyperuricemia" and"Uric acid balance".)
A causative relationship among hyperuricemia, deposition of urate crystals, and gout was
proposed by Garrod in 1859 and later [1]. However, injection of solutions of uric acid into
blood or joints failed to reproduce the clinical features of the disease, casting doubt on the
association. Freudweiler noted in 1899 that injection of tophaceous material caused
inflammation [2,3].
The identification of MSU crystals within phagocytes when synovial fluid was examined using
compensated polarized microscopy was an important advance in understanding the
pathogenesis of gout [4]. This was followed by the observation that injection of MSU
crystals into normal joints produced acute gout attacks, reestablishing the connection
between urate crystals and gout [5,6]. The outline of the pathophysiology of gout is as
follows: a period of hyperuricemia leads to MSU crystal deposition, reaction to which can
result in acute and/or chronic inflammation.
INTRODUCTION
Gout (monosodium urate crystal deposition disease) is characterized biochemically by
extracellular fluid urate saturation. The clinical manifestations include one or more of the
following:
O #ecurrent attacks of acute inflammatory arthritis
O Chronic arthropathy
O Accumulation of urate crystals in the form of tophaceous deposits
O Uric acid nephrolithiasis
O A chronic nephropathy that in gouty patients is most often due to comorbid states
All patients with gout have hyperuricemia (saturation of serum for urate) at some point in
their disease. (See"Uric acid balance".) However, most hyperuricemic individuals never
experience a clinical event resulting from urate crystal deposition. Thus, the diagnosis of
gout focuses on the fundamental pathophysiologic events defining the clinical state: tissue
deposition of urate crystals and the accompanying inflammatory and potentially destructive
consequences. Within this framework, hyperuricemia is viewed as a necessary but not
sufficient precondition for the development of urate crystal deposition disease and is to be
distinguished from gout, the clinical syndrome.
The clinical manifestations and diagnosis of gout will be reviewed here. Issues related to
asymptomatic hyperuricemia, the treatment of acute gout, and prevention of recurrent gout
are discussed separately. (See"Asymptomatic hyperuricemia" and "Treatment of acute
gout" and "Prevention of recurrent gout".)
HISTORICAL PERSPECTIVE
Descriptions of the epidemiology, clinical features, and natural history of gout evolved over
more than two millennia of study. The last half of the twentieth century produced
confirmation that the pathogenesis of gout involves urate crystal deposition. Pivotal in this
progress was the introduction of polarized light microscopy into clinical practice, providing
urate crystal identification in synovial fluid as the means to achieve rapid and definitive
diagnosis and to resolve the formerly ambiguous relationship between hyperuricemia and
gout [1].
INTRODUCTION
Acute gout is an intensely painful inflammatory arthritis that typically involves a single joint,
but can affect multiple joints. The goal of therapy in an acute gout attack is prompt and safe
termination of pain and disability. (See "Clinical manifestations and diagnosis of gout",
section on 'Acute gouty arthritis'.)
Without therapy, acute gouty arthritis usually resolves within a few days to several weeks.
However, symptoms improve more quickly with administration of any of a broad array of
antiinflammatory drugs (figure 1) [1]. Symptom resolution is more prompt and complete
with early initiation of therapy.
A number of drugs have been used for the treatment of acute gout, although randomized
trial data is surprisingly limited [2]:
O onsteroidal antiinflammatory drugs
O Colchicine
O Intraarticular or systemic glucocorticoids
O Interleukin 1 beta inhibition (investigational)
Upon resolution of the acute attack, the patient is said to have entered the intercritical
(between attacks) period, which may or may not require prophylactic therapy to prevent
recurrent gout and chronic tophaceous disease.
