Documente Academic
Documente Profesional
Documente Cultură
A Preliminary Bacteriological Study of Refrigerated Channerl Catfish Sperm
Încărcat de
Orlando Karim Shiro Jr.Descriere originală:
Drepturi de autor
Formate disponibile
Partajați acest document
Partajați sau inserați document
Vi se pare util acest document?
Este necorespunzător acest conținut?
Raportați acest documentDrepturi de autor:
Formate disponibile
A Preliminary Bacteriological Study of Refrigerated Channerl Catfish Sperm
Încărcat de
Orlando Karim Shiro Jr.Drepturi de autor:
Formate disponibile
JOURNALOFTHE WORLD AQUACULTURE SOCIETY
Vol. 28, No. 3 September, 1997
A Preliminary Bacteriological Study of Refrigerated Channel Catfish Sperm
JILL JENKINS' A.
Southeastern Fish Cultural Laboratory, United States Department of the Interior, Route 3, Box 86, Marion, Alabama 36756 USA
TERRENCETIERSCH~ R.
School o Forestry, Wildlife, and Fisheries, Louisiana Agricultural Experiment Station, f Louisiana State University Agricultural Center, Baton Rouge, Louisiana 70803 USA Abstract.-This study was designed to simulate conditions encountered routinely during refrigerated storage of channel catfish sperm. Sperm samples were stored at 4 C in non-sterile and sterile Hanks' balanced salt solution (HBSS). Non-sterile HBSS was prepared with distilled water stored for 2 wk in a plastic carboy prior to use. Observations were made on the frequency and abundance of bacteria in samples, and on changes in sperm motility and quality. Sperm samples stored in non-sterile HBSS had a complete loss of motility within 72 h. Samples maintained in sterile HBSS showed an initial decrease in motility between 48 and 72 h. and a complete loss of motility within 10 d. Quality of the sperm in each buffer decreased as motility decreased; morphologic changes and reduced motility of sperm were coincident with increased bacterial numbers. Bacteria were cultured on tryptic soy agar and Pseudomonas F agar (PFA) spread-plating by IO+L aliquots from each sample onto bacteriologic media and incubating for 5 d. The dominant bacteria observed were members of the genus Pseudomonas. representing 67% of the total bacteria identified. The dominant pseudomonad (Pseudomonas sp.) cultured from sperm samples stored in sterile buffer produced caseinase, lecithinase, and was P-hemolytic, whereas the dominant bacteria (P. putida) cultured from samples stored in the non-sterile buffers did not. Highly motile pseudomonads, present in two samples stored in sterile buffer, colonized below the surface of the PFA media at 4 C. The attributes of the bacterial contaminants that likely contributed to the decrease in sperm quality were production of extracellular enzymes, consumption of oxygen, and a high level of motility. Potential sources of degradative bacteria were commensal flora of channel catfish and the water used in preparing the storage buffer.
I Present address: National Wetlands Research Center, Biological Resources Division, United States Geological Survey, United States Department of the Interior, Lafayette. Louisiana 70506 USA. * Corresponding author.
Artificial spawning is used routinely for study and production of fishes. Standard methods include the collection and storage of gametes (Huner and Dupree 1984). Typically, eggs are collected from a ripe female, mixed with a sperm suspension, and fertilized by the addition of water or an activating solution. Storage of gametes prior to fertilization is useful in a number of techniques including polyploidization (Benfey 1989), hybridization (Goudie et al. 1993), and gynogenesis (Ihssen et al. 1990). Effective storage of gametes is also a prerequisite for the establishment of cryopreserved gene banks from improved strains or endangered wild stocks. Problems with the collection and viability of gametes limit artificial spawning of channel catfish Zctalurus punctatus, the most important food fish cultured in the United States. Because the eggs rapidly become inviable after stripping, the time interval for fertilization is narrow. Sperm cannot be stripped from male channel catfish, and the testis must be removed by dissection. The testis is mechanically disrupted to collect sperm which are suspended in buffers for storage (Tiersch et al. 1994). Thus, sperm samples are generally collected and stored in advance to ensure availability when female channel catfish spawn. Catfish sperm can be stored refrigerated at 4 C (Christensen and Tiersch 1996) or cryopreserved (Guest et al. 1976; Tiersch et al. 1994), although in our experience refrigerated storage appears to be limited by bacterial growth. The presence of bacteria has
0 Copynghl by the World Aquaculture Society 1997
282
BACTERIOLOGY OF CATFISH SPERM
283
been shown to decrease fertility rates of salmon sperm (Stoss and Refstie 1983) and motility and fertility of carp sperm (Saad et al. 1988); however, microbial contamination of fish sperm has received little attention. A few studies have addressed optimization of refrigerated storage of fish sperm by use of antibiotics but typically without bacteriologic analysis (Scott and Baynes 1980; Stoss 1983). The present study was designed to simulate conditions encountered during the routine storage of channel catfish sperm. It represents a preliminary attempt to identify dominant bacterial contaminants and to evaluate changes in sperm motility and quality over time. The objectives were to compare sperm motility with the occurrence of bacteria during storage at 4 C and to identify the predominant bacteria found during storage.
