Documente Academic
Documente Profesional
Documente Cultură
Urea Plant Design
Încărcat de
Aamli AgarwalDescriere originală:
Drepturi de autor
Formate disponibile
Partajați acest document
Partajați sau inserați document
Vi se pare util acest document?
Este necorespunzător acest conținut?
Raportați acest documentDrepturi de autor:
Formate disponibile
Urea Plant Design
Încărcat de
Aamli AgarwalDrepturi de autor:
Formate disponibile
1
CHAPTER 1
INTRODUCTION
2
Urea is an oraganic compound with the chemical formula (NH
2
)
2
CO. Urea is also
known by the International Nonproprietary Name (INN) carbamide, as established by
the World Health Organization. Other names include carbamide resin, isourea,
carbonyl diamide, and carbonyldiamine.
Synthetic urea
It was the first organic compound to be artificially synthesized from
inorganic starting materials, in 1828 by Friedrich Whler, who prepared it by the
reaction of potassium cyanate with ammonium sulfate. Although Whler was
attempting to prepare ammonium cyanate, by forming urea, he inadvertently
discredited vitalism, the theory that the chemicals of living organisms are
fundamentally different from inanimate matter, thus starting the discipline of organic
chemistry.
This artificial urea synthesis was mainly relevant to human health
because of urea cycle in human beings. Urea was discovered; synthesis in human liver
in order to expel excess nitrogen from the body. So in past urea was not considered as
a chemical for agricultural and industrial use. Within the 20
th
century it was found to be
a by far the best nitrogenic fertilizer for the plants and became widely used as a
fertilizer. Urea was the leading nitrogen fertilizer worldwide in the 1990s.Apart
from that urea is being utilized in many other industries.
Urea is produced on a scale of some 100,000,000 tons per year worldwide.
For use in industry, urea is produced from synthetic ammonia and carbon dioxide. Urea
can be produced as prills, granules, flakes, pellets, crystals, and solutions.More than
90% of world production is destined for use as a fertilizer. Urea has the highest
nitrogen content of all solid nitrogenous fertilizers in common use (46.7%).
Therefore, it has the lowest transportation costs per unit of nitrogen nutrient. Urea is
highly soluble in water and is, therefore, also very suitable for use in fertilizer
solutions (in combination with ammonium nitrate).
Commercial production of urea
Urea is commercially produced from two raw materials, ammonia,
and carbon dioxide. Large quantities of carbon dioxide are produced during the
manufacture of ammonia from coal or from hydrocarbons such as natural gas and
3
petroleum-derived raw materials. This allows direct synthesis of urea from these raw
materials. The production of urea from ammonia and carbon dioxide takes place in an
equilibrium reaction, with incomplete conversion of the reactants. The various urea
processes are characterized by the conditions under which urea formation takes
place and the way in which unconverted reactants are further processed.
Unconverted reactants can be used for the manufacture of other products, for
example ammonium nitrate or sulfate, or they can be recycled for complete
conversion to urea in a totalrecycle process. Two principal reactions take place in the
formation of urea from ammonia and carbon dioxide. The first reaction is exothermic:
2 NH
3
+ CO
2
H
2
N-COONH
4
(ammonium carbamate)
Whereas the second reaction is endothermic:
H
2
N-COONH
4
(NH
2
)
2
CO + H
2
O
Both reactions combined are exothermic.
Properties of urea
Mol. Formula -NH
2
CO NH
2
Melting Point -132.6
0
C
Sp. Gravity - 1.335@20
0
C
Heat of Fusion - 57.08 Cal/gm
Heat of Combustion - 2531 Cal/gm
Crystal Form - Tetragonal
Nitrogen Content - 46.6%
Boiling Point - Decompose on boiling
Colour - White
Bulk Density - 740 to 750 kg/m
3
Angle of Repose - 23 to 30
0
C
Viscosity - 2.58@132.7
0
C
Triple point - 102
0
C
Dielectric Constant -3.52 to 0.2
4
Sp. Heat - 0.42Kcal/cm
2
Raw materials of urea manufacturing
1. Ammonia
Ammonia, NH
3
, is a comparatively stable, colourless gas at ordinary
temperatures, with a boiling point of -33 C. Ammonia gas is lighter than air, with a
density of approximately 0.6 times that of air at the same temperature. Ammonia is
highly soluble in water, although solubility decreases rapidly with increased
temperature. Ammonia reacts with water in a reversible reaction to produce
ammonium (NH4)
+
and hydroxide (OH)
-
ions, as shown in
equation. Ammonia is a weak base, and at room temperature only about 1 in 200
molecules are present in the ammonium form (NH
4
)
+
. The formation of
hydroxide ions in this reaction increases the pH of the water, forming an alkaline
solution.
NH
3
+ H
2
O (NH
4
)
+
+ OH
-
.
Ammonia Production
Essentially all the processes employed for ammonia synthesis are
variations of the Haber-Bosch process, developed in Germany from 1904-1913.
This process involves the reaction of hydrogen and nitrogen under high
temperatures and pressures with an iron based
catalyst.
N2 +3 H2 2NH3
The source of nitrogen is always air. Hydrogen can be derived from a
number of raw materials including water, hydrocarbons from crude oil refining,
coal, and most commonly natural gas. Hydrogen rich reformer off-gases from oil
refineries have also been used as a source of hydrogen. Steam reforming is generally
employed for the production of hydrogen from these raw materials. This process also
generates carbon dioxide, which can then be used as a raw material in the production
of urea.
Ammonia storage
Anhydrous ammonia is usually stored as a liquid in refrigerated tanks at
5
-33.3 C and atmospheric pressure, often in doubled-walled tanks with the capacity for
hundreds or thousands of tonnes. The low temperature is usually maintained by the
venting of ammonia gas.
2. Carbon Dioxide
CO
2
is a odourless and colourless gas which contain 0.03% in the
atmosphere. It is emitted as a pollutant from number of industries. CO2 can be
obtained from ammonia production process as a by product.
Applications of urea
1. Agricultural use
More than 90% of world production is destined for use as a fertilizer. Urea
is used as a nitrogen-release fertilizer, as it hydrolyses back to ammonia and carbon
dioxide, but its most common impurity, biuret, must be present at less than 2%, as it
impairs plant growth. Urea has the highest nitrogen content of all solid nitrogeneous
fertilizers in common use (46.4%N.) It therefore has the lowest transportation costs
per unit of nitrogen nutrient. In the past decade urea has surpassed and nearly replaced
ammonium nitrate as a fertilizer
In the soil, urea is converted into the ammonium ion form of nitrogen.
For most floras, the ammonium form of nitrogen is just as effective as the nitrate
form. The ammonium form is better retained in the soil by the clay materials than the
nitrate form and is therefore less subject to leaching. Urea is highly soluble in water and
is therefore also very suitable for use in fertilizer solutions, e.g. in foliar feed
fertilizers.
2. Industrial use
Urea has the ability to form 'loose compounds', called clathrates, with
many organic compounds. The organic compounds are held in channels formed by
interpenetrating helices comprising of hydrogen-bonded urea molecules. This
behaviour can be used to separate mixtures, and has been used in the production of
aviation fuel and lubricating oils. As the helices are interconnected, all helices in a
crystal must have the same 'handedness'. This is determined when the crystal is
nucleated and can thus be forced by seeding. This property has been used to separate
6
racemic mixtures.
3. Further commercial uses
A stabilizer in nitrocellulose explosives
A component of fertilizer and animal feed, providing a relatively
cheap source of nitrogen to promote growth
A raw material for the manufacture of plastics, to be specific,
urea-formaldehyde resin
A raw material for the manufacture of various glues (urea-formaldehyde or
urea-melamine-formaldehyde); the latter is waterproof and is used for marine
plywood
An additive ingredient in cigarettes,designed to
enhance flavor
An ingredient in some hair conditioners, facial cleansers, bath oils, and lotions
A flame-proofing agent (commonly used in dry chemical fire extinguishers as Urea-
potassium bicarbonate)
An ingredient in many tooth whitening products
A cream to soften the skin, especially cracked skin on the bottom of
one's feet
An ingredient in dish soap.
4. Medical use
Urea is used in topical dermatological products to promote rehydration of
the skin. If covered by an occlusive dressing, 40% urea preparations may also be
used for nonsurgical debridement of nails. This drug is also used as an earwax
removal aid. Like saline, urea injection is used to perform abortions. It is also the main
component of an alternative medicinal treatment referred to as urine therapy.
7
5. Textile use
Urea is a raw material for urea-formaldehyde resins production in the adhesives
and textile industries. A significant portion of urea production is used in the
preparation of urea- formaldehyde resins. These synthetic resins are used in the
manufacture of adhesives, moulding powders, varnishes and foams. They are also
used for impregnating paper, textiles and leather. In textile laboratories they are
frequently used both in dyeing and printing as an important auxiliary, which provides
solubility to the bath and retains some moisture required for the dyeing or
printing process.
8
CHAPTER 2
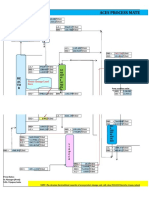
PROCESS SELECTION
9
Process Selection
Several processes are used to urea manufacturing. Some of them are used
conventional technologies and others use modern technologies to achieve high efficiency. These
processes have several comparable advantages and disadvantages based on capital cost,
maintenance cost, energy cost, efficiency and product quality. Some of the widely used urea
production processes are
1. Conventional processes
2. Stamicarbon CO
2
- stripping process
3. Snamprogetti Ammonia and self stripping processes
4. Isobaric double recycle process
5. ACES process
Snamprogetti Ammonia and self stripping processes
In the first generation of NH
3
and self strip ping processes, ammonia was used as
stripping agent. Because of the extreme solubility of ammonia in the urea containing synthesis
fluid, the stripper effluent contained rather large amount s of dissolved ammonia, causing
ammonia overload in down stream section of the plant. Later versions of the process abandoned
the idea of using ammonia as stripping agent; stripping was achieved only by supply of heat.
