Documente Academic
Documente Profesional
Documente Cultură
MS2 Complete Study Guide
Încărcat de
cornejo10 evaluări0% au considerat acest document util (0 voturi)
15 vizualizări20 paginiDrepturi de autor
© Attribution Non-Commercial (BY-NC)
Formate disponibile
PDF, TXT sau citiți online pe Scribd
Partajați acest document
Partajați sau inserați document
Vi se pare util acest document?
Este necorespunzător acest conținut?
Raportați acest documentDrepturi de autor:
Attribution Non-Commercial (BY-NC)
Formate disponibile
Descărcați ca PDF, TXT sau citiți online pe Scribd
0 evaluări0% au considerat acest document util (0 voturi)
15 vizualizări20 paginiMS2 Complete Study Guide
Încărcat de
cornejo1Drepturi de autor:
Attribution Non-Commercial (BY-NC)
Formate disponibile
Descărcați ca PDF, TXT sau citiți online pe Scribd
Sunteți pe pagina 1din 20
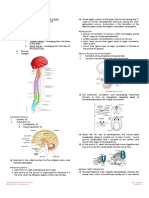
1.) GAMETOGENESIS AND FERTILIZATION
Primordial germ cells (PGCs):
~ Originate from epiblasts (pluripotential embryonic cells which can become either germ/somatic)
ation based on:
~ Location (posterior primitive streak region of embryo that's fated to become hindgut)
~ Signaling factors e.g. BMP (bone morphogenetic proteins) from extraembryonic ectoderm
~ Suppression of somatic cell genes e.g. Blimp-1 (transcriptional repressor) from extraembryonic tissue
- Migration from yolk sac endoderm to gonadal ridge (3-5 wks gestation)
~ Controlled by various molecular mechanisms e.g, distant soluble factors, local signals, interactions with
ECM molecules
- Any PGCs that are left along migratory route (i.e. don’t reach ridge) are killed by apoptosis,
+ ifnot eliminated, pediatric midline germ cell tumors e.g. dysgerminoma
~ PGC at gonadal ridge don’t die b/c steel factor on somatic cells in ridge binds cKit receptor on PGCs
Sdownregulates pro-apoptotic genes
~ Type of somatic cell in gonad determines whether PGC become oogonia vs. spermatogonia
‘Spermatogenesis (64 days/cycle, occurs in seminiferous tubules of testes)
- At puberty, PGCs will differentiate into spermatogonia, seminiferous tubules mature
Spermatagonia divide mitotically
Spermatagonia > Primary spermatocytes
Primary spermatocytes enter Meiosis | > Secondary spermatocytes
Secondary spermatocytes enter Meisosi Il > Spermatids
Spermatids undergo spermiogenesis (loss of cytoplasm, development of sperm tail and acrosome) > Mature
sperm (aka spermatozoa)
6) Spermatozoa break connection with Sertoli cells, released into lumen of seminiferous tubules (spermiation)
- Spermatocytes joined by cytoplasmic bridges as they divide
~ Division takes place btwn Sertoli cells (large cells transversing entire seminiferous tubule; tight junctions btwn
them form blood testis barrier; nurture developing sperm)
- _ Acrosome derived from Golgi of spermatid, contains enzymes needed to penetrate secondary oocyte
- Tail has three segments, middle part has mitochondria/ATP
- Spermatogonia can self-renew (<<1%) or differentiate into Primary spermatocytes
~ SSC (Spermatogonial Stem Cells) allows for continual sperm production through lifetime
- Sertoli cells secrete GDNF (glial cell line-derived growth factor) > binds GRA1 or RET receptors on
spermatogonia, induces SSC formation
- “In vivo” sperm culturing - implant male germline stem cells into another male carrier
Ysune
ogenesis
- PGCs divide mitoticaly and differentiate into oogonia and enter meiosis by 5 months gestation
1) Oogonia > Primary oocytes (9 wks gestation)
‘a. No primary oocytes formed after 20 wks gestation
b. High oocyte attrition: 7 million > 2 million during fetal period; then > 400,000 by puberty
2), Primary oocytes enter Meiosis I, arrest in prophase (13 wks gestation)
3) Stromal cells form single layer flat epithelial cells (granulosa cells) around oocyte = primordial follicle
4) Primordial follicle > Primary follicle as primary oocyte grows, granulosa cells become columnar
