Documente Academic
Documente Profesional
Documente Cultură
Molecular Geometry Memorize Shape
Încărcat de
osvaldocossioDescriere originală:
Drepturi de autor
Formate disponibile
Partajați acest document
Partajați sau inserați document
Vi se pare util acest document?
Este necorespunzător acest conținut?
Raportați acest documentDrepturi de autor:
Formate disponibile
Molecular Geometry Memorize Shape
Încărcat de
osvaldocossioDrepturi de autor:
Formate disponibile
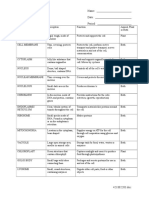
Molecular Geometry Memorize Shape Electron Density Regions 2 3 3 4 4 4 5 5 5 5 6 6 Lone Pairs 0 0 1 0 1 2 0 1 2 3 0 1 Example CO2 BF3 SO2 CH4 NH3
H2O PF5 SF4 ICl3 I3SCl6 XeF5+ Molecular Geometry (Bond Angles) Linear (180) Trigonal Planar (120) Bent (117) Tetrahedral (109.5) Trigonal Pyramidal (107) Bent (105) Trigonal Bipyramidal See Saw T-shaped Linear Octahedral Square Pyramidal Hybridization sp sp2 sp2 sp3 sp3 sp3 sp3d sp3d sp3d sp3d sp3d2 sp3d2
**Base geometries in bold print 1. Once you are able to predict geometry, it is then possible to understand polarity of molecules - polarity refers to the uneven sharing of electrons in a covalent bond - polarity can lead to polar molecules - all polar molecules must contain polar bonds - some nonpolar molecules can contain polar bonds - dipole moments (difference between electronegativities) can be added together as vector quantities to determine the net polarity of a molecule 2. polarity is important because it too (like the geometry of the molecule) determines how a molecule will interact with other molecules
II.
Hybridization of Orbitals Based on carbons electron configuration and the fact that only lone electrons in an orbital can contribute to covalent bonding, how then does carbon create four single bonds? ANSWER: the electron configuration does not explain the bonding properties of carbon; only, hybridization can explain how carbon forms its four single bonds A. Hybridization 1. Hybridization of orbitals is the mixing of two or more atomic orbitals from similar energies on the same atom to produce new hybrid atomic orbitals of equal energy (degenerate orbitals) 2. hybridization of orbitals explains carbon (and other atoms) bonding properties 3. Hybrid orbitals are what we call the degenerate orbitals 4. for hybrid orbitals, the number of hybrid orbitals produced is equal to the number of orbitals combined to create them 5. Types of hybrid orbitals: Combining Orbitals s,p s,p,p s,p,p,p s,p,p,p,d s,p,p,p,d,d Type of Hybridization sp sp2 sp3 sp3d sp3d2 Number of Hybrid orbitals 2 3 4 5 6 Base Geometry Shape Linear Trigonal planar Tetrahedral Trigonal bipyramidal Octahedral
6. hybridization can be determined by looking at the lewis structure of a cpd 7. to determine hybridization, simply count the electron density regions around an atom , the number of electron density regions is equal to the number of hybrid orbitals as noted above
Electron Density Regions 2
Lone Pairs 0
Example CO2
Molecular Geometry (Bond Angles) Linear (180)
Hybridization sp
Electron Density Regions 3
Lone Pairs 0
Example BF3
Molecular Geometry (Bond Angles) Trigonal Planar (120)
Hybridization sp2
Electron Density Regions 3
Lone Pairs 1
Example SO2
Molecular Geometry (Bond Angles) Bent (117)
Hybridization sp2
Electron Density Regions 4
Lone Pairs 0
Example CH4
Molecular Geometry (Bond Angles) Tetrahedral (109.5)
Hybridization sp3
Electron Density Regions 4
Lone Pairs 1
Example NH3
Molecular Geometry (Bond Angles) Trigonal Pyramidal (107)
Hybridization sp3
Electron Density Regions 4
Lone Pairs 2
Example H2O
Molecular Geometry (Bond Angles) Bent (105)
