Documente Academic
Documente Profesional
Documente Cultură
Hospital Disclosure Policy
Încărcat de
vks7876Descriere originală:
Drepturi de autor
Formate disponibile
Partajați acest document
Partajați sau inserați document
Vi se pare util acest document?
Este necorespunzător acest conținut?
Raportați acest documentDrepturi de autor:
Formate disponibile
Hospital Disclosure Policy
Încărcat de
vks7876Drepturi de autor:
Formate disponibile
Hospital Disclosure Policy
Introduction Reducing medical errors has become an international concern. Population-based studies from a number of nations around the world have consistently demonstrated unacceptably high rates of medical injury and preventable deaths. In seeking to improve safety, one of the most frustrating aspects for patients and professionals alike is the apparent failure of health-care systems to learn from their mistakes. Too often neither health-care providers nor health-care organizations advise others when a mishap occurs, nor do they share what they have learned when an investigation has been carried out. As a consequence, the same mistakes occur repeatedly in many settings and patients continue to be harmed by preventable errors. One solution to this problem is reporting and disclosure: by the doctor, nurse, or other provider within the hospital or health-care organization. Some believe that an effective incident disclosure and reporting system is the cornerstone of safe practice and,within a hospital or other health-care organization, a measure of progress towards achieving a safety culture. Expectations of disclosure are challenging and have given rise to a new mindset which states that Disclosure is an ethical obligation, not just a regulatory requirement. The precept of autonomy would require that caregivers not only seek permission to provide specific treatment, but that they give patients (and their families) the array of information needed to make decisions about care. Outcomes of care, including death, require decisions on the part of patients and/or their families. Disclosure provides patients as well as caregivers the opportunity to recover from the devastating effect of an unanticipated outcome. Disclosure, properly managed and controlled, can potentially lessen the frequency and severity of litigation. At a minimum, disclosure and reporting can help identify hazards and risks, and provide information as to where the system is breaking down. This can help target improvement efforts and systems changes to reduce the likelihood of injury to future patients. Definitions Safety : Freedom from accidental injuries . Error : The failure of a planned action to be completed as intended (i.e. error of execution) or the use of a wrong plan to achieve an aim (i.e. error of planning).Errors may be errors of commission or omission, and usually reflect deficiencies in the systems of care.
Adverse event: An injury related to medical management, in contrast to complications of disease. Medical management includes all aspects of care, including diagnosis and treatment, failure to diagnose or treat, and the systems and equipment used to deliver care. Preventable adverse event: An adverse event caused by an error or other type of systems or equipment failure. Near-miss or close call: Serious error or mishap that has the potential to cause an adverse event but fails to do so because of chance or because it is intercepted. Also called potential adverse event. Adverse drug event: A medication-related adverse event. Hazard: Any threat to safety, e.g. unsafe practices, conduct, equipment, labels, names. System: A set of interdependent elements (people, processes, equipment) that interact to achieve a common aim. Latent error (or latent failure): A defect in the design, organization, training or maintenance in a system that leads to operator errors and whose effects are typically delayed. Objective To assure that patients and, where appropriate, their families are fully informed about the outcomes of diagnostic tests, medical treatment, and surgical intervention, whether the outcomes are expected or unanticipated. It will be our endeavour to always have open, honest and constant communication with our patients Purpose To address the issue of unanticipated outcomes To describe how and when unanticipated outcomes are disclosed to patients, and when appropriate, their families. To educate physicians and staff on the disclosure of unanticipated outcomes
Primary values Providing Safe, Compassionate, Competent and Ethical care Promoting Health and well being Promoting and respecting informed decision making Preserving Dignity Maintaining Privacy and Confidentiality Promoting Justice Being Accountable
APPLICATION OF POLICY: This policy applies to all Medical Officers, staff (regulated and non-regulated health care providers), Nursing officers, Paramedical Staff and Class III Employees Responsibilities :
(a) Team
Health and Safety Committee ( will review, discuss and analyse the statistical data on the adverse event reports to identify trends or areas of concerns). Quality and Risk Committee ( will provide quarterly reports on adverse events and will receive a prcised narrative report quarterly to provide assurance that adverse events are being reported, managed and lessons learned)
(b) Individuals
HODs / Sr Advisors :
promote a culture where it is acceptable and safe for their staff to report all adverse events, including near misses promote a culture where adverse events can be openly discussed and have a system in place to ensure that lessons are learned and communicated following an event. ensure that there is full compliance with the Adverse Event Policy and reporting procedure and that all areas within their responsibility are reporting adverse events and near misses. review and analyse in one to one basis with senior management, the quarterly adverse event report received from the Health and
Safety Advisor to identify trends and review that appropriate action has been taken and lessons learned disseminated to staff ensure a member of the Quality and Risk Team has been notified of any adverse event with an actual severity of major or catastrophic event. Sr Clinicians / MOi/c
