Documente Academic
Documente Profesional
Documente Cultură
Tutorial 5 Chem Bond
Încărcat de
aribniminnakDrepturi de autor
Formate disponibile
Partajați acest document
Partajați sau inserați document
Vi se pare util acest document?
Este necorespunzător acest conținut?
Raportați acest documentDrepturi de autor:
Formate disponibile
Tutorial 5 Chem Bond
Încărcat de
aribniminnakDrepturi de autor:
Formate disponibile
Tutorial 5- chemical bonding
Activity 1: Formation of chemical bonds Choose the correct answer from the table
Sharing monoatomic
ionic arrangement
stable inert
chemical bonds covalent duplet
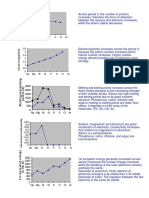
Noble gases are gases. They exist as.. gases and are chemically unreactive . They have ... octet or .. electron ..
Other atoms besides noble gases tend to achieve the stable electron arrangement through the formation of
Two types of chemical bonds : (i) .. bond - formed when atoms join together by transferring of electrons (ii) .. bond - formed when atoms join together by ..of electrons
Activity 2 1 Underline the correct answer. To achieve a stable electron arrangement : (i) A metal atom (donates / accepts) electrons , forming a (positive / negative) ion called cation . (ii) A (non-metal / metal) atom accepts electrons , forming a (positive / negative) ion called anion .
Complete the diagram below.
(a)
(b)
Activity 3 1 Formation of ionic compound, sodium chloride ( NaCl ) Electron arrangement of sodium atom is ............................................................. A sodium atom .one electron to achieve the electron
arrangement which is 2.8. Sodium ion, .......... is formed Electron arrangement of chlorine atom is.. Electron from sodium atom is transferred to a .atom A chlorine atom electron from sodium atom to .. the stable electronwhich is 2.8.8 Chloride ion,.. is formed The sodium ion, Na and chloride ion, Cl formed are ..to one another to form an ionic compound .., NaCl . The strong .forces between the opposite-charged ions is called .bond.
Complete the diagram below.
2.8.1 Sodium atom,Na
2.8.7 Chlorine atom,Cl
............. Sodium ion,
............... Chloride ion, ..
Activity 4 : Formation of covalent bonds Fill in the blanks with the correct words. 1 Covalent bonds are formed when .. achieve . electron arrangements . 2 Types of covalent bonds:(i) (ii) atoms .. electrons to
(iii) 3 A single bond is formed when of electrons is shared between two atoms. A double bond is formed when ..of electrons is shared between two atoms. A triple bond is formed when .of electrons is shared between two atoms. 4 Formation of hydrogen molecules, H2 : A hydrogen atom has valence electron, with an electron arrangement of... It needs .. more electron to achieve the .. electron arrangement ..hydrogen atoms one electron each for Shared-paired electrons forms a .. bond in the hydrogen molecule, H2 Single bond holds the two hydrogen atoms together because the shared-pair of electrons is attracted to the.. of both atoms 5 Complete the diagram below. (a)
(b) A covalent bond can be illustrated by using.
Formation of oxygen molecules, O2 : An oxygen atom has valence electron, with an electron arrangement of.. It needs .more electrons to achieve the .. electron arrangement ....oxygen atoms sharepairs of electrons forming a..bond
2 Draw the electron arrangement for the formation of oxygen molecule. (a) [Proton number : O, 8 ;]
(b) Illustrate the formation of oxygen molecule using the Lewis structure.
Activity 5 Ionic bond Valence electrons Characteristic Electrons involved Elements Electron transfer to achieve stable electron arrangement Bond formation Particles Non-metals atom and non metal atoms Covalent bond
Activity 6 Ionic compound Properties Physical states at room temperature Melting points Boiling points Electrical Conductivity Solubility in water Solubility in organic solvent Covalent compound
Activity 7 1
Atom A (a) Write the electron arrangement for atom A.