a plastic carboy for 2 wk prior to use. We chose to use aged, non-sterile water because the distilled water used to prepare buffers in fish hatcheries is often stored in a similar manner. The osmolality of each buffer (290 mOsm/kg) was measured by a vapor pressure osmometer (Model 5500, Wescor Corp., Logan, Utah, USA) to ensure sufficient osmotic pressure to prevent activation of sperm during storage (Bates et al. 1996). In a second experiment, three sperm samples were collected aseptically and suspended in sterile HBSS as described above. These samples were collected outside of the spawning season (December 1994) and were assessed for sperm motility and the presence of bacteria, which were identified biochemically. The initial motility of the samples was 80%, 80%, and 5%. Sperm Storage and Motility Estimates
The sperm suspensions in experiments one and two were stored refrigerated at 4 C in sterile 50-mL tissue culture flasks (CornPreparation of Sperm ing, Inc., Coming, New York, USA) with Two experiments were conducted. In the loosened caps. Samples were removed first, conducted during July 1993, healthy, aseptically once every 24 h for 10 d to esmature, 4-yr-old male channel catfish (2- timate sperm motility and to culture bacte2.5 kg) were killed and their testes re- ria. To estimate the percentage of motile moved. Using aseptic technique, adherent sperm in each sample, a 1-pL aliquot was tissue was dissected away, and the testes added to 40 p,L of distilled water on a miwere blotted dry and weighed. Three sperm croscope slide, and the mixtures were samples were chosen to represent a range viewed immediately for 15 s using darkfield of motility values (i.e., initial motilities of microscopy at 1OOX. A minimum of three 85%, 60% and 15%) encountered com- motility estimates were made for each sammonly in sperm used for artificial spawning. ple. Paired samples from the testis of each fish Bacterial Cultures were placed into sterile or non-sterile conditions as follows. One gram of anterior tesBecause this was a preliminary bacterial tis (Sneed and Clemens 1963) from each survey, we chose to perform a large number fish was dissociated by crushing, and the of bacterial cultures (with repeated samak spermatozoa suspended in 20 mL of H n s pling, various media and two temperatures) balanced salt solution (HBSS) (Tiersch et and biochemical tests (over 40) on a small al. 1994) sterilized by passage through a number of representative testis samples. We 0.22-pm filter (Gelman Sciences, Ann Ar- chose this design for two reasons: 1) the bor, Michigan, USA). Another gram of tes- patterns of occurrence of bacterial contamtis was dissociated, and the sperm suspend- ination and sperm morphology described in ed in identical fashion in HBSS prepared this paper are representative of those obwith distilled water that had been stored in served by us in hundreds of channel catfish
Materials and Methods
284
JENKINS AND TIERSCH
sperm samples studied over the last 5 yr; ) and 2 future studies can focus on fewer specific bacteria and biochemical tests with larger numbers of catfish. Bacteria from the samples were cultured at 4 C and 24 C by spread-plating IO-FL aliquots onto each of two types of bacteriologic media: 1) tryptic soy agar (TSA) (Difco, Detroit, Michigan, ) USA), a general nutrient medium; and 2 Pseudomonas F agar (PFA) (Difco). The PFA medium enhances production of the pigment fluorescein which differentiates some pseudomonads. The plates were examined at 5 d (4C incubation) or at 24 h (24C), after maximal growth had occurred. Viable organisms were counted as colonyforming units (CFU). Selected colonies from TSA and PFA were subcultured on PFA for 24 h at 24 C before transfer to blood agar (TSA supplemented with 5% sheep blood, Carolina Biological Supply Company, Burlington, North Carolina, USA) and incubation for 24 h at 24 C. Bacteria were first identified by Gram staining; all Gram-negative bacteria were identified further by biochemical characterization (Palleroni 1984) using standard tube tests incubated at 24 C. Bacterial flagella were identified with a standard flagellar stain (Cam Scarborough Microbiologicals, Inc., Decatur, Georgia, USA).