promote a culture where it is acceptable and safe for their staff to report all adverse events, including near misses and where adverse events can be openly discussed have a system in place to ensure that lessons are learned and communicated following an adverse event. investigate and take action, when requested or required to do so, on all adverse events referred to them regularly review all adverse events that occur within their areas of responsibility with the immediate manager of the department/service, including action taken to prevent recurrence to ensure that all reasonable steps have been taken to prevent a recurrence. notify the HOD / Sr Advisor and the Quality and Risk Team immediately of any adverse event with an actual severity of major or catastrophic within their area(s) of responsibility. discuss and analyse with their line mangers the quarterly adverse event report received from the Health and Safety Advisor to identify trends and review that appropriate action has been taken and lessons learned are disseminated to staff
NO i/c promote a culture where it is acceptable and safe for their staff to report all adverse events, including near misses and where adverse events can be openly discussed notify to MOi/c immediately of all serious adverse events i.e. with an actual severity of major or catastrophic or if the seriousness of previously reported adverse events change to move into this category. ensure that all adverse events within their area of responsibility are reported using the Adverse Event report form. forward the Adverse Event Report Form to the Quality and Risk Team within
48 hours of the event. ensure that Adverse Event Reporting is included in the local induction for all staff
Permanent staff. duty to follow this Policy and procedure by reporting all adverse events and near misses promptly completing an adverse event report form within stipulated time frame
Reporting of Incidents : All hospital employees will participate in the hospital-wide incident reporting program. All incidents such as those listed as follows will be reported 1. Incidents involving inconsistencies with written hospital policies and procedures informed consent, bedrails, patient restraints, nursing physician professional relationships, confidential patient information released to the public, etc. 2. Non-anticipated and non-routine employee, contract service personnel, patient, visitor, and volunteer injuries resulting from accidents or errors such as: falls for any reason, with or without injuries, medication errors, needle punctures and/or occupational exposures, diagnostic/therapeutic procedures performed on wrong patients, burns from heating pads, x-rays, broken teeth, pressure sores, etc. 3. Mishaps due to faulty/defective equipment or adverse conditions in our physical facility. 4. Sudden, unexpected adverse results of professional care and treatment , death, brain damage, physical, mental, or emotional loss or impairment, cardiac/respiratory arrests, or any occurrence or situation which necessitates additional hospitalization or a dramatic change in patient treatment regimens. 5. All vocal or written expressions of dissatisfaction from the patient or patient families concerning the professional and non-professional services. 6. All incidents involving patient, employee, visitor, or volunteer property claimed to be lost, stolen, or damaged. Severity of event For the purposes of disclosure of unanticipated outcomes, the following grading system can be utilized: Level 1: An event occurred but the patient was not harmed Level 2: An event occurred that resulted in the need for increased patient assessment/monitoring, but no change in vital signs and no patient harm
Level 3: An event occurred that resulted in the need for treatment and/or intervention and caused temporary patient harm Level 4: An event occurred that resulted in initial or prolonged hospitalization, and caused temporary patient harm Level 5: An event occurred that resulted in permanent patient harm or near death event, such as anaphylaxis Level 6: An event occurred that resulted in patient death
Disclosure of Incidents 1. What events should be disclosed? Disclosure of unanticipated outcomes should be performed in occurrences that are scored a Level of Severity (see above) from 3 to 6. For example, this would include an unexpected admission to intensive care, unexpected patient death, returns to the OR, wrong site surgery, or a medication error that results in death or patient harm. Errors that do not harm patients and do not have the potential to do so (insignificant or minor incidents) may not require immediate disclosure to the patient, however the same has to be disclosed to the higher authorities of the Hospital and their views to be obtained for disclosure of the event to the patient. 2. To whom should disclosure be made? Disclosure of unanticipated outcomes should be made to the patient, and when appropriate, the patients family or personal representative (i.e. parent, guardian, durable power of attorney). 3. When should disclosure take place? Disclosure of unanticipated outcomes should take place as soon as practical after the event has been identified. Disclosure to the patient should occur when the patient is stable and/or able to comprehend the information. Disclosure to the patients family or personal representative may occur sooner depending on the incidents severity and their need to know this information. 4. Who should disclose events to patients? Prior to disclosure, the concerned physician and will consult with higher medical /and administrative authorities regarding what should be disclosed and how disclosure is made. In some circumstances further investigation may be required to determine which individual(s) should be involved.
The attending physician and the higher authorities will consider involving representatives from nursing, allied health professionals, social workers or staff members known to and trusted by the patient/family. If the attending physician is unwilling or unable to disclose the event, or if investigation determines that his involvement could exacerbate the problem, the administration will identify the appropriate person to handle this responsibility.
