Documente Academic
Documente Profesional
Documente Cultură
Dermeng 060703
Încărcat de
J.V. Siritt ChangDescriere originală:
Titlu original
Drepturi de autor
Formate disponibile
Partajați acest document
Partajați sau inserați document
Vi se pare util acest document?
Este necorespunzător acest conținut?
Raportați acest documentDrepturi de autor:
Formate disponibile
Dermeng 060703
Încărcat de
J.V. Siritt ChangDrepturi de autor:
Formate disponibile
J U N E / J U L Y
2 0 0 3
Vo l u m e 2 ,
I s s u e
DERMATOLOGY
An Overview of Basal Cell Carcinoma
By GHANIMA ALOMER, MD, and ALFRED BALBUL, MD, FRCPC
AS
TM
PRESENTED IN THE ROUNDS OF
Rounds
THE
D IVISION
OF
D ERMATOLOGY,
M C G ILL U NIVERSITY H EALTH C ENTRE
Members of the Division of Dermatology
Denis Sasseville, MD, Director Editor, Dermatology Rounds Alfred Balbul, MD Alain Brassard, MD
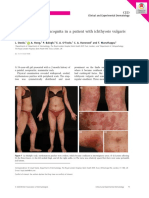
Basal cell carcinoma (BCC) is the most common malignancy in humans.1-3 It typically occurs in areas that are chronically exposed to the sun.3,4 In general, BCC is a slow-growing tumour;2 however, it has the potential for causing disgurement, especially if neglected or treated inadequately. With proper management, it usually carries an excellent prognosis. BCC generally grows by contiguous extension and metastatic BCC (MBCC) is rare.1,3,5,6 The rate of metastasis for primary BCC ranges from 0.0028% to 0.5%. Once it metastasizes, BCC follows an aggressive course and < 20% of patients who develop MBCC survive 1 year.2 BCC is generally seen in Caucasians, especially those with very fair skin. It is rare in individuals with dark skin. A male predominance with a ratio of 3:2 has been reported.3 The estimated annual incidence in the United States is 900,000 people (550,000 male, 350,000 female).7 The estimated lifetime risk of BCC in the white population is 33%-39% in men and 23%-28% in women. BCC most commonly occurs in adulthood, especially among the elderly. This issue of Dermatology Rounds describes the various clinical and histological modes of presentation, as well as the different modalities of treatment of BCC.
Judith Cameron, MD Wayne D. Carey, MD Ari Demirjian, MD Anna Doellinger, MD John D. Elie, MD Odette Fournier-Blake, MD Roy R. Forsey, MD William Gerstein, MD David Gratton, MD Raynald Molinari, MD Brenda Moroz, MD Khue Huu Nguyen, MD Elizabeth A. OBrien, MD Maria Rozenfeld, MD Wendy R. Sissons, MD Marie St-Jacques, MD Beatrice Wang, MD Ralph D. Wilkinson, MD
Pathophysiology
BCC is believed to arise from pluripotent cells within the basal layer of the epidermis or its appendages. During a lifetime, these cells are forming continuously and are capable of forming hair, sebaceous glands, and apocrine glands. Tumours usually arise from the epidermis and occasionally from the outer root sheath of a hair follicle,3 specically, from cells residing just below the sebaceous gland duct in an area called the bulge. Damage to DNA caused by ultraviolet light is thought to be the most common cause of BCC. Shorter wavelength ultraviolet (UV) radiation (290-320 nm, sunburn rays) is believed to play a greater role in BCC formation than ultraviolet A (UVA) radiation (320-400 nm, tanning rays). A long latency period of 20 to 50 years is typical between the time of UV damage and clinical onset of BCC. Ultraviolet light-induced mutations in the p53 tumour suppressor gene, which resides on chromosome 17p, are frequently seen in BCC.8,9 Additional risk factors are listed in Table 1.
Centre universitaire de sant McGill McGill University Health Centre
McGill University Health Centre Division of Dermatology Royal Victoria Hospital 687 Pine Avenue West Room A 4.17 Montreal, Quebec H3A 1A1 Tel.: (514) 934-1934, local 34648 Fax: (514) 843-1570
The editorial content of Dermatology Rounds is determined solely by the Division of Dermatology, McGill University Health Centre.
Mortality/morbidity
BCC can cause signicant morbidity if allowed to progress. Cosmetic disgurement is not uncommon since this cancer most commonly affects the head and neck, occurring more rarely on the arms, hands, and buttocks.15 In addition, these neoplasms are often very friable and prone to ulcerate, providing a nidus for infection. Death due to BCC is extremely rare.