INTRODUCTION
Pharmacologic modalities employed in the treatment of gout can be classified as
antiinflammatory, prophylactic, and antihyperuricemic. These designations reflect the three
distinctive aims of treatment:
O Antiinflammatory therapy for prompt and safe termination of the acute arthritic
attack
O Antiinflammatory prophylaxis for prevention of recurrences of acute gouty arthritis
O Antihyperuricemic (urate-lowering) therapy for prevention and reversal of the
consequences of urate crystal deposition in joints (gouty arthropathy), urinary tract
(nephrolithiasis), renal interstitium (rarely producing renal failure due to urate
nephropathy), and tissues and parenchymal organs (tophi)
Upon resolution of an acute gouty attack, the patient is said to have entered an intercritical
(between attacks) period. Intervals between attacks of acute gouty arthritis are of variable
duration. Most untreated patients with gout will experience a second episode within two
years. (See "Clinical manifestations and diagnosis of gout", section on 'Intercritical gout and
recurrent gouty arthritis'.)
The following preventive issues should be addressed during the intercritical period:
O ifestyle modification and agents for risk reduction
O Comorbid disease management
O The need for pharmacologic urate-lowering therapy
O Prophylactic nonsteroidal antiinflammatory drug or colchicine therapy, primarily to
reduce the recurrence of acute attacks of gout during initiation of urate-lowering
therapy
INTRODUCTION
Asymptomatic hyperuricemia is the term applied to settings in which the serum urate
concentration is elevated, but neither symptoms nor signs of urate crystal deposition (gout)
have occurred. Although gout may develop in a hyperuricemic individual at any point, it is
likely that two-thirds or more of hyperuricemic individuals will remain asymptomatic [1-5].
The implications of hyperuricemia may be broadly regarded as those related to urate or uric
acid crystal deposition and those emerging from crystal deposition-unrelated associations of
hyperuricemia with important disorders, including hypertension, chronic kidney disease,
cardiovascular disease, and the insulin resistance syndrome.
The etiology and management of asymptomatic hyperuricemia will be reviewed here. Gout,
uric acid renal diseases, and uric acid nephrolithiasis are discussed separately. (See "Clinical
manifestations and diagnosis of gout" and "Treatment of acute gout" and "Prevention of
recurrent gout" and "Uric acid renal diseases" and"Uric acid nephrolithiasis".)
DEFINITIONS
The statistical definition of hyperuricemia is difficult to accept because of non-normal
distribution of serum urate concentrations in most populations. For purposes relating to
urate crystal deposition, a definition of hyperuricemia based on the solubility limit of urate
in body fluids (ie, the concentration at which a state of supersaturation for urate is reached
in the serum) is appropriate. This physicochemical definition corresponds to urate
concentrations exceeding about 7.0 mg/d (416 micromol/), as measured by automated
enzymatic (uricase) methods in routine clinical laboratory use. These values are
approximately 1 mg/d (60 micromol/) lower than those obtained with colorimetric
methods.
A definition of hyperuricemia appropriate to the non-crystal deposition-associations of
hyperuricemia with the conditions listed above is more problematic for two reasons: first,
the high prevalence of urate values exceeding saturation but within 2 standard deviations of
the population mean (for example, an estimated 5 to 8 percent in adult white males in the
US and 25 percent in Taiwan Chinese males [6]); and second, the fact that associations of
serum urate levels with cardiovascular and other disorders are manifested at concentrations
that are clearly subsaturating [7].
Classification - Persistent hyperuricemia is a common biochemical abnormality that
results from excessive urate production and/or diminished renal uric acid excretion [8].
6|rrhos|s - a Hajor L|ver 0|sease
CIrrIosIs Is u cIronIc IIver dIseuse LIuL ends up IeuvIng LIe scur und dumugIng LIe IIver
LIssues cenLrIc Lo body IuncLIons. Once dumuged by CIrrIosIs LIe ceIIs und LIssues In LIe
IIver ure dIIIIcuIL Lo repuIr und wIen regeneruLed susLuIns u permunenL scurs und
sLrunds LIuL creuLes probIem In bIood cIrcuIuLIon process LIrougI IIver.