5) After puberty, FSH (follicle stimulating hormone, from pituitary) promotes growth of several Primary follicles
(granulose cells divide to form lots layers around oocyte, closest layer called cumulus cells) -> Secondary follicle
Only one follicle is ovulated per month, the rest degenerate
Zona pellucida (an ECM made from glycoproteins secreted by oocytes) forms around oocyte
Secondary follicle forms antrum (fluid filled cavity) surrounding oocyte
Follicle enlarges until forms swelling on ovary surface > mature or Graafian follicle
LH (luteinizing hormone, from pituitary) promotes Primary oocyte to resume and finish Meiosis | > Secondary
oocyte and first polar body (asymmetric division)
a Secondary oocyte gets all the cytoplasm, but chromosomes split evenly
10) Secondary oocyte enters Meiosis l, arrests at metaphase until fertilization
- Complex factors governing oocyte development e.g, TGFB subfamilies, Steel/c-Kit
- Oocyte arrest is maintained by complex processes:
= Cross talk btwn oocyte and pregranulosa cells
- _ Steel/c-kit protect preantral follicles from apoptosis
- Anti-mullerian hormone secreted by follicle suppresses growth of surrounding primordial follicles
‘Ovulation
1) At mid-cycle (14 days), mature follicle growth spurt, high estrogen conc causes increase in LH
2), LH induces ovulation = oocyte w/cumulus cells expelled from ovary into Fallopian tube (coaxed by fingerlike
fimbriae)
3) Remaining follicle tissue becomes corpus luteum
‘a. Corpus luteum secretes progesterone to prepare endometrium for implantation
b. if oocyte fertilized, CL enlarges and increases hormone production, embryo's extraembryonic tissues
secrete hCG (human chorionic gonadotropin) to prevent CL degeneration
c_fnot fertilized, CL degenerates after 10-12 days, estrogen and progesterone decrease
~ Oocyte to zygote transition requires: synthesis of new proteins, removal of mRNA that maintain prophase arrest,
protein degradation, reshuffling organelles
Fertilization (occurs in ampulla of Fallopian tube)
1) 200-600 million sperm deposited, but only 200 survive trip to ampulla, takes min-1hr
2), Sperm undergo capacitation before they can fertilize
‘a. Takes 7hrs, increased sperm motility, loss of glycoprotein coat and seminal proteins in acrosome
3) Acrosomerxn
a sperm contacts the cumulus cells and zona pellucida around oocyte
b, acrosome membrane perforates and fuses w/sperm plasma membrane
proteolytic enzymes e.g, hyaluronidase and acrosin released, help sperm penetrate cumulus cells and
zona pellucida
d. glycoprotein ZP3 found in ZP can initiate acrosome rxn
4) Sperm fuses with egg plasma membrane, mediated through CD9 (egg) and Izumo (sperm)
5) Sperm head decondenses, releases phospholipase C-zeta -> Ca2+ increase in egg > cortical granule exocytosis
(granules release enzymes to modify ZP to prevent polyspermy)
6) Ca2+ increase induces finish of Meiosis ll > Secondary oocyte emits second polar body
7) Male and female pronucleiform, replicate DNA, chromosomes condense, first mitosis
~ _ Infertility = inability to conceive after 1 yr of trying; 20% and rising in US
- “functionally sterile” = sperm counts below 20 million/mL semen
- Invitro fertilization more successful if woman younger
~ ICSI (intracytoplasmic sperm injection) ~ sperm injected directly into egg cytoplasm; circumvents sperm motility
defects, low sperm count
(CLEAVAGE AND IMPLANTATION
Early Cleavage
- _ Embryo surrounded by zona pellucida, first cleavages as travel thru Fallopian, no overall size increase after
divisions; these cells are truly totipotent
- Compaction at & cell stage
- Ca2+-dep event, mediated by E-cadherin (aka uvomorulin)
~ First event in embryo that causes cells to differ from one another — cells no longer totipotent
~ Cells on inside become inner cell mass (ICM) (forms embryo)
~ Cells on outside become trophectoderm (forms extraembryonic membranes, implantation)
- Cell differentiation based on LOCATION
- Embryo now a morula (borders of individual cells not distinguishable now)