Clinical features History
Patients often present with a nonhealing sore of varying duration. These lesions are typically seen on the head and neck area or upper trunk. Minimal trauma, such as face washing, may initiate bleeding. A history of chronic recreational or occupational sun exposure is commonly elicited.
t ne r e a Int nds.c e h ou n t logyr o le ato b a rm ail w.de v A w
w
Table 1: Risk factors for BCC Ultraviolet radiation: UVB > UVA10 Other radiation: X-rays and grenz rays2 Arsenic exposure: medicinal, occupational, or dietary [ie, contaminated water supply]11 Immunosuppression: Modest increase in the risk of BCC3 History of previous nonmelanoma skin cancer (BCC or squamous cell (SC) carcinoma): Incidence of new non-metastatic SC is 35% at 3 years and 50% at 5 years after initial skin cancer diagnosis12-14
Morpheaform and inltrating BCC
These represent aggressive forms of BCC that present with sclerotic plaques or papules.16 Usually they have ill-dened borders and often extend well beyond the clinically discernable margins. In addition, since ulceration, bleeding, and crusting are uncommon, they can easily be mistaken for scar tissue.
Cystic BCC
Lesions of cystic BCC are translucent blue-gray nodules that may mimic benign cystic lesions.16
Fibroepithelioma of Pinkus
This is an unusual variant of BCC that is most often seen on the lower trunk, particularly the lumbosacral area.3 Lesions may be single or multiple and take the form of raised, moderately rm, slightly pedunculated nodules, covered by smooth, slightly reddened skin. Clinically, they resemble bromas and usually run an indolent course, but they may become invasive.
Physical examination
Several clinical and histological subtypes of BCC have been identied. These exhibit different patterns of behaviour. Recognizing the various types is important since more aggressive therapy is often necessary for aggressive variants of BCC (eg, micronodular, inltrating, and morpheaform BCC). Use of good lighting and magnication are very important in the examination of such lesions. The affected skin should be stretched and palpated to better estimate tumour size and depth.
Clinical syndromes with BCC Xeroderma pigmentosum
This autosomal-recessive disease predisposes to rapid aging of sun-exposed skin, starting with pigmentary changes and progressing to BCC, squamous cell carcinoma, and malignant melanoma. The effects are due to an inability to repair UV-induced DNA damage.3 Other features include corneal opacities leading to blindness and neurologic decits.
Clinical Variants Nodular BCC
This is the most common variety of BCC.1,3,16 It occurs most frequently on the head, neck, and upper back, and exhibits some of the following features: waxy papules with central depression or pearly appearance, erosions or ulceration, crusting, rolled (raised) border, translucency and telangiectases over the surface. The patient frequently gives a history of bleeding with minor trauma.
Nevoid BCC syndrome (basal cell nevus syndrome, Gorlin syndrome)
This syndrome is an autosomal-dominant condition, characterized by early and continued appearance of many BCCs. Clinical features include odontogenic keratocysts, palmoplantar pitting, intracranial calcication, and rib anomalies. Various tumours, (eg, medulloblastomas, meningiomas, fetal rhabdomyomas, and ameloblastomas) can also occur. Some data support mutation of a tumour suppressor gene on band 9q22 as the cause of the nevoid BCC syndrome (PTCH GENE).3,8,9
Pigmented BCC
As the name implies, these lesions contain increased brown or black pigment and are seen more commonly in individuals with dark skin;1,3,16 otherwise, they have similar features to nodular BCCs. At times, they may be mistaken for melanocytic nevi or malignant melanomas.
Bazex syndrome
Features of this syndrome include follicular atrophoderma (ice pick marks, especially on the dorsum of the hands), multiple BCCs, and local anhidrosis.17
Supercial BCC
This variety presents as scaly plaques or papules that are pink- to red-brown in colour, with a threadlike border, and often with central clearing.3,16 Erosions are seen less commonly than in nodular BCC. It occurs most commonly on the trunk and has little tendency to become invasive. It may mimic psoriasis, eczema, or irritated seborrheic keratosis (SK), but is slowly progressive and not prone to uctuate in appearance. Occurrence of numerous supercial BCCs may raise the suspicion of arsenic exposure.
Histological ndings Nodular BCC
Tumour cells of nodular BCC sometimes called basalioma cells typically have large, hyperchromatic, oval nuclei with scant cytoplasm. Cells appear uniform and mitotic gures are usually few in number.3,18,19 Nodular tumour aggregates may be of varying sizes, but
Table 2: Differential diagnosis of BCC Actinic keratosis Bowens disease Fibrous papule Keratoacanthoma Melanocytic nevi and melanoma Sebaceous hyperplasia Seborrheic keratosis Squamous cell carcinoma Trichoepithelioma
Micronodular BCC
This aggressive variant appears as small nodular aggregates of basaloid cells.18 Retraction artifact tends to be less pronounced than in the nodular form of BCC and subclinical involvement is often signicant.