Iver Is un ImporLunL IuncLIonury oI body und Is responsIbIe Ior cerLuIn essenLIuI body
IuncLIons sucI us bIood porLuI cIrcuIuLIon, BIIIrubIn SecreLIon und bIocIemIcuI IuncLIons
(drugs & LoxIns meLuboIIzuLIon, bIood cIoLLIng, CIoIesLeroI, BIood pressure eLc). And u
sIIgIL dysIuncLIonIng oI IIver muyuIIecL LIese IuncLIons und cun Ieud Lo severe
compIIcuLIons.
CIrrIosIs cun be IdenLIIIed wILI sympLoms IIke Iever, VomILIng, ResLIessness, SIeepIng
dIsorders, oss oI uppeLILe, unusuuI weIgIL guInJIoss, Lender muscIes und muscIe puIn
eLc.Some oI LIe oLIer mujor CIrrIosIs sympLoms LIuL ure Indeed compIIcuLIons ure
DIseuses IIke DIurrIeu, BIood cIoLLIng probIem, HemorrIoIds, AbdomInuI sweIIIng und
puIn due Lo enIurge IIver, ReucLIon Lo medIcuLIons, drugs eLc due Lo non-IuncLIonIng
bIocIemIcuI ucLIon oI IIver, JuundIce rIses due Lo BIIIrubIn secreLIon muIIuncLIonIng In
IIver und Is IdenLIIIed by yeIIowIng oI skIn und eyes, ow sex drIve und ImpoLence
S-ar putea să vă placă și
- Kristal Artropati KuliahDocument36 paginiKristal Artropati KuliahRoby KieranÎncă nu există evaluări
- Gout and Othercrystal-Associated ArthropathiesDocument31 paginiGout and Othercrystal-Associated ArthropathiesLia pramitaÎncă nu există evaluări
- Got ADocument5 paginiGot AViribu GaliciaÎncă nu există evaluări
- Akram Et AlDocument3 paginiAkram Et AlElok IzawatiÎncă nu există evaluări
- Gout DLL ArtikelDocument74 paginiGout DLL ArtikelFawzia Haura FathinÎncă nu există evaluări
- Gout & PseudogoutDocument14 paginiGout & PseudogoutPatrick CommettantÎncă nu există evaluări
- Gout AmanDocument39 paginiGout AmanSandip VaghelaÎncă nu există evaluări
- Jurnal Gout EnglishDocument8 paginiJurnal Gout EnglishPutri ArtikaÎncă nu există evaluări
- Gout&Pseudogout UpdateDocument54 paginiGout&Pseudogout UpdateSandy AgustianÎncă nu există evaluări
- NEJM GoutDocument9 paginiNEJM GoutAnonymous G26HIUtzV100% (1)
- Name: ID:: Madiha Sayed Nagy. 51859Document12 paginiName: ID:: Madiha Sayed Nagy. 51859drng48Încă nu există evaluări
- Lect # 2 Care of Patients With Gout and Paget's DiseaseDocument21 paginiLect # 2 Care of Patients With Gout and Paget's DiseaseShayan ShayanÎncă nu există evaluări
- GoutDocument5 paginiGoutTeslim RajiÎncă nu există evaluări
- Hiperuricemia AsintomaticaDocument1 paginăHiperuricemia AsintomaticaElizabethÎncă nu există evaluări
- Gout (Also Known As Podagra When It Involves The Big ToeDocument54 paginiGout (Also Known As Podagra When It Involves The Big ToearunshreerajendranÎncă nu există evaluări
- Part 1 Introduction BDocument5 paginiPart 1 Introduction BGeevee Naganag VentulaÎncă nu există evaluări
- How To Treat: BackgroundDocument6 paginiHow To Treat: BackgroundmeeandsoeÎncă nu există evaluări
- GOUT PresentationDocument24 paginiGOUT Presentationtasneemsofi100% (1)
- Gout and Hyperuricemia: A Peer-Reviewed Bulletin For The Family PhysicianDocument6 paginiGout and Hyperuricemia: A Peer-Reviewed Bulletin For The Family PhysicianRhoda LicardoÎncă nu există evaluări