Atom B
.. (b) A and B can form a compound (i) What type of bond holds atom A and B together ? .. What will happen to atom A during the formation of the compound with atom B? ..
(ii)
(iii)
Draw the electron arrangement of the compound formed in (b)(ii).
(iv) State one physical property of the compound formed. ..
S-ar putea să vă placă și
- Formation of Chemical BondsDocument11 paginiFormation of Chemical BondsNovah GurulooÎncă nu există evaluări
- Chapter 5: Chemical Bonds: A Formation of Compounds Learning OutcomesDocument9 paginiChapter 5: Chemical Bonds: A Formation of Compounds Learning OutcomesWong Wai Lun100% (1)
- Chemical Bonds ExplainedDocument10 paginiChemical Bonds ExplainedNor Fairul Bin SudarmanÎncă nu există evaluări
- F4 Che C5 ExeDocument11 paginiF4 Che C5 ExeVulcan Chong100% (1)
- Tutorial Chemical Bond 1Document9 paginiTutorial Chemical Bond 1Izhan 7658Încă nu există evaluări
- Electrolysis of Aqueous SolutionsDocument20 paginiElectrolysis of Aqueous SolutionshanifzainolÎncă nu există evaluări
- 3.4 Electrolytic and Voltaic CellsDocument3 pagini3.4 Electrolytic and Voltaic CellsSY ChowÎncă nu există evaluări
- IGCSE 1 37to1 50DA BondingexerciseDocument2 paginiIGCSE 1 37to1 50DA Bondingexerciseseth sheldrakeÎncă nu există evaluări
- Test On Electrolysis Grade IXDocument13 paginiTest On Electrolysis Grade IXkrisnuÎncă nu există evaluări
- Class 1-4 chemical bondsDocument2 paginiClass 1-4 chemical bondsMohamed AitaÎncă nu există evaluări
- Test Grade 8 (Ionic Bonding)Document4 paginiTest Grade 8 (Ionic Bonding)widya sari100% (1)
- TR - Dominic s2Document112 paginiTR - Dominic s2hervemanzi498Încă nu există evaluări
- Electrolysis of Molten and Aqueous SolutionsDocument20 paginiElectrolysis of Molten and Aqueous SolutionsahlonggÎncă nu există evaluări
- Chem Ex6answersDocument7 paginiChem Ex6answersVarshLokÎncă nu există evaluări
- Chemical Bonding PropertiesDocument20 paginiChemical Bonding PropertiesHema LataÎncă nu există evaluări
- Newdocument1 6Document16 paginiNewdocument1 6Magd OsamaÎncă nu există evaluări
- 5.0 Module Pahang For Chemical BondingDocument19 pagini5.0 Module Pahang For Chemical Bondingkhayranizam0% (1)
- GCSE C2 Revision + Exam Questions (1) - Chemi-BondingDocument35 paginiGCSE C2 Revision + Exam Questions (1) - Chemi-BondingPrincess KimÎncă nu există evaluări
- Formation of Ionic BondsDocument8 paginiFormation of Ionic BondsangjellyÎncă nu există evaluări
- Lesson 3 Period 7Document6 paginiLesson 3 Period 7Jackson LtorishaÎncă nu există evaluări
- Objectives Questions: Chemical BondingDocument10 paginiObjectives Questions: Chemical BondingFary SaidinÎncă nu există evaluări
- Topic 4 Structure and Properties of Materials, Ionic Bonding, Covalent BondingDocument33 paginiTopic 4 Structure and Properties of Materials, Ionic Bonding, Covalent BondingKaixin HuangÎncă nu există evaluări
- Chapter 5: Chemical Bonds: Ionic Bonds (Metal + Non Metal)Document10 paginiChapter 5: Chemical Bonds: Ionic Bonds (Metal + Non Metal)Irfan FarhanÎncă nu există evaluări
- PteDocument11 paginiPteDanica PamilÎncă nu există evaluări