Even without using ammonia as a stripping agent, the NH
3
:CO
2
ratio in the stripper effluent is
relatively high. So the recirculation section of the plant requires an ammonia-carbomate
separation section
The process uses a vertical layout in the synthesis section. Recycle within the
synthesis section, from the stripper via the high pressure carbamate condenser, through the
carbamate separator back to the reactor, is maintained by using an ammonia-driven liquid-liquid
ejector. In the reactor, which is operated at 150 bars, NH
3
:CO
2
molar feed ratio of 3.5 is applied.
The stripper is of the falling film type. Since stripping is achieved thermally, relatively high
temperatures (200-210
0
C) are required to obtain a reasonable stripping efficiency. Because of
this high temperature, stainless steel is not suitable as a construction material for the stripper
from a corrosion point of view; titanium and bimetallic zircornium - stainless steel tubes have
been used Off gas from the stripper is condensed in a kettle type boiler. At the tube side of this
condenser the off gas is absorbed in recycled liquid carbamate from the medium pressure
recovery section. The heat of absorption is removed through the tubes, which are cooled by the
10
production of low pressure steam at the shell side. The steam produced is used effectively
in the back end of the process.In the medium pressure decomposition and recirculation section ,
typically operated at 18 bar, the urea solution from the high pressure stripper is subjected to the
decomposition of carbamate and evaporation of ammonia. The off gas from this medium pressure
decomposer is
rectified. Liquid ammonia reflux is applied to the top of this rectifier; in this way a top product
consisting of pure gaseous ammonia and a bottom product of liquid ammonium carbamate are
obtained. The pure ammonia off gas is condensed and recycled to the synthesis section. To
prevent solidification of ammonium carbamate in the rectifier, some water is added to the bottom
section of the column to dilute the ammonium carbamate below its crystallization point. The
liquid ammonium carbamate-water mixture obtained in this way is also recycled to the synthesis
section. The purge gas of the ammonia condenser is treated in a scrubber prior to being purged to
the atmosphere.
The urea solution from the medium pressure decomposer is subjected to a second low
pressure decomposition step. Here further decomposition of ammonium carbamate is achieved,
so that a substantially carbamate -free aqueous urea solution is obtained. Off gas from this low
pressure decomposer is condensed and recycled as an aqueous ammonium carbamate solution to
the synthesis section via the medium pressure recovery section.
Concentrating the urea water mixture obtained from the low pressure decomposer is
preformed in a single or double evaporator depending on the requirement of the finishing
section. Typically, if prilling is chosen as the final shaping procedure, a two stage evaporator is
required, whereas in the case of a fluidized bed granulator a single evaporation step is sufficient to
achieve the required final moisture content of the urea melt. In some versions of the process, heat
exchange is applied between the off gas from the medium pressure decomposer and the aqueous
urea solution to the evaporation section. In this way, the consumption of low pressure steam by the
process is reduced.
The process condensate obtained from the evaporation section is subjected to a
desorption hydrolysis operation to recover the urea and ammonia contained in the process
condensate.
11
CHAPTER 3
PROCESS DESCRIPTION AND
FLOW SHEET
12
PROCESS DESCRIPION
The process which is used in formation of urea is Snam Pragetti Process at IFFCO Plant.
This is self-stopping process.
The basic raw material for the formation of urea is Ammonia & Carbon Dioxide . The
formation of urea is taking place in following manner:-
2NH
3
+ CO
2
NH
4
COONH
2
+ Heat (1)
(ammonium carbamate)
NH
4
COONH
2
NH
2
CONH
2
+ H
2
O - Heat (2)
(UREA)
First reaction is takes place at high pressure and temperature that is P=150kg/cm
2
(g) & T= 170
0
C.
In this reaction carbamate is formed. At high pressure reaction is taking place at in forward
direction and at low pressure reaction is taking place in backward direction. It is exothermic
reaction. In the 2
nd
reaction carbamate is dehydrated to form Urea. This is endothermic process.
The heat which is generated in reaction first is utilised in reaction two. At a very high temperature
reaction two proceed backward direction.
The process root is summarised in the following steps:-
1. COMPRESSION OF CARBON DIOXIDE
In this step carbon dioxide is compressed through compressor. The carbon dioxide enters in the
compressor at a 1.4 ata & temp. is around 40
0
C for increasing the pressure up to 155kg/cm
2
(g).
This is achieved by using two centrifugal pumps driven by an extraction cum condensing turbine.
Ammonia is comes from the Ammonia Plant or from the Ammonia Storage Tank. The ammonia is
passed through the preheated tank to high pressure synthesis loop. The high pressure synthesis
loop is combination of booster centrifugal pump and reciprocating pressure pump. The pressure of
ammonia comes out from the high pressure synthesis loop is 240kg/cm
2
. The high pressure liquid
ammonia is also provided for motive force for ejector, which recycles carbamate solution to urea
reactor. The ammonia is kept in excess for the complete conversion of carbon dioxide. The ration
of ammonia to carbon dioxide is 3.33:1.
13
2. UREA SYNTHESIS AND HIGH PRESSURE RECOVERY
This section consist of reactor, high pressure stripper, horizontal carbamate condenser (two unit
placed in series). The compressed carbon dioxide and excess ammonia is entered in the reactor to
form the urea at the temp. 190
0
C & pressure 150kg/cm
2
(g). the concentration of urea formed in the
reactor is nearly 32%. The effluent of reactor is consisting of ammonia. Carbon dioxide,
carbamate,vapour and urea. This effluent is passed to stripper in which CO
2
is absorbed according
to the henery law. Heat required for stripping is supplied by 26kg/cm
2
(g) steam obtained from
extraction of carbon dioxide compression turbine. The concentration of urea obtained from the
stripper is 45%. The off gases obtained from the stripper ammonia, CO2 and vapour is entered
into horizontal carbamate condenser where the total mixture ,except for some inert ,is condensed
as carbamate and recycled to the reactor by means of ejector.
3. UREA PURIFICATION AND LOW PRESSURE RECOVERIES
Urea purification takes place in two stages at decreasing pressures as follows:
Medium Pressure at 18 ata pressure
Low Pressure at 4.5 ata pressure
Medium Pressure Purification and Recovery at 18ata
The solution, with a low residual CO
2
content, leaving the bottom of the stripper at synthesis
pressure is let down to18 ata and enters medium pressure decomposer The M.p decomposer and
divided in two parts
1.Top separated :where the released flash gases are removed before the solution enters the
tube bundle
2. Decomposition section (falling film type): where residual carbamate is decomposed and the
heat require for the decomposition is applied by means of 26 ata steam condensate flowing out
of the shell side of stripper
The NH
3
and CO
2
rich gases leaving the top separator are sent to medium pressure condenser
where they are partially absorbed in aqueous carbonate solution coming from low pressure
recovery section .The absorption heat is removed by tempered cooling water circulation in the
tube side of the medium pressure condenser. In the M.P condenser CO
2
is almost totally
absorbed. The effluents flow to medium pressure absorber. The gaseous phase enters the
14
rectification section of the M.P absorber. The rectification section has bubble trays. The bubble
cap trays are fed by pure reflux ammonia at the top trays which eliminates residual CO
2
and
H
2
O from gases leaving M.P absorber. The reflux ammonia is pumped to rectification
column.NH
3
with inert gases leaving the M.P absorber is condensed in ammonia condenser.
The inert gases , saturated with ammonia enter ammonia preheater where an additional
amount of ammonia is condensed by heating cold ammonia coming from ammonia storage
area and used as make up feed to Urea plant
The inert gases with residual ammonia content are sent to medium pressure ammonia
absorber, which is a falling film type and where they meet a condensate flow which absorbs
ammonia From bottom of ammonia absorber the water ammonia solution is pumped to
medium pressure absorber.The inerts leaving the top are free from ammonia.
Low pressure purification and recovery stage(at 4.5 ata)
Low pressure decomposer consists of:
1.Top separator: where the released gases are removed before the solution enters the lower tube
bundle
2. Decomposition section (falling film type):where residual carbamate is decomposed and the heat
require for the decomposition is applied by means of saturated steam at 4.5 ata
The urea solution from the M.P decomposer bottom enters the L.P decomposer after expansion
through a level controller. Consequently most of the residual carbamate is decomposed and in the
process urea solution gets concentrated. The remaining carbamate is decomposed in a falling film
exchanger, which is a part of L.P. decomposer.
The vapors from the L.P decomposer enter the L.P. condenser where they get cooled and liquefied.
Prior to the entry of L.P off gases in L.P condenser the vapor gets mixed with the aqueous solution
from waste water section.The vapor thus formed get condensed in L.P condenser goes to carbonate
solution tank from where it is send back to MP condenser.The inert gases in the tank contains
considerable amount of ammonia and thus are absorbed in cool condensate before being sent to
vent stack.
The urea solution at the bottom of the L.P. decomposer is sent to pre vacuum concentrator through
a level control valve.
15
Urea concentration section:
As it is necessary in order to prill urea ;to concentrate urea solution up to 99.8 % wt, a vaccum
concentration section in two stages is provided.
The two concentrator use saturated steam at 4.5 ata the liq. Vapor phase coming out of second
vacuum concentrator enters gas- liq. Separator where the vapors are extacted by second vacuum
system.
First vacuum system:
First evaporator is operated at 130C and 0.3 Kg/cm2 pressure. Over head vapor from the top of the
first vacuum separator is directed to the shell side of pre condenser and heat of condensation is
removed by cooling water in the tube side. Ammonia vapor and residual CO2 is absorbed in
condensate forming dil. Ammonium carbonate sol. and flows down through barometric leg of
waste water tank.
Uncondensed gases are sucked by the ejector (motive fluid being 44.5 ata steam) and discharged in
the shell side after condenser , which also receives uncondensed gases from second vacuum
system.Heat of condensation is removed by cooling water in the tube side.
Second vacuum system:
It operates at 1400 C and 0.03 Kg/cm2 pressure. Over head gases from second vacuum separator
are sucked by a booster ejector and discharged at slightly higher pressure where heat of
condensation is removed by cooling water in the tube side.
Uncondensed gases are drawn by ejector and discharged to shell side of second inter condenser
where heat of condensation is again removed by cooling water.