- Regulation — before compaction, all cells same, so can remove one and still be normal; take ZP from three 8-cell
embryos and aggregate them, they'll compact into one morula and form a normal mouse
Blastocyst Formation
~ Outer cells of morula pump fluid towards embryo center to form blastocoele (fluid-filled cavity); embryo now
called blastocyst
- Two distinct cell types: outer = trophectoderm; inner = ICM
- ICM cells are pluripotent (can make germ line, endoderm, ectoderm, mesoderm but NOT placental or
extraembryonic cells), but can’t self-renew; ICM is what’s used to make ES cells in vitro
- Hatching -ZP degenerates, blastocyst free floats in uterus
Implantation
- _ Blastocyst attaches to endometrial epithelium of uterine wall
- Implantation occurs in superior (upper) part, posterior wall of uterus (6 days after fertilization)
- _ HB-EGF (heparin-binding EGF-like growth factor) induces attachment of embryo
- Regulated by growth factors that are selectively expressed in luminal epithelium
- _ Embryonic pole of blastocyst attached to uterine wall now defines dorsal-ventral axis of embryo
- Trophoblast cells differentiate into: syncytiotrophoblast (directly above ICM) and cytotrophoblast (around
blastocoele}
- _ Syncytiotrophoblast secrete enzymes to let embryo embed further into endometrium
- _ Syncytiotrophoblast secrete HCG that enters maternal bloodstream to maintain corpus luteum, but
placenta will ake over this role and secrete progesterone
= Decidual cells (maternal stromal cells) loaded w/elycogen and lipids, provide nutrients for embryo
- Implantation regulated by uterine expression of LIF (leukemia inhibitory factor) and L-selectin on trophoblasts
- May failed pregnancies spontaneously terminate around time of implantation
- Ectopic pregnancy = implantation outside uterus, usually in uterine tube, due to inflammation/scarring of tube
S-ar putea să vă placă și
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeDe la EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeEvaluare: 4 din 5 stele4/5 (5794)
- The Little Book of Hygge: Danish Secrets to Happy LivingDe la EverandThe Little Book of Hygge: Danish Secrets to Happy LivingEvaluare: 3.5 din 5 stele3.5/5 (400)
- Shoe Dog: A Memoir by the Creator of NikeDe la EverandShoe Dog: A Memoir by the Creator of NikeEvaluare: 4.5 din 5 stele4.5/5 (537)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceDe la EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceEvaluare: 4 din 5 stele4/5 (895)
- The Yellow House: A Memoir (2019 National Book Award Winner)De la EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Evaluare: 4 din 5 stele4/5 (98)
- The Emperor of All Maladies: A Biography of CancerDe la EverandThe Emperor of All Maladies: A Biography of CancerEvaluare: 4.5 din 5 stele4.5/5 (271)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryDe la EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryEvaluare: 3.5 din 5 stele3.5/5 (231)
- Never Split the Difference: Negotiating As If Your Life Depended On ItDe la EverandNever Split the Difference: Negotiating As If Your Life Depended On ItEvaluare: 4.5 din 5 stele4.5/5 (838)
- Grit: The Power of Passion and PerseveranceDe la EverandGrit: The Power of Passion and PerseveranceEvaluare: 4 din 5 stele4/5 (588)
- On Fire: The (Burning) Case for a Green New DealDe la EverandOn Fire: The (Burning) Case for a Green New DealEvaluare: 4 din 5 stele4/5 (74)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureDe la EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureEvaluare: 4.5 din 5 stele4.5/5 (474)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaDe la EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaEvaluare: 4.5 din 5 stele4.5/5 (266)
- The Unwinding: An Inner History of the New AmericaDe la EverandThe Unwinding: An Inner History of the New AmericaEvaluare: 4 din 5 stele4/5 (45)
