Documente Academic
Documente Profesional
Documente Cultură
Organic Chem Exam 3
Încărcat de
Alyssa McCallTitlu original
Drepturi de autor
Formate disponibile
Partajați acest document
Partajați sau inserați document
Vi se pare util acest document?
Este necorespunzător acest conținut?
Raportați acest documentDrepturi de autor:
Formate disponibile
Organic Chem Exam 3
Încărcat de
Alyssa McCallDrepturi de autor:
Formate disponibile
Organic Chemistry 2 Major
Mid-Term Exam 3
April 18, 2011
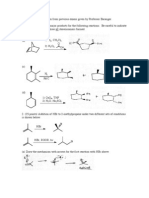
Instructions. Answer all questions on the answer sheets in the back of the exam with the letter, number, or drawing that corresponds to the best answer. All questions are 10 points each. At the end of the exam, turn in the answer sheets only. You may keep the exam. Important! Before you begin, put your name and ID number on all of the answer sheets. 1. Write the structures of the organometallic compounds (A and B) formed from the following reactions.
a) H3C CH3 Br + 2Li diethyl ether
b) H3C
CH3 Br
Mg
diethyl ether
2. Give the major organic product (A and B) of each of the following reactions.
O a) MgBr ND3 1) MgBr 2) H3O+ H
b)
3. Give the best reagent(s) for step 1 (A) and the major organic product for step 2 (B).
Reagent(s) OH H O NaBD4, H2O step 2
A
step 1
major organic product
4. Give the major organic product (A and B) of each of the following reactions.
nol etha H 4, m Na B
O H O OH
ss exce
1) ex c 2) H ess LiAlH O
2
4,
dieth y
l ethe r
5. Give the organometallic intermediate (A) and the major organic product (B) of the following reaction.
O 1) H 3C H CH3CH2MgBr diethyl ether
2) H3O+
Organic Chemistry 2 Major
Mid-Term Exam 3
April 18, 2011
6. Give the major organic product (A) including stereochemistry of the following reaction.
MgBr CH3 CH3 CH3 1) 2) H3O+ CH3
7. Give the organometallic intermediate (A) and the major organic product (B) including stereochemistry of the following reaction.
CH3 H O O S O
2 H3C
Li
1 CuI diethyl ether
H 3C
8. Predict the principle organic product (A and B) of each of the following reactions (14.24 b, f).
+ O CH3CH2Li 1) diethyl ether 2) H3O+
A
H3CO
LiCu(CH3)2
9. Give the intermediate (A) and the major organic product (B) including stereochemistry of the following reaction.
CH3 O OH HO SOCl2
A
(pyridine) N
10. Suggest reaction sequences and reagents suitable for carrying out the following conversion. Two synthetic operations are required in each case (15.30 c).
OH to OH OH (racemic)
Organic Chemistry 2 Major
Mid-Term Exam 3
April 18, 2011
11. Give the major organic product (A) of the following reaction.
OH (racemic) OH HIO4
12-13. Give the major organic product (A to D) of each step of the following reaction sequence.
1) B2H6, diglyme 2) H2O2, HO-
K2Cr2O7 H2SO4, H2O
CH3OH H2SO4, heat
excess CH3CH2CH2CH2MgBr
14. Give the major organic product (A) of the following reaction.
O O H3CO OCH3 1 equiv Br2 FeBr3
15. Give the major organic product (A) of the following reaction.
O OH O 1 equiv.Br2 acetic acid
16-17. Draw MAJOR resonance form(s) leading to MAJOR product(s) in the reactions of bromobenzene (1) and benzonitrile (2) with 1 equiv Cl2 using FeCl3 as catalyst. If more than one product is formed, draw ALL major resonance forms involved.
Br 1 equiv Cl2 FeCl3 1 2 CN 1 equiv Cl2 FeCl3
18. Starting from benzene, propose a synthesis (specify the reagent(s) required) for the following compound (1). If more than one step is required, draw the major organic product for each step. There is more than one possible answer to this question.
CH3 from Br 1 benzene
(a mixture of E/Z isomers)
Organic Chemistry 2 Major
Mid-Term Exam 3
April 18, 2011
19. Suggest a suitable series of reactions for carrying out each of the following synthetic transformations (12.47 d).
OCH3 O2N to OCH3 H3C OCH3 CH CH3 3 OCH3
20. Rank the following aromatic compounds by INCREASING reactivity toward electrophilic aromatic substitution reactions. Use inductive effects and/or resonance effects to explain the order. (q 2, quiz 7).
H N O CH3 a) b) H N CH3 N CH 3 CH3 c)
21 (bonus question quiz 8). Use retrosynthetic analysis to determine ONE possible route to the following tertiary alcohol. You can use benzene, anisole, organic molecules with no more than FOUR carbons, and inorganic reagents.