- GoutDocument12 paginiGoutEarle Jimenez Niervo RN100% (1)
- Penyakit GoutDocument34 paginiPenyakit Goutluh komang ari trisna jayantiÎncă nu există evaluări
- Approach To A Case of Hyperuricemia: Singh V, Gomez VV, Swamy SGDocument7 paginiApproach To A Case of Hyperuricemia: Singh V, Gomez VV, Swamy SGsharu4291Încă nu există evaluări
- Classification and External Resources: Icd 10 Icd 9 Omim Diseasesdb Medlineplus 000422 Emedicine Patient Uk MeshDocument9 paginiClassification and External Resources: Icd 10 Icd 9 Omim Diseasesdb Medlineplus 000422 Emedicine Patient Uk MeshBecky NekesaÎncă nu există evaluări
- Gout Arthritis Dan PseudogoutDocument53 paginiGout Arthritis Dan PseudogoutCavaliere MascheratoÎncă nu există evaluări
- Gout: The Radiology and The Clinical ManifestationsDocument9 paginiGout: The Radiology and The Clinical ManifestationsDian YazmiatiÎncă nu există evaluări
- GoutDocument5 paginiGoutDe Sesto Rhys Carlo100% (2)
- International Journal of Cardiology: Micaela Gliozzi, Natalia Malara, Saverio Muscoli, Vincenzo MollaceDocument5 paginiInternational Journal of Cardiology: Micaela Gliozzi, Natalia Malara, Saverio Muscoli, Vincenzo MollaceRashmeeta ThadhaniÎncă nu există evaluări
- Gout and Hyperuricemia: EpidemiologyDocument8 paginiGout and Hyperuricemia: Epidemiologywahyu santikaÎncă nu există evaluări
- Gout - A Guide For The General and Acute Physicians: Authors: Abhishek AbhishekDocument6 paginiGout - A Guide For The General and Acute Physicians: Authors: Abhishek AbhishekMaretha NazhifahÎncă nu există evaluări
- GoutDocument10 paginiGoutAmberÎncă nu există evaluări
- Review On Treatment of Gout & HyperuricemiaDocument11 paginiReview On Treatment of Gout & HyperuricemiaMirza ProthikhaÎncă nu există evaluări
- Clinpharm M4 ReviewerDocument10 paginiClinpharm M4 ReviewerCHARLES RONALD GENATOÎncă nu există evaluări
- Kuliah Gouty ArthritisDocument56 paginiKuliah Gouty Arthritischrysandre100% (1)
- Clinical Practice: Robert A. Terkeltaub, M.DDocument9 paginiClinical Practice: Robert A. Terkeltaub, M.DUdsanee SukpimonphanÎncă nu există evaluări
- Biomedicines 11 01258Document15 paginiBiomedicines 11 01258Ramadan PhysiologyÎncă nu există evaluări
- Crystal Deposition DisordersDocument4 paginiCrystal Deposition DisordersShuvashishSunuwarÎncă nu există evaluări
- Gout 130718051351 Phpapp01Document47 paginiGout 130718051351 Phpapp01Manurun Londong AlloÎncă nu există evaluări
- Artritis Inducida Por Depósito de CristalesDocument10 paginiArtritis Inducida Por Depósito de CristalesAlejandro GuzmánÎncă nu există evaluări
- GoutDocument6 paginiGoutNader Smadi100% (1)
- GoutDocument6 paginiGoutJokevin PrasetyadhiÎncă nu există evaluări
- Clinical PharmacyDocument4 paginiClinical PharmacysadiaÎncă nu există evaluări
- PBL Nyeri SendiDocument9 paginiPBL Nyeri SendiNUR ZAMZAM AZIZAHÎncă nu există evaluări