5. How should disclosure occur? The nature, severity and cause (if known) of the unexpected outcome should be presented in a straight-forward and non-judgmental fashion. An expression of sorrow is often appropriate and not an admission of guilt. Speculation should be avoided and focus placed on what is known at the time of the discussion. Answering of questions and providing assurance that unanswered questions will be investigated further will be part of disclosure. Describe what, if anything, can be done to correct the consequences of the unanticipated outcome. 6. How is disclosure documented? Relevant information on the unanticipated outcome should be well documented. This documentation should include:
Documentation of the time, date, and place that disclosure took place. The name and relationships of those present. Documentation in appropriate cases that as further information becomes
available, this information will be shared with the patient, and when appropriate, with their representative. Documentation of an offer to be of assistance and the response to it. Documentation of any questions posed by the patient, family, or legally authorized representative, and the answers provided by the caregiver. In specific cases in which a decision is made to withhold some or all information, appropriate documentation is made of the reason(s) for this decision. It is acknowledged that in some cases the documentation may be separate from the medical record to protect the safety or welfare of the patient or to prevent interference in law enforcement investigations.
Support : Patients Support of the patient should be psychological, social, and in some cases, financial. following a medical injury, fear, anxiety, depression, anger, frustration, loss of trust, and feelings of isolation are common reactions. Inadequate or insensitive management of incidents may cause further emotional trauma, while open acknowledgement of error
and harm, sensitivity, good communication, and skillful management of corrective actions may reduce emotional trauma. All Patients / family concerns to be taken seriously and addressed completely Therapeutic relationship with the patient and family should not be compromised Patients and families will be provided with contact information for clinical as well as psychological counseling and support. Patients will be provided with adequate contact information to facilitate communication after discharge Staff Like patients and families, caregivers are significantly impacted, emotionally and functionally, following an adverse event. They should be provided with institutional support that enables them to recover, to communicate and apologize effectively to the patient, and to return rapidly to their professional duties. Frequently they are unrecognized second victims in these events, and receive little understanding or support. program should be designed to provide aid to normal people who are experiencing normal stress after experiencing highly abnormal events. The objective should be to help professionals manage the stress of the adverse event so that they can better care for their patients private and group counseling and short- and long-term counseling can be arranged for affected staff. appropriate adjustment of responsibilities and time off if needed should be provided to the affected staff member Structured assistance should be provided for reporting of adverse events by staff. Training and retraining, for communication, quality tools and means to provide support to colleagues. Training Training and education of Hospital staff will entail the following few aspects: All staff should be trained on the existence and purpose of the policy. Development of programs in communication with patients and families for all levels with emphasis on training on the techniques of disclosing unanticipated events. Training the doctors and nurses in dealing with their own feelings. Educating administrative and Senior Clinical staff in their responsibilities as handlers of sensitive information and expert analysis. Provision of Initial orientation training and thereafter regular update by means of workshops/ Conferences/ CMEs etc. A session in all CMEs should be devoted to aspects of Patient Safety and promotion of culture of fair disclosure without any blame and shame. Provision of short intensive just-in-time capsule training for busy clinicians. Whistle Blower / Open Disclosure