Urea prilling:
The molten urea leaving second vacuum separator is pumped to the prilling bucket by means of
centrifugal pump.
The molten urea coming out of the prilling bucket in the form of drops fall along the prilling tower
and encounters air flow which causes its solidification and subsequent cooling solid prills are sent
to the conveyer belt by rotary scraper which carries urea to bagging plant or storage. The heated air
containing few ppm of NH
3
is released from the top into the atmosphere.
16
PROBLEM STATEMENT:
To design a UREA PLANT of capacity 1000 ton/day using ammonia and
carbon di-oxide as raw material
17
CHAPTER 4
MATERIAL BALANCE
18
MATERIAL BALANCE
Plant Capacity =1000 ton/day of urea
Taking 10% over the design factor,
Capacity =1.1*1000 =1100 tons/day
=1100*1000 =45833.33 Kg/h
r
24
=763.89 Kmol/h
r
1. II
nd
VACCUM CONCENTRATOR
X
3
U
=0.989 (given) X
3
B
=0.002 (assume)
: W
3
=46343.0107 Kg/h
r
4 X
NH3
=0.2722
X
3
H2O
=0.011 X
CO2
= 0.128
X
U
5
=0.94 (given)
Material balance: 5
W
3
X
3
U
= W
5
X
U
5
X
U
=0.94
46343.1075*0.989 = W
5
*0.94 X
H2O
=0.040
W
5
=48758.865 Kg/h
r
X
NH3
=0.0135
W
4
= W
5
W
3
X
CO2
=0.00634
=2415.76 Kg/h
r
X
B
=
0.0019
3 X
U
=0.989
Let X
4
NH3
=0.2722 X
4
CO2
=0.128 X
H2O
=0.011
:0.2722*2415.76 = X
5
NH3
*48758.865 X
B
=0.002
: X
5
NH3
= 0.1035
: 0.128 *2415.76 = X
5
CO2
*48758.865
X
5
CO2
= 0.00634
X
5
H2O
= 0.040
19
2 .I
st
VACCUM CONCENTRATOR
X
U
7
=0.70 , X
U
5
=0.94(given)
Material balance
: 0.94 *48758.865 =0.7 * W
7
W
7
=65476.19 Kg/h
r
W
6
= W
7
W
5
= 16717.325 Kg/h
r
W
6
X
5
CO2
= 0.00634 X
B
=0.001415 X
H2O
=0.76
X
5
NH3
=0.0135 X
U
=0.7 X
NH3
=0.17
X
B
5
=
0.0019
X
NH3
=0.0534 X
CO2
=0.0726
Let water evaporated =12683.7973, W
7
CO
2
from top =1214.65, X
CO2
=0.023
NH
3
from top =2841.945 X
H2O
=0.22 X
U
=0.94
X
6
CO2
=0.0126, X
6
NH3
=0.17 , X
6
H2O
=0.76 W
5
X
B
=0.0019
: X
7
NH3
*65476.19 =0.17*16717.325 +0.0135*48758.865 X
NH3
=0.0135
X
7
NH3
= 0.0534 X
CO2
=0.00634
X
7
B
*65476.19 =0.0019 *48758.865 X
H2O
=0.040
X
7
B
=0.001415
X
7
CO2
*65476.19 =1214.65+0.00634*48758.865
X
7
CO2
=0.023
X
7
H2O
= 0.22
20
3 L.P DECOMPOSER
X
U
9
=0.63 , X
U
7
=0.70 (given)
Material balance
0.63 *W
9
= 0.7 *65476.19
W
9
= 72751.322
Let carbamate =72.28 Kmol/h
r
As NH
2
COONH
4
2NH
3
+ CO
2
NH
3
produced =2 * 72.28
=144.56 Kmol/h
r
=2457.58
Kg/h
r
:CO
2
produced =3180.32 Kg/h
r
W
8
72751.132 =65476.19 +W
8
X
NH3
=0.517
W
8
=7275.132 X
H2O
=0.252
CO
2
in vapour =3180.32 -0.023*65476.19 X
CO2
=0.23
=1674.367 Kg/h
r
W
9
Let H
2
O evaporated =1838.77 Kg/h
r
X
NH3
=0.0997
NH
3
evaporated =1304.475 Kg/h
r
X
B
=0.00127
W
8
=1304.475 +2457.52 +1838.77 +1674.367 X
CARB
=0.0775
=7275.132 X
H2O
=0.1928
0.001415 *65476.19 = X
9
B
*72757.322 W
7
X
NH3
=0.0534
X
9
B
=0.00127 X
B
=0.00415
X
9
NH3
*72757.322 =0.0534 *65476.19 +1304.475 +2457.52 X
CO2
=0.023
X
9
NH3
=0.0997 X
H2O
=0.22
X
9
CARB
= 72.28*78 X
U
=0.70
72757.322
21
= 0.0775
: X
9
H2O
= 0.1928
4 .VACCUM SYSTEMS
W
10
= W
6
+ W
4
=19133.085 Kg/h
r
W
6
+ W
4
X
10
NH3
= 2841.945 +(0.2722*2415.76)
19133.085
=0.183
X
10
H2O
= 12683.7973 +0.599*2415.76
19133.085 X
NH3
=0.183, X
H2O
=0.739
= 0.739 W
10
X
CO2
=0.078
: X
10
CO2
=0.0783
5.DISTILLATION COLUMN
NH
3
distilled = 17% W
11
X
NH3
=0.289
= 3252.62 Kg/h
r
X
H2O
=0.576
Water distilled = 32.8% X
CO2
=0.133
=6466.98 Kg/h
r
W
10
CO
2
at top =1498.12 X
NH3
=0.183
W
11
=11217.72 X
H2O
=0.739
W
10
W
11
= W
12
X
CO2
=0.078
W
12
=19133.085 -11217.72
=7915.365 Kg/h
r
W
12
X
NH3
=0.031
X
NH 3
=0.031 X
H2O
=0.968
X
H2O
=0.968
22
6.REFLUX ACCUMULATOR
Assume CO
2
evaporation =52.7% W
13
=789.196 Kg/h
r
; W
13
=789.196 W
11
W
14
For W
15
, NH
3
=2663.06 X
NH3 =
0.289
CO
2
=652.88 X
H2O
=0.576 X
CO2
=0.076
H
2
O=5240.22 X
CO2
=0.133 W
15
X
H2O
=0.615
: W
15
=8590.52 X
NH3
=0.309
W
14
= W
11
- W
15
- W
13
= 1838.004 Kg/h
r
W
13
X
14
X
NH3
= W
11
*0.29 - W
15
*0.31 W
11
W
14
X
CO2
=0.46
X
14
NH3
= 0.32 X
NH3
=0.32
W
14
X
14
CO2
= W
11
(0.1325) - W
15
(0.076) W
15
: X
14
CO2
=0.46
7. L. P CONDENSER
Assume:
W
8
+ W
14
=9113.136 X
CO2
=0.0873
NH
3
=4350.16 W
16(g)
X
NH3
=0.912
CO
2
=2519.85
H
2
O =2243.13
Assume: W
14(g)
+ W
8(g)
NH
3
in vapour = 526.37
CO
2
in vapour =50.397
: W
16
=576.767 Kg/h
r
W
17
= W
14
+ W
8
- W
16
W
17(l)
X
CO2
=0.29,
X
NH3
=0.145, X
H2O
=0.565
23
= 8536.369 Kg/h
r
W
17
X
NH3
= W
8
(0.577) + W
14
(0.32) -526.37
: X
17
NH3
=0.45
W
17
X
CO2
= W
8
X
8
CO2
+ W
14
X
14
CO2
-50.397
: X
CO2
=0.29
8. L.P NH
3
ABSORBER
NH
3
added =526.37
Let H
2
O added =440 Kg/h
r
vent(g) 50.397Kg/h
r
:
W
18
=966.37 Kg/h
r
W
16(g)
H
2
O(440 Kg/h
r
)
W
16
+440 =966.37 +vent X
NH3
=0.912 X
NH3
=0.544
:vent =50.397 Kg/h
r
X
CO2
=0.0873
W
18(l)
X
H2O
=0.455
9. CARBAMATE SOLUTION TANK
W
21
= W
17
+ W
18
X
NH3
=0.544
=9502.739 Kg/h
r
W
18(l)
X
H2O
=0.45
W
21
X
NH3
= W
18
*0.544 + W
17
*0.45 W
17(L)
X
21
NH3
=0.46 X
NH3
=0.145 W
21(l)
W
21
X
CO2
= W
17
*0.29 X
CO2
=0.29 X
NH3
=0.46
X
21
CO2
=0.26 X
H2O
=0.563 X
CO2
= 0.26
X
H2O
=0.28
24
10.M .P DECOMPOSER
Let 64% of carbamate decomposed
:cabamate decomposed =130 Kmol/h
r
=10140 Kg/h
r
W
23(L)
W
22(G)
X
NH3
=0.307
NH
2
COONH
4
2NH
3
+CO
2
X
H2O
=0.299
NH
3
formed =2*130 =260 Kmol/h
r
X
CO2
=0.393
=4420 Kg/h
r
CO
2
formed =130 Kmol/h
r
=5720 Kg/h
r
Let 0.5% of urea be converted into biuret W
9(L)
X
NH3
=0.0997
2NH
2
CONH
2
NH
2
CONHCONH
2
+NH
3
X
B
=0.00127
Urea converted = 4072
Kmol/h
r
X
CARB
=0.0775
=286.46 Kg/h
r
X
H2O
=0.1928
Biuret formed =2.385 Kmol/h
r
W
23(L)
W
22(G)
=245.69 Kg/h
r
X
NH3
=0.134
NH
3