Fibroepithelioma of Pinkus
Histologically, in this variant, there are features of both supercial BCC and reticulated seborrheic keratosis. Long, branched strands of basaloid cells are connected to the overlying epidermis and embedded in a bromyxoid stroma.3,18
Basosquamous carcinoma
These tumours exhibit features of both BCC and squamous cell carcinoma;18 they are also considered aggressive skin cancers.
tumour cells tend to align more densely in a palisade pattern at the periphery of these nests. Early lesions may exhibit some connection to the overlying epidermis, but such contiguity may be difcult to appreciate in more advanced lesions. Increased mucin may be present in the surrounding dermal stroma. Cleft formation commonly occurs between BCC nests and surrounding stroma. Known as retraction artifact, it occurs during tissue xation and staining. Some lobules may exhibit areas of pseudoglandular change and this is the predominant change in adenoid BCC. In other instances, larger tumour lobules may degenerate centrally, forming pseudocystic spaces lled with debris. These changes are seen in the nodulocystic variant of BCC.
Diagnosis
Under its various guises, BCC may mimic other cutaneous tumours, as shown in Table 2. Skin biopsy is the gold standard to conrm the diagnosis and to identify the histologic subtype of the BCC. Additional workup is rarely necessary unless a genetic disorder is suspected. A skin biopsy may be performed by one of the following techniques. Shave biopsy: This simple technique is sufcient to diagnose most BCCs.20 It is best to avoid an exceedingly supercial biopsy, because it is possible to miss the tumour. For example, an ulcerated BCC may reepithelialize with normal epidermis, while a tumour still exists at a deeper level.21-23 While part or all of the BCC may be sampled, going beyond the clinical margins is unnecessary if the biopsy is performed for diagnostic purposes only. Punch biopsy: The most suspicious area of a lesion may be sampled or multiple biopsies may be taken if the tumour has different appearances in different areas.20,21 This method allows for a thicker specimen. A punch biopsy is to be avoided if curettage is planned as the denitive treatment. Curettage biopsy: This is a preferred method among several of the clinicians in our division. It has the advantage of selectively sampling the tumour, while sparing normal skin;21 however, it is less useful for lesions with a sclerotic component.
Pigmented BCC
In this variant, benign melanocytes in and around the tumour produce large amounts of melanin18,19 and these melanin granules are picked up by surrounding tumour cells.
Supercial BCC
These appear as buds of basaloid cells attached to the undersurface of the epidermis or hair follicles.18 Nests of various sizes are often seen in the upper dermis. The tumour cell aggregates typically show peripheral palisading with cleft formation.18,19
Morpheaform BCC and inltrating BCC
Morpheaform BCC arises as thin strands of tumour cells (often only 1 cell in thickness) that are embedded in a dense, brous stroma. Inltrating BCC usually does not exhibit the scar-like stroma seen in morpheaform BCC.18 The cellular strands tend to be somewhat thicker and they have a spiky, irregular appearance. Peripheral palisading and retraction are much less pronounced in morpheaform and inltrating BCC than in less aggressive forms of the tumour, and subclinical involvement is often extensive.
Treatment
The choice of a given modality of treatment depends on many factors (eg, size and location of the tumour, age of the patient, presence of associated medical conditions, and concomitant medication). Furthermore, the physicians experience will often weigh heavily in the selection of the preferred mode of treatment. Techniques most commonly used by dermatologists include curettage,
excision with margin examination, and Mohs micrographic surgery. Curettage and electrodesiccation: This is the most commonly used method, usually for relatively small lesions (<2 cm) located in low-risk areas.2 A looped blade (curette) is used to vigorously scrape the tumour away from adjacent normal skin.21,24 Start with a larger curette to debulk the tumour and follow with a smaller curette to better remove smaller fragments of tumour from surrounding stroma. Thorough curettage is followed by hemostasis and electrodesiccation. This technique works best in nodular or supercial BCCs because these tumours tend to be friable and tend not to be embedded in brous stroma. The success of this treatment, even in nonaggressive tumours in low-risk sites, is highly dependent on operator experience and technique. Many recurrences after curettage are believed to be due to insufcient aggressiveness on the part of the surgeon. The overall cure rate exceeds 90% for lowrisk BCCs.25 The method is quick and simple and is less expensive than most other methods. Drawbacks include the blind nature of the technique since the specimen cannot be examined for margin control. In addition, while