Hybridization sp3
Electron Density Regions 5
Lone Pairs 0
Example PF5
Molecular Geometry (Bond Angles) Trigonal Bipyramidal
Hybridization sp3d
Electron Density Regions 5
Lone Pairs 1
Example SF4
Molecular Geometry (Bond Angles) See Saw
Hybridization sp3d
Electron Density Regions 5
Lone Pairs 2
Example BrF3
Molecular Geometry (Bond Angles) T-shaped
Hybridization sp3d
Electron Density Regions 5
Lone Pairs 3
Example ICl2-
Molecular Geometry (Bond Angles) Linear
Hybridization sp3d
Electron Density Regions 6
Lone Pairs 0
Example PCl6-
Molecular Geometry (Bond Angles) Octahedral
Hybridization sp3d2
Electron Density Regions 6
Lone Pairs 1
Example XeF5+
Molecular Geometry (Bond Angles) Square Pyramidal
Hybridization sp3d2
Electron Density Regions 6
Lone Pairs 2
Example XeF4
Molecular Geometry (Bond Angles) Square Planar
Hybridization sp3d2
S-ar putea să vă placă și
- AP Chemistry Solubility Rules Equations SheetDocument8 paginiAP Chemistry Solubility Rules Equations SheetssÎncă nu există evaluări
- Chemical Equilibrium NotesDocument4 paginiChemical Equilibrium NotesHaile CordaÎncă nu există evaluări
- Solving Exponential Equations Using Logarithms - ChiliMathDocument6 paginiSolving Exponential Equations Using Logarithms - ChiliMathMelanie AbaldeÎncă nu există evaluări
- Solubility Rules: Summary of Strong and Weak ElectrolytesDocument1 paginăSolubility Rules: Summary of Strong and Weak ElectrolytesOwie Toong0% (1)
- Chemical KineticsDocument169 paginiChemical KineticsHope WorldÎncă nu există evaluări
- Krebs Cycle: CHEM 160Document47 paginiKrebs Cycle: CHEM 160Gianna Kristen MirandaÎncă nu există evaluări
- Stoichiometry ProblemsDocument4 paginiStoichiometry Problemsphilippeprean0% (1)
- Organic Chemistry 1Document110 paginiOrganic Chemistry 1Mahmoud RslanÎncă nu există evaluări
- Chemistry - MCQDocument30 paginiChemistry - MCQjoydeep_d32320% (1)
- Regents Living Environment Practice Questions: New York Regents Living Environment Practice Questions with Detailed ExplanationsDe la EverandRegents Living Environment Practice Questions: New York Regents Living Environment Practice Questions with Detailed ExplanationsÎncă nu există evaluări
- Acids & BasesDocument32 paginiAcids & BasesLechimiÎncă nu există evaluări
- Mercury FulminateDocument11 paginiMercury FulminateAnoshän IndreswaränÎncă nu există evaluări
- Cell Organelles ReviewDocument5 paginiCell Organelles ReviewvictoriaÎncă nu există evaluări
- Organic ChemistryDocument9 paginiOrganic ChemistryLinda Aida100% (1)
- SKO 3013 B O C: Asic Rganic HemistryDocument53 paginiSKO 3013 B O C: Asic Rganic HemistryLuxemberg Ng100% (1)
- VSEPRDocument1 paginăVSEPRĐan KhanhÎncă nu există evaluări
- Chemical Bonding ChemistryDocument101 paginiChemical Bonding ChemistryYoshitha Kuntumalla100% (2)
- Essentials of Inorganic Chemistry: For Students of Pharmacy, Pharmaceutical Sciences and Medicinal ChemistryDe la EverandEssentials of Inorganic Chemistry: For Students of Pharmacy, Pharmaceutical Sciences and Medicinal ChemistryÎncă nu există evaluări
- Biotest 4Document7 paginiBiotest 4T.SonÎncă nu există evaluări
- Acid Base HomeworkDocument5 paginiAcid Base HomeworkAriel ChuÎncă nu există evaluări
- Analytical NotesDocument25 paginiAnalytical NotesRyan BoodramlallÎncă nu există evaluări
- ELECTROCHEMISTRYDocument10 paginiELECTROCHEMISTRYISLAM I. Fekry100% (2)
- Review of Fundamental Concepts F12Document7 paginiReview of Fundamental Concepts F12DerrickGMcCoyÎncă nu există evaluări
- Alcohols, Phenols and Ethers NotesDocument8 paginiAlcohols, Phenols and Ethers Notesmajji satishÎncă nu există evaluări
