Documente Academic
Documente Profesional
Documente Cultură
Isotonicfluids: Hypotonicfluids
Încărcat de
shveys_mDescriere originală:
Titlu original
Drepturi de autor
Formate disponibile
Partajați acest document
Partajați sau inserați document
Vi se pare util acest document?
Este necorespunzător acest conținut?
Raportați acest documentDrepturi de autor:
Formate disponibile
Isotonicfluids: Hypotonicfluids
Încărcat de
shveys_mDrepturi de autor:
Formate disponibile
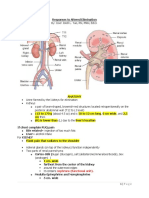
IsotonicFluids Isotonic fluids are close to the same osmolarity as serum.
They remain inside the intravascular compartment, thus expanding it. They can be helpful in hypotensive or hypovolemic patients, however, they can also be harmful. There is a risk of fluid overloading, especially in patients with congestive heart failure and hypertension. HypotonicFluids Hypotonic fluids have less osmolarity than serum (i.e. less sodium ion concentration than serum). It dilutes the serum, which decreases serum osmolarity. Water is then pulled from the vascular compartment into the interstitial fluid compartment. As the interstitial fluid is diluted, its osmolarity decreases, which draws water into the adjacent cells. Hypotonic fluids can be helpful when cells are dehydrated, such as those of a dialysis patient on diuretic therapy. They may also be used for hyperglycemic conditions such as diabetic ketoacidosis, in which high serum glucose levels draw fluid out of the cells and into the vascular and interstitial compartments. Hypotonic fluids can be dangerous to use because of the sudden fluid shift from the intravascular space to the cells. This can cause cardiovascular collapse and increased intracranial pressure in some patients. HypertonicFluids Hypertonic fluids have a higher osmolarity than serum. They pull fluid and electrolytes from the intracellular and
interstitial compartments into the intravascular compartment and can help stabilize blood pressure, increase urine output and reduce edema. They are rarely used in the pre-hospital setting and care must be taken with their use. Hypertonic fluids can be dangerous in the setting of cell dehydration. There are two main groups of fluids: crystalloid and colloid Crystalloid Crystalloids are isotonic and remain isotonic and are, therefore, effective volume expanders for a short period of time. However, both the water and the electrolytes in the solution can freely cross the semipermeable membranes of the vessel walls (but not the cell membranes) into the interstitial space and will achieve equilibrium in two to three hours. They are ideal for patients who need fluid replacement. When using an isotonic crystalloid for fluid replacement to support blood pressure from blood loss, it should be borne in mind that three milliliters of isotonic crystalloid solution are needed to replace one milliliter of patient blood. This is because approximately two-thirds of the infused crystalloid solution will leave the vascular spaces by about one hour. Generally, a good rule of thumb is that initial crystalloid replacement should not exceed three liters before whole blood is instituted. Continued use of crystalloids runs the very real risk that the fluid that has leaked into the interstitial space will result in edema, primarily in the lungs (pulmonary edema).
