Documente Academic
Documente Profesional
Documente Cultură
Dative Bonding 2008
Încărcat de
Elias VouzounisDescriere originală:
Drepturi de autor
Formate disponibile
Partajați acest document
Partajați sau inserați document
Vi se pare util acest document?
Este necorespunzător acest conținut?
Raportați acest documentDrepturi de autor:
Formate disponibile
Dative Bonding 2008
Încărcat de
Elias VouzounisDrepturi de autor:
Formate disponibile
2008 AS-Level Chemistry
Coordinate or Dative Covalent Bonding
NH4+ CO
Al2Cl6 H3O+
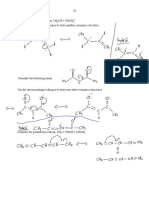
Co-ordinate (DativeCovalent) Bond = a covalent bond formed when both shared electrons are provided by only one atom. e- pair comes from a lone pair on the donor atom eg N of NH3, O of H2O the atom accepting and sharing the lone pair must have an incomplete outer energy level eg H+, Al in AlCl3, C in CO Dative (co-ordinate) bonds are physically and chemically identical to ordinary covalent bonds - only the method of formation differs. Represent by X: Y instead of X Y
1. Ammonium ion : NH4+
NH3(g) + HCl(g) NH4+Cl-(s)
H .. . . H . N . .. H
+ H+
H .. . . H . N . H .. H
2. Hydronium ion : H3O+
H2O(l) + HCl(g) H3O+(aq) + Cl-(aq)
.. . . H . O. .. H
+ H+
.. . . H . O. .. H
H4O2+ unlikely to form via the second lone pair because the second H+ is repelled by H3O+
3.
Aluminium chloride dimer, Al2Cl6
Cl .. . Cl . Al .. . Cl.
.. . Al . Cl .. Cl
. .Cl
4.
Carbon monoxide, CO
X X
C N
X X
O N
Isoelectronic with N2 :
X X X X X
Why does NH3 react readily with BF3 to form a stable compound ? N of NH3 has a lone pair on the N atom. The B atom of BF3 has an incomplete energy level and can form a dative bond with the N atom.
H .. . . H . N . .. H
F .. . B . F .. F
ALL atoms now have a noble gas configuration
The End
S-ar putea să vă placă și
- JB CI 13.1 HalogenoalkanesDocument6 paginiJB CI 13.1 HalogenoalkanesOCRChemistrySaltersÎncă nu există evaluări
- Perceptual Development (Cross Cultural Studies)Document14 paginiPerceptual Development (Cross Cultural Studies)Elias VouzounisÎncă nu există evaluări
- UKCAT StuffDocument2 paginiUKCAT StuffElias Vouzounis0% (1)
- Kimia Organik 1 Stuktur Atom: - Jurusan/Program Studi: Kimia/Pendidikan KimiaDocument50 paginiKimia Organik 1 Stuktur Atom: - Jurusan/Program Studi: Kimia/Pendidikan Kimiatimbul wibowoÎncă nu există evaluări
- CHAPTER 1 EditedDocument105 paginiCHAPTER 1 EditedSyafiqah SuhaimiÎncă nu există evaluări
- Putting Together Organic Molecules: Death Taxes andDocument31 paginiPutting Together Organic Molecules: Death Taxes andIvan BuljanÎncă nu există evaluări
- 10.covalent Bonding and Octet RuleDocument44 pagini10.covalent Bonding and Octet RuleX-sins Hsand Iceberg WolfÎncă nu există evaluări
- Xi Chem CH 4Document24 paginiXi Chem CH 4LUCKY BHARADWAJÎncă nu există evaluări
- Química Inorgánica II - Parte 4 - Tipos de Enlace y Reactividad - 21PDocument91 paginiQuímica Inorgánica II - Parte 4 - Tipos de Enlace y Reactividad - 21Pandrea davila alvarezÎncă nu există evaluări
- Neutrophiles and ElectrophilesDocument2 paginiNeutrophiles and Electrophileswhydaspam joeÎncă nu există evaluări