formed =2.385 *17 =40.545 Kg/h
r
X
CARB
=0.18
Total NH
3
in vapour =4420+40.545 Kg/h
r
X
H2O
=0.156
Water evaporated =4356.4 Kg/h
r
X
U
=0.53
W
9(L)
W
22
=4356.4 +4460.545 +5720
=14536.945 Kg/h
r
W
23
- W
22 =
W
9
:
W
23
=72751.322+14536.945
=87288.267 Kg/h
r
W
23
X
U
= W
9
*0.63 +urea converted
=45833.33 +286.46
=46119.79
25
: X
23
U
=0.53
W
23
X
carb
= W
9
*0.078 +carbamate decomposed
X
23
carb
=0.18
W
23
X
NH3
= W
9
*0.0997 +4460.545
X
23
NH3 =
0.134
11. M.P CONDENSER
CO
2
in (W
22
+ W
21
) X
NH3
=0.46
=5720 +2470.712 W
21(l)
X
CO2
=0.26
=8190.712 W
22(g)
X
H2O
=0.28
NH
3
=4460.545 +4371.26 X
NH3
=0.307 W
25(l)
=8831.8 X
H2O
=0.299
W
25
= W
23
+ W
22
X
CO2
=0.393
=9502.739+14536.945
=24039.684 Kg/h
r
98% of CO
2
converted back to carbamate through condensation
2NH
3
+CO
2
NH
2
COONH
4
Carbamate formed =0.98 *8190.712/44
=182.45 Kmol/h
r
W
21(l)
=14229.5 Kg/h
r
W
25(l)
W
25
X
carb
=14229.5 W
22(g)
X
NH3
=0.27
: X
25
carb
=0.341 X
CO2
=0.241
W
25
X
NH3
= W
21
X
21
NH3
+ W
22
X
22
NH3
X
CARB
=0.42
=4460.545 +0.46 *9502.739
26
: X
25
NH3
= 0.37
12.M.P ABSORBER
NH
3
=2400 Kg/h
r
W
26(g)
W
28(g)
W
26
=2400
Kg/h
r
X
NH3
=1
Water in W
27
=3900 Kg/h
r
W
25
W
27(l)
& NH
3
in W
27
= 475 Kg/h
r
X
H2O
=0.003 X
NH3
=0.108
: W
27
=4375 X
NH3
=0.27 X
H2O
=0.891
CO
2
absorbed =113.23 Kg/h
r
X
CO2
=
0.241
W
29(l)
NH
3
absorbed =85% X
CARB
=0.492
Total ammonia in =11769.68
NH
3
absorbed =10023.68 Kg/h
r
NH
3
not
absorbed =8894.68 +2400+475 -10013.68 W
26(g)
W
28(g)
X
NH3
=0.98
=1746.0 Kg/h
r
W
25
X
H2O
=0.011
CO
2
not absorbed=9215.74 W
27(l)
: W
28
=10961.74
W
29
= W
25
+ W
26
+ W
27
+ W
28
W
29(l)
X
CO2
=0.0059
, X
NH3
=
0.42
=19852.9 Kg/h
r
X
CARB
=0.50
W
29
X
29
CO2
= W
25
X
25
CO2
- W
28
X
28
CO2
:
X
29
CO2
=0.0057
W
29
X
29
carb
= W
25
X
25
carb
X
29
carb
=0.50
W
29
X
29
NH3
= W
26
X
26
NH3
+ W
27
X
27
NH3
+8894.62 - W
28
X
28
NH3
=2400+475+8894.62-1746
=10023.68
:
X
29
NH3
=0.32
27
13.AMMONIA CONDENSER
Let ammonia condensed =25% W
30(g)
X
NH3
=0.1244
=0.25*1746 X
CO2
=0.876
=436.5 W
28(g)
: W
31
=436. X
NH3
=0.98 W
31(l)
W
28
= W
30
+ W
31
X
CO2
=0.011 X
NH3
=1
:
W
30
=10961.74 -436.5
=10525.24
W
30
X
30
NH3
= W
28
X
28
NH3
NH
3
condensed
=1746 -436.5
=1309.5
: X
30
NH3
=0.1244
W
30
X
30
CO2
= W
28
X
28
CO2
=9215.74
:
X
30
CO2
=0.876
14.AMMONIA RECEIVER
W
30
+ W
31
=1746
Let ammonia leaving top =454
Let CO
2
leaving top =45 X
NH3
=1, W
32(l)
W
34(g)
X
NH3
=0.909
NH
3
leaving bottom =34000 Kg/h
r
X
CO2
=0.090
: W
34
=499 & W
33
=34000 W
31(l)
+ W
30(g)
W
30
+ W
31
+ W
32
= W
33
+W
34
1746+ W
32
=34000+499
: W
32
=32753 W
33(L)
X
NH3
=1
28
15.M.P ABSORBER
Let ammonia absorbed =464.5 Kg/h
r
Water added =4000
Vent = W
34
+water added W
27
=499 +4000 -4375
=124 Kg/h
r
16.STRIPPER
Let 80% conversion of carbamate is taken place at stripper
W
23
=87288.267 Kg/h
r
W
35(g)
X
NH3
=0.750
NH
3
23
=11696.63 Kg/h
r
X
CO2
=0.24
Carb
23
=15711.9 Kg/h
r
W
36(g)
Carbamate decomposed = 930 Kg/h
r
X
NH3
=0.222
NH
2
COONH
4
2NH
3
+CO
2
X
N2
=0.00193
NH
3
formed =70000 Kg/h
r
X
CARB
=0.58
CO
2
formed =35000 Kg/h
r
X
U
=0.199 W
23(l)
X
NH3
=0.134
NH
3
boiled in vapour =40000 Kg/h
r
X
H2O
=0.156
Total NH
3
boiled in vapour =40000 +70000 =110000 Kg/h
r
X
CARB
=0.18
NH
3
in vapour =430 Kg/h
r
X
U
=0.53
W
35
=110000 +35000 +430
=145430 Kg/h
r
W
36
= W
35
+ W
23
=145430+87288.267
=232718.27 Kg/h
r
W
36
X
U
= W
23
(0.53)
: X
36
U
=0.199
29
W
36
X
36
N2
= W
35
X
35
N2
=450
: X
36
N2
=0.00193
W
36
X
NH3
= W
23
X
23
NH3
+ W
35
X
35
NH3
- NH
3
formed
=11696.6+110000-70000
=57696.6 Kg/h
r
X
NH3
=0.222
W
36
X
CARB
= W
23
X
23
CARB
: X
36
CARB
= 0.58
17 CARBAMATE CONDENSER
NH
3
+ CO
2
NH
4
COONH
2
X
NH3
=0.759 W
35(g)
Let CO
2
condensed =64000 X
CO2
=0.24
NH
3
condensed =59100 W
29
X
NH3
=0.42
:carbamate formed =113454.54 X
CO2
=0.0059
W
37
= W
35
+ W
29
X
CARB
=0.50
=
145430+19852.9 X
NH3
= 0.25 W
37
= 165282.9
Kg/h
r
X
CARB
=0.73, X
CO2
=0.00069, X
H2O
=0.0693
W
37
X
CARB
= W
29
X
29
CARB
+ W
29
(0.32) NH
3
condensed
=11000+6352.9 -59100
=0.25
W
37
X
CO2
=64000+113.23-64000
=0.00069
30
18.CARBAMATE SEPARATOR
NH
3
evaporated =1500 Kg/h
r
vent(g) X
NH3
=0.43,X
H2O
=0.52
H
2
O evaporated =1800 Kg/h
r
X
CO2
=0.048
CO
2
in vent = 150 Kg/h
r
W
38
W
37
Vent =3450 Kg/h
r
X
NH3
=0.17 X
NH3
=0.25
W
37
= W
38
+vent X
H2O
=0.09 X
CARB
=0.73
W
38
=165282.9 3450 X
CARB
=0.74 X
CO2
=0.00069
= 161832.9 X
H2O
=0.0193
W
38
X
CARB
= W
37
X
37
CARB
+vent X
CARB
=41320.725 +1500
X
38
NH3
=0.17
X
38
H2O
=0.09
19.UREA REACTOR
W
40
= W
33
- W
26
W
36(g)
X
U
=0.199
= 34000 -2400 X
NH3
=0.222
=31600 Kg/h
r
X
CARB
=0.58
W
36
= W
39
+ W
38
+ W
40
W
39
X
N2
=0.00193
= 232718.27 -161832.9 -31600 X
CO2
=0.9854
=39285.37 Kg/h
r
X
N2
=0.01145
Known amount of N
2
in W
36
=430
Corresponding O
2
=143.3 W
40
W
38
X
CARB
=0.74
Air used =573.2 X
NH3
=0.17, X
H2O
=0.09
Amount of CO
2
coming in reactor with W
39
steam=39285.37-573.2
=38712.17 Kg/h
r
31
W
39
X
CO2
=38712.17
: X
39
CO2
=0.9854
W
39
X
N2
= W
36
X
36
N2
: W
39
X
N2
=0.01145
2NH
3
+CO
2
NH
2
COONH
4
For 100% CO
2
conversion,
NH
3
required =W
32
= 32753 Kg/h
r
=1926.65 Kmol/h
r
UREA FORMED =771.85 Kmol/h
r
32
CHAPTER 5
ENERGY BALANCE
33
ENERGY BALANCE
Energy balance across stripper:
Now, here the solution enters at 190Cand leaves at 210C.Also a part of carbamate decomposes
into NH
3
and CO
2
.The decomposition is as follows;
NH
4
COONH
2
2NH
3
+CO
2:
H= -38.Kcal/mol
The vapour and gases product from the top at 190C.Now we assume an average temperature of
200Cand find the final heat capacity of the solution.