Raising a Concern(Whistle blowing) Policy, which provides a system where staff have the opportunity to raise concerns without fear of suffering any adverse consequences as a result of disclosure. Formal disciplinary action should not routinely follow an adverse event even if a mistake has been made. On rare occasions it may be necessary to attach blame if the outcome of investigation identifies that there has been an act of malice, criminal or gross (or repeated) professional misconduct. Formal disciplinary action will only result where: 1. An individual persists in unsafe practice 2. There is deliberate failure to report, or an attempt to cover up, an adverse event 3. A breach of criminal law of professional conduct has occurred 4. This Policy has not been complied with and an adverse event has occurred and intentionally not been reported. Feedback and Monitoring Feedback to staff : Senior administrators should ensure that a system is in place to provide feedback at a local level to staff that have reported adverse events Monitoring effectiveness : The effectiveness of the reporting policy will me monitored by the Health and Safety Advisor along with the the monitoring teams as mentioned above on a quarterly basis when statistical reports are circulated. Performance Indicators The following performance indicators will be used to measure the success of the policy and culture of reporting adverse events : Number of adverse events reported Evidence of decrease in severity of adverse events year on year Evidence of adverse event reporting from all areas of Hospital Non Punitive Organisational Culture In the end the policy emphasizes the need to develop an organizational culture where there is no Blame Shame game. The Disclosure Policy encourages an environment of reporting and disclosure of adverse events. Disclosure of an adverse event shall be treated in a non-punitive manner wherever possible. The disclosure policy and procedure supports physicians, staff and students honesty, professionalism and efforts to continually improve health care safety and quality. The Reporting of Adverse Events will have the following characteristics :-
Non-punitive. The most important characteristic for success of a patient safety reporting system is that it must be non-punitive. Neither reporters nor others involved in the incidents will be punished as a result of reporting. For public systems, this requirement is the most difficult to achieve, since the public often assumes an individual is to blame, and there can be strong pressure to punish the culprit. While perhaps temporarily emotionally satisfying, this approach is doomed to fail. People will not report any errors they can hide. It is important for us to protect reporters from blame. The best way to do this is by keeping the reports confidential. Confidential. The identities of the patient and reporter must never be revealed to any third party. At the institutional level, confidentiality also refers to not making public specific information that can be used in litigation. Concern about disclosure is a major factor inhibiting reporting for many voluntary reporting programmes and same needs to be looked into. Independent. The reporting system will be independent of any authority with the power to punish the reporter or organization with a stake in the outcome. Maintaining a firewall between the reporting agency and the disciplinary agency is difficult, but it is essential if trust in reporting is to be maintained. Expert analysis. Reports will be evaluated by experts who understand the clinical circumstances under which the incidents occur and who are trained to recognize underlying systems causes. It seems obvious that collecting data and not analyzing it is of little value. Expertise is a major, and essential, resource requirement for any reporting system. Training of Senior management in certain tools like RCA, Peer review, Quality Assurance and Quality Improvement will be undertaken to ensure that quality analysis is done in most of the cases. Credible. The combination of independence and the use of content experts for analysis is necessary if recommendations are to be accepted and acted upon. Timely. Reports will be analysed without delay, and recommendations must be promptly disseminated to those who need to know. When serious hazards are identified, notification should take place rapidly Systems-oriented. Recommendations should focus on changes in systems, processes or products, rather than being targeted at individual performance. This is a cardinal principle of safety that must be reinforced by the nature of recommendations that come from any reporting system. It is based on the concept that even an apparently egregious individual error results from systems defects, and will recur with another person at another time if those systems defects are not remedied. Responsive. For recommendations to result in widespread systems changes, the organization receiving reports must be capable of making and disseminating effective recommendations, and target organizations must make a commitment to implement recommendations. Hence Organizational commitment is a must for successful implementation of Hospital Disclosure Policy.