- Agis Mira Dewi, S.kedDocument35 paginiAgis Mira Dewi, S.kedAgiish EMdeÎncă nu există evaluări
- Gout and HyperuricemiaDocument70 paginiGout and HyperuricemiaThe AbyssinicansÎncă nu există evaluări
- A2 Group 3 GOUTDocument83 paginiA2 Group 3 GOUTArt Buenaventura0% (1)
- Gout Is Usually Characterized by Recurrent Attacks ofDocument15 paginiGout Is Usually Characterized by Recurrent Attacks ofJimmy KusumaÎncă nu există evaluări
- Gout Is Usually Characterized by Recurrent Attacks ofDocument17 paginiGout Is Usually Characterized by Recurrent Attacks ofJimmy KusumaÎncă nu există evaluări
- Gout Is Usually Characterized by Recurrent Attacks ofDocument14 paginiGout Is Usually Characterized by Recurrent Attacks ofJimmy KusumaÎncă nu există evaluări
- Moving The Needle: Improving The Care of The Gout Patient: Jon Golenbiewski Robert T. KeenanDocument15 paginiMoving The Needle: Improving The Care of The Gout Patient: Jon Golenbiewski Robert T. KeenanArfan HakimÎncă nu există evaluări
- Diagnosisandtreatmentof Goutandpseudogoutfor EverydaypracticeDocument24 paginiDiagnosisandtreatmentof Goutandpseudogoutfor EverydaypracticePutri Aswariyah RamliÎncă nu există evaluări
- Treatment of Acute GoutDocument38 paginiTreatment of Acute GoutAnoop PkÎncă nu există evaluări
- Hyperurecimia and GoutDocument3 paginiHyperurecimia and GoutSunshine_Bacla_4275Încă nu există evaluări
- Cirrhosis Hours: 5. Working Place: Classroom, Hospital Wards. QuestionsDocument5 paginiCirrhosis Hours: 5. Working Place: Classroom, Hospital Wards. QuestionsNahanÎncă nu există evaluări
- Gouty ArthritisDocument12 paginiGouty ArthritisManoj KandoiÎncă nu există evaluări
- Management of Oliguria: Understanding The DiseaseDocument4 paginiManagement of Oliguria: Understanding The DiseaseJhosep SilvestreÎncă nu există evaluări
- Anti GoutDocument41 paginiAnti GoutGareth BaleÎncă nu există evaluări
- Pemicu 4 Putu GOUTDocument31 paginiPemicu 4 Putu GOUTTirtha MaharaniÎncă nu există evaluări
- Anti GoutDocument41 paginiAnti GoutGareth BaleÎncă nu există evaluări
- Guideline of GoutDocument28 paginiGuideline of GoutAhmad Fathira Fitra100% (1)
- The Evolving Landscape of Liver Cirrhosis ManagementDe la EverandThe Evolving Landscape of Liver Cirrhosis ManagementHitoshi YoshijiÎncă nu există evaluări
- Acute Myocardial InfarctionDocument35 paginiAcute Myocardial Infarctionvirnzrobz80% (10)
- Achieve Your Goals. Make A Difference. Join A Community of CaringDocument64 paginiAchieve Your Goals. Make A Difference. Join A Community of CaringvirnzrobzÎncă nu există evaluări
- Imci Care For DevelopmentDocument2 paginiImci Care For DevelopmentvirnzrobzÎncă nu există evaluări
- Drug Study and NCP For Eamc Ob-Gyne Ward Case PresDocument4 paginiDrug Study and NCP For Eamc Ob-Gyne Ward Case PresvirnzrobzÎncă nu există evaluări
- Acute Myocardial InfarctionDocument35 paginiAcute Myocardial Infarctionvirnzrobz80% (10)