C
p
of water =4.278 KJ/KgC
C
p
of ammonia =8.851 KJ/KgC
C
p
of carbamate =2.682
KJ/KgC
C
p
of urea =2.331 KJ/KgC
C
p
mean :
[ ( 4.278 *42510.27) +(8.851 *15711.9) +(2.682 *11696.63) +(2.331 *17370.365)]
(42510.27 +15711.9 +11696.63 +17370.365)
C
p
mean =4.499 KJ/KgC
Energy consumed in raising solution temperature is
=87288.267 *4.499*(210-190)
=7854198.265KJ/h
r
Energy consumed in carbamate decomposition is
=(930/78) *159 *10
3
=1895769.23 KJ/h
r
Total energy consumed =9749967.495 KJ/h
r
Saturated steam at 26 bar and266C is used for heating, =1827.914 KJ/kg
Steam flow rate =5333.93 Kg/h
r
34
Energy balance across M.P Decomposer
Now, here the solution enters from the stripper, the solution is at 145 Kg/cm
2
and 210C, and it is
flashed to 17 Kg/cm
2
before it enters the decomposer.Thus the temperature falls to 147C.Here the
first solution is heated to155C then water is evaporated from the solution.Also carbamate is
decomposed producing NH
3
and CO
2 .The
energy is supply by steam condensate at 225C at 26 bar,
which gets cooled to 210C in this process
C
p
mean =[ (4.27*14026.45) + (8.85*7253.306) + (2.68*5638.23) + (2.33*45833.33)]
72751.322
Energy consumed in raising solution temperature :
=72751.322*3.38(155-147)
=3.38 KJ/KgC
Energy consumed in carbamate decomposition :
=130*159*10
3
=20670000 KJ/h
r
Total energy consumed =22637894.57 KJ/h
r
Hot water at 225C is used to provide the heat ,which get cooled to 210C
Water flow rate =22637894.57/(4.2*(225-210))
=359331.66 Kg/h
r
Energy balance across L.P Decomposer:
Now the solution enters from the M.P Decomposer at 147C and 16.5 Kg/cm
2
and it is flashed to
13.5 Kg/cm
2
before it enters the decomposer.Thus the temperature falls to 100C.
Here all the carbamate isdecomposed and liquid stream flows out at a temperature of138C.The
top gases have NH
3
, CO
2
and water vapour. The energy is supplied by steam condensate at 148C
at 4.5 bar.
C
p
mean=[(2.33*45833.33) +(4.278*14404.76) +(8.85*3496.43)]
65476.19
=3.045 KJ/KgC
Energy consumed in carbamate decomposition :
35
=159*10
3
*72.28
=11492520 KJ/h
r
Energy consumed in evaporation of water:
=1838.77*2149
=3951516.73 KJ/h
r
Energy consumed in raising solution temperature :
=65476.19*3.045*38
=7576249.9 KJ/h
r
Total energy consumed:
=20123979.16 KJ/h
r
Energy balance across L.P ammonia absorber:
Ammonia is absorbed in water and the residue O
2
is vented to the atmosphere
Here ammonia dissolved is =526.37Kg/h
r
Heat released due to ammonia absorption =526.37/17 *34.79*10
3
=1077200.724 KJ/h
r
Cooling water enters at 32C and leaves at 40C
Cooling water required =1077200.724 / 4.2*(40-32)
=32059.5437Kg/h
r
Energy balance across I
st
vaccum concentrator separator:
In this due to flashing the temperature of the solution drop to 90cwhile effluent stream goes out at
130C.This heat is required to raise the temoerature and vaporize the water.The system operates at
130C and 0.3 Kg/cm
2
pressure.
C
p
mean=[(4.278*1958.156) +(8.851*658.2) +(2.331*45833.33)]
48449.6891
=2.5 KJ/KgC
Heat to raise the solution temperature =4878.865*2.5(130-90)
36
=4872504.618 KJ/h
r
Heat required to vaporize water =12683.7973*2337
=29642034.29 KJ/h
r
Total heat required =34514538.9 KJ/h
r
Steam at 4.5 bar is used as heating
=2119.77KJ/kg
Steam required =34514538.91
2119.7
=16282.75Kg/hr
Energy balance across II
nd
vaccum concentrator separator:
The unit operates at0.03 Kg/cm
2
and 140C.The incoming feedis at 130C due to
flashing.Thusheat is required to first raise the feed temperature to 140C and to vaporize water.
C
p
mean=[(4.278*509.77) +(2.33*45833.33) ]
46343.10
=2.35 KJ/KgC
Heat to raise the solution temperature =46343.10*2.35*10
=1089062.55 KJ/h
r
Heat required to vaporize water =2445.1*1448.97
=3542883.511 KJ/h
r
Total heat required =4631946.361 KJ/h
r
Steam at 4.5bar is used as heating media
37
=2119.7KJ/kg
Steam required = 4631946.361
2119.7
=2185.19Kg/hr
Energy balance across reactor:
2NH
3
+CO
2
NH
2
COONH
4
38.1Kcal/mol
NH
2
COONH
4
NH
2
COONH
2
+H
2
O -7.1Kcal/mol
Carbamate formed =1730.47 Kmol/h
r
Urea formed =771.85 Kmol/h
r
=46313.94Kg/hr
Energy released in carbamate formation:
=1730.47*100*159
=27514460.77 KJ/hr
Energy consumed in urea formation :
= 46310.94*30
=1389328.2 KJ/hr
Net energy released =26125132.57 KJ/hr
38
DESIGN OF AMMONIA PREHEATER
Ammonia inlet flow rate = 32753 kg/hr
Specific heat of ammonia = 1.23 kcal/kg c
Inlet ammonia temp.= 86 F = 30 c
Outlet ammonia temp.= 230 F= 110 c
Heat required by ammonia= (32753 kg/ 3600 sec) * 5.14 KJ/kg c * 80 c
= 3741.12 KJ/sec
= 3741.12 KW
Latent heat of steam = 503.7 cal/mol= 2105.466 J/mol
Therefore, m*2105.466 = 3741.12
m = (3741.12*1000)/2105.466
m = 1776.86 mol/sec =98.7 kg/sec
LMTD = {(304 - 86) (304 230 )}/ ln (218/74) = 133.28
Assume U = 200 W/ m
2
c
Area A = Q/(U*LMTD) = (3741.12*1000)/(200*133.28)= 140.35 m
2
Choose 20mm O.D.,16mm I.D.,4.88m long tubes, cupro nickel
L= 4.83m
Area of one tube = 3.14*d*l= 3.14*4.83*(20/1000)= 0.303 m
2
No.of tubes= 140.5/0.303 = 463
Use 1.25 triangular pitch
Bundle diameter, D
b
= d
0
(N
t
/k
1
) where, N
t
= no.of tubes
D
b
= bundle dia in mm
d
0
= tube outside dia in mm
Therefore, D
b
= 20*(43/0.249) = 605.897mm
39
Using a split ring floating head type,
From the graph bundle diameter clearance= 62mm
Therefore, Shell diameter, D
s
= 605.897+62 = 667.897mm
COLD FLUID TUBE SIDE:--
Mean ammonia temp.= (230+86)/2 =158 F= 70 c
Tube cross-sectional area =(3.14/4)*16
2
= 201mm
2
Tubes per pass =no.of tubes/2 = 463/2 = 231.5 = 231
Tube flow area = (231*201)/1000000= 0.046m
2
Ammonia mass velocity= 32753/(60*60*0.046)= 197.78 kg/sec m
2
Density of ammonia = 0.618 g/ml= 618 kg/m
3
Ammonia linear velocity, u
t
= 197.78/618 = 0.32 m/sec
From the relation,
Viscosity of ammonia= 0.19 cp=(0.19/100) N/m
2
K of ammonia= 0.29 Btu/hr ft
2
= 0.29*1.729 = 0.50141 W/m c
Specific heat of ammonia= 1.02 cal/gm c= 4.2636 KJ/kg c
Reynold no.= (d
i
*v*p)/viscosity= (618*0.32*16)/(0.19*10)= 1665.35
Prandtl no.=(c
p
*viscosity)/K
f
=(4.2636*10*0.19)/ 0.50141= 16.156
L/ d
i
= 4.83*1000/16= 302
From the fig., j
h
= 2.2*10
-3
Therefore, h
i
= 2.2*10
-3
* 1665.35* (16.156)
0.33
*0.50141 / 16*10
-3
=
287.575 W/m
2
c
40
HOT FLUID SHELL SIDE:--
Choose baffle spacing = D
S
/5 =667.897/5 = 133.6mm
Tube pitch = 1.25*20 = 25mm
Cross sectional area, A
S
= (P
T
-d
0
) D
s*
l
b
/ P
t
={(25-20)*667.897*133.6*10
-6
}/25
= 0.0178 m
2
Mass velocity, G
S
= 98.7 kg/s*(1/0.0178)m
2
= 5544.9 kg/m
2
s
Equivalent diameter, d
e
= 1.1/d
o
( P
t
2
- 0.917d
0
2
)
= 1.1/20( 25
2
-0.917*20
2
)= 14.4mm
Mean shell side temp.=340 F= 151.11 c
K of steam = 700*10
-3
W/m c
Viscosity of steam = 1860*10
-6
Ns/m
2
Re= (G
s
*d
e
)/ viscosity= 5544.9*14.4*10
-3
/ 1860*10
-6
= 42928.26
Pr= C
p
*viscosity/ K= 4.21*1860*10
-6
/0.700 = 0.011
Choose 25% baffle cut, from fig.
