Documente Academic
Documente Profesional
Documente Cultură
Mgcl2 Vs Mgso4
Încărcat de
Cholid RiskiantoDescriere originală:
Titlu original
Drepturi de autor
Formate disponibile
Partajați acest document
Partajați sau inserați document
Vi se pare util acest document?
Este necorespunzător acest conținut?
Raportați acest documentDrepturi de autor:
Formate disponibile
Mgcl2 Vs Mgso4
Încărcat de
Cholid RiskiantoDrepturi de autor:
Formate disponibile
Magnesium Research 2005; 18 (3): 187-92
ORIGINAL ARTICLE
Review paper
Magnesium chloride or magnesium sulfate: a genuine question
J. Durlach1, A. Guiet-Bara2, N. Pags3, P. Bac4, M. Bara2 SDRM, 64 rue de Lonchamp, 92200 Neuilly, France ; 2 Laboratoire de physiologie et physiopathologie, Universit P. et M. Curie, Paris, France ; 3 Laboratoire de toxicologie, Facult de pharmacie, Universit Louis Pasteur, Strasbourg, 67400 IllkirchGrafenstaden, France ; 4 Laboratoire de pharmacologie, Facult de pharmacie, Paris XI, Pierre Maurois, 92290 Chatenay-Malabry, France
Correspondence: Dr. Michel Bara, Direction des tudes de lUniversit Pierre et Marie Curie, 4 place Jussieu, 75252 Paris Cedex 05, France. Tel.: (+ 00 33) 1 44 27 38 57 ; fax: (+ 00 33) 1 44 27 25 71. <michel.bara@upmc.fr>
1
Abstract. MgSO4 is routinely used in therapeutics despite its toxicity. The aim of the present review was to compare MgSO4 and MgCl2 effects in order to answer the question whether MgSO4 could be or not replaced by MgCl2. Considering that the two salts have both similar and proper effects, a clear-cut conclusion is not easy to draw. However, choosing MgCl2 seems advisable because of its more interesting clinical and pharmacological effects and its lower tissue toxicity as compared to MgSO4.
Keywords: chloride, magnesium, sulfate
Magnesium ions are known to play a central role in cellular function and to strongly influence the cardiovascular and neuromuscular excitability. They are also an important factor in both the growth and maintenance of living cells and an essential co-factor for many intracellular enzymes involved in both glycolytic metabolism and ion movements mediated by Na and Ca pumps [1]. Magnesium sulphate (MgSO4, 7H2O) is commonly used, in the United States, as prophylactic and clinical treatment of eclamptic seizures whereas in Europe, its use is highly debated in that indication. Conversely, magnesium chloride is not frequently used in either physiology or physiopathology. Nonetheless, it has been postulated, but not established, that the anion associated with magnesium other than sulphate could have a less toxic, or even beneficial effects on neonate health outcomes. Several scholarly reviews have concluded that MgSO4 was not an effective tocolytic agent, and have recommended to ban it in that indication [2]. Nevertheless, in spite of the lack of supporting data and the ongoing absence of an international consensus, MgSO4 remains the first line pharmacological agent
employed for tocolysis in North America. Finally, it seems that the large clinical use of MgSO4 results more from routine habits than from clinical, pharmacological or toxicological data. However, other magnesium compounds such as oxide, gluconate and chloride may be successfully used in various pathological situations. For example, these magnesium salts are effective in promoting continued uterine quiescence in patients recently treated for preterm labor [2]. In addition they are generally less toxic than MgSO4. All these considerations led us to highlight the differences between the pharmacological and toxicological properties of MgSO4 and MgCl2. MgSO4 has the least interesting properties. Its absorption, cellular penetration, membrane effects and antihypoxic properties are low. Comparative studies between MgSO4 and MgCl2 have shown that absorption and retention are more efficient with MgCl2 than with MgSO4 [3]. Consequently, the aim of the present review was to compare the properties and the effects of both magnesium sulphate and chloride.
187
J. DURLACH, ET AL.
Physical comparison The main physical and chemical properties of each magnesium salts are summarized in the table 1. Examples of physiological and clinical utilisation of MgSO4 and MgCl2 Some recent MgSO4 and MgCl2 uses are reported hereafter. Magnesium sulphate alone In the last decade, many papers were published, dealing with MgSO4 uses. They may be classified as clinical and pharmacological data. Pharmacological uses MgSO4 shows vaso- and neuro-protective properties after spinal cord injury [4]; Postnatal MgSO4 infusion is safe and can improve short-term outcome in infants with severe birth asphyxia [5]; MgSO4 has an effective antithrombotic activity in vivo, and treatment with MgSO4 may lower the risk of thromboembolic-related disorders [6]; MgSO4 is an effective and safe antiarrhythmic agent for arrhythmias developed after open-heart surgery. Its antiarrhythmic effect may be related to its pharmacological properties and not to the normalization of the circulating magnesium concentrations [7]; MgSO4 is given in cardioplegia [8]; The mitochondrial respiratory function which decreases significantly after traumatic brain injury can be improved after MgSO4 infusion as shown by electron microscopy [9]; A beneficial effect of MgSO4 has been also reported after severe traumatic brain injury in rats [10].