"1
11
rcnyr of
(dayI)
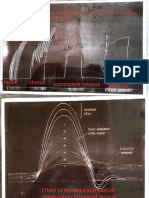
FIGURE Percent motility of channel catjish sperm 1. stored a f 4 C in sterile Hanks' balanced salt solution (HBSS) (open symbols), or in HBSS prepared with aged distilled water (closed symbols). Each point represents the mean value of three fish.
ReSUltS A complete loss of motility occurred within 72 h of storage in the samples stored in non-sterile HBSS (Fig. 1). In the samples maintained in sterile HBSS, motility began to decrease between 48 and 72 h, and complete loss of motility occurred within 10 d. With sterile or non-sterile buffers, the quality of sperm samples decreased as motility decreased. Initially the samples were consistent liquid suspensions and sperm that were not motile appeared structurally intact. At as early as 2 d, the spermatozoa in non-sterile buffer began to clump and could not be collected with a micropipette (Fig. 2A); few motile bacteria could be observed under darkfield microscopy. Mor-
phologic changes included entangled flagella and sperm heads without flagella. Complete loss of motility was associated with degenerated spermatozoa and the presence of a noticeable odor. Motility losses of sperm corresponded to the appearance of bacterial growth on TSA and PFA, and CFU were equivalent on each medium ( X O CFU at day 6). A high level of bacterial motility was indicated by colonies of pseudomonads from two of the samples (with initial motilities of 15% and 80%) that formed below the surface of the PFA when incubated at 4 C. When cultured at 24 C on PFA, these isolates did not colonize below the agar surface. Gram-positive streptococci and bacilli occurred at 0 d and 1 d in all sperm samples stored in non-sterile buffer. Because bacterial results obtained for incubation at 4 C were qualitatively identical to those at 24 C on both media, we chose to report only the biochemical results from the lower temperature which was most relevant to refligerated storage (Table 1). The dominant bacteria cultured from the samples in experiment one were members of the genus Pseudomonas (Fig. 2B) representing 67% (10 out of 15) of the total bacteria identified. Bacteria (and number of samples in which they occurred) that were isolated from the samples stored in sterile HBSS were: Pseudomonas spp. (3/10), Pseudomonas paucimobilis (l/lO), Pseu-
BACTERIOLOGY OF CATFISH SPERM
285
FIGURE Channel carJish sperm diluted in Hanks balanced salt solution and stored for 2 d ( A ) viewed with 2. darkfeld microscopy (bar = 5 p n ; total magnifcation = 576X). Members of the genus Pseudomonas ( B ) were the dominant bacteria found in refrigerated sperm samples viewed with brighrfield microscopy (bar = 2 pn: total magnifcation = 1440X). Note the presence o bacterial figella (arrowheads). f
domonas putida (l/lO), Pantoea agglomerans (l/lO), Aeromonas hydrophila (l/lO), and Klebsiella oxytoca (140). The most frequently encountered pseudomonad cultured from these sperm samples produced proteases (Table 1). Bacteria (and number of samples in which they occurred) isolated from sperm samples stored in non-sterile HBSS were: P. putida (3/10) and P. Juorescens (l/lO). In all cultures, pseudomonads were the most numerous microorganism present. Pseudomonas was the sole genus observed at day 10 on either medium. Bacteria in experiment two (and number
of samples in which they occurred) were: Pseudomonas paucimobilis (U3) and Pseudomonas maltophilia (1/3). Two gram-positive, non-spore-forming rods and one strain of streptococci were present in 2 of 3 samples. No bacteria were cultured from the third sperm sample. By day 6, four times more CFU were present in the sample with 5% initial motility than in the samples with 80% initial motility (data not shown).
Discussion
Studies addressing sperm quality during storage have been conducted extensively in
286
JENKINS AND TIERSCH
TMLE 1. Nutritional and physiological characteristicsp of the dominant pseudomonads cultured from samples o channel catJishsperm stored at 4 C in sterile (S)and non-sterile (N)Hanks' balanced salt solution. f
Characteristic Enzyme function Arginine decarboxylase Lysine decarboxylase Ornithine decarboxylase Urease Nitrate reductase Nitrite reductase P-galactosidase Cytochrome oxidase Lecithinase Gelatinase Phenylalanine deamidase Utilization of citrate Esculin hydrolysis Blood agar hemolysis Starch hydrolysis Casein hydrolysis Other characteristics Motility Gram reaction Fluorescein production Hydrogen sulfide Vogues-Proskauer Indole production
a
Characteristic Carbohydrate utilization xylose ma1onate adonitol arabinose cellibiose dextrose dextrose (anaerobic) dulcitol galactose glycerol inositol lactose levulose maltose mannitol mannose melibiose raffinose rhamnose salicin sorbitol sucrose trehalose
S
-
+
-
+
-
+ +
-
+ +
+ +
-
+ +
+ +
-
+
P
+
-
+
-
Results of biochemical tests: "+", positive or present; "-", negative or absent.