H 3C O OCH3 OH from
anisole
benzene
CH3
S-ar putea să vă placă și
- Test Bank For Organic Chemistry A Short Course 13th by Hart DownloadDocument12 paginiTest Bank For Organic Chemistry A Short Course 13th by Hart Downloaddannyriddle05051994ieq100% (23)
- Exam Answers Quiz KeysDocument24 paginiExam Answers Quiz Keysseanra23Încă nu există evaluări
- Organic Chemistry Additional Problems Final Exam Part2Document6 paginiOrganic Chemistry Additional Problems Final Exam Part2John SmithÎncă nu există evaluări
- Ch1b Ps3 Key SerDocument7 paginiCh1b Ps3 Key SerRichard ZhuÎncă nu există evaluări
- Chapter 8 Nucleophilic Substitution: Answers Prof. Sivaguru JayaramanDocument16 paginiChapter 8 Nucleophilic Substitution: Answers Prof. Sivaguru JayaramanRahma AshrafÎncă nu există evaluări
- Midterm III A Key 2321 F13'Document8 paginiMidterm III A Key 2321 F13'acb4039Încă nu există evaluări
- Additional Problems Final Exam Part 2 AnswersDocument10 paginiAdditional Problems Final Exam Part 2 AnswersJohn SmithÎncă nu există evaluări
- Che 232 Test 1 Sptember 2007Document16 paginiChe 232 Test 1 Sptember 2007BONOLO RANKOÎncă nu există evaluări
- Chem52 Su13 PracticeExam1ADocument11 paginiChem52 Su13 PracticeExam1Aamarka01Încă nu există evaluări
- CHM 1321 Assignment 5 Answers: 1) Name The Following CompoundsDocument15 paginiCHM 1321 Assignment 5 Answers: 1) Name The Following CompoundsSara Yuen100% (1)
- Practice 4ADocument22 paginiPractice 4ACamha NguyenÎncă nu există evaluări
- Practice 3CDocument13 paginiPractice 3CCamha NguyenÎncă nu există evaluări
- 12 Revision TestDocument5 pagini12 Revision TestHeartykingnkÎncă nu există evaluări
- CHE 232 Test 1 2015 AnsDocument12 paginiCHE 232 Test 1 2015 AnsBONOLO RANKOÎncă nu există evaluări
- Organic Chemistry 3Rd Edition Gorzynski Test Bank Full Chapter PDFDocument38 paginiOrganic Chemistry 3Rd Edition Gorzynski Test Bank Full Chapter PDFdannyblackerzawcfyni100% (9)
- 432georgia Spring 2018 Practice MT 2 - REVISEDDocument14 pagini432georgia Spring 2018 Practice MT 2 - REVISEDChemist MeÎncă nu există evaluări
- Organic Chemistry Questions 3Document12 paginiOrganic Chemistry Questions 3Ram KrishnaÎncă nu există evaluări
- Exam Three Practice TestDocument13 paginiExam Three Practice TestBUCH203100% (1)
- BIOKMOR N01 3rd ExamDocument8 paginiBIOKMOR N01 3rd ExamMacy MarianÎncă nu există evaluări
- Chem e TermDocument6 paginiChem e TermchituÎncă nu există evaluări
- Chapter 08 MergedDocument38 paginiChapter 08 MergedreemÎncă nu există evaluări
- Moon - Exam 2 - Summer 2011Document10 paginiMoon - Exam 2 - Summer 2011Andres Pena100% (2)
- 231 F 2010 Practice MT4 - Pp1to12Document12 pagini231 F 2010 Practice MT4 - Pp1to12Chemist MeÎncă nu există evaluări
- Che 232 Test 1b 2016 AnsDocument12 paginiChe 232 Test 1b 2016 AnsBONOLO RANKOÎncă nu există evaluări
- Chapter 4 Alcohols and Alkyl Halides: Answers Prof. Sivaguru JayaramanDocument13 paginiChapter 4 Alcohols and Alkyl Halides: Answers Prof. Sivaguru JayaramanCeseley HaynesÎncă nu există evaluări
- 2007 ADocument4 pagini2007 AAmiro MayraÎncă nu există evaluări
- Set 2Document6 paginiSet 2sanjith4arisÎncă nu există evaluări
- Do Not Open This Test Until Everyone Has OneDocument12 paginiDo Not Open This Test Until Everyone Has OneChemist MeÎncă nu există evaluări
- Exam Three Practice Test Answers PDFDocument19 paginiExam Three Practice Test Answers PDFBUCH203100% (1)
- Iit 2011 FST1 QNS P1Document25 paginiIit 2011 FST1 QNS P1grdgerÎncă nu există evaluări