- Team of Rivals: The Political Genius of Abraham LincolnDe la EverandTeam of Rivals: The Political Genius of Abraham LincolnEvaluare: 4.5 din 5 stele4.5/5 (234)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyDe la EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyEvaluare: 3.5 din 5 stele3.5/5 (2259)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreDe la EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreEvaluare: 4 din 5 stele4/5 (1090)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersDe la EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersEvaluare: 4.5 din 5 stele4.5/5 (344)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)De la EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Evaluare: 4.5 din 5 stele4.5/5 (121)
- Her Body and Other Parties: StoriesDe la EverandHer Body and Other Parties: StoriesEvaluare: 4 din 5 stele4/5 (821)
- Secrets of Pediaatric OrthopaedicsDocument610 paginiSecrets of Pediaatric OrthopaedicsPanagiotis Poulios100% (9)
- Brain Biochemistry and DisordersDocument191 paginiBrain Biochemistry and DisordersTrajce PasowskyÎncă nu există evaluări
- Neural Tube DefectsDocument12 paginiNeural Tube Defectsdaniel_1592Încă nu există evaluări
- Human BrainDocument45 paginiHuman BrainNirmal BhowmickÎncă nu există evaluări
- Yakovlev - 1989 ObituaryDocument20 paginiYakovlev - 1989 ObituaryDonovan Roediger100% (1)
- Development of The Nervous SystemDocument5 paginiDevelopment of The Nervous SystemChaGonzalesÎncă nu există evaluări
- Labexercises3 4Document11 paginiLabexercises3 4Sittie Rainnie BaudÎncă nu există evaluări
- Embryo and Fetal PathologyDocument20 paginiEmbryo and Fetal PathologyAldo RdzÎncă nu există evaluări
- 24 Hour Chick Embryo - Embryology LabDocument3 pagini24 Hour Chick Embryo - Embryology LabIvy Cruz50% (2)
- DEVBIOL LE 1 ReviewerDocument43 paginiDEVBIOL LE 1 ReviewernamkimseoÎncă nu există evaluări
- Embryo JohariDocument25 paginiEmbryo JohariTejas PLÎncă nu există evaluări
- Developmental Neuro LectureDocument53 paginiDevelopmental Neuro Lectureapi-3784483100% (1)
- Basic Embryology Series PDFDocument252 paginiBasic Embryology Series PDFGrace Nduta0% (1)
- Developmental Biology of Frog: SpermDocument21 paginiDevelopmental Biology of Frog: SpermRavindra MadurÎncă nu există evaluări
- Neuroanatomy Quiz BeeDocument55 paginiNeuroanatomy Quiz BeeJulienne Sanchez-Salazar100% (3)
- Biol127s - EXERCISE 5 10Document53 paginiBiol127s - EXERCISE 5 10Shell Mae CarcuebaÎncă nu există evaluări
- Nervous System: Third EditionDocument36 paginiNervous System: Third EditionAlexis Tobar100% (1)
- HDTD-E - 6 - Embryonic PeriodDocument33 paginiHDTD-E - 6 - Embryonic PeriodMariam QaisÎncă nu există evaluări
- Group 6 - Nervous Sytem PresentationDocument157 paginiGroup 6 - Nervous Sytem PresentationNgirlÎncă nu există evaluări
- NeurulationDocument9 paginiNeurulationaesha890% (1)
- Neural Tube Defects (NTDS)Document59 paginiNeural Tube Defects (NTDS)Syed ShahÎncă nu există evaluări
- Animal Development: Neurulation and Extra-Embryo MembranDocument45 paginiAnimal Development: Neurulation and Extra-Embryo MembranNurul JasminÎncă nu există evaluări
- Developmental Biology of Frog: SpermDocument21 paginiDevelopmental Biology of Frog: SpermRavindra MadurÎncă nu există evaluări
- Embryonic Period 4-8weekDocument25 paginiEmbryonic Period 4-8weekaimi BatrisyiaÎncă nu există evaluări
- General Embryology PDFDocument9 paginiGeneral Embryology PDFmedpgnotesÎncă nu există evaluări
- NeuroembryologyDocument7 paginiNeuroembryologykidÎncă nu există evaluări
- General Embryology 2022Document130 paginiGeneral Embryology 2022KAMAL ALSOFIANYÎncă nu există evaluări
- HAMILDocument82 paginiHAMILNandya CarolineÎncă nu există evaluări
- L1 Introduction 17Document35 paginiL1 Introduction 17Lisa NguyenÎncă nu există evaluări
- Embyology UG Part II Note 2 - Development of Amphioxus Drprity Mam2Document8 paginiEmbyology UG Part II Note 2 - Development of Amphioxus Drprity Mam2Adwika DeoÎncă nu există evaluări