J
h
= 3*10
-3
Therefore, (h
s
*d
e
)/ K
f
= J
h*
Re* Pr
0.33
h
s
=3*10
-3
* 42928.26*0.011
0.33
*0.700/ 14.4*10
-3
h
s
=1413.38 W/m
2
c
OVERALL COEFFICIENT;
K of cupro nickel alloys =50 W/m c
Take the fouling coefficient from the table;
Steam condensate= 5000 W/m
2
c
Ammonia= 10,000 W/m
2
c
41
i i id i
i
o
o
od
h d
d
h d
d
U
d
d
d
h h U
1 1
ln
1 1 1
0 0
0 0 0
+ +
|
|
.
|
\
|
+ + =
1/U
O
= 1/1413.38+1/5000+20*10
-3
ln(20/16)/(2*50)+(20/16)*(1/10000)+1/287.575
U
O
= 219.58 = 220 W/m
2
c
PRESSURE DROP:
TUBE SIDE-
2
5 . 2 8
2
t
m
w i
P t
u
d
L
jf N P
(
(
+
|
|
.
|
\
|
|
|
.
|
\
|
= A
From fig, for Re= 1.67*10
3
J
f
= 7.1*10
-3
Therefore, P
t
= 2*[8*7.1*10
3
(4.83*10
3
)/16 + 2.5] *618*(0.32
2
)/2
= 1243.29 N/m
2
= 1.24 KPa
SHELL SIDE-
Linear velocity = G
s
/p = 5544.9/618 = 8.97 m/s
From the graph, j
f
= 4*10
-2
14 . 0
2
2
8
|
|
.
|
\
|
|
|
.
|
\
|
|
|
.
|
\
|
= A
w
s
B
s
e
s
s
u
I
L
d
D
jf P
42
Therefore, P
s
= 8*4*10
-2
*(667.897/14.4)*(4.83*10
3
/133.6)*(618* 8.97
2
)/2
= 1334.07 N/m
2
43
44
BELLS METHOD
No.of tubes = 463
Shell i.d = 667.897 mm
Bundle diameter= 605.897 mm
Tube o.d.= 20 mm
Tube length = 4830 mm
1.HEAT TRANSFER COEFFICIENT
A
s
= (25-20)*667.897*133.6*10
-6
/ 25
= 0.0178 m
2
G
s
= 98.7/0.0178 = 5544.94 ks/s m
2
Re= G
s
*d
o
/ viscosity = 5544.94*20*10
-3
/(1860*10
-6
)= 59623
From fig., j
h
= 4.1*10
-3
Prandtl no.= 0.011
Neglect viscosity correction factor,
h
oc
*d
o
/K = j
h
*Re*Pr
0.33
h
oc
= 4.1*10
-3
*59623*0.011
0.33
*0.700/ (20*10
-3)
h
oc
= 1931.636vW/m
2
c
2. TUBE ROW CORRECTION FACTOR
Tube vertical pitch, P
t
= 25* 0.87 =21.8 mm
Baffle cut height, H
c
=0.25*667.897= 166.97mm
Height between baffle tips= 667.897-(2*166.97)=333.957 mm
N
cv
= 333.957/ Pt = 15.32
45
From fig., F
n
=1.02
3. WINDOW CORRECTION FACTOR
H
b =
D
b
/2 D
si
(0.5 baffle cut)
=605.897/2 -667.897(0.5 -0.25)
=302.9485-166.974
=135.974 mm
Bundle cut =H
b
/D
b
= 135.97/605.897 =0.22
From the fig.12.41 at cut of 0.22
R
a
1
=0.16
Tubes in one window area, N
w
=Nt*R
a
=463*0.16= 74.08=74
Tubes in cross flow area, N
c
=N
t
-2N
w
=463-2*74
= 315
R
w
=2N
w
/N
t
=2*74/463= 0.32
From fig., F
w
= 1.07
4.BYPASS CORRECTION FACTOR,F
b
A
b
=(D
i
-D
b
)B
s
= (667.897-605.897)*267.2*10
-6
=0.0166 m
2
A
b
/A
s
=0.0166/0.0178=0.93
F
b
= exp[-1.35*0.39]=0.59
Very low sealing strips needed; try one strip for each 5 vertical rows
N
s
/N
w
=1/5
F
b
= exp[-1.35*0.93{1-(2/5)0.33}]=0.72
46
5. LEAKAGE CORRECTION FACTOR,F
L
Using clearances as specified in the standards
Tube to baffle 1/32inch =0.8 mm
Baffle to shell 3/16inch =4.8mm
Atb=(0.8/2)*20**(463-74)=9771.68 mm
2
=0.0098m
2
From fig., 25% baffle cut,
b
=2.1 rads
A
sb
=(C
s
*D
s
/2)*(2-
b
)=(4.8/2)*667.897*(2-2.1)
= 6700.34mm
2
=0.006m
2
A
t
=A
tb
+A
sb
=0.0098+0.006=0.0158 m
2
A
l
/A
s
=0.0158/0.0178=0.89
From fig,
L
=0.43
F
l
=1-0.43{0.0098+2*0.006}/0.0158=0.41
SHELL SIDE COEFFICIENT:
h
s
= h
oc
F
n
F
w
F
b
F
L
=1931.636*1.02*1.07*0.72*0.41
= 622.34 W/m
2
c
Appreciably lower than that predicted by Kerns method.
PRESSURE DROP--------
1.CROSS FLOW ZONE:
From fig.,at Re=59623,for 1.25pitch,
J
f
= 5*10
-2
U
s
=G
s/
p=5544.94/618=8.97 m/s
P
i
=8*j
f
*N
cv
*pu
s
2
/2=8*5*10
-2
*15.32*618*(8.97
2
)/2=152356.9 N/m
2
=4.0 for Re>100,
47
Therefore, F
b
=exp[-4*0.93{1-(2/5)
0.33
}]=0.38
From fig.,
L
=0.7
F
L
=1-0.7[(0.0098+2*0.006)/0.0158]=0.034
P
c
=1523569*0.38*0.034=1968.45N/m
2
2. WINDOW ZONE:
From fig. for 25% baffle cut, R
a
=0.19
A
w
=[(D
s
2
/4)*R
a
(N
W
**d
O
2
/4)]
=[(*667.897
2
*0.19/4) (74**20
2
/4)]=43297.8 mm
2
=0.043m
2
U
w
=(98.7/618)/0.043=3.7m/s
U
z
=( U
w
*U
s
)
1/2
= (3.7*8.97)
1/2
=5.8m/s
N
wv
=H
b
/ P
t
=135.97/21.8 = 6.24
P
w
=F
L
(2+0.6N
wv
)pU
z
2
/2=2030.055 N/m
2
3.END ZONE:
P
e
=P
c
[(N
wv
+N
cv
)/N
cv
]*F
b
= 152356.9[(6.24+15.32)/15.32]*0.38=81477.13N/m
2
TOTAL PRESSURE DROP:--
No.of baffles, N
b
= (4830/267.2)-1=17
P
s
=2*81477.13+1968.45(17-1)+17*2030.055
= 228960.39 N/m
2
= 228.96 KPa
48
DESIGNING OF UREA REACTOR
The liquid mixture of NH
3
and carbamate (100C) and gaseous CO
2
(140C) are fed to reactor
where they meet 180C temperature and 150 atm pressure and form ammonia carbamate .This
carbamate dehydrates and forms Urea.The reactions taking place in the reactor are given as:
2NH
3
+CO
2
NH
2
COONH
4
NH
2
COONH
4
NH
2
COONH
2
+H
2
O
KINETIC DESIGN
49
The predominant rate controlling mechanism is the reaction kinetics.The first reaction occurs
rapidly and goes to completion, the second reaction occurs slowly and determines the reaction rate.
Assuming order of reaction is first order reaction. Then rate of reaction is
-r =kC
A
1+X
A
From material balance;
Mole ratio = NH
3
=2.55 , H
2
O =0.37
CO
2
CO
2
Assuming overall carbamate conversion to be 42%
Now , = 2.55 3.55 = -0.28
3.55
Performance equation of an ideal plug flow reactor
o
V =F
AO
I =
Value of integral is solved by Simpsons 1/3 rule:
S.No. X
A
(1-0.28X
A
)/(1-X
A
)
1 0 1
2 0.05 1.04
3 0.1 1.08
4 0.15 1.13
50
5 0.20 1.18
6 0.25 1.24
7 0.30 1.31
8 0.35 1.39
9 0.40 1.48
10 0.45 1.59
Using Simpsons rule:
= h/3 [y
0
+4 (y
1
+y
3
+ y
5
+ y
7
+.. ) +2(y
2
+y
4
+y
6
+ y
8
+ y
10
..) +y
n
]
=0.059/3[1 +4(1.04 + 1.13 + 1.24 + 1.39) +2(1.08 +1.18 +1.31 +1.48) +1.59]
=0.627
F
AO
=(4333.91/17) +(14564.96/18)+(120727.34/78)
=254.9+809.164+1547.79
=2611.85 Kmol/h
r
0
= 4333.91 +14564.96 +120727.34
890 1000 910
= 4.87 +14.565+132.667
=152.10 m
3
/h
r
C
AO
= F
AO
/
0
= 2611.85/152.10
=17.172 Kmol/m
3
DETERMINATION OF RATE CONSTANT K:
Reactor temperature= T
2
=180C= 453 K
R=1.985 cal K
-1
mol
-1
At temperature T
1
=140
C =413 K, K=0.0134 min
-1
for 1
st
order reaction(reference)
From the reaction, E=38.1-7.1= 31 Kcal/mol=31000 cal/mol
51
ln K
2
=E{(1/T
1
)
(1/T
2
)}
K
1
R
Therefore, K
2
= 0.0378 min
-1
=2.27 hr
-1
Volume of reactor V =
F
AO
* I
K* C
AO
V= 2611.85*0.627 / (2.27*17.172)
V=42.01 m
3
Length and diameter of urea reactor
Assuming L/D =20
V=( /4) *D
2
*20D
D
i
=1.39 m
L =27.8 m
52
COST ESTIMATION
Acceptable plant design must present a process that is capable of operating
under conditions, which will yield profit. Since net profit equals total value minus
all expenses, it is essential that the chemical engineer be aware of the many
different types of cost involved in the manufacturing processes. Capital must
allocate for the direct, plant expenses, such as those for raw material, labor and
equipment.
Besides direct expenses many others indirect expenses are incurred, and these
must be included if a complete analysis of the total cost is to be obtained. Some
examples of these indirect expenses are administrative salary, product
distribution cost and cost for interplant communication. A capital investment is
required for every industrial process and determination of necessary investment
is an important part of a plant design process. The total investment for any
process consist fixed capital investment for practical equipment and facilities in
the plant plus working capital, which must be available to pay salaries, keep raw
material and products on hand, and handle other special items requiring the
direct cost outline.
When the cost for any type of commercial process is to be determined, sufficient
accuracy has to be provided for reliable decision. There are many factors
affecting investment and production cost. These are;
1. Source of equipment
2. Price fluctuation
3. Company policies
4. Operating and rate of production
5. Governmental policies
Before an industrial plant can be put into operation, a large sum of money must
be supplied to purchase and install the necessary machinery and equipment.
53
Land and service facilities must be obtained, and the plant must be erected completely
with all piping, controls and services.