S-ar putea să vă placă și
- Dr. D's Pain Treatment Manual: Practical Pain Management Solutions (REVISED NEW EDITION 2024)De la EverandDr. D's Pain Treatment Manual: Practical Pain Management Solutions (REVISED NEW EDITION 2024)Evaluare: 5 din 5 stele5/5 (1)
- Perioperative Preparation and Management: Pre-OperativeDocument14 paginiPerioperative Preparation and Management: Pre-OperativeRed StohlÎncă nu există evaluări
- An Examiner’s Guide to Professional Plastic Surgery ExamsDe la EverandAn Examiner’s Guide to Professional Plastic Surgery ExamsÎncă nu există evaluări
- The Gross Room/surgical Cut-Up Including Sample HandlingDocument8 paginiThe Gross Room/surgical Cut-Up Including Sample Handlingo.zuletaaÎncă nu există evaluări
- Day Surgery Update 2019Document15 paginiDay Surgery Update 2019O Mei NeilÎncă nu există evaluări
- IHOP - 9.13.25 - Time Out Universal Protocol For Invasive and Surgical ProceduresDocument3 paginiIHOP - 9.13.25 - Time Out Universal Protocol For Invasive and Surgical Proceduresmaxxer1985Încă nu există evaluări
- Day Case For WebDocument34 paginiDay Case For WebDavid CruzÎncă nu există evaluări
- 2008, Vol.92, Issues 5, Women's HealthDocument312 pagini2008, Vol.92, Issues 5, Women's HealthHussain OudahÎncă nu există evaluări
- Evaluation of The Endometrium For Malignant or Premalignant DiseaseDocument10 paginiEvaluation of The Endometrium For Malignant or Premalignant Diseasenautilus81Încă nu există evaluări
- Dilation and CurettageDocument9 paginiDilation and CurettagedenekeÎncă nu există evaluări
- Day Care SurgeryDocument5 paginiDay Care SurgeryazharmoÎncă nu există evaluări
- Australian Regulatory Response To Genome Edited Plants, Karinne LudlowDocument30 paginiAustralian Regulatory Response To Genome Edited Plants, Karinne LudlowJournal of Law, Information & ScienceÎncă nu există evaluări
- Endometrial Study by TVS and It's Correlation With Histopathology in Abnormal Uterine BleedingDocument12 paginiEndometrial Study by TVS and It's Correlation With Histopathology in Abnormal Uterine BleedingIOSRjournalÎncă nu există evaluări
- Gynecology and ObstetricsDocument190 paginiGynecology and Obstetricsbhesh_seanÎncă nu există evaluări
- Operational Guidelines For Clients' Rights and Providers' Rights-DutiesDocument34 paginiOperational Guidelines For Clients' Rights and Providers' Rights-DutiesVichhai100% (1)
- Eras GynDocument6 paginiEras GynFirah Triple'sÎncă nu există evaluări
- Postmenopausal Bleeding Assessment G39v2Document7 paginiPostmenopausal Bleeding Assessment G39v2clement_sethÎncă nu există evaluări
- Manejo Da MenopausaDocument16 paginiManejo Da MenopausaGe NomÎncă nu există evaluări
- Art 20177509Document4 paginiArt 20177509Jashashree SaikiaÎncă nu există evaluări
- PBM Module1 MTP Template 0Document2 paginiPBM Module1 MTP Template 0Daniela Marie RonquilloÎncă nu există evaluări
- Surgical Site MarkingDocument12 paginiSurgical Site Markingh1m4w4nÎncă nu există evaluări
- Monitored Anesthesia Care in Adults: Considerations During Covid-19 PandemicDocument29 paginiMonitored Anesthesia Care in Adults: Considerations During Covid-19 PandemicGanda Impola TBAÎncă nu există evaluări
- Obtaining Valid Consent: Clinical Governance Advice No. 6Document9 paginiObtaining Valid Consent: Clinical Governance Advice No. 6Ywagar YwagarÎncă nu există evaluări
- Blair Smith Documentation 2012Document11 paginiBlair Smith Documentation 2012Eni SartikaÎncă nu există evaluări
- WA Health Clinical Risk Management GuidelinesDocument46 paginiWA Health Clinical Risk Management GuidelinesRuli Nurul Aman100% (1)
- PPG GDCH Nur 44 Effective Communication Read Back OrderDocument5 paginiPPG GDCH Nur 44 Effective Communication Read Back OrderKenny JosefÎncă nu există evaluări
- Pre Op 2010Document37 paginiPre Op 2010Tawona DhlakamaÎncă nu există evaluări
- Vaishali Syal Moderator - Prof. J. R. ThakurDocument34 paginiVaishali Syal Moderator - Prof. J. R. ThakurTasha NurfitrianiÎncă nu există evaluări
- Aldrete Discharge Scoring - Appropriate For Post Anesthesia PhaseDocument28 paginiAldrete Discharge Scoring - Appropriate For Post Anesthesia PhaseHeather PorterÎncă nu există evaluări
- Principles of Balanced AnesthesiaDocument2 paginiPrinciples of Balanced AnesthesiaZahra ZakiyyahÎncă nu există evaluări
- 11 Uterine CancerDocument43 pagini11 Uterine Cancerclaire yowsÎncă nu există evaluări
- Grossing Uterus Presentation1Document19 paginiGrossing Uterus Presentation1jayesh saha100% (1)
- Endometrial Malignancies 1Document41 paginiEndometrial Malignancies 1jerrydanfordfxÎncă nu există evaluări