sparing normal skin, healing by second intention often leads to atrophic white scars that may not be satisfactory in aesthetically important areas;21 however, it is less useful for lesions with a sclerotic component. These more aggressive subtypes of BCC, such as morpheaform, inltrating, micronodular, and recurrent tumours, tend to be less friable and therefore, less likely to be removed by the curette.2 Cryosurgery: This treatment was originally described for relatively small, early BCC in non high-risk areas, as well as for the supercial types of BCC. Cryosurgery is also an effective treatment for most nonaggressive BCCs, with cure rates approaching 90%.21 Optimal cure rates are obtained when depth, duration, and temperature of treatment are measured with special instrumentation (eg, cryoprobes). However, successful treatment is highly dependent on the experience of the operator. Patients must be willing to endure the immediate post-treatment swelling, resultant necrosis of treated areas, and unpredictable scarring that can occur with this approach. This method is not commonly used for treatment of BCC, except by a few very experienced cryosurgeons. Curettage followed by cryosurgery: This method is a combination of curettage as outlined earlier, followed by hemostasis (typically with aluminum chloride), and completed with cryosurgery using a cryospray.21 The tumour is rst removed macroscopically with the curette.26 This is followed by
freezing any microscopic foci that may persist. This method has the advantage of being less destructive to the surrounding stroma and, with modern wound care techniques, leads to a much better cosmetic result than traditional curettage and electrodesiccation. In our hands, recurrences have been remarkably uncommon. Surgical excision: This modality entails surgical excision of the clinically apparent tumour and a margin of clinically normal-appearing skin.3,21,27 Usually, this method can be performed in an ambulatory setting and provides the pathologist with a specimen for examination of tissue margins. Healing time is generally shorter with sutured closure than with secondary intention, and cosmesis compares favorably with curettage. However, surgical excision is more time consuming and costly than curettage. Also, this method requires sacrice of normal tissue to obtain acceptable cure rates. At least 4-mm margins are recommended in even the least aggressive BCCs to achieve 95% cure rates. Frequently, this is not performed and so recurrence rates are > 5%.3 Another drawback is related to the processing of the pathological specimen; if a standard bread-loaf tissue sectioning is used, areas of margin involvement may be missed microscopically, since only a small sampling of the specimen is evaluated. Mohs micrographic surgery: This method, described in detail in the March 2003 issue of Dermatology Rounds, is reserved for high-risk areas, large BCC (> 2 cm), recurrent lesions,2,3 and is performed by a specially-trained dermatosurgeon. Mohs surgery implies removal of the clinically apparent tumour together with a thin rim of normal-appearing skin.21 This saucer-shaped tissue specimen represents tissue adjacent to the tumour or the margin surrounding the tumour. This margin specimen is sectioned and marked such that the entirety of the undersurface and outer edges of the tumour are microscopically examined and sampling error is minimized.28 Use of the frozen section technique allows examination of tissue while the patient is in the ofce. Tissue is mapped microscopically, such that if any foci of tumour persist, further excision can be directed only to those areas, sparing normal tissue. The Mohs technique examines almost 100% of the tissue margins,28 compared to standard vertical (bread-loaf) sectioning that examines < 1% of the outer margins. Because relatively thin layers are taken only in areas of proven tumour, this technique is tissue sparing. Usually, excision and repair can be performed on the same day. Most importantly, Mohs micrographic surgical excision results in the best long-term cure rates of any treatment modality for BCC. Cure rates for primary BCC are 98%-99%
DERMATOLOGY
Rounds
Table 3: Recurrence rates after treatment of primary BCC3 Treatment modality Surgical excision Radiation therapy Cryotherapy All non-Mohs modalities Mohs micrographic surgery 5-year recurrence rate 10% 8-9% 7-7.5% 8-9% 1.0%
margins. Following radiotherapy, patients must be followed closely for recurrence and for late radiation-induced malignancy.31
Prognosis
BCC that is treated incompletely can recur, therefore, all treated sites must be monitored following therapy.32,33 The recurrence rate after seemingly adequate treatment is shown in Table 3. Individuals who are diagnosed with BCC have an approximately 30% greater risk than the general population of being diagnosed with another BCC unrelated to the previous lesion.14
Curettage and electrodesiccation 7-8%