domesticated farm animals (Bartlett et al. 1976), but few are available for fish sperm (e.g., Sneed and Clemens 1956; Scott and Baynes 1980; Saad et al. 1988). No data are available on the bacteriology of stored catfish sperm. Bacterial contamination can alter the success of artificial spawning of fishes. Decreases in motility have been related directly to decreased fertilizing capacity (Stoss and Refstie 1983; Saad et al. 1988). In our study, the decreased motility of sperm stored at 4 C was associated directly with an increased prevalence of bacteria. Morphologic changes of catfish spennatozoa were observed during storage, similar to those identified for carp sperm stored at 4 C (Saad et al. 1988). The initial differences in motility of sperm samples could be attributed to maleto-male variability. Alternatively, initial motility could be influenced by microoru ganisms derived from commensal flora. O r
results indicate that the flora of the testes of channel catfish is composed of organisms representing the genera Pseudomonas, Pantoea, Aeromonas, and Klebsiella. Some gram-positive rods and cocci were also present. Pseudomonads were indigenous to five of the six channel catfish samples examined, and they were the most numerous bacteria cultured from the samples. The Pseudomonas sp. derived from sperm samples stored in sterile HBSS produced proteolytic enzymes not produced by the Pseudomonas putida obtained from the samples stored in non-sterile HBSS. Organisms producing proteolytic enzymes are more likely to invade tissues (Gilardi 1985). Upon death and rupture of spermatozoa, the proteins released could provide nutrients for bacteria, and proteases could degrade other spermatozoa. The use of the non-sterile aged distilled water in HBSS could have introduced Pseu-
BACTERIOLOGY OF CATFISH SPERM
287
domonas putida as a buffer contaminant. Members of the genus Pseudomonas can originate from the natural environment because they are motile, free-living bacteria ubiquitous in aquatic habitats (Thune et al. 1993). Pseudomonads are non-fermentative, obligate aerobes that can multiply rapidly in nutrient-rich and confined environments (Gilardi 1985). They can affect spermatozoa directly or compete for substrate in storage media. Oxygen consumption by these bacteria was implicated in reduced survival of carp sperm samples (Saad et al. 1988). As opportunistic pathogens pseudomonads can employ high levels of motility as a virulence mechanism. McManus et al. (1980) found that mutants of Pseudomonas aeruginosa displaying a loss of spreading in soft agar had diminished virulence in sepsis of burn wounds. In our study, the migration of Pseudomonas spp. below the surface of PFA indicated a high level of motility at 4 C. The motility displayed at this temperature likely contributed to bacterial invasiveness and subsequent colonization of refrigerated sperm samples. It should also be noted that given the high level of motility of Pseudomonas and their similarity in size to catfish sperm (Fig. 2) inexperienced hatchery personnel could mistake bacterial motility for sperm motility. Use of poor quality sperm samples for fertilization could result in the loss of valuable eggs. Multiplication of microorganisms may be suppressed by refrigeration, but it is not stopped, and microorganisms are not necessarily killed by freezing. For example, Pseudomonas aeruginosa which produces exotoxin A has been associated with low fertility in bulls and can be transmitted in frozen semen (Getty and Ellis 1967). To control infectious agents that might be transmissible by sperm, the addition of antibiotics is common in breeding practices with farm animals (Hafez 1993). With regard to fishes, this practice has been studied most in salmonids (Stoss 1983) and has in-
creased storage time of refrigerated channel catfish sperm (Christensen and Tiersch 1996). Cryopreservation of sperm has become an accepted technique for selective breeding and genetic improvement in livestock industries. This technology is being developed rapidly for use in aquaculture species and would enable widespread distribution and exchange of sperm samples and, potentially, of disease organisms. Cryopreservation of sperm is especially valuable for channel catfish because sperm cannot be stripped, and it is necessary to remove the testis or kill valuable broodstock to collect sperm. In addition, gene banks are needed for protecting endangered fish species and for producing improved stocks for aquaculture. Therefore, research is required to identify non-spermicidal antibiotics that will control the microorganisms that occur in fish sperm. This study represents the first bacteriological evaluation of catfish sperm samples and one of the few for fish sperm in general. With increased numbers of bacteria, the motility, quality, and viability of the channel catfish sperm decreased. Morphologic changes and reduced motility of sperm were coincident with increased bacterial numbers. The characteristics of the bacteria that likely contributed to reduced sperm quality were production of proteolytic enzymes and metabolic by-products, consumption of oxygen, and a high level of motility. Protocols for the collection and storage of sperm free from bacteria would minimize vertical and horizontal transmission of microorganisms, and improve fertilization success in the intensive culture conditions used in hatcheries. By defining microbial populations, antibiotics can be selected to test against target species of bacteria. Because incubation at 4 C and 24 C yielded identical bacterial results in this study, it would seem most useful to incubate cultures at 24 C to speed diagnosis of contaminants in refrigerated sperm samples. The availability of these baseline data allows development of improved gamete stor-