- Chemistry Sample PaperDocument145 paginiChemistry Sample Paperseemantalukdar4Încă nu există evaluări
- Chem 222 Sample Exam 3Document8 paginiChem 222 Sample Exam 3Yarys YauÎncă nu există evaluări
- Chapter 6: Reactions of Alkenes - Addition Reactions: Trans-1,2-Dimethylcyclopentane Cis-1,2-DimethylcyclopentaneDocument21 paginiChapter 6: Reactions of Alkenes - Addition Reactions: Trans-1,2-Dimethylcyclopentane Cis-1,2-DimethylcyclopentaneRahma AshrafÎncă nu există evaluări
- Qoii0708 CO 17 TIFDocument34 paginiQoii0708 CO 17 TIFLovely Joysweet100% (2)
- 235practice Exam 3 AnswerDocument4 pagini235practice Exam 3 Answersowmmiya karuppiahÎncă nu există evaluări
- Ann QP 11Document4 paginiAnn QP 11technical SiteÎncă nu există evaluări
- Review Questions: Medicinal Chemistry 300550Document49 paginiReview Questions: Medicinal Chemistry 300550vanyarufusÎncă nu există evaluări
- II PUCmid Term 23Document4 paginiII PUCmid Term 23Varun. B. CÎncă nu există evaluări
- Organic Chemistry 1 Multiple Choice: Cis TransDocument4 paginiOrganic Chemistry 1 Multiple Choice: Cis Transacb4039Încă nu există evaluări
- I IIT (IRP) Chemistry Worksheet - 16Document9 paginiI IIT (IRP) Chemistry Worksheet - 16Ashwin KumarÎncă nu există evaluări
- Grade12Pre Boardexamination QPChemistryQPSET1Document7 paginiGrade12Pre Boardexamination QPChemistryQPSET1BigsmokeÎncă nu există evaluări
- Reactions of AlkenesDocument37 paginiReactions of Alkenesadamkassas1967Încă nu există evaluări
- Tugas - 6 Senyawa AlkunaDocument3 paginiTugas - 6 Senyawa AlkunaBaiq ArinÎncă nu există evaluări
- Q4 06710-12-14 Qs and AsDocument13 paginiQ4 06710-12-14 Qs and AsGhadeer M HassanÎncă nu există evaluări
- QP 3 Xi Chem Paper 3Document5 paginiQP 3 Xi Chem Paper 3technical SiteÎncă nu există evaluări
- Test Problems For Chapter 18 With AnswersDocument27 paginiTest Problems For Chapter 18 With AnswersJosh Wishman50% (2)
- XII CHEMISTRY Pre Board 2 - 2023Document6 paginiXII CHEMISTRY Pre Board 2 - 2023VOLTZÎncă nu există evaluări
- ExamDocument10 paginiExamEllen MarksÎncă nu există evaluări
- QP-Chemistry-12-Common Exam-Set-1Document6 paginiQP-Chemistry-12-Common Exam-Set-1Vijayaraj DuraiÎncă nu există evaluări
- Test1 342 PracticeV1Document5 paginiTest1 342 PracticeV1Camha NguyenÎncă nu există evaluări
- Pentadienyl CationDocument7 paginiPentadienyl CationAbhishek SardaÎncă nu există evaluări
- Por Jorge L: Uis Breña OréDocument32 paginiPor Jorge L: Uis Breña OréAlexa TorresÎncă nu există evaluări
- Organic Exam Answer.Document11 paginiOrganic Exam Answer.S JÎncă nu există evaluări
- Alkene Rxns SV Part 1Document5 paginiAlkene Rxns SV Part 1stephenmichaelramdeenÎncă nu există evaluări
- CHM 241-Practice Exam FinalDocument12 paginiCHM 241-Practice Exam FinalPreeti SharmaÎncă nu există evaluări
- Chapter 9 Alkynes: Answers Prof. Sivaguru JayaramanDocument11 paginiChapter 9 Alkynes: Answers Prof. Sivaguru JayaramanRahma AshrafÎncă nu există evaluări
- TBA Chapter9Document23 paginiTBA Chapter9Ha Vi100% (1)
- Organic Ps Chapter 7Document33 paginiOrganic Ps Chapter 7Mond DamascoÎncă nu există evaluări
- College Organic Chemistry Semester II: Practice Questions with Detailed ExplanationsDe la EverandCollege Organic Chemistry Semester II: Practice Questions with Detailed ExplanationsÎncă nu există evaluări
- 4th Conference ParticipantsDocument14 pagini4th Conference ParticipantsmaxÎncă nu există evaluări
- Antibiotic I and II HWDocument4 paginiAntibiotic I and II HWAsma AhmedÎncă nu există evaluări