with Mohs excision and 94%-96% for recurrent BCC.3 The chief disadvantages of Mohs surgery are greater expense and time compared to curettage. Topical 5-uorouracil (Efudex 5% cream): This topical treatment may be tried for small BCC, especially supercial types on the trunk.29 It may also be used with patients for whom surgery is not practical. Our experience with this modality is very limited. Topical imiquimod (Aldara 5% cream): Recent studies show that this topical treatment is effective in supercial BCC. Imiquimod, which induces the release of cytokines involved in cell-mediated immune responses including interferon, was effective in curing almost 90% of supercial BCCs in a multicentre trial in Australia. This recently introduced method is used in different settings such as for small lesions and supercial BCC where scarring is a concern (eg, in a young patient with a small lesion on the face). In some cases, there is virtually no scar after the lesion has resolved. We routinely use Aldara for patients where a small lesion is biopsied and pathology conrms the diagnosis of BCC, but no residual tumour mass is left. The factors limiting its use are irritation and cost (1 box costs about $160-180.). In conclusion, this is a promising new modality for the treatment of selected cases of BCCs. The optimal treatment protocols (eg, frequency of application and duration of treatment) have yet to be determined. Radiation therapy: Radiation therapy is rarely used in our centre. Primarily, it is used when surgery is difcult (patients are elderly and debilitated, and therefore, not good surgical candidates) or contraindicated. It also is useful for patients who refuse surgery because of the size of a lesion or its proximity to vital structures.2 Radiotherapy is effective as primary treatment for most nonaggressive BCCs, but it is not the optimal therapy for aggressive types.30 With fractionation of the radiation dose, an excellent cosmetic result is the rule. Disadvantages include the potential for late radiation changes in the skin, as well as the inability to examine skin
Patient education
Avoiding exposure to UV radiation is encouraged. Helpful preventive measures include carefully planning outdoor activities, wearing a broadbrimmed hat during outdoor activities, and using sunscreens with SPF 15 or over.34
References 1. Lang PG, Maize JC. Basal cell carcinoma. In: Friedman RJ, Rigel, Kopf AW, Harris MN, Baker D, eds. Cancer of the Skin 1991: 35-73. 2. Schoelch SB. Recognition and management of high-risk cutaneous tumors. Dermatol Clin 1999;17(1):93-111, viii-ix . 3. Basal Cell Carcinoma. In: Freedberg IM, Fitzpatrick TB, et al. Eds. Fitzpatricks Dermatology In General Medicine. 5th ed , 2 vol. New York: McGraw-Hill Health Professions Division; 1998:857-863. 4. Woolley T. Sun-related behaviours of outdoor workingmen with a history of non-melanoma skin cancer. J Occup Environ Med 2002; 44(9):847-54 . 5. Padgett JK. Cutaneous malignancies and their management. Otolaryngol Clin North Am 2001;34(3):523-53. 6. Preston DS, Stern RS. Nonmelanoma cancers of the skin. N Engl J Med 1992;327(23):1649-62. 7. Miller DL, Weinstock MA. Nonmelanoma skin cancer in the United States: incidence. J Am Acad Dermatol 1994;30(5 Pt 1): 774-8. 8. Ratner D. UV-specic p53 and PTCH mutations in sporadic basal cell carcinoma of sun-exposed skin. J Am Acad Dermatol 2001; 44(2):293-7. 9. Mirowski GW. Nevoid basal cell carcinoma syndrome. J Am Acad Dermatol 2000;43(6):1092-3. 10. Pennello G. Association of surface ultraviolet B radiation levels with melanoma and nonmelanoma skin cancer in the United States blacks. Cancer Epidemiol Biomarkers Prev 2000;9(3):291-7. 11. Boonchai W. Basal cell carcinoma in chronic arsenicism occurring in Queensland, Australia, after ingestion of an asthma medication. J Am Acad Dermatol 2000;43(4):664-9. 12. Schinstine M. Risk of synchronous and metachronous second nonmelanoma skin cancer when referred for Mohs micrographic surgery. J Am Acad Dermatol 2001;44(3):497-9. 13. Frankel DH, Hanusa BH, Zitelli JA. New primary nonmelanoma skin cancer in patients with a history of squamous cell carcinoma of the skin. Implications and recommendations for follow-up. J Am Acad Dermatol 1992;26(5 Pt 1):720-6. 14. Karagas MR. Occurrence of cutaneous basal cell and squamous cell malignancies among those with a prior history of skin cancer. The Skin Cancer Prevention Study Group. J Invest Dermatol 1994;102(6):10S-13S. 15. Herzinger T. Basal cell carcinoma of the toenail unit. J Am Acad Dermatol 2003;48(2):277-8. 16. Maloney ME, Miller SJ. Aggressive versus nonaggressive subtypes (basal cell carcinoma). In: Miller SJ, Maloney ME, Eds. Cutaneous Oncology: pathophysiology, diagnosis, and management. Malden, MA: Blackwell Science; 1998:609-613. 17. Metry D, Guill C. Generalized basaloid follicular hamartoma syndrome. J Am Acad Dermatol 2001;45(4):644-6. 18. Kirkham N. Tumors and cysts of the epidermis. In: Elder D, Elenitsas R, Jaworsky C, Johnson B Jr, Eds. Levers Histopathology of the Skin. 8th ed. Philadelphia, PA: Lippincott-Raven; 1997:719-746.