The capital needed to supply the necessary manufacturing and plant facilities is called the
fixed-capital investment, while that necessary for the operation of plant is termed the
working capital. The sum of the fixed capital investment and the working is known as the
total capital investment. Generally, the working capital amounts 10-20% of the total capital
investment. Following is the breakdown of the fixed capital investment for a chemical
process.
DIRECT COST:
1. purchased equipments
2. purchased equipment installation
3. instrumentation and control
4. piping
5. electrical equipment and material
6. building (including services)
7. yard improvement
8. land
INDIRECT COST:
1. engineering supervision
2. construction expenses
3. contractors fee
4. contingency
TYPES OF CAPITAL COST ESTIMATE:
Order of magnitude estimate (ratio estimate) based on similar cost data;
probable accuracy of this estimate over 30%.
54
Study estimate based on knowledge of major items of equipment,
probable accuracy of this estimate up to 30%.
Preliminary estimate( budget authorization estimate scope method): based
on sufficient data to permit the estimate to the budget, probable accuracy
of this estimate is within 20%.
Detailed estimate based on complete engineering drawing, specifications
and site survey, probable accuracy of this estimate within 10%.
COST ESTIMATION OF 1000 TONS/DAY OF UREA PLANT:
Urea plant size = 1000 T/day
Fixed capital investment for cost index of 130 = Rs 2.05110
7
Cost index for 2002 = 402
Therefore present fixed capital investment = 2.05110
7
(402/130)
=Rs 63,42,32,31
Estimation of total investment cost :
1) Direct cost:
a) Purchased equipment cost:(15 - 40% of FCI ) Assume
40% of FCI
=Rs 25369292
b) Installation cost :(35 - 45% of PEC)
Assume 45%
=Rs 11416181
c) Instrument and control installed :(6 -30% of PEC)
Assume 30% of PEC
=Rs 7610787
d) Piping installation cost :(10 -80% of PEC)
Assume 80%
=Rs.20295433
e) Electrical installation cost:(10 - 40% of PEC)
55
Assume 40% of PEC
=Rs 10147716
f) Building process and auxilliary:(10-70% of PEC)
Assume 70%
=Rs 17758504
g) Service facilities:(30-80% 0f PEC)
Assume 80%
=Rs 20295433
h) Yard improvement :(10-15% of PEC)
Assume 15%
=Rs 3,805,393
i) Land:(4-8% of PEC)
Assume 8%
=Rs 2029543
Therefore direct cost =Rs. 118728282
Indirect cost:
Expenses which are not directly involved with material and labour of actual installation or complete
facility
a) Engineering and supervision :(5-30% of DC)
Assume 30%
=Rs 35618485
b)Construction expenses:(10% of DC)
=Rs 11872828
c) Contractors fee:(2-7% 0f DC)
Assume 7%
=Rs 8310979
d) Contingency :(8-20% of DC)
Assume 18%
=Rs 21371091
Therefore total indirect cost =Rs 77,173,383
Fixed capital investment:
56
Fixed capital investment (FCI) = DC+IC
= Rs 195,901,665
Working capital investment:
10 -20% of FCI
Assume 18%
=Rs 35262299
2) Total capital investment:
= FCI + WC
=Rs 231163965
Estimation of total product cost(TPC):
Fixed charges:
a) Depreciation :(10% of FCI for machinery)
=Rs 19590166
b) Local taxes :(3-4% of FCI)
Assume 4%
=Rs 7836067
c) Insurances :(0.4-1% of FCI)
Assume 0.9%
=Rs 1763115
d) Rent :(8-12% of FCI)
Assume 12%
=Rs 23508199
Therefore total fixed charges =Rs 52697547 But,
Fixed charges = (10-20% of TPC)
Assume 20%
Therefore Total product cost =52697547/0.20
=Rs 263487735
Direct production:
a) Raw material :(10-50% 0f TPC)
Assume 50%
57
=Rs 131743867
b) Operating labour(OL):(10-20% of TPC)
Assume 20%
=Rs 52697547
c) Direct supervisory and electric labour:(10-25% of OL) Assume
25%
=Rs 13174387
b) Utilities :(10-20% of TPC)
Assume 20%
=Rs 52697547
c) Maintenance :(2-10% of FCI)
Assume 9%
=Rs 17631149
d) Operating supplies (OS):(10-20% of maintenance) Assume
20%
=Rs 3526229
e) Laboratory charges :(10-20% of OL)
Assume 18%
=Rs 9485558
f) Patent and royalties :(2-6% of TPC)
Assume 6%
=Rs 15809264
Plant overhead cost:
50-70% of (OL+OS+M = 73854925)
Assume 70%
=Rs 51698447
General expenses:
a) Administration cost:(40-60% of OL)
Assume 60%
=Rs 31618528
b) Distribution and selling price:(2-30% of TPC)
58
Assume 30%
=Rs 79046320
c) Research and development cost
:(3% of TPC)
Rs= 7904632
Therefore general expenses (GE) =Rs 118569480
Therefore manufacturing cost (MC)= Product cost+fixed charges+Plant
overhead expenses
=Rs 367883729
Total production cost:
Total production cost =MC + GE
=Rs 486453209
Gross earnings and rate of return:
The plant is working for say 340 days a year
Selling price =Rs. 15 /kg for a Urea
Total income =100340100015
=Rs 510000000
Gross income =Total income - total product cost
=Rs 23546791
Tax =50%
Net profit =Rs 11773395
Rate of return = net profit/total capital investment
=5.1%
59
REFERENCES
IFFCO( Phulpur unit) manual
Peters, Max S. and Timmerhaus, Klaus D., Plant Design & Economics, 4
th
edition,
McGraw Hill, Inc. (1980)
Wilbrant, Frank C. & Dryden, Charles E., Chemical Engineering Plant Design, 4
th
edition, McGraw Hill, Inc. (1980)
Coulson, J. M. & Richardson, J. F., Chemical Engineering, Volume 6
Perry, J.H., Chemical Engineers Handbook, 7
th
edition, McGraw Hill, Inc. (1985)
Joshi, M.V. and Mahajani, V.V, Process Equipment Design,3
rd
edition, Macmillan
India ltd.(2007)
McCabe, Warren L., Smith, Julian C., and Harriot, Peter, Unit Operations of
Chemical Engineering, 5
th
edition, Pergamon Press (1983)
Kern, D.Q, Process Heat Transfer , J. A., 4
th
Edition , McGraw Hill International
Edition .
Shreeve R . N. & Brink J A , Chemical Process Industries , 5
th
Edition , McGraw
Hill International Edition .
Sahu, J.N ,Patwardhan,A.V and Meiko, B.C (IIT Kharagpur) Equilibrium and
kinetic studies of generation of urea in areactor
60
APPENDIXES
61
62
63
64
65
66
67
68
69
70
S-ar putea să vă placă și
- Adiabatic Fixed-Bed Reactors: Practical Guides in Chemical EngineeringDe la EverandAdiabatic Fixed-Bed Reactors: Practical Guides in Chemical EngineeringÎncă nu există evaluări
- Urea Plant DesignDocument70 paginiUrea Plant Designravichem823Încă nu există evaluări
- Urea Project Report 1 PDFDocument9 paginiUrea Project Report 1 PDFVirendra RathvaÎncă nu există evaluări
- Full Report UreaDocument103 paginiFull Report Ureanisasoberi100% (1)
- Nirbhay Urea Final PDFDocument99 paginiNirbhay Urea Final PDFHimanshu vikram100% (1)
- Aces 4 Complete Urea ProjectDocument92 paginiAces 4 Complete Urea ProjectAyat100% (1)
- Urea Manufacturing Plant: CH 4200 - Comprehensive Design ProjectDocument124 paginiUrea Manufacturing Plant: CH 4200 - Comprehensive Design Projectwaqas83% (18)
- Urea Plant Material Balance (ACES Process)Document7 paginiUrea Plant Material Balance (ACES Process)muks19950% (2)
- Reactor Kinetics of Urea FormationDocument21 paginiReactor Kinetics of Urea Formationtitas5123100% (1)
- Final ThesisDocument33 paginiFinal Thesisusama100% (2)
- Urea PDFDocument11 paginiUrea PDFSteve WanÎncă nu există evaluări
- Engineers Guide - The Snamprogetti Urea Process DescriptionDocument2 paginiEngineers Guide - The Snamprogetti Urea Process DescriptionBalas43Încă nu există evaluări
- Snamprogetti Urea ProcessDocument106 paginiSnamprogetti Urea ProcessHeba Ramadan95% (19)
- Designing Urea ReactorDocument21 paginiDesigning Urea ReactorAdawiyah Al-jufri100% (4)
- Draft Report For Urea ProductionDocument59 paginiDraft Report For Urea ProductionBryan Jesher Dela CruzÎncă nu există evaluări
- Urea Production ReportDocument38 paginiUrea Production ReportAbhijit NathÎncă nu există evaluări
- Ammonia Synthesis Loops Variables Investigated by Steady-State SimulationDocument14 paginiAmmonia Synthesis Loops Variables Investigated by Steady-State Simulationpolonium2310Încă nu există evaluări
- Nitric Acid Overall Material BalanceDocument4 paginiNitric Acid Overall Material Balanceyogeshdama100% (1)
- Plant Design Project Report-Group 6BDocument275 paginiPlant Design Project Report-Group 6BNana kwadwo100% (2)
- Manufacture of UreaDocument86 paginiManufacture of UreamohamedÎncă nu există evaluări
- P Urea Smcarb 2018 Ok Ok OkDocument143 paginiP Urea Smcarb 2018 Ok Ok Okهشام حدودÎncă nu există evaluări
- Urea Manufacturing Plant-StamicarbonDocument4 paginiUrea Manufacturing Plant-StamicarbonRadhika PillayÎncă nu există evaluări
- Urea ProjectDocument17 paginiUrea ProjectAbdo Shaaban100% (2)
- Urea Project ReportDocument107 paginiUrea Project Reportmuks19995% (57)