Examples are Lactated Ringers, normal saline. Colloid Colloids contain molecules (usually proteins) that are too large to pass out of the capillary membranes and therefore remain in the vascular compartment. The large protein molecules give colloid solutions a very high osmolarity. As a result, they draw fluid from the interstitial and intracellular compartments into the vascular compartment. They work well in reducing edema (as in pulmonary or cerebral edema) while expanding the vascular compartment. Colloids can produce dramatic fluid shifts and place the patient in considerable danger if they are not administered in a controlled settings. Examples are albumin and steroids
S-ar putea să vă placă și
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryDe la EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryEvaluare: 3.5 din 5 stele3.5/5 (231)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)De la EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Evaluare: 4.5 din 5 stele4.5/5 (119)
- Never Split the Difference: Negotiating As If Your Life Depended On ItDe la EverandNever Split the Difference: Negotiating As If Your Life Depended On ItEvaluare: 4.5 din 5 stele4.5/5 (838)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaDe la EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaEvaluare: 4.5 din 5 stele4.5/5 (265)
- Overcoming Crystal Meth Addiction An Essential Guide To Getting Clean From CM AddictionDocument444 paginiOvercoming Crystal Meth Addiction An Essential Guide To Getting Clean From CM Addictionrey_colón_5100% (1)
- The Little Book of Hygge: Danish Secrets to Happy LivingDe la EverandThe Little Book of Hygge: Danish Secrets to Happy LivingEvaluare: 3.5 din 5 stele3.5/5 (399)
- Grit: The Power of Passion and PerseveranceDe la EverandGrit: The Power of Passion and PerseveranceEvaluare: 4 din 5 stele4/5 (587)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyDe la EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyEvaluare: 3.5 din 5 stele3.5/5 (2219)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeDe la EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeEvaluare: 4 din 5 stele4/5 (5794)
- Team of Rivals: The Political Genius of Abraham LincolnDe la EverandTeam of Rivals: The Political Genius of Abraham LincolnEvaluare: 4.5 din 5 stele4.5/5 (234)
- Shoe Dog: A Memoir by the Creator of NikeDe la EverandShoe Dog: A Memoir by the Creator of NikeEvaluare: 4.5 din 5 stele4.5/5 (537)
- The Emperor of All Maladies: A Biography of CancerDe la EverandThe Emperor of All Maladies: A Biography of CancerEvaluare: 4.5 din 5 stele4.5/5 (271)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreDe la EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreEvaluare: 4 din 5 stele4/5 (1090)
- Her Body and Other Parties: StoriesDe la EverandHer Body and Other Parties: StoriesEvaluare: 4 din 5 stele4/5 (821)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersDe la EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersEvaluare: 4.5 din 5 stele4.5/5 (344)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceDe la EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceEvaluare: 4 din 5 stele4/5 (890)
- The China Study PDFDocument7 paginiThe China Study PDFPatrice108365Încă nu există evaluări
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureDe la EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureEvaluare: 4.5 din 5 stele4.5/5 (474)
- The Unwinding: An Inner History of the New AmericaDe la EverandThe Unwinding: An Inner History of the New AmericaEvaluare: 4 din 5 stele4/5 (45)
- The Yellow House: A Memoir (2019 National Book Award Winner)De la EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Evaluare: 4 din 5 stele4/5 (98)
- Physical Examination AbdomenDocument249 paginiPhysical Examination AbdomenDrbee10100% (1)
- The Brigham Intensive Review of Internal Medicine Q&A Companion, 2eDocument227 paginiThe Brigham Intensive Review of Internal Medicine Q&A Companion, 2eMalueth Angui100% (4)