- Airway Management Inside and Outside Operating Rooms 2018 British Journal ofDocument3 paginiAirway Management Inside and Outside Operating Rooms 2018 British Journal ofSeveNÎncă nu există evaluări
- 02.surgical EthicsDocument28 pagini02.surgical EthicsHassan Altyib100% (1)
- Postoperative Nausea and VomitingDocument33 paginiPostoperative Nausea and Vomitingferlina100% (1)
- Handout and Questions of HysterosalpingDocument12 paginiHandout and Questions of Hysterosalpingahmad shaltout100% (2)
- Who Guidelines For Safe SurgeryDocument173 paginiWho Guidelines For Safe SurgeryAhmed M Frieg100% (1)
- Diagnostic Work Up of Ovarian CystsDocument12 paginiDiagnostic Work Up of Ovarian CystsAnshul KumarÎncă nu există evaluări
- Preoxygenation During Induction of Anesthesia in Noncritically Ill PatientsDocument6 paginiPreoxygenation During Induction of Anesthesia in Noncritically Ill PatientsasfwegereÎncă nu există evaluări
- Anesthesia For The Patient With Peripartum Hemorrhage - UpToDateDocument25 paginiAnesthesia For The Patient With Peripartum Hemorrhage - UpToDateZurya UdayanaÎncă nu există evaluări
- Sop Number Title of SOP Author Consultation With StakeholdersDocument23 paginiSop Number Title of SOP Author Consultation With StakeholdersMr. BamsÎncă nu există evaluări
- Chest Drains GuidanceDocument14 paginiChest Drains Guidancenob2011nobÎncă nu există evaluări
- Day Surgery in AustraliaDocument0 paginiDay Surgery in Australiamonir61Încă nu există evaluări
- Millers Anesthesia - Sixth Edition - Chapter 66 - Anesthesia For Robotic Surgery - 233Document44 paginiMillers Anesthesia - Sixth Edition - Chapter 66 - Anesthesia For Robotic Surgery - 233Adriana VickÎncă nu există evaluări
- Standards of PracticeDocument9 paginiStandards of PracticeAlfitoHarfahGiffaryÎncă nu există evaluări
- DR - Mohammed Abdalla Egypt. Domiat G. Hospital: Controversies in GynecologyDocument66 paginiDR - Mohammed Abdalla Egypt. Domiat G. Hospital: Controversies in Gynecologymadmax500Încă nu există evaluări
- Massive Blood Transfusion ProtocolDocument1 paginăMassive Blood Transfusion ProtocolTheNurseelleÎncă nu există evaluări
- Ambulatory Hysteroscopy Evidence-Based Guide To Diagnosis and TherapyDocument23 paginiAmbulatory Hysteroscopy Evidence-Based Guide To Diagnosis and TherapyAngela EstevesÎncă nu există evaluări
- HysterosDocument17 paginiHysterosAnto PopaÎncă nu există evaluări
- Operating Room Care of The Surgical Patient: Teaching PlanDocument9 paginiOperating Room Care of The Surgical Patient: Teaching PlanscribdnijoizÎncă nu există evaluări
- 3rd Edition NABH Guidebook 2012 New PDFDocument258 pagini3rd Edition NABH Guidebook 2012 New PDFraamki_99Încă nu există evaluări
- Eras Bps Final Nov2017 2Document24 paginiEras Bps Final Nov2017 2Wardah Fauziah El SofwanÎncă nu există evaluări
- Never Events Fact Sheet PDFDocument2 paginiNever Events Fact Sheet PDFThanh Doan Thi100% (2)
- Safe Sedation Practice For Healthcare Procedures: Standards and GuidanceDocument39 paginiSafe Sedation Practice For Healthcare Procedures: Standards and GuidanceAlaa ShnienÎncă nu există evaluări
- Doctrines Applied in Medical Malpractice CasesDocument20 paginiDoctrines Applied in Medical Malpractice CasesMJ CarreonÎncă nu există evaluări
- Hospital Ethics HandbookDocument47 paginiHospital Ethics HandbookZiaul Haque100% (1)
- MS Obst & GynaeDocument77 paginiMS Obst & GynaeAmna MunawarÎncă nu există evaluări
- (Current Clinica (Current - Clinical - Strategies) - Gynecology - and - Obstetrics - 2004l Strategies) - Gynecology and Obstetrics 2004Document125 pagini(Current Clinica (Current - Clinical - Strategies) - Gynecology - and - Obstetrics - 2004l Strategies) - Gynecology and Obstetrics 2004Eliza Stochita100% (1)
- BMS OverviewDocument1 paginăBMS OverviewEngr Dil KhurshidÎncă nu există evaluări
- Application For Registration of Marriage Under Section 8 of The Hindu Marriages ActDocument2 paginiApplication For Registration of Marriage Under Section 8 of The Hindu Marriages Actvks7876Încă nu există evaluări
- Health Care in IndiaDocument2 paginiHealth Care in Indiavks7876Încă nu există evaluări
- RCH & Uip: Maj Vinay Kumar SharmaDocument67 paginiRCH & Uip: Maj Vinay Kumar Sharmavks7876Încă nu există evaluări
- African Journal of Microbiology Research VolDocument2 paginiAfrican Journal of Microbiology Research Volvks7876Încă nu există evaluări
- Adult Immunization ScheduleDocument5 paginiAdult Immunization Schedulejack01236Încă nu există evaluări
- Clerkship Survival Guide 7.2015 PDFDocument65 paginiClerkship Survival Guide 7.2015 PDFAffan HaqÎncă nu există evaluări
- MaciasDocument2 paginiMaciasapi-365473208Încă nu există evaluări
- Daniela Daviddiculescu enDocument4 paginiDaniela Daviddiculescu enDany David-DiculescuÎncă nu există evaluări