- Simulation of A Urea Synthesis Reactor. 1. ThermodynamicDocument10 paginiSimulation of A Urea Synthesis Reactor. 1. ThermodynamicYaraKanawatiÎncă nu există evaluări
- Stamicarbon Project PDFDocument30 paginiStamicarbon Project PDFMir Hasib Ul Latif100% (7)
- Production of Urea by ACES ProcessDocument157 paginiProduction of Urea by ACES ProcessAriel Sandoval100% (1)
- Urea Written ReportDocument64 paginiUrea Written Reportnitish100% (3)
- Proposal For UreaDocument24 paginiProposal For UreaUmar ZamanÎncă nu există evaluări
- Urea PlantDocument80 paginiUrea Plantharsha100% (2)
- Ammonia Plant Material BalanceDocument66 paginiAmmonia Plant Material Balancesagar dasgupta100% (1)
- Ammonia SynthesisDocument43 paginiAmmonia Synthesissorincarmen88100% (2)
- Major Ammonia Leak From HP Ammonia Feed Pump: P.Hari Narayana Reddy, R. Raghavan and Ramashray SinghDocument10 paginiMajor Ammonia Leak From HP Ammonia Feed Pump: P.Hari Narayana Reddy, R. Raghavan and Ramashray Singhvaratharajan g rÎncă nu există evaluări
- ToyoDocument17 paginiToyoZeeshan Khan100% (1)
- Urea Plant Nangal 2014Document8 paginiUrea Plant Nangal 2014Ishan HaiderÎncă nu există evaluări
- Project 0n UreaDocument38 paginiProject 0n UreaVishal SinghÎncă nu există evaluări
- Designing Urea ReactorDocument20 paginiDesigning Urea ReactordcobasbÎncă nu există evaluări
- Stamicarbon Urea Process Data PDFDocument1 paginăStamicarbon Urea Process Data PDFtreyzzztylerÎncă nu există evaluări
- Block Diagram of The Ammonium Sulphate Production PlantDocument3 paginiBlock Diagram of The Ammonium Sulphate Production PlantAkuwh SyaSya100% (1)
- Cumene Project ReportsDocument33 paginiCumene Project ReportsDiv Savaliya100% (2)
- Ammonia Urea Mass BalanceDocument44 paginiAmmonia Urea Mass BalanceSenthil NathanaÎncă nu există evaluări
- 2000 Development of The ACES 21 ProcessDocument17 pagini2000 Development of The ACES 21 ProcessTTaddictÎncă nu există evaluări
- Revamping of Ammonia Plant - OptionsDocument11 paginiRevamping of Ammonia Plant - OptionsrvnesariÎncă nu există evaluări
- 2008 Morikawa TEC IFA ACES21 Advanced Urea Production Technology - 2Document15 pagini2008 Morikawa TEC IFA ACES21 Advanced Urea Production Technology - 2MubasharÎncă nu există evaluări
- Plant Design For The Production of Nitric Acid PDFDocument59 paginiPlant Design For The Production of Nitric Acid PDFحاتم غيدان خلفÎncă nu există evaluări
- Topsoe Steam Reform SolutionsDocument4 paginiTopsoe Steam Reform Solutionsusman_hafeez86100% (2)
- Urea Technology, Toyo Eng. CompnayDocument20 paginiUrea Technology, Toyo Eng. CompnaySteve WanÎncă nu există evaluări
- Dimethyl Aniline PDFDocument68 paginiDimethyl Aniline PDFVirendra RathvaÎncă nu există evaluări
- Design Project On Nitric Acid ProductionDocument170 paginiDesign Project On Nitric Acid ProductionKamran Malik75% (8)
- Production of Maleic Anhydride PresentationDocument15 paginiProduction of Maleic Anhydride PresentationNqobile LowakwaMkhize100% (1)
- Co2 Absorption and V2o5Document9 paginiCo2 Absorption and V2o5sivsyadavÎncă nu există evaluări
- Ammonia ProductionDocument28 paginiAmmonia ProductionMuhammad Ali HashmiÎncă nu există evaluări
- Ammonium Sulphate ModifiedDocument13 paginiAmmonium Sulphate ModifiedAkuwh SyaSyaÎncă nu există evaluări
- Aces Process Material Balance: RE AC TO RDocument4 paginiAces Process Material Balance: RE AC TO Rwaheed ahmadÎncă nu există evaluări
- Process Intensification: Engineering for Efficiency, Sustainability and FlexibilityDe la EverandProcess Intensification: Engineering for Efficiency, Sustainability and FlexibilityÎncă nu există evaluări
- Reaction Kinetics for Chemical Engineers: Butterworths Series in Chemical EngineeringDe la EverandReaction Kinetics for Chemical Engineers: Butterworths Series in Chemical EngineeringEvaluare: 4 din 5 stele4/5 (3)
- Direct Methane to Methanol: Foundations and Prospects of the ProcessDe la EverandDirect Methane to Methanol: Foundations and Prospects of the ProcessÎncă nu există evaluări
- Coulson and Richardson’s Chemical Engineering: Volume 2A: Particulate Systems and Particle TechnologyDe la EverandCoulson and Richardson’s Chemical Engineering: Volume 2A: Particulate Systems and Particle TechnologyÎncă nu există evaluări
- Industrial Chemical Process Analysis and DesignDe la EverandIndustrial Chemical Process Analysis and DesignEvaluare: 4 din 5 stele4/5 (2)
- NCH Tech Sheet Deox ThailandDocument1 paginăNCH Tech Sheet Deox Thailandson.brbÎncă nu există evaluări
- MFC Hubli Final Bill Jan 2013Document12 paginiMFC Hubli Final Bill Jan 2013Nagaraj PatilÎncă nu există evaluări
- Bob Neal Overunity Compression Unit - US2030759Document5 paginiBob Neal Overunity Compression Unit - US2030759John CarterÎncă nu există evaluări
- Bromine New PDFDocument1 paginăBromine New PDFSuhasÎncă nu există evaluări
- 01 Introduction To Press ToolsDocument9 pagini01 Introduction To Press ToolsAngelo De DominicisÎncă nu există evaluări
- CLASS - X CHEMISTRY Important QuestionsDocument17 paginiCLASS - X CHEMISTRY Important Questionsvt654009Încă nu există evaluări
- 3 Methods of Determining Workability of ConcreteDocument4 pagini3 Methods of Determining Workability of Concretesuryakantame100% (1)
- Is 6158 - 1984 Recommended Practice For Safeguarding Against Embrittlement of Hot-Dip Galvanized Iron and Steel ProductsDocument10 paginiIs 6158 - 1984 Recommended Practice For Safeguarding Against Embrittlement of Hot-Dip Galvanized Iron and Steel ProductsNadeem KhanÎncă nu există evaluări
- Furanic Compounds PDFDocument6 paginiFuranic Compounds PDFAbdullah GhannamÎncă nu există evaluări
- Energy Demand in Wood Processing Plants: Jingge Li, Murray Mccurdy, Shusheng PangDocument9 paginiEnergy Demand in Wood Processing Plants: Jingge Li, Murray Mccurdy, Shusheng PangsabrahimaÎncă nu există evaluări
- Municipal Solid Waste Management in Moradabad City, IndiaDocument11 paginiMunicipal Solid Waste Management in Moradabad City, IndiaJames Konthou50% (2)
- All Formulas HvacDocument11 paginiAll Formulas HvacrockÎncă nu există evaluări
- Coagulation FlocculationDocument71 paginiCoagulation FlocculationDeepa Singh100% (1)
- Pip Ctse1000-2018Document82 paginiPip Ctse1000-2018John BuntalesÎncă nu există evaluări
- Paschen's LawDocument6 paginiPaschen's LawKhan YousafzaiÎncă nu există evaluări
- Tranportation All ExperimentDocument55 paginiTranportation All ExperimentMaiwand KhanÎncă nu există evaluări
- Draka 2Document260 paginiDraka 2Sakinah Cik KinoÎncă nu există evaluări
- CH10 CFLDocument11 paginiCH10 CFLNoman KhanÎncă nu există evaluări
- Materials and Design: Ping Duan, Chunjie Yan, Wei Zhou, Wenjun Luo, Chunhua ShenDocument13 paginiMaterials and Design: Ping Duan, Chunjie Yan, Wei Zhou, Wenjun Luo, Chunhua ShenJack LinÎncă nu există evaluări
- Week 2-Basic Cost ManagementDocument21 paginiWeek 2-Basic Cost ManagementRichard Oliver CortezÎncă nu există evaluări
- Materials Chemistry and Physics: Jure Zigon, Matja Z Pavli C, Marko Petri C, Sebastian DahleDocument12 paginiMaterials Chemistry and Physics: Jure Zigon, Matja Z Pavli C, Marko Petri C, Sebastian DahleAromoÎncă nu există evaluări
- Chapter 4 Forming and ShapingDocument147 paginiChapter 4 Forming and Shapingquan quanÎncă nu există evaluări
- Dow Coring Asia ManualDocument69 paginiDow Coring Asia ManualGULJAR SINGHÎncă nu există evaluări
- Materials Properties Handbook Titanium Alloys CompressDocument788 paginiMaterials Properties Handbook Titanium Alloys CompressJordan PowellÎncă nu există evaluări
- Automobile Waste and Its Management PDFDocument7 paginiAutomobile Waste and Its Management PDFBojan TanaskovskiÎncă nu există evaluări
- Herramientas de Diagnostico CaterpillarDocument24 paginiHerramientas de Diagnostico CaterpillarGuido Giovanni Franco Rodriguez100% (1)
- Type Mao Type Mbo Type Mci: W.AriyaratneDocument1 paginăType Mao Type Mbo Type Mci: W.AriyaratneLeon ZhouÎncă nu există evaluări
- Contractor Job Safety PlanDocument15 paginiContractor Job Safety PlanAnonymous ocCa18RÎncă nu există evaluări
- Concrete Terminology PDFDocument20 paginiConcrete Terminology PDFamirthraj74Încă nu există evaluări
- PB Filter Press Sidebar Me1500 Me2500 en Web DataDocument8 paginiPB Filter Press Sidebar Me1500 Me2500 en Web DataTiago J C MachadoÎncă nu există evaluări