- On Fire: The (Burning) Case for a Green New DealDe la EverandOn Fire: The (Burning) Case for a Green New DealEvaluare: 4 din 5 stele4/5 (73)
- Acute Kidney Injury W/ Hyperkalemia NCPDocument5 paginiAcute Kidney Injury W/ Hyperkalemia NCPMyrvic Ortiz La OrdenÎncă nu există evaluări
- Abg InterpretationDocument14 paginiAbg Interpretationmara5140100% (7)
- Hangnails and HomoeopathyDocument7 paginiHangnails and HomoeopathyDr. Rajneesh Kumar Sharma MD HomÎncă nu există evaluări
- DRUG STUDY PsycheDocument1 paginăDRUG STUDY Psychejulesubayubay5428100% (1)
- Drug Study - Nifedipine PODocument1 paginăDrug Study - Nifedipine POJet BautistaÎncă nu există evaluări
- Nephrons (Functional Unit)Document44 paginiNephrons (Functional Unit)Nur SanaaniÎncă nu există evaluări
- Lecture - 7 - Work - Capacity, - Stress - and - Fatigue (Autosaved)Document51 paginiLecture - 7 - Work - Capacity, - Stress - and - Fatigue (Autosaved)Aizat RomainoÎncă nu există evaluări
- ETHICON Encyclopedia of Knots (Noduri Chirurgicale PDFDocument49 paginiETHICON Encyclopedia of Knots (Noduri Chirurgicale PDFoctav88Încă nu există evaluări
- 49+Treacher+Collins+SyndromeDocument22 pagini49+Treacher+Collins+Syndromeshveys_mÎncă nu există evaluări
- DexametasonaDocument5 paginiDexametasonashveys_mÎncă nu există evaluări
- Evaluation of Healing Criteria For Success After Periapical SurgeryDocument5 paginiEvaluation of Healing Criteria For Success After Periapical Surgeryshveys_mÎncă nu există evaluări
- ShineDocument37 paginiShineMohd SaifÎncă nu există evaluări
- Oral Pathology Hereditary Conditions: Group IDocument74 paginiOral Pathology Hereditary Conditions: Group IFatima CarlosÎncă nu există evaluări
- It 18 - Neonatal Seizure - HerDocument35 paginiIt 18 - Neonatal Seizure - HerRurie Awalia SuhardiÎncă nu există evaluări
- Bull InfertilDocument29 paginiBull InfertilYonaaasÎncă nu există evaluări
- A Descriptive Study To Assess The Knowledge Regarding Management of Hyperemesis Gravidarum Among PostDocument6 paginiA Descriptive Study To Assess The Knowledge Regarding Management of Hyperemesis Gravidarum Among PostIJRASETPublicationsÎncă nu există evaluări
- ACTIVIDAD INGLES Reading ComprehensionDocument3 paginiACTIVIDAD INGLES Reading Comprehensionyulis mesaÎncă nu există evaluări
- Introduction To Nursing PharmacologyDocument5 paginiIntroduction To Nursing PharmacologyJon Adam Bermudez SamatraÎncă nu există evaluări
- Pgmcet 2014-15 ListDocument6 paginiPgmcet 2014-15 ListEdward FieldÎncă nu există evaluări
- C-Section Guide: What to Expect with Cesarean DeliveryDocument3 paginiC-Section Guide: What to Expect with Cesarean DeliveryFEALABREPORTSÎncă nu există evaluări
- Prescriptions On Asthma & APDDocument9 paginiPrescriptions On Asthma & APDeesha shahÎncă nu există evaluări
- Full Body To Body Massage Centre in MG Road Gurgaon Delhi NCRDocument11 paginiFull Body To Body Massage Centre in MG Road Gurgaon Delhi NCRFlip Body SpaÎncă nu există evaluări
- Effects of Malnutrition Among ChildrenDocument3 paginiEffects of Malnutrition Among ChildrenDesiree Aranggo MangueraÎncă nu există evaluări
- Autism and Dietary Therapy: Case Report and Review of The LiteratureDocument6 paginiAutism and Dietary Therapy: Case Report and Review of The LiteratureAntiopi PanteliÎncă nu există evaluări
- Lesson 3 ReviewDocument4 paginiLesson 3 ReviewHo Yong WaiÎncă nu există evaluări
- Elevated BilirubinDocument5 paginiElevated BilirubinNovita ApramadhaÎncă nu există evaluări
- Literature Review Type 2 DiabetesDocument4 paginiLiterature Review Type 2 Diabetesafmaadalrefplh100% (1)
- Ir Medical 2017 A3 PDFDocument25 paginiIr Medical 2017 A3 PDFHeidi BlueÎncă nu există evaluări
- Diet PlanDocument6 paginiDiet Plantrical27 tricalÎncă nu există evaluări
- Basic pharmacology of anaesthesia drugs in 40 charactersDocument56 paginiBasic pharmacology of anaesthesia drugs in 40 charactersrajvikram87Încă nu există evaluări
- A Case Report On Sickle Cell Disease With Hemolyti PDFDocument4 paginiA Case Report On Sickle Cell Disease With Hemolyti PDFAlhaji SwarrayÎncă nu există evaluări