288
JENKINS AND TERSCH
techniques for the culture of flathead catfish and other catfishes. Pages 44-82 in H K.Dupree and . J. V Huner. editors. Third rewrt to the fish fann. em: The sktus of warmwakr fish farming and Acknowledgments progress in fish fanning research. U.S. Fish and Wildlife Service, Washington, D.C., USA. This work was supported in part by the Ibssen, P. E., L. R McKay, I. McMillan and R B. Louisiana Catfish Promotion and Research Phillips. 1990. Ploidy manipulation and gynoBoard, and USDA w t 93-34310-9057. genesis in fishes: cytogenetic and fisheries appliWe thank D. Leedy for assisting with baccations. 'Itansactions of the American Fisheries Society 119:698-717. terial identification, K. Barton for help in preparing the manuscript, and P. Mazik, I? McManus, A. T., E. E. Moody and A. D. Mason. 1980. Bacterial motility: a component in experiTaylor and L. Torrans for critical review. e mental Pseudomonas aeruginosa burn wound s p sis. Burns 6:235-239. Literature Cited PaUeroni, N. J. 1984. Family I. Pseudomonadaceae Winsloe, Broadhurst, Buchanan, Krumwiede, Barlett, D. E., L. L. Lanson,W. G. Parker and T. Rogers and Smith 1917, 5 5 W . Pages 141-199 in H. Howard. 1976. Specific pathogen free (SPF) N. R. Krieg and J. G.Holt editors. Bergey's manfrozen bovine semen: A goal? Pages 11-22 in ual of systematic bacteriology, volume 1. The Proceedings of the Sixth T c n c l Conference on ehia Williams and Wilkins Company, Baltimore, Artificial Insemination and Reproduction. ColumMaryland, USA. bus, Missouri, USA. saad, A., R BWard, M. C. "heron and M. G. HolBates, M. C., W. R Wayman and T. R Tiersch. lebecq. 1988. Short-term preservation of carp 1996. Effect of osmotic pressure on the activation Cyprinus carpi0 semen. Aquaculture 71:133-150. and storage of channel catfish sperm. 'hnsactions Scott, A. P. and S. M. Baynes. 1980. A review of of the American Fisheries Society 125:798-802. the biology, handling and storage of salmonid Bedey, T. J. 1989. A bibliography of triploid fish, spermatozoa. Journal of Fish Biology 17:7071943 to 1988. Canadian Technical Report of Fish739. eries and Aquatic Sciences, No. 1682. Sneed, K E and H P. Clemens. 1956. Survival of .. . Christensen, J. M. and T. R T i e d 1996. Refrigfish sperm after freezing and storage at low temerated storage of channel catfish sperm. Journal of peratures. Progressive Fish Culturist 1899-103. the World Aquaculture Society 27:340-346. Sneed, K. E and H. P. Clemens. 1963. The mor. Getty, S. M. and D. J. EW. 1967. The experimental phology of the testes and accessory reproductive use of bull semen contaminated with Pseudomonglands of the catfishes Zctaluridue. Copeia 1963: as aeruginosa organisms. Journal of the American 606-611. Veterinary Medical Association 151:1688-1691. Stoss, J. 1983. Fish gamete preservation and sperGuardi, G. L. 1985. Nonfermentative gram-negative matozoan physiology. Pages 305-350 in W. S. rods: laboratory identificationand clinical aspects. Hoar. D. J. Randall and E. M. Donaldson, editors. Marcel Dekker, Inc., New York, USA. Fish physiology, volume I ,part B. Behavior and X Goudie, C. A., T. R Tiersch, B. A. Slmco, K. B. fertility control. Academic Press, Orlando. FloriDavis and Q. Liu. 1993. Early growth and morda, USA. phology among hybrids o ictalurid catfishes. Stoaa, J and T Refstie. 1983. Short-term cryopref . . Journal of Applied Aquaculture 3:235-255. servation of milt from Atlantic salmon and sea Guest, W. C., J. W. Avadt and J. D. R o d . 1976. trout. Aquaculture 30:229-236. Preservation of channel catfish sperm. Itansac- Thune, R L., L. A. Stanley and R K. Cooper. 1993. tions of t e American Fisheries Society 105:469h Pathogenesis of Gram-negative bacterial infec474. tions in wannwater fish. Annual Review of Fish Hafa, E S. E 1993. Preservation and cryopreser. . Diseases 3:37-68. vation o gametes and embryos. Pages 503-525 f Tiersch, T. R, C. A. Goudie and G. J. Cadchael. 1994. Cryopreservation of channel catfish sperm: in E. S. E. Hafez, editor. Reproduction in farm Storage in cryoprotectants. fertilization trials, and animals. Lea and Febiger, Malvern, Pennsylvania, growth of channel catfish produced with cryopreUSA. served sperm. 'hnsactions of the American FishHuner, J. V. and H. K Dupree. 1984. Methods and . eries Society 123580-586. economics of channel catfish production, and
age techniques, such as cryopreservation, and benefits artificial breeding programs.