- Cuerpos Extraños Origen FDADocument30 paginiCuerpos Extraños Origen FDALuis GallegosÎncă nu există evaluări
- Zoomlion Gulf FZE Introduction: 1.1 ME Service Support 1.2 Construction CasesDocument13 paginiZoomlion Gulf FZE Introduction: 1.1 ME Service Support 1.2 Construction CasesArk TradingÎncă nu există evaluări
- Cavitation in Francis PDFDocument373 paginiCavitation in Francis PDFAlberto AliagaÎncă nu există evaluări
- Lesson Plan Cot1Document9 paginiLesson Plan Cot1Paglinawan Al KimÎncă nu există evaluări
- A Modified Linear Programming Method For Distribution System ReconfigurationDocument6 paginiA Modified Linear Programming Method For Distribution System Reconfigurationapi-3697505Încă nu există evaluări
- Check Out The Buyers Guide On FacebookDocument28 paginiCheck Out The Buyers Guide On FacebookCoolerAdsÎncă nu există evaluări
- HearstDocument16 paginiHearstapi-602711853Încă nu există evaluări
- Where Business Happens Where Happens: SupportDocument19 paginiWhere Business Happens Where Happens: SupportRahul RamtekkarÎncă nu există evaluări
- Denso - History PDFDocument5 paginiDenso - History PDFVenkateswaran KrishnamurthyÎncă nu există evaluări
- Comparativa Microplex F40 Printronix P8220 enDocument1 paginăComparativa Microplex F40 Printronix P8220 enangel ricaÎncă nu există evaluări
- 11 Stem P - Group 2 - CPT First GradingDocument7 pagini11 Stem P - Group 2 - CPT First GradingZwen Zyronne Norico LumiwesÎncă nu există evaluări
- The Community Reinvestment Act in The Age of Fintech and Bank CompetitionDocument28 paginiThe Community Reinvestment Act in The Age of Fintech and Bank CompetitionHyder AliÎncă nu există evaluări
- Inkolo Namasiko Kuyamakha Umuntu - Brainly - inDocument1 paginăInkolo Namasiko Kuyamakha Umuntu - Brainly - inxqxfkqpy5qÎncă nu există evaluări
- Val Ed SyllabusDocument25 paginiVal Ed Syllabusroy piamonteÎncă nu există evaluări
- Jose André Morales, PH.D.: Ingeniería SocialDocument56 paginiJose André Morales, PH.D.: Ingeniería SocialJYMYÎncă nu există evaluări
- PDF RR Grade Sep ProjectsDocument46 paginiPDF RR Grade Sep ProjectsjunqiangdongÎncă nu există evaluări
- LOMA FLMI CoursesDocument4 paginiLOMA FLMI CoursesCeleste Joy C. LinsanganÎncă nu există evaluări
- 033 - Flight Planning Monitoring - QuestionsDocument126 pagini033 - Flight Planning Monitoring - QuestionsEASA ATPL Question Bank100% (4)
- Role of ACT, S & WHO Guidlines For The Treatment of MalariaDocument34 paginiRole of ACT, S & WHO Guidlines For The Treatment of MalariasalmanÎncă nu există evaluări
- Macro Economics A2 Level Notes Book PDFDocument33 paginiMacro Economics A2 Level Notes Book PDFMustafa Bilal50% (2)
- Group 9Document1 paginăGroup 9Kyla Jane GabicaÎncă nu există evaluări
- Chiba International, IncDocument15 paginiChiba International, IncMiklós SzerdahelyiÎncă nu există evaluări
- Topic 3 Module 2 Simple Annuity (Savings Annuity and Payout Annuity)Document8 paginiTopic 3 Module 2 Simple Annuity (Savings Annuity and Payout Annuity)millerÎncă nu există evaluări
- Mineral Claim Purchase and Sale Agreement FinalDocument5 paginiMineral Claim Purchase and Sale Agreement Finaldaks4uÎncă nu există evaluări
- DGA Furan AnalysisDocument42 paginiDGA Furan AnalysisShefian Md Dom100% (10)
- Ga-z68p-Ds3 v2.x eDocument104 paginiGa-z68p-Ds3 v2.x ejohnsonlimÎncă nu există evaluări
- CFA L1 Ethics Questions and AnswersDocument94 paginiCFA L1 Ethics Questions and AnswersMaulik PatelÎncă nu există evaluări
- Iot Practical 1Document15 paginiIot Practical 1A26Harsh KalokheÎncă nu există evaluări