Clinical uses First of all, MgSO4 has been often used in case of preeclamptic-eclamptic seizures [11-13] but it has been also successfully used in other clinical indications, as reported hereafter. MgSO4 may be beneficial in the control of ventricular ectopy and supraventricular tachyarrhythmias after coronary artery bypass graft surgery [14]; MgSO4 is used clinically to induce smooth muscle relaxation, mainly in airway smooth muscles [15]; MgSO4 administration may lead to a significant reduction of anaesthetic drugs during total intravenous anaesthesia with propofol, remifentanil and vecuronium [16-17]. In addition, the intraoperative use of MgSO4 as an adjunct to the conventional use of nicardipine has been effective to manage a pediatric patient undergoing a laparoscopic operation [18]; MgSO4 may be considered as an alternative treatment in persistent pulmonary hypertension of the newborn when no other modalities exist since it is a non aggressive and low cost treatment [15]. Magnesium chloride clinical uses MgCl2 has been used less frequently. However, its usefulness was reported in various indications. MgCl2 is an efficient anaesthetic and narcotic agent for cephalopod molluscs [19]; It has a valuable role as cardioprotective agent in rabbits [20]; MgCl2 is a more advisable salt to use in cerebral palsy [21]; Oral supplementation with MgCl2 solution restored serum magnesium levels, improving insulin sensitivity and metabolic control of type 2 diabetic patients with decreased serum magnesium levels [22].
Table 1. Comparison of each magnesium salts properties.
Magnesium salts Elemental Mg++/dose (mg) Solubility in water Chloride MgCl2, 6H2O 25.54 1 g dissolves in: - 0.6 ml water - 0.3 ml boiling water - 2 ml alcohol 1.56 good 19.68 (1.04) 2 176 mg/kg (rats) Sulphate MgSO4, 7H2O 20.2 1g dissolves in: - 0.8 ml water - 0.2 ml boiling water - slightly in alcohol 1.67 low 4 (oral dose), limited and variable extent 5 750 mg/kg (dogs)
Density Bioavailability Oral absorption % (mEq) H2O molecules lost at 100C Lethal doses (LD 50) (i.v.)
188
MAGNESIUM CHLORIDE OR MAGNESIUM SULFATE
Consequently, it appears very interesting to review, in the literature, the studies comparing the respective effects of MgSO4 and MgCl2, the magnesium salt which has the best fits with MgSO4 in regard to elemental Mg++/dose (in mEq). Comparison between magnesium chloride and magnesium sulphate The publications comparing the two magnesium salts are very scarce but allow to distinguish similar and proper effects to each magnesium salt. Similar effects In the literature, there are some examples of similar pharmacological and clinical effects between MgCl2 and MgSO4. Pharmacological effects The comparison of the muscle relaxant activity of equimolar solutions of MgCl2 and MgSO4 using the head drop method in rabbits shows no statistical difference between the two salts with regard to their potency and duration of action, suggesting that these activities are not influenced by the anion associated with the Mg2+ cation [23]; No significant difference was seen between MgCl2 and MgSO4 infusions on the duration of epinephrine-induced cardiac arrhythmia [24]; The two salts decrease the aldosterone production in a dose-dependent manner [25]; MgCl2 and MgSO4 have similar effects on the isolated and perfused rat heart: decreasing heart rate, left ventricular systolic pressure, voltage epicardial electrogram and increasing coronary flow rate [26]; MgCl2 or MgSO4 treatments are equally effective on diffuse axonal injury [27]. Clinical effects Both salts have a similar oral tocolytic role [28]. Compared with MgSO4 and ritodrine, enteric-coated MgCl2 was as effective in prolonging pregnancy and preventing recurrent preterm labor. MgCl2 and MgSO4 penetrate the blood-brain barrier after brain damage, enter injured tissue and improve neurologic outcomes [29]. These results demonstrate the possible use of MgCl2 instead of MgSO4 and reciprocally in pharmacological and clinical indications. Different effects Some recent studies indicate different or/opposite effects between MgCl2 and MgSO4.
MgSO4 > MgCl2 The poliovirus and measles vaccines [30] are stabilized by incorporating molar MgCl2 or MgSO4 respectively. The MgCl2-stabilized poliovirus vaccine loses 0.5 log10 units in three hours at 45C whereas MgSO4-stabilized measles vaccine loses only 0.3 log10 in 30 minutes at 50C. The effect of precalving magnesium source (MgO, MgSO4 and MgCl2) and post calving calcium supplementation were examined on calcemia and calciuria: postcalving plasma calcium concentration was affected by precalving magnesium source with MgSO4 > MgCl2 > MgO sequence [31]. MgCl2 > MgSO4 Nishio et al. [32] studied the influence of magnesium salts (MgCl2, MgSO4, Mg aspartate HCl and Mg acetate) on rat mesenteric arteriole and venule reactivity to standard constrictor doses of epinephrine and BaCl2 and showed that arteriolar constrictions were attenuated by systemic intravenous infusion of each Mg salt tested, except MgSO4 which was insufficient. Grin et al. [33] studied, in dogs fed a normal diet, the antiarrhythmic and proarrythmic effects of MgCl2 and MgSO4 intravenous infusions and showed that infusion of MgSO4 solution decreased plasma sodium, potassium and ventricular fibrillation threshold (VFT) (a proarrhythmic effect), prolonged the ventricular effective refractory period (VERP) and increased the urinary excretion of potassium. By contrast, infusion of MgCl2 solution did not affect VFT and plasma potassium levels and prolonged also VERP. Durlach et al. [3] have pointed out that MgCl2 was both more effective and less toxic than MgSO4 in maintaining optimal aquaculture of scallops. From these data, they have indicated that MgCl2 had a better therapeutic ratio (LD50/ED50) than MgSO4, which could be relevant even in human beings. Such data raised the question as to whether the magnesium cation might be responsible for MgSO4 toxicity or if such toxicity was instead attributable to the associated sulphate anion. The reason of the toxicity of magnesium pharmacological doses of magnesium using the sulphate anion rather than the chloride anion may perhaps arise from the respective chemical structures of both the two magnesium salts. Chemically, both MgSO4 and MgCl2 are hexa-aqueous complexes. However MgCl2 crystals consist of dianions with magnesium coordinated to the six water molecules as a complex, [Mg(H2O)6]2+ and two independent chloride anions, Cl-. In MgSO4, a seventh water molecule is associated