S-ar putea să vă placă și
- Japan Math SyllabusDocument33 paginiJapan Math SyllabuskalloliÎncă nu există evaluări
- Clonally Transmissible Cancers in Dogs and Tasmanian Devils: EP MurchisonDocument12 paginiClonally Transmissible Cancers in Dogs and Tasmanian Devils: EP MurchisonOrlando Karim Shiro Jr.Încă nu există evaluări
- Surgical Treatment of Transmissible Venereal Tumor (Sticker Sarcoma)Document5 paginiSurgical Treatment of Transmissible Venereal Tumor (Sticker Sarcoma)Orlando Karim Shiro Jr.Încă nu există evaluări
- Estimating Canine Cancer Incidence Italy 2017Document9 paginiEstimating Canine Cancer Incidence Italy 2017Orlando Karim Shiro Jr.Încă nu există evaluări
- The Complete Mitochondrial Genome of The PirarucuDocument10 paginiThe Complete Mitochondrial Genome of The PirarucuOrlando Karim Shiro Jr.Încă nu există evaluări
- Advances in Prostatic Diagnostics in Dogs 2018Document4 paginiAdvances in Prostatic Diagnostics in Dogs 2018Orlando Karim Shiro Jr.Încă nu există evaluări
- S0213925104729020 PDFDocument1 paginăS0213925104729020 PDFOrlando Karim Shiro Jr.Încă nu există evaluări
- Mortality in North American Dogs From 1984 To 2004 Fleming - Et - Al-2011-Journal - of - Veterinary - Internal - Medicine PDFDocument12 paginiMortality in North American Dogs From 1984 To 2004 Fleming - Et - Al-2011-Journal - of - Veterinary - Internal - Medicine PDFOrlando Karim Shiro Jr.Încă nu există evaluări
- Folder RaivaDocument4 paginiFolder RaivaOrlando Karim Shiro Jr.Încă nu există evaluări
- Genetic Variability of Two Populations of Pseudoplatystoma Reticulatum From The Upper Paraguay River BasinDocument6 paginiGenetic Variability of Two Populations of Pseudoplatystoma Reticulatum From The Upper Paraguay River BasinOrlando Karim Shiro Jr.Încă nu există evaluări
- Genetic Structure of The Migratory Catfish Pseudoplatystoma Corruscans (Siluriformes Pimelodidae) Suggests Homing BehaviourDocument11 paginiGenetic Structure of The Migratory Catfish Pseudoplatystoma Corruscans (Siluriformes Pimelodidae) Suggests Homing BehaviourOrlando Karim Shiro Jr.Încă nu există evaluări
- Genetic Variability of Two Populations of Pseudoplatystoma Reticulatum From The Upper Paraguay River BasinDocument6 paginiGenetic Variability of Two Populations of Pseudoplatystoma Reticulatum From The Upper Paraguay River BasinOrlando Karim Shiro Jr.Încă nu există evaluări
- In Vitro Evaluation of Ram Sperm Frozen With Glycerol, Ethylene Glycol or AcetamideDocument15 paginiIn Vitro Evaluation of Ram Sperm Frozen With Glycerol, Ethylene Glycol or AcetamideOrlando Karim Shiro Jr.Încă nu există evaluări
- Sperm DNA Fragmentation Induced by Cryopreservation New Insights and Effect of A Natural Extract From Opuntia Ficus IndicaDocument8 paginiSperm DNA Fragmentation Induced by Cryopreservation New Insights and Effect of A Natural Extract From Opuntia Ficus IndicaOrlando Karim Shiro Jr.Încă nu există evaluări
- Parte1 Online Ingles Prof Rene Maas1 PDFDocument8 paginiParte1 Online Ingles Prof Rene Maas1 PDFOrlando Karim Shiro Jr.Încă nu există evaluări
- Manual On The Production and Use of Live Food For Aquaculture (Tugas Planktonologi)Document305 paginiManual On The Production and Use of Live Food For Aquaculture (Tugas Planktonologi)asepirwan100% (1)
- Oxidative Stressisinvolvedinage-DependentspermatogenicdamageofDocument11 paginiOxidative Stressisinvolvedinage-DependentspermatogenicdamageofOrlando Karim Shiro Jr.Încă nu există evaluări