- Metals and Nonmetals Classified by Their Properties and Electron StructureDocument2 paginiMetals and Nonmetals Classified by Their Properties and Electron StructureMohamed AitaÎncă nu există evaluări
- Sec 3 Chemistry Practice QuestionsDocument4 paginiSec 3 Chemistry Practice Questionschong56100% (1)
- TT2.1 - Ionic and Covalent BondDocument9 paginiTT2.1 - Ionic and Covalent BondDaniel VictoriaÎncă nu există evaluări
- Electrolysis Worksheet 1: 1. A) Complete The Sentences Using Words From The Box BelowDocument1 paginăElectrolysis Worksheet 1: 1. A) Complete The Sentences Using Words From The Box BelowMenaga A/P IlangkovanÎncă nu există evaluări
- Chapter2 ChemicalbondingDocument23 paginiChapter2 ChemicalbondingAbbyÎncă nu există evaluări
- Lab Manual 02Document158 paginiLab Manual 02Stephen VivekÎncă nu există evaluări
- Ionic Compound HomeworkDocument2 paginiIonic Compound HomeworkJOSH FITZPARICKÎncă nu există evaluări
- C2 - Revision Booklet - CombinedDocument17 paginiC2 - Revision Booklet - Combinedvarguard101Încă nu există evaluări
- Class X Chemistry Sample Paper IDocument11 paginiClass X Chemistry Sample Paper IshomitaÎncă nu există evaluări
- Grade 8 Test 1Document5 paginiGrade 8 Test 1dowanahamidÎncă nu există evaluări
- Chapter 5Document14 paginiChapter 5Ishraqi IlyasÎncă nu există evaluări
- CompleteDocument17 paginiCompleteTelÎncă nu există evaluări
- Waja Chemistry Chapter 2: Structure of The AtomDocument10 paginiWaja Chemistry Chapter 2: Structure of The AtomChewfun KhooÎncă nu există evaluări
- CHEMISTRY REVIEWDocument5 paginiCHEMISTRY REVIEWWhitneyÎncă nu există evaluări
- BondingDocument11 paginiBondingMaku MichaelÎncă nu există evaluări
- 1-Atoms and CompoundsDocument4 pagini1-Atoms and CompoundsAllie DarlingÎncă nu există evaluări
- Chapter 5: Chemical Bonds 5.1 Understanding Formation of CompoundsDocument3 paginiChapter 5: Chemical Bonds 5.1 Understanding Formation of CompoundsMSKÎncă nu există evaluări
- Revision For First Term 9GCE 2010 11Document30 paginiRevision For First Term 9GCE 2010 11Anonymous 8VJhV1eI2yÎncă nu există evaluări
- Science-10-1Unit 2 - Review BookletDocument35 paginiScience-10-1Unit 2 - Review BookletMosarrat QureshiÎncă nu există evaluări
- CHEMISTRY SPM FORM 4 Short Notes Chapter 5 CHEMICAL BONDSDocument4 paginiCHEMISTRY SPM FORM 4 Short Notes Chapter 5 CHEMICAL BONDSJay Bee88% (8)
- Worksheet IGCSEDocument6 paginiWorksheet IGCSEsiennaÎncă nu există evaluări
- Chapter 5: Chemical Bond Stability of Noble GasesDocument20 paginiChapter 5: Chemical Bond Stability of Noble GasesLuna LatisyaÎncă nu există evaluări
- Important Question ICSE 2010 Class 10th Chemical BondingDocument3 paginiImportant Question ICSE 2010 Class 10th Chemical BondingYash KapoorÎncă nu există evaluări
- Class 10 Concise Chemistry Chemical Bonding SolutionsDocument30 paginiClass 10 Concise Chemistry Chemical Bonding SolutionsPIYUSH DikshitÎncă nu există evaluări
- 2nd Sem Chemistry Revision PaperDocument5 pagini2nd Sem Chemistry Revision PaperChamiru RathnasekaraÎncă nu există evaluări
- Form 4 Chemistry Chapter 5Document37 paginiForm 4 Chemistry Chapter 5SF CHENGÎncă nu există evaluări
- SNC1D7 Ionic Compounds NameDocument3 paginiSNC1D7 Ionic Compounds NameSutanga FreansÎncă nu există evaluări