DERMATOLOGY
Rounds
19. Lowe L. Histology (basal cell carcinoma). In: Miller SJ, Maloney ME, Eds. Cutaneous Oncology: pathophysiology, diagnosis, and management. Malden, MA: Blackwell Science; 1998:633-645. 20. Russell EB. Basal cell carcinoma: a comparison of shave biopsy versus punch biopsy techniques in subtype diagnosis. J Am Acad Dermatol 1999; 41(1):69-71. 21. Orengo I. Techniques in the removal of skin lesions. Otolaryngol Clin North Am 2002;35(1):153-70, vii. 22. Michael E. Lutz MD. Surgical Pearl Curettage before cutaneous surgery to identify an unidentiable biopsy site. J Am Acad Dermatol 2002;46: 591-2. 23. Holmkvist KA. Incidence of residual basal cell carcinoma in patients who appear tumor free after biopsy. J Am Acad Dermatol 1999;41(4):600-5. 24. Goldman G. The current status of curettage and electrodesiccation. Dermatol Clin 2002;20(3):569-78, ix. 25. Silverman MK, Kopf AW, Grin CM, et al. Recurrence rates of treated basal cell carcinomas. Part 2: Curettage- electrodesiccation. J Dermatol Surg Oncol 1991;17(9):720-6. 26. Nordin P. Five-year results of curettage-cryosurgery for 100 consecutive auricular non-melanoma skin cancers. J Laryngol Otol 2002;116(11):893-8. 27. Ratner D, Skouge JW. Surgical management of local disease (basal cell carcinoma). In: Miller SJ, Maloney ME, Eds. Cutaneous Oncology: pathophysiology, diagnosis, and management. Malden, MA: Blackwell Science; 1998:664-671. 28. Florell SR. A comparison of touch imprint cytology and Mohs frozensection histology in the evaluation of Mohs micrographic surgical margins. J Am Acad Dermatol 2001;44(4):660-4. 29. Kerr C. Rub on treatment for basal-cell carcinoma. Lancet Oncol 2002; 3(4):201. 30. Habeck M. Good results with radiotherapy for BCCs. Lancet Oncol 2001;2(6);326. 31. Little MP. Cancer after exposure to radiation in the course of treatment for benign and malignant disease. Lancet Oncol 2001;2(4):212-20. 32. Robinson JK. Recurrent basal cell carcinoma after incomplete resection. Arch Dermatol 2000;136(11):1318-24. 33. Robinson JK. What are adequate treatment and follow-up care for nonmelanoma cutaneous cancer? Arch Dermatol 1987;123(3):331-3. 34. Ramirez R. Practical guide to sun protection. Surg Clin North Am 2003; 83(1);97.
frequently, he disagrees with some of the truisms expressed in the literature about CE and attempts to dene carefully what he believes are the strengths and limitations of this technique. Dermatol Clin 2002;20(3):569-78, ix.
Cutaneous malignancies and their management
PADGETT JK, H ENDRIX JD J R , C HARLOTTESVILLE , VA Skin cancer is the most common malignancy occurring in humans, and the incidence of basal cell carcinoma, squamous cell carcinoma, and melanoma continues to rise. Advances in the diagnosis and treatment of skin cancer have led to more successful management of these tumors. A number of options for the treatment of skin cancer are available to the patient and physician, allowing for high cure rates and excellent functional and cosmetic outcomes. Otolaryngol Clin North Am 2001;34(3):523-53.
Upcoming Scientic Meetings
25-29 July 2003 ACADEMY 03 Summer Meeting of the American Academy of Dermatology Hyatt Regency, Chicago, IL CONTACT: AAD www.aad.org Tel: 847-330-0230 Fax: 847-330-1090 19-20 September 2003 Twenty-fourth Paul A. OLeary Meeting and Minnesota Dermatologic Society Rochester, Minnesota CONTACT: Mayo Clinic Dept. of Dermatology Tel: 507-284-3668 Fax: 507-284-2072 16-19 October 2003 Fall Clinical Dermatology Conference Luxor, Las Vegas CONTACT : Center for Bio-Medical Communication Tel: 201-883-5874 Fax: 201-342-7555 www.cbcbiomed.com 15-19 October 2003 Dermatology Chicago Wyndham Chicago Hotel CONTACT : Skin Disease Education www.sdefderm.com Fax: 312-988-7759
Abstracts of Interest
The current status of curettage and electrodesiccation
G OLDMAN G., B URLINGTON, VT Curettage and electrodesiccation (CE) is a technique widely used in the destruction of benign and selected malignant cutaneous neoplasms. CE is used mainly by dermatologists and family practice physicians, whereas plastic surgeons and other surgeons excise most benign and malignant lesions. The use of CE for the treatment of skin cancer has been widely extolled and also fervently criticized. Some practitioners treat most non-melanoma skin cancers (NMSC) with CE, and others have called for abandoning the technique in the treatment of such lesions. A thorough review of the literature reveals that CE has both virtues and aws. In taking a rational approach to the treatment of benign and malignant cutaneous lesions, it is essential to learn the basis for CE, the likely cure rates for given lesions, the proper technique, and the expected level of cosmesis. As the surgical treatment of skin cancer has become rmly entrenched in the eld of dermatology, it is valuable to examine this technique in depth and to come to some thoughtful conclusions about its use for patients with skin cancer and assorted benign skin lesions. Last year, a remarkably complete and exhaustive favorable review of curettage, electrosurgery, and skin cancer was published by Sheridan and Dawber. This article is a must read for anyone performing curettage; however, the author is writing with a slightly different perspective, that of a dermatologic surgeon. Although the author believes that CE has value and he uses this technique
Change of address notices and requests for subscriptions for Dermatology Rounds are to be sent by mail to P.O. Box 310, Station H, Montreal, Quebec H3G 2K8 or by fax to (514) 932-5114 or by e-mail to info@snellmedical.com. Please reference Dermatology Rounds in your correspondence. Undeliverable copies are to be sent to the address above.
This publication is made possible by an educational grant from
Novartis Pharmaceuticals Canada Inc.