- H.XHH H: Structure and Bonding 39Document3 paginiH.XHH H: Structure and Bonding 39Gregorio ValllejoÎncă nu există evaluări
- Periodic Properties-02 Solved ProblemsDocument10 paginiPeriodic Properties-02 Solved ProblemsRaju SinghÎncă nu există evaluări
- 6 Chemical Bonding and Structures of Environmental PollutantsDocument20 pagini6 Chemical Bonding and Structures of Environmental PollutantsMaaz WaseemÎncă nu există evaluări
- Chapter 3 Chemical Bonding and StructureDocument11 paginiChapter 3 Chemical Bonding and Structurenesrine boufadenÎncă nu există evaluări
- Compounds: Carbon andDocument17 paginiCompounds: Carbon andankitrathoreagentÎncă nu există evaluări
- Chemical Bonding - Lewis TheoryDocument7 paginiChemical Bonding - Lewis TheoryJubairÎncă nu există evaluări
- H H - H - C C - H H - BR: Lectrophilic Ddition Carbocation IntermediateDocument12 paginiH H - H - C C - H H - BR: Lectrophilic Ddition Carbocation IntermediateWalid Ebid ElgammalÎncă nu există evaluări
- 1 AllDocument18 pagini1 AllMarcos ViníciusÎncă nu există evaluări
- CH 14 Solutions ManualDocument21 paginiCH 14 Solutions Manuallmbrn0415Încă nu există evaluări
- Organic Chem. NotesDocument117 paginiOrganic Chem. NoteselcarlsansÎncă nu există evaluări
- CH1O3 Questions PDFDocument52 paginiCH1O3 Questions PDFPrince T MashandaÎncă nu există evaluări
- PhenolDocument48 paginiPhenolyazara90Încă nu există evaluări
- Bonding in Carbonyl CompoundsDocument11 paginiBonding in Carbonyl CompoundsRohini SelvarajahÎncă nu există evaluări
- Lecture 5 Chemical Bonding and StructureDocument33 paginiLecture 5 Chemical Bonding and StructurekedirÎncă nu există evaluări
- Solution & Answer For Kcet-2009 Version - A-3: ChemistryDocument5 paginiSolution & Answer For Kcet-2009 Version - A-3: ChemistryRahul DubeyÎncă nu există evaluări
- Assignment 2 AnswerDocument5 paginiAssignment 2 AnswerVaibhav BacchavÎncă nu există evaluări
- PDF CH 2 C Chemical BondingDocument21 paginiPDF CH 2 C Chemical Bondingarnavpatel138Încă nu există evaluări
- Introduction To Organic ChemistryDocument92 paginiIntroduction To Organic ChemistryNUR HAZWANI BINTI MOHAMAD SANI / UPMÎncă nu există evaluări
- Ionic Equlibrium FinalDocument66 paginiIonic Equlibrium Finalshreyas bulbuleÎncă nu există evaluări
- Assignment 7 and Practice Third Exam Solutions: C N S C N SDocument6 paginiAssignment 7 and Practice Third Exam Solutions: C N S C N SJoshua OndiegiÎncă nu există evaluări
- Pentadienyl CationDocument7 paginiPentadienyl CationAbhishek SardaÎncă nu există evaluări
- MCQ & A - R of Class - 12 (P - Block)Document6 paginiMCQ & A - R of Class - 12 (P - Block)assentialÎncă nu există evaluări
- Matriculation Chemistry (Introduction To Organic Compound) Part 4Document40 paginiMatriculation Chemistry (Introduction To Organic Compound) Part 4ridwanÎncă nu există evaluări
- Haloalkanes and HaloarenesDocument8 paginiHaloalkanes and HaloarenesYash RajÎncă nu există evaluări
- Basic Concepts of Chemical BondingDocument34 paginiBasic Concepts of Chemical BondingAwais altafÎncă nu există evaluări
- Class XII Chemistry 23-24 Pre-Board SolDocument14 paginiClass XII Chemistry 23-24 Pre-Board Solap1124214Încă nu există evaluări