189
J. DURLACH, ET AL.
with the sulphate anion, [Mg(H2O)6]2 +[SO4. H2O]. Consequently, the more hydrated MgSO4 molecule may have chemical interactions with paracellular components, rather than with cellular components, presumably potentiating toxic manifestations while reducing therapeutic effect [11]. Finally, to corroborate these explanations, two experimental in vitro studies in the physiology of human gestational physiology amniotic membrane and allantochorial placental vessels must be considered. Since 1984, the research group associating M. Bara, A. Guiet-Bara and J. Durlach has studied the interrelation between Mg salts and various elements of the placental unit [34-41]. Human amniotic membrane [34-37] These experiments associated electrophysiological and morphological studies. The main results were as follows. Scanning and transmission electron microscopy results, analysed by a stereological method which indicates the ratio between the volume of the intercellular space (R1), the microvilli (R2) and the podocytes (R3) versus the cell volume, showed that (i) at low concentration, MgSO4 increased R1 and R2 but decreased R3, whereas MgCl2 decreased R1 and R3 and had no significant effect on R2; (ii) at high concentration, MgCl2 decreased R1 and increased R2 and R3, while MgSO4 had no significant effect on R1, increased R2 and decreased R3 [34]. The study of the ionic fluxes in the two directions between the mother and the fetus indicated that, when the MgCl2 concentration increased, the ratio between influx and efflux being was two-fold increased, whereas when the MgSO4 concentration increased, influx and efflux became equal. Consequently, MgSO4 could not guarantee the fetal needs in sodium and potassium provided across the human amnion [35]. In addition, it has been demonstrated that MgCl2 interacts with all the exchangers in the membrane, while the effect of MgSO4 effect is limited to paracellular components without interaction with cellular components, with exception of the antiport Na/H [36, 37]. To sum up, MgCl2 interacts with all exchangers while the interaction of MgSO4 is limited to paracellular exchangers, and MgCl2 increases the flux ratio between mother to fetus while MgSO4 decreases it. Human allantochorial placental vessels [38-42] MgCl2 or MgSO4 added in vitro induced a depolarization of the human placental chorionic cells (from arteries and veins with or without endothelium), but the depolarization thresholds were differ-
ent and higher with MgSO4. This difference indicated that MgCl2 influences the cell membrane potential directly, whereas MgSO4 interferes first with endothelial cells and then with muscle cells [38, 39]. MgCl2 and MgSO4 both regulated the cell tonus and the Ca2+ influx through voltage-gated Ca2+ channels in smooth muscle and endothelial cells but the depolarization reduction was more important with MgCl2 than with MgSO4 [40]. Moreover, MgCl2 blocked the ATP-dependent K+ channels and opened the delayed (K(df)) K+ channels, while MgSO4 blocked the current through voltage-gated and ATP dependent K+ channels but had no effect on K(df) [41]. These results were confirmed by micro-particle induced X-ray emission studies [42]. To sum up, the differences between MgCl2 and MgSO4 were less important in allantochorial vessels than in amniotic membranes, but the effects of MgCl2 effects seemed more interesting than those of MgSO4 ones. The comparison reveals that it is very difficult to reach a conclude conclusion on the possible utilisation of a one salt instead of another but it indicates that MgSO4 is not always the appropriate salt in clinical therapeutics and that MgCl2 seems the better anion-cation association to be used in many clinical and pharmacological indications.
Conclusions This review showed shows that it is difficult to elicit identify MgSO4 or MgCl2 as the reference magnesium salt to be used as a therapeutic agent, since each salt displayed valuable clinical properties in various cases situations. The comparison did not allow to a preference of one salt to over the another other, but the studies indicated that MgSO4 is not the alone only magnesium salt which may be used in severe injuries. It is attractive tempting to explain the differences between MgCl2 and MgSO4 by their crystal structures. Magnesium chloride forms a stable hexahydrate, while magnesium sulphate forms a stable heptahydrate [43]. In the two crystals, magnesium has the same complex form. Consequently, the different effects observed might be attributed to the anions. As a result, the biological properties of magnesium salts might depend on their interactions with water and with the polar groups at the membrane surface by screening and/or binding processes [44].