2003 Division of Dermatology, McGill University Health Centre, Montreal, which is solely responsible for the contents. The opinions expressed in this publication do not necessarily reect those of the publisher or sponsor, but rather are those of the authoring institution based on the available scientic literature. Publisher: SNELL Medical Communication Inc. in cooperation with the Division of Dermatology, McGill University Health Centre. Dermatology Rounds is a Trade Mark of SNELL Medical Communication Inc. All rights reserved. The administration of any therapies discussed or referred to in Dermatology Rounds should always be consistent with the recognized prescribing information in Canada. SNELL Medical Communication Inc. is committed to the development of superior Continuing Medical Education.
SNELL
124-010
S-ar putea să vă placă și
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeDe la EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeEvaluare: 4 din 5 stele4/5 (5794)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreDe la EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreEvaluare: 4 din 5 stele4/5 (1090)
- Never Split the Difference: Negotiating As If Your Life Depended On ItDe la EverandNever Split the Difference: Negotiating As If Your Life Depended On ItEvaluare: 4.5 din 5 stele4.5/5 (838)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceDe la EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceEvaluare: 4 din 5 stele4/5 (895)
- Grit: The Power of Passion and PerseveranceDe la EverandGrit: The Power of Passion and PerseveranceEvaluare: 4 din 5 stele4/5 (588)
- Shoe Dog: A Memoir by the Creator of NikeDe la EverandShoe Dog: A Memoir by the Creator of NikeEvaluare: 4.5 din 5 stele4.5/5 (537)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersDe la EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersEvaluare: 4.5 din 5 stele4.5/5 (345)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureDe la EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureEvaluare: 4.5 din 5 stele4.5/5 (474)
- Her Body and Other Parties: StoriesDe la EverandHer Body and Other Parties: StoriesEvaluare: 4 din 5 stele4/5 (821)
- The Emperor of All Maladies: A Biography of CancerDe la EverandThe Emperor of All Maladies: A Biography of CancerEvaluare: 4.5 din 5 stele4.5/5 (271)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)De la EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Evaluare: 4.5 din 5 stele4.5/5 (121)
- The Little Book of Hygge: Danish Secrets to Happy LivingDe la EverandThe Little Book of Hygge: Danish Secrets to Happy LivingEvaluare: 3.5 din 5 stele3.5/5 (400)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyDe la EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyEvaluare: 3.5 din 5 stele3.5/5 (2259)
- The Yellow House: A Memoir (2019 National Book Award Winner)De la EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Evaluare: 4 din 5 stele4/5 (98)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaDe la EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaEvaluare: 4.5 din 5 stele4.5/5 (266)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryDe la EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryEvaluare: 3.5 din 5 stele3.5/5 (231)
- Team of Rivals: The Political Genius of Abraham LincolnDe la EverandTeam of Rivals: The Political Genius of Abraham LincolnEvaluare: 4.5 din 5 stele4.5/5 (234)
- On Fire: The (Burning) Case for a Green New DealDe la EverandOn Fire: The (Burning) Case for a Green New DealEvaluare: 4 din 5 stele4/5 (74)
- Cidesco Case Histories BookletDocument11 paginiCidesco Case Histories Bookletmatina papaspyrouÎncă nu există evaluări
- The Unwinding: An Inner History of the New AmericaDe la EverandThe Unwinding: An Inner History of the New AmericaEvaluare: 4 din 5 stele4/5 (45)
- U.S. Type Identification Guide: KP Numismatics Digital DownloadDocument8 paginiU.S. Type Identification Guide: KP Numismatics Digital DownloadJ.V. Siritt ChangÎncă nu există evaluări
- Epidemiology and Bio StatisticsDocument108 paginiEpidemiology and Bio Statisticssaeedoof100% (15)
- Building Online Employability: A Guide For Academic DepartmentsDocument33 paginiBuilding Online Employability: A Guide For Academic DepartmentsJ.V. Siritt ChangÎncă nu există evaluări
- OMS Urinary System and Male Genital OrgansDocument354 paginiOMS Urinary System and Male Genital OrgansMaggy Melpómene100% (1)