- Chapter 7 (Acids & Bases)Document15 paginiChapter 7 (Acids & Bases)amin_zamanÎncă nu există evaluări
- Molecular GeometryDocument21 paginiMolecular GeometryCacey Daiwey CalixtoÎncă nu există evaluări
- DAT QuizletDocument78 paginiDAT QuizletJihee YoonÎncă nu există evaluări
- PH and BufferDocument68 paginiPH and BufferDileesha WeliwaththaÎncă nu există evaluări
- Sch4uc Unit 2 Lesson 05Document28 paginiSch4uc Unit 2 Lesson 05Luis David Lazo CondoriÎncă nu există evaluări
- C F C CL C - BR: HalogenoalkanesDocument11 paginiC F C CL C - BR: HalogenoalkanesMufaro MutotiÎncă nu există evaluări
- Lewis StructureDocument5 paginiLewis StructureGiuliano CiolacuÎncă nu există evaluări
- Advanced ChemistryDocument137 paginiAdvanced ChemistryMaheshÎncă nu există evaluări
- Concise Inorganic Chemistry (4th Edition) by J.D.Lee PDFDocument342 paginiConcise Inorganic Chemistry (4th Edition) by J.D.Lee PDFNeelima JainÎncă nu există evaluări
- CHEM 1110 Practice FinalDocument14 paginiCHEM 1110 Practice FinalEric CabarloÎncă nu există evaluări
- Study Guide Nuclear ChemistryDocument4 paginiStudy Guide Nuclear ChemistryAdam100% (1)
- Chem Final Exam Rev Fall 2017Document6 paginiChem Final Exam Rev Fall 2017fdlsdfsÎncă nu există evaluări
- PH CalculationsDocument2 paginiPH CalculationsEnriqueFariazÎncă nu există evaluări
- Chapter 2. Molecular Structure and Bonding: 3.1 The Octet RuleDocument89 paginiChapter 2. Molecular Structure and Bonding: 3.1 The Octet RuleAnn BorromeoÎncă nu există evaluări
- Writing Lewis StructuresDocument10 paginiWriting Lewis StructuresnÎncă nu există evaluări
- Valence Bond TheoryDocument11 paginiValence Bond TheoryGenien HongÎncă nu există evaluări
- StoichiometryDocument10 paginiStoichiometryvanditÎncă nu există evaluări
- SNAP Challenge Full InformationDocument4 paginiSNAP Challenge Full InformationAnonymous pR2QW345BÎncă nu există evaluări
- 1520 Solution 20 EquilibriaDocument25 pagini1520 Solution 20 EquilibriaNguyễn Minh AnhÎncă nu există evaluări
- Multiple Choice QuestionsDocument39 paginiMultiple Choice QuestionsFatma JamalÎncă nu există evaluări
- DesigningSpeechAssignments PDFDocument4 paginiDesigningSpeechAssignments PDFJohn MarkÎncă nu există evaluări
- Bio AR 2 - Plant & Animal KingdomDocument48 paginiBio AR 2 - Plant & Animal KingdomFazlur RehmanÎncă nu există evaluări
- Principles of SpeechwritingDocument10 paginiPrinciples of SpeechwritingJulieSanchezErsandoÎncă nu există evaluări
- Applications of Aqueous EquilibriaDocument24 paginiApplications of Aqueous EquilibriaEuler MendozaÎncă nu există evaluări
- Halogenoalkanes NotesDocument5 paginiHalogenoalkanes NotesAgustina Tedja100% (1)
- 15 Buffers Made EasyDocument8 pagini15 Buffers Made Easyapi-287405319Încă nu există evaluări
- Acids Base Equilibria NEHDocument123 paginiAcids Base Equilibria NEHLulwa KhaskiehÎncă nu există evaluări
- 7 Resonance Structure AnsDocument3 pagini7 Resonance Structure AnsJesus Eddy Peña MelissaratosÎncă nu există evaluări
- AP Bio Big Study GuideDocument33 paginiAP Bio Big Study GuideHayden CaseyÎncă nu există evaluări
- Organic Chemistry II Chapter22Document8 paginiOrganic Chemistry II Chapter22RangikaÎncă nu există evaluări
- How To Study Biochemistry?Document1 paginăHow To Study Biochemistry?Prof.PTS96% (28)
- Stereochemistry QuestionsDocument7 paginiStereochemistry Questionsalyson_lÎncă nu există evaluări
- Electrochemistry 494 PDFDocument55 paginiElectrochemistry 494 PDFHarsh SaxenaÎncă nu există evaluări
- Summary Buffer SolutionDocument3 paginiSummary Buffer Solutionelcha_putraÎncă nu există evaluări
- Bio Lab 12 (Submit)Document8 paginiBio Lab 12 (Submit)Nor Ashikin IsmailÎncă nu există evaluări