- In Vitro Survivalandlipidperoxidationstatusofrabbitspermatozoa After BothchilledandfrozenstorageinlycopeneenrichedextendersDocument4 paginiIn Vitro Survivalandlipidperoxidationstatusofrabbitspermatozoa After BothchilledandfrozenstorageinlycopeneenrichedextendersOrlando Karim Shiro Jr.Încă nu există evaluări
- Effect of Nonylphenol On Male Reproduction Analysis of Rat Epididymal Biochemical Markers and Antioxidant Defense EnzymesDocument8 paginiEffect of Nonylphenol On Male Reproduction Analysis of Rat Epididymal Biochemical Markers and Antioxidant Defense EnzymesOrlando Karim Shiro Jr.Încă nu există evaluări
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeDe la EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeEvaluare: 4 din 5 stele4/5 (5784)
- The Yellow House: A Memoir (2019 National Book Award Winner)De la EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Evaluare: 4 din 5 stele4/5 (98)
- Never Split the Difference: Negotiating As If Your Life Depended On ItDe la EverandNever Split the Difference: Negotiating As If Your Life Depended On ItEvaluare: 4.5 din 5 stele4.5/5 (838)
- Shoe Dog: A Memoir by the Creator of NikeDe la EverandShoe Dog: A Memoir by the Creator of NikeEvaluare: 4.5 din 5 stele4.5/5 (537)
- The Emperor of All Maladies: A Biography of CancerDe la EverandThe Emperor of All Maladies: A Biography of CancerEvaluare: 4.5 din 5 stele4.5/5 (271)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceDe la EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceEvaluare: 4 din 5 stele4/5 (890)
- The Little Book of Hygge: Danish Secrets to Happy LivingDe la EverandThe Little Book of Hygge: Danish Secrets to Happy LivingEvaluare: 3.5 din 5 stele3.5/5 (399)
- Team of Rivals: The Political Genius of Abraham LincolnDe la EverandTeam of Rivals: The Political Genius of Abraham LincolnEvaluare: 4.5 din 5 stele4.5/5 (234)
- Grit: The Power of Passion and PerseveranceDe la EverandGrit: The Power of Passion and PerseveranceEvaluare: 4 din 5 stele4/5 (587)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaDe la EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaEvaluare: 4.5 din 5 stele4.5/5 (265)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryDe la EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryEvaluare: 3.5 din 5 stele3.5/5 (231)
- On Fire: The (Burning) Case for a Green New DealDe la EverandOn Fire: The (Burning) Case for a Green New DealEvaluare: 4 din 5 stele4/5 (72)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureDe la EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureEvaluare: 4.5 din 5 stele4.5/5 (474)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersDe la EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersEvaluare: 4.5 din 5 stele4.5/5 (344)
- The Unwinding: An Inner History of the New AmericaDe la EverandThe Unwinding: An Inner History of the New AmericaEvaluare: 4 din 5 stele4/5 (45)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyDe la EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyEvaluare: 3.5 din 5 stele3.5/5 (2219)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreDe la EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreEvaluare: 4 din 5 stele4/5 (1090)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)De la EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Evaluare: 4.5 din 5 stele4.5/5 (119)
- Her Body and Other Parties: StoriesDe la EverandHer Body and Other Parties: StoriesEvaluare: 4 din 5 stele4/5 (821)
- University of Phoenix Anatomy and Physiology Week 2 QuizDocument2 paginiUniversity of Phoenix Anatomy and Physiology Week 2 QuizSophia FHSÎncă nu există evaluări
- Lesson Plan On RhinitisDocument15 paginiLesson Plan On Rhinitiskiran mahal100% (4)