- CH 18 SummaryDocument5 paginiCH 18 Summaryalstjq1003Încă nu există evaluări
- Coordination CompoundDocument34 paginiCoordination CompoundsukoyoÎncă nu există evaluări
- 133 AcylationDocument4 pagini133 AcylationAnonymous vRpzQ2BLÎncă nu există evaluări
- Problems For Chapter 1 & 2 ANSWERS: 2xH 2 2xN 10 O 6Document6 paginiProblems For Chapter 1 & 2 ANSWERS: 2xH 2 2xN 10 O 6JibrilAttawarahÎncă nu există evaluări
- Bonding, Molecular Shape & Structure: Dr. Fawaz AldabbaghDocument54 paginiBonding, Molecular Shape & Structure: Dr. Fawaz AldabbaghHoney Jyl DucusinÎncă nu există evaluări
- Problem Set 1 PDFDocument2 paginiProblem Set 1 PDFLouisÎncă nu există evaluări
- Electron Delocalization (Resonance) : CH CH CL .. ..Document34 paginiElectron Delocalization (Resonance) : CH CH CL .. ..Ephraim Remann D. GarciaÎncă nu există evaluări
- 12th CBSE Chemistry SP SolutionsDocument10 pagini12th CBSE Chemistry SP SolutionsRohith MungathÎncă nu există evaluări
- Tutorial Letter 201/1/2017: General Chemistry 1BDocument23 paginiTutorial Letter 201/1/2017: General Chemistry 1BLeigh MakanÎncă nu există evaluări
- Focus 3 Chemical BondingDocument10 paginiFocus 3 Chemical BondingHengLow100% (1)
- Chemical Bonding PQPDocument6 paginiChemical Bonding PQPHarsh KumarÎncă nu există evaluări
- Allen Allen330Document192 paginiAllen Allen330Fatimah Ali AfrozÎncă nu există evaluări
- Practicetopic 4 Paper 1Document10 paginiPracticetopic 4 Paper 1api-312595005Încă nu există evaluări
- Chem Topic 4 QuestionsDocument19 paginiChem Topic 4 QuestionsOscarHigson-SpenceÎncă nu există evaluări
- Chemical Bonding and Chemical StructureDocument93 paginiChemical Bonding and Chemical StructureGita AmaliaÎncă nu există evaluări
- Hcno C H H H C H H H C H H H: Remains +1 ThroughoutDocument4 paginiHcno C H H H C H H H C H H H: Remains +1 ThroughoutpÎncă nu există evaluări
- Presentation 1Document60 paginiPresentation 1Mezgebu BiresawÎncă nu există evaluări
- Chemical BondingDocument5 paginiChemical BondingYanti FarhanaÎncă nu există evaluări
- Covalent Bonding: Gilbert N. Lewis 1875-1946Document36 paginiCovalent Bonding: Gilbert N. Lewis 1875-1946ade dosmariaÎncă nu există evaluări
- Thing To Remember - Haloalkane - StudentsDocument10 paginiThing To Remember - Haloalkane - StudentspoornaÎncă nu există evaluări
- Inter Conversions of Functional GroupsDocument9 paginiInter Conversions of Functional Groupssaqib sulmanÎncă nu există evaluări
- Consider The Following Anion CH CH CHCHDocument13 paginiConsider The Following Anion CH CH CHCHbobÎncă nu există evaluări
- 2010 Chem Bond Tut Ans AllDocument37 pagini2010 Chem Bond Tut Ans AllDarren LimÎncă nu există evaluări
- Ln-2 Idle Mind SolutionsDocument22 paginiLn-2 Idle Mind Solutionssanjay975Încă nu există evaluări
- End of IMF WorksheetDocument2 paginiEnd of IMF WorksheetAnshu MovvaÎncă nu există evaluări
- Practice Makes Perfect in Chemistry: Oxidation-ReductionDe la EverandPractice Makes Perfect in Chemistry: Oxidation-ReductionEvaluare: 5 din 5 stele5/5 (1)
- PBL Write-Up 3Document7 paginiPBL Write-Up 3Elias VouzounisÎncă nu există evaluări
- Psychology AggressionDocument15 paginiPsychology AggressionElias VouzounisÎncă nu există evaluări