- AP Chemistry FR Test BankDocument7 paginiAP Chemistry FR Test BankzeustamÎncă nu există evaluări
- Thrifty Meal PlanDocument9 paginiThrifty Meal Planapi-239706380Încă nu există evaluări
- Tips & Tricks Inorganic Chemistry FlashCardsDocument25 paginiTips & Tricks Inorganic Chemistry FlashCardsseemagoyal0206Încă nu există evaluări
- 6 C15 Notes CH4 Chemical BondsSTEM StudentsDocument12 pagini6 C15 Notes CH4 Chemical BondsSTEM StudentsDONNA JEAN ACOJEDOÎncă nu există evaluări
- Ebook Chemistry and Chemical Reactivity 9Th Edition Kotz Solutions Manual Full Chapter PDFDocument44 paginiEbook Chemistry and Chemical Reactivity 9Th Edition Kotz Solutions Manual Full Chapter PDFMrNicolasGuerraJrnsadz100% (12)
- Bonding Theories: Presented By: Nimra Nasir (2016-2313) Presented To: Respected Mam TayyabaDocument27 paginiBonding Theories: Presented By: Nimra Nasir (2016-2313) Presented To: Respected Mam TayyabaNimra MalikÎncă nu există evaluări
- Worksheet 15 VSEPR PDFDocument5 paginiWorksheet 15 VSEPR PDFAbdur RehmanÎncă nu există evaluări
- Chem1011 Exam Practice Test 2Document27 paginiChem1011 Exam Practice Test 2Chirisuu PantsuÎncă nu există evaluări
- Topic 4 Bonding SL AnswersDocument48 paginiTopic 4 Bonding SL AnswersŁØÎncă nu există evaluări
- Polarity of Molecules - For Aui-Video PresentationDocument25 paginiPolarity of Molecules - For Aui-Video PresentationDelson SonÎncă nu există evaluări
- Laboratory Activity 3 - Group 10Document6 paginiLaboratory Activity 3 - Group 10Reinier FrancoÎncă nu există evaluări
- CH 3. Chemical Bonding (Chem +1)Document52 paginiCH 3. Chemical Bonding (Chem +1)nitinÎncă nu există evaluări
- Chemical Bonding KRR PDFDocument40 paginiChemical Bonding KRR PDFggk201367% (3)
- I6 VSEPR Effectoflonepairstheorysheet - 000Document2 paginiI6 VSEPR Effectoflonepairstheorysheet - 000Anuj MalaraÎncă nu există evaluări
- Chemical Bonding HWDocument17 paginiChemical Bonding HWAayush PawarÎncă nu există evaluări
- PMDC MDCAT Curriculum 2023Document30 paginiPMDC MDCAT Curriculum 2023Muhammad Haris AzharÎncă nu există evaluări
- Module 1 Properties and Structure of MatterDocument17 paginiModule 1 Properties and Structure of Matterisaheqq12Încă nu există evaluări
- Molecular Geometry and Bonding TheoriesDocument5 paginiMolecular Geometry and Bonding TheoriesPineraserÎncă nu există evaluări
- VSEPR ShortcutDocument3 paginiVSEPR ShortcutSubhojyotiDasÎncă nu există evaluări
- Competency Test 1Document15 paginiCompetency Test 1Uhu UhuÎncă nu există evaluări
- Module 1 - ChemistryDocument48 paginiModule 1 - ChemistryShapnil FinneyÎncă nu există evaluări
- Chem Notes (Yr11)Document46 paginiChem Notes (Yr11)nguyenbohaeÎncă nu există evaluări
- Chemical Bonding Class11th by PS Sir IIT JEEDocument44 paginiChemical Bonding Class11th by PS Sir IIT JEEMahendra PandaÎncă nu există evaluări
- Q2 Molecular Geometry and PolarityDocument50 paginiQ2 Molecular Geometry and PolarityTosee istoseeÎncă nu există evaluări
- PKT Bonding2 Student NotesDocument40 paginiPKT Bonding2 Student NotesrajaijahÎncă nu există evaluări
- 2018 Singapore-Cambridge A Level H2 Chemistry P2 Suggested Answer Key (9729)Document14 pagini2018 Singapore-Cambridge A Level H2 Chemistry P2 Suggested Answer Key (9729)Imagreenbucklegirl SGÎncă nu există evaluări
- Ib PPT 4 SL PDFDocument103 paginiIb PPT 4 SL PDFzarna nirmal rawalÎncă nu există evaluări
- (Chemical Bonding) H2 Chem NotesDocument11 pagini(Chemical Bonding) H2 Chem Notesblah blehÎncă nu există evaluări
- Chemical Bonding - (DPP)Document4 paginiChemical Bonding - (DPP)Dipendra KumarÎncă nu există evaluări