190
MAGNESIUM CHLORIDE OR MAGNESIUM SULFATE
References
1. Durlach J, Bara M. Le Magnsium en biologie et en mdecine. Cachan (France): Ed. Med. Int., 2000; 443 pp. 2. Pryde PG, Besinger RE, Gianopoulos JG, Mittendorf R. Adverse and beneficial effects of tocolytic therapy. Semin Perinatol 2001; 25: 316-40. 3. Durlach J, Bara M, Theophanides T. A hint on pharmacological and toxicological differences between magnesium chloride and magnesium sulphate, or of scallops and men. Magnesium Res 1996; 9: 217-9. 4. Kaptanoglu E, Beskonakli E, Solaroglu I, Kilinc A, Taskin Y. Magnesium sulfate treatment in experimental spinal cord injury: emphasis on vascular changes and early clinical results. Neurosurg Rev 2003; 29: 283-7. 5. Daffa SH, Milaat WA. Role of magnesium sulphate in treatment of severe persistent pulmonary hypertension of the newborn. Saudi Med J 2002; 23: 1266-9. 6. Sheu JR, Hsiao G, Shen MY, Lee YM, Yen MH. Antithrombotic effects of magnesium sulphate in in vivo experiments. Int J Hematol 2003; 77: 414-9. 7. Kiziltepe U, Eyliten ZB, Sirlak M, Tasoz R, Aral A, Eren NT, Uysalel A, Akalin H. Antiarrhythmic effect of magnesium sulphate after open heart surgery: effect of blood levels. Int J Cardiol 2003; 89: 153-8. 8. Roscoe A, Ahmed AB. A survey of peri-operative use of magnesium sulphate in adult cardiac surgery in the UK. Anaesthesia 2003; 58: 363-5. 9. Xu M, Dai W, Deng X. Effects of magnesium sulfate on brain mitochondrial respiratory function in rats after experimental traumatic brain injury. Chin J Traumatol 2002; 5: 361-4. 10. Esen F, Erdem T, Aktan D, Kalayci R, Cakar N, Kaya M, Telci L. Effects of magnesium administration on brain edema and blood brain barrier breakdown after experimental traumatic brain injury in rats. J Neurosurg Anesthesiol 2003; 15: 119-25. 11. Mittendorf R, Pryde PG, Elin RJ, Gianopoulos JG, Lee KS. Relationship between hypermagnesaemia in preterm labour: adverse health outcomes in babies. Magnes Res 2002; 15: 253-61. 12. Linvingston JC, Livingston LW, Ramsey R, Mabie BC, Sibai BM. Magnesium sulphate in women with mild preeclampsia: a randomized controlled trial. Obstet Gynecol 2003; 101: 217-20. 13. Durlach J, Bac P, Bara M, Guiet-Bara A. Is the pharmacological use of intravenous magnesium before preterm cerebroprotective or deleterious for premature infants? Possible importance of the use of magnesium sulphate. Magnesium Res 1998; 11: 323-5. 14. Wistbacka JO, Koistinen J, Karlqvist KE, Lepojarvi MV, Hanhela R, Laurila J, Nissinen J, Pokela R, Salmela E, Ruokonen A. Magnesium substitution in elective coronary artery surgery: a double-blind clinical study. J Cardiothorac Vasc Anesth 1995; 9: 140-6. 15. Kumasaka D, Lindeman KS, Clancy J, Lande B, Croxton TL, Hirshman CA. MgSO4 relaxes porcine air-
way smooth muscle reducing Ca2+ entry. Am J Physiol 1996; 270: L469-L474. 16. Ichiba H, Tamai H, Negishi H, Ueda T, Kim TJ, Sumida Y, Takahashi Y, Fujinaga H, Minami H. Randomized controlled trial of magnesium sulphate infusion for severe birth asphyxia. Pediatr Int 2002; 44: 505-9. 17. Telci L, Esen F, Akcora D, Erden T, Candolat AT, Akpir K. Evaluation of effects of magnesium sulphate in reducing intraoperative anaesthetic requirements. Br J Anaesth 2002; 89: 594-8. 18. Minami T, Adachi T, Fukuda K. An effective use of magnesium sulfate for intraoperative management of laparoscopic adrenalectomy for pheochromocytoma in a pediatric patient. Anesth Analg 2002; 95: 1243-4. 19. Messenger JB, Nixon M, Ryan KP. Magnesium chloride as an anesthetic in cephalopods. Comp Biochem Physiol C 1985; 82: 203-5. 20. Naik P, Malati T, Ratnakar KS, Naidy MUR, Rajasekhar A. Cardioprotective effect of magnesium chloride in experimental acute myocardial infarction. Ind J Exp Biol 1999; 37: 131-7. 21. Oorschot DE. Cerebral palsy and experimental hypoxiainduced perinatal brain injury: is magnesium protective? Magnes Res 2000; 13: 265-73. 22. Rodriguez-Moran M, Guerrero-Romero F. Oral magnesium supplementation improves insulin sensitive and metabolic control in type 2 diabetic subjects: a randomized double-blind controlled trial. Diabetes Care 2003; 26: 1147-52. 23. Plume C. Dpression neuromusculaire provoque par le chlorure de Mg et le sulphate de Mg. Arch Int Pharmacodynam Ther 1972; 195: 411-4. 24. Mayer DB, Miletich DJ, Feld JM, Albrecht RF. The effects of magnesium salts on the duration of epinephrine-induced ventricular tachyarrhythmias in anesthetized rats. Anesthesiology 1989; 71: 923-8. 25. Atarashi K, Matsuoka H, Takagi M, Sugimoto T. Magnesium ion: a possible physiological regulator of aldosterone production. Life Sci 1989; 44: 1483-9. 26. Farrara N, Abete P, Longobardi G, Leosco D, Caccese P, Leonarda De Rosa M, Orlando M, Fittipaldi R, Rengo F. Action of magnesium salts on the toxic effects of calcium overload in the isolated and perfused rat heart. G Ital Cardiol 1988; 18: 605-14. 27. Heath DL, Vink R. Optimization of magnesium therapy after severe diffuse axonal brain injury in rats. J Pharmacol Exp Ther 1999; 288: 1311-6. 28. Ricci JM, Hariharan S, Helfgott A, Reed K, OSullivan MJ. Oral tocolysis with magnesium chloride: a randomized controlled prospective clinical trials. Am J Obstet Gynecol 1991; 165: 603-10. 29. Heath DL, Vink R. Neuroprotective effects of MgSO4 and MgCl2 in closed head injury: a comparative phosphorus NMR study. J Neurotrauma 1998; 15: 183-9. 30. Melnick JL. Thermostability of poliovirus and measles vaccines. Dev Biol Scand 1996; 87: 155-60.