- 4 High Alert Medication FinalDocument4 pagini4 High Alert Medication Finalrini setyawatiÎncă nu există evaluări
- Blue Royale 66 100 BrochureDocument2 paginiBlue Royale 66 100 BrochureAna Patricia SanchezÎncă nu există evaluări
- News in PhysiotherapyDocument2 paginiNews in PhysiotherapyDobreanu BiancaÎncă nu există evaluări
- Health 1st Quarter Reviewer Incomplete 1Document5 paginiHealth 1st Quarter Reviewer Incomplete 1Hana ZaneÎncă nu există evaluări
- Diversity MeaningDocument2 paginiDiversity MeaningChareeseÎncă nu există evaluări
- Integrated Care Applying Theory To PracticeDocument429 paginiIntegrated Care Applying Theory To PracticeMyriam Hernández Núñez100% (1)
- EFLM COLABIOCLI Recommendation For Venous Blood SamplingDocument24 paginiEFLM COLABIOCLI Recommendation For Venous Blood SamplingErick2007Încă nu există evaluări
- Amang Rodriguez For ADB PDFDocument67 paginiAmang Rodriguez For ADB PDFKristine Presbitero100% (1)
- Legal and Ethical Implication in NursingDocument17 paginiLegal and Ethical Implication in NursingSanjay Kumar Sanju0% (1)
- Mas Yuhanies BT Mat Yaziz 2012987921 CS2446ADocument7 paginiMas Yuhanies BT Mat Yaziz 2012987921 CS2446AEngkuZainÎncă nu există evaluări
- New Digital Transformation in HealthcareDocument95 paginiNew Digital Transformation in HealthcarejoelÎncă nu există evaluări
- TMP - 12206 AnnualReport14 15 709362705Document213 paginiTMP - 12206 AnnualReport14 15 709362705shakilsaiÎncă nu există evaluări
- Bioethics Semi-FinalsDocument151 paginiBioethics Semi-FinalsTrishaÎncă nu există evaluări
- Legal Medicine Review PDFDocument10 paginiLegal Medicine Review PDFGhie TangonanÎncă nu există evaluări
- Out Patient Department OPD PDFDocument64 paginiOut Patient Department OPD PDFDAD SHOTS100% (1)
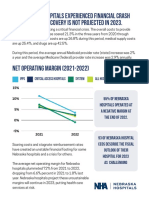
- Nebraska Hospitals Experienced Financial Crash in 2022Document2 paginiNebraska Hospitals Experienced Financial Crash in 2022FOX42 NewsÎncă nu există evaluări
- Caregiving For Grades 7 To 10Document26 paginiCaregiving For Grades 7 To 10Yeshua YeshaÎncă nu există evaluări
- Apollo Hospitals - Differentiation Through HospitalityDocument20 paginiApollo Hospitals - Differentiation Through Hospitalityanjali sharmaÎncă nu există evaluări
- Fundamentals of Nursing Transes 3Document4 paginiFundamentals of Nursing Transes 3Louise TorresÎncă nu există evaluări
- Record ChecklistDocument2 paginiRecord ChecklistEduardo AnerdezÎncă nu există evaluări
- Clinical Coach For Effective Perioperative Nursing Care@2009 PDFDocument374 paginiClinical Coach For Effective Perioperative Nursing Care@2009 PDFAdindaÎncă nu există evaluări
- Synthesis Draft 6Document5 paginiSynthesis Draft 6api-315984538Încă nu există evaluări
- DIAS Week 5a - Career Opportunities of Counsellor - Responsibilities of CounsellorsDocument3 paginiDIAS Week 5a - Career Opportunities of Counsellor - Responsibilities of CounsellorsRoxanne Gensaya0% (1)
- 2 B 65 A 6Document3 pagini2 B 65 A 6Marvin ThomasÎncă nu există evaluări
- Standard Booklet (Updated) NewDocument20 paginiStandard Booklet (Updated) NewFakrulÎncă nu există evaluări
- 7-Learning Portfolio-HomeworkDocument13 pagini7-Learning Portfolio-Homeworkbushra asad khanÎncă nu există evaluări
- Deficiencies Observed During Pre-Assessment - Paf 2 Hospital (Full Accreditation) : Date(s) of VisitDocument8 paginiDeficiencies Observed During Pre-Assessment - Paf 2 Hospital (Full Accreditation) : Date(s) of Visitpranit mÎncă nu există evaluări
- No Bad Parts: Healing Trauma and Restoring Wholeness with the Internal Family Systems ModelDe la EverandNo Bad Parts: Healing Trauma and Restoring Wholeness with the Internal Family Systems ModelEvaluare: 5 din 5 stele5/5 (4)
- Eat That Frog!: 21 Great Ways to Stop Procrastinating and Get More Done in Less TimeDe la EverandEat That Frog!: 21 Great Ways to Stop Procrastinating and Get More Done in Less TimeEvaluare: 4.5 din 5 stele4.5/5 (3226)
- Think This, Not That: 12 Mindshifts to Breakthrough Limiting Beliefs and Become Who You Were Born to BeDe la EverandThink This, Not That: 12 Mindshifts to Breakthrough Limiting Beliefs and Become Who You Were Born to BeEvaluare: 2 din 5 stele2/5 (1)
- Indistractable: How to Control Your Attention and Choose Your LifeDe la EverandIndistractable: How to Control Your Attention and Choose Your LifeEvaluare: 3 din 5 stele3/5 (5)
- Summary of Atomic Habits: An Easy and Proven Way to Build Good Habits and Break Bad Ones by James ClearDe la EverandSummary of Atomic Habits: An Easy and Proven Way to Build Good Habits and Break Bad Ones by James ClearEvaluare: 4.5 din 5 stele4.5/5 (560)
- Summary of Dan Martell's Buy Back Your TimeDe la EverandSummary of Dan Martell's Buy Back Your TimeEvaluare: 5 din 5 stele5/5 (1)