- 4 Chemical Bonding - After Review - 8!10!2019Document24 pagini4 Chemical Bonding - After Review - 8!10!2019AFAQ HYDÎncă nu există evaluări
- Term 1 Exam 2022-2023 Gr. 8 ChemistryDocument12 paginiTerm 1 Exam 2022-2023 Gr. 8 ChemistryMatthew EdbertÎncă nu există evaluări
- Chemistry Test 2Document2 paginiChemistry Test 2Daniel Ngenokesho WandyaÎncă nu există evaluări
- Worksheet Chapter 5Document9 paginiWorksheet Chapter 5Maktok Azi RahimÎncă nu există evaluări
- Noble Gases Stability Due to Duplet and Octet Electron ArrangementDocument30 paginiNoble Gases Stability Due to Duplet and Octet Electron ArrangementAim1111Încă nu există evaluări
- First Tier Icp Agreement: BetweenDocument2 paginiFirst Tier Icp Agreement: BetweenaribniminnakÎncă nu există evaluări
- Chap 07 ControlDocument6 paginiChap 07 ControlaribniminnakÎncă nu există evaluări
- Salt 2Document3 paginiSalt 2Sulaiman MohamadÎncă nu există evaluări
- QPCR Optimization 2011.unlockedDocument22 paginiQPCR Optimization 2011.unlockedaribniminnakÎncă nu există evaluări
- 1way ANOVA of Data 1Document1 pagină1way ANOVA of Data 1aribniminnakÎncă nu există evaluări
- 76 Antitumor-Promoting Effects of Cyclic Diarylheptanoids On Epstein Barr Virus Activation and Two Stage Mouse Skin CarcinogenesisDocument6 pagini76 Antitumor-Promoting Effects of Cyclic Diarylheptanoids On Epstein Barr Virus Activation and Two Stage Mouse Skin CarcinogenesisaribniminnakÎncă nu există evaluări
- Formula KimiaDocument1 paginăFormula KimiaShamshul DidarellyÎncă nu există evaluări
- Topic3 Periodic TableDocument66 paginiTopic3 Periodic TableNana SazanaÎncă nu există evaluări
- Journal Free With Good IntegrityDocument2 paginiJournal Free With Good IntegrityaribniminnakÎncă nu există evaluări
- Periodic TableDocument8 paginiPeriodic TableKhairiyah AbdullahÎncă nu există evaluări
- Teaching Plan SBP3112 Semester 1 2013-2014Document4 paginiTeaching Plan SBP3112 Semester 1 2013-2014aribniminnakÎncă nu există evaluări
- NDU Referee Report FormDocument2 paginiNDU Referee Report FormaribniminnakÎncă nu există evaluări
- Ace To Genin As Natural EsDocument31 paginiAce To Genin As Natural EsNathaly Jiménez DíazÎncă nu există evaluări
- SBP 3114-Salivary SecretionDocument3 paginiSBP 3114-Salivary SecretionaribniminnakÎncă nu există evaluări
- 30 The Malignant Conversion Step of Mouse Skin CarcinogenesisDocument3 pagini30 The Malignant Conversion Step of Mouse Skin CarcinogenesisaribniminnakÎncă nu există evaluări
- 38 Bullatacin, A Potent Antitumor Annonaceous Acetogenin, Induces Apoptosis Through A Reduction of Intracellular CAMP and CGMP Levels in Human Hepatoma 2.2.15 CellsDocument9 pagini38 Bullatacin, A Potent Antitumor Annonaceous Acetogenin, Induces Apoptosis Through A Reduction of Intracellular CAMP and CGMP Levels in Human Hepatoma 2.2.15 CellsaribniminnakÎncă nu există evaluări
- 26 Mechanisms of Inhibitors of Mutagenesis and CarcinogenesisDocument8 pagini26 Mechanisms of Inhibitors of Mutagenesis and CarcinogenesisaribniminnakÎncă nu există evaluări