191
J. DURLACH, ET AL.
31. Roche JR, Morton J, Kolver ES. Sulfur and chlorine play a non-acid base role in periparturient calcium homeostasis. J Dairy Sci 2002; 85: 3444-52. 32. Nishio A, Gebrewold A, Altura BT, Altura BM. Comparative effects of magnesium salts on reactivity of arterioles and venules to constrictor agents: an in situ study on microcirculation. J Pharmacol Exp Ther 1988; 246: 859-65. 33. Grin J, Pellizon OA, Raynald A. Mechanisms involved in the antiarrhymtic and proarrhythmic effects of magnesium. Medicina (B Aires) 1996; 56: 231-40. 34. Guiet-Bara A, Bara M. Ultrastructure effects of magnesium on human amniotic epithelial cells. Magnes Res 1990; 3: 23-9. 35. Bara M, Guiet-Bara A, Durlach J. Comparative effects of MgCl2 and MgSO4 on monovalent cations transfer across isolated human amnion. Magnesium Bull 1984; 6: 36-40. 36. Guiet-Bara A, Bara M, Durlach J. Cellular and shunt conductances of human isolated amnion. II. Comparative effects of MgCl2 and MgSO4: electrophysiological studies. Magnesium Bull 1985; 7: 16-9. 37. Bara M, Guiet-Bara A, Durlach J. Comparative effects of MgCl2 and MgSO4 on the ionic transfer components through the isolated human amniotic membrane. Magnes Res 1994; 7: 11-6. 38. Ibrahim B, Guiet-Bara A, Leveteau J, Challeir JC, Vervelle C, Bara M. membrane potential of smooth
muscle cells of human placental chorionic vessels. Comparative effects of MgCl2 and MgSO4. Magnes Res 1995; 8: 127-35. 39. Ibrahim B, Leveteau J, Guiet-Bara A, Bara M. Influence of magnesium salts on the membrane potential of human endothelial placental vessel cells. Magnes Res 1995; 8: 233-6. 40. Bara M, Guiet-Bara A. Magnesium regulation of Ca2+ channels in smooth muscle and endothelial cells of human allantochorial placental vessels. Magnes Res 2001; 14: 11-8. 41. Guiet-Bara A, Bara M. Magnesium sulphate and magnesium chloride effects on K(df), K(Ca) and K(ATP) channels of smooth muscle and endothelial cells of allantochorial human placental vessels. In: Rayssiguier Y, Mazur A, Durlach J, eds. Advances in magnesium research: nutrition and health. J. Libbey Ltd, 2001: 465-7. 42. Michelet-Habchi C, Barberet P, Dutta RK, Guiet-Bara A, Bara M. Elemental maps in human allantochorial placental vessels. 2. MgCl2 and MgSO4 effects. Magnes Res 2003; 16: 171-5. 43. Theophanides T. Biological implications of magnesium salts at the molecular level. Magnes Res 1996; 9: 259-62. 44. Bara M, Guiet-Bara A, Durlach J. A qualitative theory of the screening-dinding effects of magnesium salts on epithelial cell membranes: a new hypothesis. Magnes Res 1989; 2: 243-7.