- The Coaching Habit: Say Less, Ask More & Change the Way You Lead ForeverDe la EverandThe Coaching Habit: Say Less, Ask More & Change the Way You Lead ForeverEvaluare: 4.5 din 5 stele4.5/5 (186)
- The 5 Second Rule: Transform your Life, Work, and Confidence with Everyday CourageDe la EverandThe 5 Second Rule: Transform your Life, Work, and Confidence with Everyday CourageEvaluare: 5 din 5 stele5/5 (9)
- The Compound Effect by Darren Hardy - Book Summary: Jumpstart Your Income, Your Life, Your SuccessDe la EverandThe Compound Effect by Darren Hardy - Book Summary: Jumpstart Your Income, Your Life, Your SuccessEvaluare: 5 din 5 stele5/5 (456)
- The One Thing: The Surprisingly Simple Truth Behind Extraordinary ResultsDe la EverandThe One Thing: The Surprisingly Simple Truth Behind Extraordinary ResultsEvaluare: 4.5 din 5 stele4.5/5 (709)
- Summary: The 5AM Club: Own Your Morning. Elevate Your Life. by Robin Sharma: Key Takeaways, Summary & AnalysisDe la EverandSummary: The 5AM Club: Own Your Morning. Elevate Your Life. by Robin Sharma: Key Takeaways, Summary & AnalysisEvaluare: 4.5 din 5 stele4.5/5 (22)
- Uptime: A Practical Guide to Personal Productivity and WellbeingDe la EverandUptime: A Practical Guide to Personal Productivity and WellbeingÎncă nu există evaluări
- Summary of Steven Bartlett's The Diary of a CEODe la EverandSummary of Steven Bartlett's The Diary of a CEOEvaluare: 5 din 5 stele5/5 (4)
- The Millionaire Fastlane: Crack the Code to Wealth and Live Rich for a LifetimeDe la EverandThe Millionaire Fastlane: Crack the Code to Wealth and Live Rich for a LifetimeEvaluare: 4.5 din 5 stele4.5/5 (2)
- Quantum Success: 7 Essential Laws for a Thriving, Joyful, and Prosperous Relationship with Work and MoneyDe la EverandQuantum Success: 7 Essential Laws for a Thriving, Joyful, and Prosperous Relationship with Work and MoneyEvaluare: 5 din 5 stele5/5 (38)
- Mastering Productivity: Everything You Need to Know About Habit FormationDe la EverandMastering Productivity: Everything You Need to Know About Habit FormationEvaluare: 4.5 din 5 stele4.5/5 (23)
- Own Your Past Change Your Future: A Not-So-Complicated Approach to Relationships, Mental Health & WellnessDe la EverandOwn Your Past Change Your Future: A Not-So-Complicated Approach to Relationships, Mental Health & WellnessEvaluare: 5 din 5 stele5/5 (85)
- Fascinate: How to Make Your Brand Impossible to ResistDe la EverandFascinate: How to Make Your Brand Impossible to ResistEvaluare: 5 din 5 stele5/5 (1)
- Napoleon Hill's Keys to Positive Thinking: 10 Steps to Health, Wealth, and SuccessDe la EverandNapoleon Hill's Keys to Positive Thinking: 10 Steps to Health, Wealth, and SuccessEvaluare: 5 din 5 stele5/5 (306)
- Growth Mindset: 7 Secrets to Destroy Your Fixed Mindset and Tap into Your Psychology of Success with Self Discipline, Emotional Intelligence and Self ConfidenceDe la EverandGrowth Mindset: 7 Secrets to Destroy Your Fixed Mindset and Tap into Your Psychology of Success with Self Discipline, Emotional Intelligence and Self ConfidenceEvaluare: 4.5 din 5 stele4.5/5 (561)
- Summary of The 48 Laws of Power by Robert GreeneDe la EverandSummary of The 48 Laws of Power by Robert GreeneEvaluare: 4.5 din 5 stele4.5/5 (36)
- How to Be Better at Almost Everything: Learn Anything Quickly, Stack Your Skills, DominateDe la EverandHow to Be Better at Almost Everything: Learn Anything Quickly, Stack Your Skills, DominateEvaluare: 4.5 din 5 stele4.5/5 (860)
- Leadership and Self-Deception: Getting out of the BoxDe la EverandLeadership and Self-Deception: Getting out of the BoxEvaluare: 5 din 5 stele5/5 (155)
- What's Best Next: How the Gospel Transforms the Way You Get Things DoneDe la EverandWhat's Best Next: How the Gospel Transforms the Way You Get Things DoneEvaluare: 4.5 din 5 stele4.5/5 (33)
- Summary: The Gap and the Gain: The High Achievers' Guide to Happiness, Confidence, and Success by Dan Sullivan and Dr. Benjamin Hardy: Key Takeaways, Summary & AnalysisDe la EverandSummary: The Gap and the Gain: The High Achievers' Guide to Happiness, Confidence, and Success by Dan Sullivan and Dr. Benjamin Hardy: Key Takeaways, Summary & AnalysisEvaluare: 5 din 5 stele5/5 (4)
- Summary of The 7 Habits of Highly Effective People: Powerful Lessons in Personal Change by Stephen R. CoveyDe la EverandSummary of The 7 Habits of Highly Effective People: Powerful Lessons in Personal Change by Stephen R. CoveyEvaluare: 4.5 din 5 stele4.5/5 (41)
- Summary: Hidden Potential: The Science of Achieving Greater Things By Adam Grant: Key Takeaways, Summary and AnalysisDe la EverandSummary: Hidden Potential: The Science of Achieving Greater Things By Adam Grant: Key Takeaways, Summary and AnalysisEvaluare: 4.5 din 5 stele4.5/5 (15)