- DR Anu Arasu How Trauma Affects Your HormonesDocument14 paginiDR Anu Arasu How Trauma Affects Your HormonesShalu SaharanÎncă nu există evaluări
- Dr. Sak Indriyani, Spa, Mkes: Department of Child Health Rsu MataramDocument48 paginiDr. Sak Indriyani, Spa, Mkes: Department of Child Health Rsu MataramMuhammad Bilal Bin AmirÎncă nu există evaluări
- Module 1. General and Special Questions of Clinical Laboratory Diagnostics Text Test QuestionDocument229 paginiModule 1. General and Special Questions of Clinical Laboratory Diagnostics Text Test QuestionA.h.MuradÎncă nu există evaluări
- Proteinuria and Chronic Kidney DiseaseDocument34 paginiProteinuria and Chronic Kidney DiseaseВалерий ГаврилуцаÎncă nu există evaluări
- Pathology of Neurodegenerative DiseasesDocument22 paginiPathology of Neurodegenerative DiseasesKarel GuevaraÎncă nu există evaluări
- Week 006 Animal-Immune-SystemDocument13 paginiWeek 006 Animal-Immune-SystemMark Lorens StaanaÎncă nu există evaluări
- Tissue Procurement, Processing, and Staining TechniquesDocument10 paginiTissue Procurement, Processing, and Staining TechniquesMuhammad JameelÎncă nu există evaluări
- 8 Curious Meridians Exercises for LongevityDocument6 pagini8 Curious Meridians Exercises for Longevitypeter911x100% (2)
- Burn Patient Management - Clinical Practice GuidelinesDocument47 paginiBurn Patient Management - Clinical Practice GuidelinesAna PÎncă nu există evaluări
- Delprato and MidgleyDocument14 paginiDelprato and MidgleyAna LucaÎncă nu există evaluări
- Fat Burn ConceptDocument31 paginiFat Burn ConceptManoj100% (1)
- Mind Maps in Biochemistry - (Metabolism of Carbohydrates)Document23 paginiMind Maps in Biochemistry - (Metabolism of Carbohydrates)Gus LionsÎncă nu există evaluări
- Dirty Genes Course Copy 1Document181 paginiDirty Genes Course Copy 1Rachel Bruce50% (4)
- Production and Characterization of Mushroom ChitosanDocument4 paginiProduction and Characterization of Mushroom ChitosanmicrokannanÎncă nu există evaluări
- Amphibian Graphs?Document9 paginiAmphibian Graphs?Anshumaan PatraÎncă nu există evaluări
- StomachDocument16 paginiStomachnevelle4667Încă nu există evaluări
- BTL Ultrasound 5000 - User ManualDocument16 paginiBTL Ultrasound 5000 - User ManualAlexaÎncă nu există evaluări
- แนวข้อสอบ National license - Hematology PDFDocument7 paginiแนวข้อสอบ National license - Hematology PDFTanawat SingboonÎncă nu există evaluări
- Health Benefits of Running and JoggingDocument2 paginiHealth Benefits of Running and JoggingOsamaÎncă nu există evaluări
- FloTrac SetupDocument2 paginiFloTrac SetupTheMIKI4444Încă nu există evaluări
- Ayurveda Physical BodyDocument11 paginiAyurveda Physical BodyanantÎncă nu există evaluări
- IndianJOralSci5278-1137123 030931Document5 paginiIndianJOralSci5278-1137123 030931Maqbul AlamÎncă nu există evaluări
- Surgical Resection of Cancer of The Buccal MucosaDocument21 paginiSurgical Resection of Cancer of The Buccal MucosapradeepÎncă nu există evaluări
- ELECTROPHRESISDocument66 paginiELECTROPHRESISM.PRASAD NAIDU100% (1)
- 8.sadvritta (Code of Conduct)Document47 pagini8.sadvritta (Code of Conduct)Vanisha AnoepÎncă nu există evaluări
- Cell Biology Test - 70 Possible Points: Prokaryotic and Eukaryotic Cells (2 Points Per Question)Document3 paginiCell Biology Test - 70 Possible Points: Prokaryotic and Eukaryotic Cells (2 Points Per Question)Vienne MonroidÎncă nu există evaluări
- Ebook PDF Developmental Biology 12th Edition PDFDocument41 paginiEbook PDF Developmental Biology 12th Edition PDFjanet.dicks248100% (36)
- Role Play KomkepDocument7 paginiRole Play KomkepSyubban AfifÎncă nu există evaluări