192
S-ar putea să vă placă și
- Hyperbaric Oxygenation Therapy: Molecular Mechanisms and Clinical ApplicationsDe la EverandHyperbaric Oxygenation Therapy: Molecular Mechanisms and Clinical ApplicationsNariyoshi ShinomiyaÎncă nu există evaluări
- Translational Glycobiology in Human Health and DiseaseDe la EverandTranslational Glycobiology in Human Health and DiseaseMichelle KilcoyneÎncă nu există evaluări
- MG Sulfate Pada Pencegahan EklamsiaDocument16 paginiMG Sulfate Pada Pencegahan Eklamsiaasti dÎncă nu există evaluări
- Mgso4 Mekanisme in KehamilanDocument10 paginiMgso4 Mekanisme in KehamilanIqra AnugerahÎncă nu există evaluări
- Magnesium Emerging Potentials in Anesthesia Practice 2155 6148 1000547Document6 paginiMagnesium Emerging Potentials in Anesthesia Practice 2155 6148 1000547Audra Firthi Dea NoorafiattyÎncă nu există evaluări
- Okusanya Et Al-BJOG - An International Journal of Obstetrics & GynaecologyDocument11 paginiOkusanya Et Al-BJOG - An International Journal of Obstetrics & GynaecologyImelda AtikaÎncă nu există evaluări
- 1 s2.0 S0301211520307168 MainDocument8 pagini1 s2.0 S0301211520307168 Mainagustiawan28Încă nu există evaluări
- Pharmacokinetic Profile of Oral Magnesium HydroxideDocument6 paginiPharmacokinetic Profile of Oral Magnesium HydroxideRegina Marsha XaveriaÎncă nu există evaluări
- Magnesium Sulphate (Mgso4) For Fetal Neuroprotection: DR Rajiv KumarDocument16 paginiMagnesium Sulphate (Mgso4) For Fetal Neuroprotection: DR Rajiv KumarManju KumariÎncă nu există evaluări
- Bioavailability of Magnesium SaltsDocument3 paginiBioavailability of Magnesium Saltssantana20130% (1)
- Fetal Pharmacotherapy 3: Magnesium Sulfate: Motherisk RoundsDocument4 paginiFetal Pharmacotherapy 3: Magnesium Sulfate: Motherisk RoundsCitra KristiÎncă nu există evaluări
- "Freeze Dried Plasma For Pre-Hospital Battlefield UseDocument8 pagini"Freeze Dried Plasma For Pre-Hospital Battlefield Useעדי כהןÎncă nu există evaluări
- Singulair: ® Tablets, Chewable Tablets, and Oral GranulesDocument25 paginiSingulair: ® Tablets, Chewable Tablets, and Oral GranulesGeneric WorldÎncă nu există evaluări
- MagSulfate InfoSheetDocument2 paginiMagSulfate InfoSheetRatning TitissariÎncă nu există evaluări
- 4784 17639 1 PB PDFDocument6 pagini4784 17639 1 PB PDFbaidyanath kumarÎncă nu există evaluări
- Antenatal Magnesium Sulphate (Mgso4) For Fetal Neuro-Protection Prior To Preterm Labor: Mini-ReviewDocument5 paginiAntenatal Magnesium Sulphate (Mgso4) For Fetal Neuro-Protection Prior To Preterm Labor: Mini-ReviewFadhilatul KhairÎncă nu există evaluări
- Magnesium Sulfate For The Treatment of Eclampsia: Go Red For WomenDocument8 paginiMagnesium Sulfate For The Treatment of Eclampsia: Go Red For WomenSukmaÎncă nu există evaluări
- Therapeutic Uses of Magnesium: Complementary and Alternative MedicineDocument6 paginiTherapeutic Uses of Magnesium: Complementary and Alternative MedicinedanielguerinÎncă nu există evaluări
- Sulphonamides: A Pharmaceutical ReviewDocument3 paginiSulphonamides: A Pharmaceutical ReviewinventionjournalsÎncă nu există evaluări
- Magnesium Sulfate For Acute Asthma in Adults A SysDocument11 paginiMagnesium Sulfate For Acute Asthma in Adults A SysBagusSatrioPambudiÎncă nu există evaluări
- MoleculesDocument10 paginiMoleculesWalid Ebid ElgammalÎncă nu există evaluări
- Neuroprotective MgSO4Document6 paginiNeuroprotective MgSO4Rafael JustinoÎncă nu există evaluări
- Anaesth. Pain Intensive CareDocument11 paginiAnaesth. Pain Intensive CareRizki FitriaÎncă nu există evaluări
- Simplified Treatment For Eclampsia Prevention Using Magnesium SulfateDocument1 paginăSimplified Treatment For Eclampsia Prevention Using Magnesium SulfateraniÎncă nu există evaluări
- REFERAT MgSO4Document20 paginiREFERAT MgSO4Selly ChasandraÎncă nu există evaluări
- Scientific Discussion: 1/20 EMEA 2004Document20 paginiScientific Discussion: 1/20 EMEA 2004Donny Rahman KhalikÎncă nu există evaluări
- Biodistribution of Drug/ADA Complexes: The Impact of Immune Complex Formation On Antibody DistributionDocument12 paginiBiodistribution of Drug/ADA Complexes: The Impact of Immune Complex Formation On Antibody Distributiongskcl429Încă nu există evaluări
- Characterization of Magnesium Orotate Loaded Chitosan Polymer Nanoparticles For A Drug Delivery SystemDocument10 paginiCharacterization of Magnesium Orotate Loaded Chitosan Polymer Nanoparticles For A Drug Delivery SystemHASSANI ABDELKADERÎncă nu există evaluări
- Anticonvulsant Therapy For EclampsiaDocument7 paginiAnticonvulsant Therapy For Eclampsiarafifah_nadiaÎncă nu există evaluări
- Tahara 2016Document11 paginiTahara 2016Hazmi MohamedÎncă nu există evaluări