- Origin of Mitochondria - Learn Science at ScitableDocument4 paginiOrigin of Mitochondria - Learn Science at ScitableJ.V. Siritt ChangÎncă nu există evaluări
- Benign and Premalignant Disease of The CervixDocument23 paginiBenign and Premalignant Disease of The CervixJ.V. Siritt ChangÎncă nu există evaluări
- Build Your Own Dobsonian TelescopeDocument22 paginiBuild Your Own Dobsonian TelescopeJ.V. Siritt ChangÎncă nu există evaluări
- Financing Health Care: Concepts, Challenges and Practices: April 28 - May 9, 2014Document2 paginiFinancing Health Care: Concepts, Challenges and Practices: April 28 - May 9, 2014J.V. Siritt ChangÎncă nu există evaluări
- Sky and Telescope 2012 09Document89 paginiSky and Telescope 2012 09J.V. Siritt Chang100% (1)
- OMS Urinary System and Male Genital OrgansDocument354 paginiOMS Urinary System and Male Genital OrgansMaggy Melpómene100% (1)
- Print VersionDocument5 paginiPrint VersionJ.V. Siritt ChangÎncă nu există evaluări
- Build Your Own Dobsonian TelescopeDocument22 paginiBuild Your Own Dobsonian TelescopeJ.V. Siritt ChangÎncă nu există evaluări
- Aurochem GlobalDocument16 paginiAurochem GlobalEyeberdi AllaberdiyevÎncă nu există evaluări
- QuatrocreamDocument2 paginiQuatrocreamapi-446758399Încă nu există evaluări
- Disseminated Tinea Incognita in A Patient With Ichthyosis Vulgaris and EczemaDocument3 paginiDisseminated Tinea Incognita in A Patient With Ichthyosis Vulgaris and EczemaNovia KurniantiÎncă nu există evaluări
- Skin PathologyDocument3 paginiSkin Pathologychrisp7Încă nu există evaluări
- Libro - Isdin - Curvas - Ok C01 - 13-11-20Document60 paginiLibro - Isdin - Curvas - Ok C01 - 13-11-20Miguel RomeroÎncă nu există evaluări
- SNE 7100 Pre Clinical Evaluation ReportDocument41 paginiSNE 7100 Pre Clinical Evaluation ReportMarta MüllerÎncă nu există evaluări
- Module 3 HomeworkDocument6 paginiModule 3 HomeworkCj LinceÎncă nu există evaluări
- Integumentary System DisordersDocument15 paginiIntegumentary System Disordersapi-236649988Încă nu există evaluări
- 3rd Announcement INDALAS 11 - 3Document20 pagini3rd Announcement INDALAS 11 - 3Yudha PermanaÎncă nu există evaluări
- EczemaDocument7 paginiEczemaNader SmadiÎncă nu există evaluări
- Introduction To Dermatology: Lecture-1Document33 paginiIntroduction To Dermatology: Lecture-1Latika ChoudhuryÎncă nu există evaluări
- Dermatology PDFDocument3 paginiDermatology PDFSyed Zafar Ali MeerzaÎncă nu există evaluări
- Is Keratosis Pilaris Curable - Google SearchDocument1 paginăIs Keratosis Pilaris Curable - Google Search1AB 至Încă nu există evaluări
- Nonbullous Pemphigoid: Prodrome of Bullous Pemphigoid or A Distinct Pemphigoid Variant?Document7 paginiNonbullous Pemphigoid: Prodrome of Bullous Pemphigoid or A Distinct Pemphigoid Variant?FatimaÎncă nu există evaluări
- Benefits Coffe ArticleDocument2 paginiBenefits Coffe ArticlefirdhaaliviaÎncă nu există evaluări
- Keratosis Pilaris Rubra and Keratosis PiDocument5 paginiKeratosis Pilaris Rubra and Keratosis Pidaily of sinta fuÎncă nu există evaluări
- OMOROVICZADocument10 paginiOMOROVICZARobert MarshallÎncă nu există evaluări
- Milia PaperDocument14 paginiMilia PaperWito Eka PutraÎncă nu există evaluări
- Acne Patient LeafletDocument4 paginiAcne Patient LeafletAbdul KareemÎncă nu există evaluări
- Tranacix en TelangectasiasDocument16 paginiTranacix en TelangectasiasAreli ValdiviaÎncă nu există evaluări
- Akingbola Traction Alopecia - A Neglected Entity in 2017Document6 paginiAkingbola Traction Alopecia - A Neglected Entity in 2017Ernawati HidayatÎncă nu există evaluări
- MCS 200 - 6 - Skin ConditionsDocument76 paginiMCS 200 - 6 - Skin ConditionsYotam MbaoÎncă nu există evaluări
- Prinsipal Kode Produk Nama ProdukDocument40 paginiPrinsipal Kode Produk Nama ProdukjihanvrpÎncă nu există evaluări
- Common TreatmentsDocument4 paginiCommon TreatmentsAdeline N. OmeneÎncă nu există evaluări
- A Study of The Efficacy of Cleansers For Acne VulgarisDocument5 paginiA Study of The Efficacy of Cleansers For Acne Vulgarisgran vittaÎncă nu există evaluări
- Daftar Pustaka NewDocument2 paginiDaftar Pustaka NewRini LianingsihÎncă nu există evaluări
- EfloresensiDocument3 paginiEfloresensiputriana safitriÎncă nu există evaluări
- About & Services (DR Shruti)Document2 paginiAbout & Services (DR Shruti)abhinav.arya.9822Încă nu există evaluări
- Dermatovenerology CS 253 PDFDocument36 paginiDermatovenerology CS 253 PDFIvanes IgorÎncă nu există evaluări