- Jurnal Inhibitor Enzim AlfaglukosidaseDocument13 paginiJurnal Inhibitor Enzim AlfaglukosidaseMarisa NurlitaÎncă nu există evaluări
- 10 1016@j Ejmech 2014 11 059Document102 pagini10 1016@j Ejmech 2014 11 059Matheus BertholucciÎncă nu există evaluări
- Prasugrel Hydrochloride Tablets Stabilized CompositionsDocument8 paginiPrasugrel Hydrochloride Tablets Stabilized CompositionsARUNKANTH KRISHNAKUMARÎncă nu există evaluări
- Mycophenolic Acid Chapter-1Document34 paginiMycophenolic Acid Chapter-1NabilaÎncă nu există evaluări
- Thechangingroleofmagnesium Inobstetricpractice: Wendy A. Haft,, Manuel C. VallejoDocument12 paginiThechangingroleofmagnesium Inobstetricpractice: Wendy A. Haft,, Manuel C. VallejoImelva GirsangÎncă nu există evaluări
- 3 Decision TreeDocument7 pagini3 Decision TreeMutiara SeptianiÎncă nu există evaluări
- Sodium Picosulfate, Magnesium Oxide, and Anhydrous Citric Acid (PREPOPIK) For Oral SolutionDocument27 paginiSodium Picosulfate, Magnesium Oxide, and Anhydrous Citric Acid (PREPOPIK) For Oral SolutionRichard SmithÎncă nu există evaluări
- Jepretan Layar 2022-12-03 Pada 01.42.13Document44 paginiJepretan Layar 2022-12-03 Pada 01.42.13Arumm88Încă nu există evaluări
- Molecules 26 00005Document18 paginiMolecules 26 00005elektifppra2022Încă nu există evaluări
- Magnesium Sulfate Infusion For Acute Asthma in The Emergency DepartmentDocument7 paginiMagnesium Sulfate Infusion For Acute Asthma in The Emergency DepartmentfabsscribdworksÎncă nu există evaluări
- 14 Aher EtalDocument4 pagini14 Aher EtaleditorijmrhsÎncă nu există evaluări
- Impact On Handling and Stress On MAbDocument22 paginiImpact On Handling and Stress On MAbvikram kaithwasÎncă nu există evaluări
- البراسيتول في استرالياDocument11 paginiالبراسيتول في استراليازيد محمدÎncă nu există evaluări
- Mur ADocument11 paginiMur Avivi AramieÎncă nu există evaluări
- Montelukast MedicineDocument4 paginiMontelukast MedicineAman ChoudharyÎncă nu există evaluări
- Modified Citrus Pectin Decreases Body Burden HG PDFDocument2 paginiModified Citrus Pectin Decreases Body Burden HG PDFportosinÎncă nu există evaluări
- Montelukast Medicines of Today and Tomorrow From Molecular Pharmaceutics To Technological FormulationsDocument10 paginiMontelukast Medicines of Today and Tomorrow From Molecular Pharmaceutics To Technological FormulationsAtia IftikharÎncă nu există evaluări
- Rapid Sequence Induction With A Standard.22Document10 paginiRapid Sequence Induction With A Standard.22Kian PrakashÎncă nu există evaluări
- Lipoic Acid Inhibits Cell Proliferation of Tumor Cells in Vitro and in VivoDocument11 paginiLipoic Acid Inhibits Cell Proliferation of Tumor Cells in Vitro and in VivosweetohmÎncă nu există evaluări
- Is Zuspan Regimen Adequate For Preventing EclampsiDocument3 paginiIs Zuspan Regimen Adequate For Preventing EclampsiTimpswalo EmatsuloÎncă nu există evaluări
- Weiss2009 PDFDocument7 paginiWeiss2009 PDFLuisAlbertoGtzMtzÎncă nu există evaluări
- Nirupam PresentationDocument40 paginiNirupam PresentationnirupamkuyateÎncă nu există evaluări
- Articulo en InglesDocument11 paginiArticulo en InglesLuis Fernando MattosÎncă nu există evaluări
- IbuprofenDocument11 paginiIbuprofenЖивка АнгеловаÎncă nu există evaluări
- Magnesium Sulphate in The EmergencyDocument12 paginiMagnesium Sulphate in The EmergencyNur AzizaÎncă nu există evaluări
- WNHS - og.HypertensionPregnancy MagnesiumAnticonvulsantDocument13 paginiWNHS - og.HypertensionPregnancy MagnesiumAnticonvulsantNisa UlfaturrosyidaÎncă nu există evaluări
- Benidipine, A New Ca2+ Channel Blocker With A Cardioprotective EffectDocument15 paginiBenidipine, A New Ca2+ Channel Blocker With A Cardioprotective EffectnahjegonÎncă nu există evaluări
- Magnesium Sulfate in Water For Injection: Flexible Plastic Container For Intravenous Use OnlyDocument5 paginiMagnesium Sulfate in Water For Injection: Flexible Plastic Container For Intravenous Use OnlyKN BaijuÎncă nu există evaluări
- Prostate Cancer Metabolism: From Biochemistry to TherapeuticsDe la EverandProstate Cancer Metabolism: From Biochemistry to TherapeuticsÎncă nu există evaluări
- Strogen and TelomeraseDocument5 paginiStrogen and TelomeraseCholid RiskiantoÎncă nu există evaluări
- Anatomi UterusDocument26 paginiAnatomi UterusCholid RiskiantoÎncă nu există evaluări
- AnovulDocument5 paginiAnovulCholid RiskiantoÎncă nu există evaluări
- 88120090225Document2 pagini88120090225Cholid RiskiantoÎncă nu există evaluări
- Premature Rupture of MembraneDocument7 paginiPremature Rupture of MembranedrommygreatÎncă nu există evaluări
- Report On Magnesium Sulphate and PregancyDocument5 paginiReport On Magnesium Sulphate and PregancyCholid RiskiantoÎncă nu există evaluări
- Pulmonary EdemaDocument2 paginiPulmonary EdemaMuhammad Bayu Zohari HutagalungÎncă nu există evaluări
- Hellp SyndromeDocument13 paginiHellp SyndromeCholid RiskiantoÎncă nu există evaluări
- WCHGLM0003 Pre Eclampsia Pet ProtocolDocument10 paginiWCHGLM0003 Pre Eclampsia Pet ProtocolCholid RiskiantoÎncă nu există evaluări
- Autism DyslexiaDocument2 paginiAutism DyslexiaAutismDyslexiaÎncă nu există evaluări
- PTSD TreatmentDocument2 paginiPTSD TreatmentAlesha Rose100% (1)
- Attachment and SchizophreniaDocument5 paginiAttachment and SchizophreniaAntonio TariÎncă nu există evaluări
- ALCOHOL Pharmacology: Samuel Murano. Dept. of PharmacologyDocument23 paginiALCOHOL Pharmacology: Samuel Murano. Dept. of PharmacologyGideon K. MutaiÎncă nu există evaluări
- Periapical GranulomaDocument6 paginiPeriapical GranulomaEzza RiezaÎncă nu există evaluări
- Hipnosis Con NiñosDocument17 paginiHipnosis Con NiñosAlberto Estrada0% (2)
- Positive End Expiratory PressureDocument5 paginiPositive End Expiratory PressureIrving H Torres LopezÎncă nu există evaluări
- 2019 Guidelines ATC WebDocument283 pagini2019 Guidelines ATC WebAnonymous W4wIVhA550% (1)
- Ai & MiDocument3 paginiAi & MiRadhiya devi CÎncă nu există evaluări
- Pediatric Osteomyelitis: Annisa Nur ArifinDocument18 paginiPediatric Osteomyelitis: Annisa Nur ArifinWahyu Adhitya PrawirasatraÎncă nu există evaluări
- Highlights From The Research Project On Gratitude and ThankfulnessDocument2 paginiHighlights From The Research Project On Gratitude and ThankfulnessyoumeuswerkgelukÎncă nu există evaluări
- Table of Specification: % # of Days Taught Total # of ItemsDocument7 paginiTable of Specification: % # of Days Taught Total # of Itemsrogelyn samilinÎncă nu există evaluări
- Traditional - Herbal Medicine (ST) - RevisiDocument31 paginiTraditional - Herbal Medicine (ST) - Revisiluqmanhasans100% (1)
- Postural Alignment For Voice Production PDFDocument10 paginiPostural Alignment For Voice Production PDFMarianela Guerrero Mesías100% (1)
- Nasal Polyp DR Eva 2018Document37 paginiNasal Polyp DR Eva 2018hudaÎncă nu există evaluări
- 4th International Thematic Monograph 2019 Pp. 359-370Document13 pagini4th International Thematic Monograph 2019 Pp. 359-370Adlo GeronimoÎncă nu există evaluări
- Freud's Psychosexual Stages of DevelopmentDocument2 paginiFreud's Psychosexual Stages of DevelopmentMichElle DanFord100% (2)
- Case Study (Goiter)Document41 paginiCase Study (Goiter)yasira100% (1)
- Material SelfDocument20 paginiMaterial SelfleoÎncă nu există evaluări
- Schema TherapyDocument14 paginiSchema TherapyReyaz Mohemmad100% (2)
- Cochlear Implant Surgery Treatment & Management - Medical Therapy, Surgical Therapy, Preoperative DetailsDocument9 paginiCochlear Implant Surgery Treatment & Management - Medical Therapy, Surgical Therapy, Preoperative DetailsFityah UfiÎncă nu există evaluări
- VWD in BriefDocument8 paginiVWD in Briefvinoedhnaidu_rajagopalÎncă nu există evaluări
- Family Constellations An Innovative Systemic Pheno PDFDocument9 paginiFamily Constellations An Innovative Systemic Pheno PDFFátimaA.SilvaÎncă nu există evaluări
- New Year's Resolution Why Do We FailDocument4 paginiNew Year's Resolution Why Do We FailVivitsa UpastiÎncă nu există evaluări
- Ayurvedic & Unani Tibbia College, Delhi: National Institute of Unani MedicineDocument3 paginiAyurvedic & Unani Tibbia College, Delhi: National Institute of Unani MedicineShashank JainÎncă nu există evaluări
- Category Company Locality Address Pin Email Address Virtual Number Whatsapp Phone #1 Phone #2Document22 paginiCategory Company Locality Address Pin Email Address Virtual Number Whatsapp Phone #1 Phone #2Krupam Thetenders.comÎncă nu există evaluări
- Validity of The Neck Disability Index and Neck Pain and Disability Scale For Measuring Disability Associated With Chronic, Non-Traumatic Neck PainDocument12 paginiValidity of The Neck Disability Index and Neck Pain and Disability Scale For Measuring Disability Associated With Chronic, Non-Traumatic Neck PainDESY NATALIANA PUTRIÎncă nu există evaluări
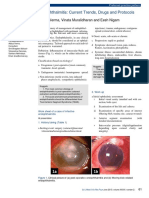
- Endophthalmitis: Current Trends, Drugs and Protocols: Aditya Verma, Vinata Muralidharan and Eesh NigamDocument10 paginiEndophthalmitis: Current Trends, Drugs and Protocols: Aditya Verma, Vinata Muralidharan and Eesh NigamHerman Kurt Ludvik100% (1)
- Schizoaffective Disorder Veteran and Family Handout-1Document7 paginiSchizoaffective Disorder Veteran and Family Handout-1Maghfirani Faroh Fauzia100% (1)
- Psychology, Counseling Psychology, and Professional Counseling: Shared Roots, Challenges, and OpportunitiesDocument22 paginiPsychology, Counseling Psychology, and Professional Counseling: Shared Roots, Challenges, and OpportunitiesGusti NguRah Suwidiya PÎncă nu există evaluări