- 29 Identifying The Cellular Origin of Squamous Skin TumorsDocument6 pagini29 Identifying The Cellular Origin of Squamous Skin TumorsaribniminnakÎncă nu există evaluări
- 25 ChemopreventionDocument19 pagini25 ChemopreventionaribniminnakÎncă nu există evaluări
- 24 Antioxidants and Multistage Carcinogenesis in Mouse SkinDocument32 pagini24 Antioxidants and Multistage Carcinogenesis in Mouse SkinaribniminnakÎncă nu există evaluări
- Adjuvant PeDocument1 paginăAdjuvant PeAlexandru IacobanÎncă nu există evaluări
- 23 A Collaborative Methodology For Developing A Semantic Model For Interlinking Cancer Chemoprevention Linked Data SourcesDocument18 pagini23 A Collaborative Methodology For Developing A Semantic Model For Interlinking Cancer Chemoprevention Linked Data SourcesaribniminnakÎncă nu există evaluări
- Year 2 Pupil Conversation Topics and InstructionsDocument9 paginiYear 2 Pupil Conversation Topics and InstructionsaribniminnakÎncă nu există evaluări
- 29 Identifying The Cellular Origin of Squamous Skin TumorsDocument6 pagini29 Identifying The Cellular Origin of Squamous Skin TumorsaribniminnakÎncă nu există evaluări
- Tox 26 293Document7 paginiTox 26 293aribniminnakÎncă nu există evaluări
- Cover Manual - Sbp3105Document1 paginăCover Manual - Sbp3105aribniminnakÎncă nu există evaluări
- 37 Bullatacin SintesisDocument4 pagini37 Bullatacin SintesisaribniminnakÎncă nu există evaluări
- Group Band Four Picnic OutingDocument6 paginiGroup Band Four Picnic OutingaribniminnakÎncă nu există evaluări
- Teacher's Classroom Activities to Improve Students' Communication SkillsDocument7 paginiTeacher's Classroom Activities to Improve Students' Communication SkillsaribniminnakÎncă nu există evaluări
- Hello, Hello My Dear Friend, My Dear Friend, My Dear Friend, Hello, Hello My Dear FriendDocument14 paginiHello, Hello My Dear Friend, My Dear Friend, My Dear Friend, Hello, Hello My Dear FriendaribniminnakÎncă nu există evaluări
- Unit 3 Test Study Guide AnswersDocument1 paginăUnit 3 Test Study Guide Answersapi-305204604Încă nu există evaluări
- McMurry Chapter 2: Polar Covalent Bonds and Acid-Base ConceptsDocument44 paginiMcMurry Chapter 2: Polar Covalent Bonds and Acid-Base ConceptsKirilKocevskiÎncă nu există evaluări
- LGO by Intutive MethodDocument7 paginiLGO by Intutive MethodlingalayaminiÎncă nu există evaluări
- 3 Factors That Stabilize CarbocationsDocument13 pagini3 Factors That Stabilize CarbocationsmridulkhandelwalÎncă nu există evaluări
- Tengisnamuun Tumenjargal - Period 3 Trend GraphsDocument1 paginăTengisnamuun Tumenjargal - Period 3 Trend GraphstenaÎncă nu există evaluări
- Exercise 22 - Hyperchem 8 04 MM Calculations Energy of Rotation Round Single BondsDocument5 paginiExercise 22 - Hyperchem 8 04 MM Calculations Energy of Rotation Round Single Bondsapi-235187189Încă nu există evaluări
- Periodic Trends and Classification of ElementsDocument32 paginiPeriodic Trends and Classification of ElementsVaibhav KargetiÎncă nu există evaluări
- Dipole Moments and Molecular StructureDocument4 paginiDipole Moments and Molecular Structurevenkitheboss100% (1)
- 12th Chemistry Full Study Material em PDFDocument258 pagini12th Chemistry Full Study Material em PDFSONAÎncă nu există evaluări
- BondingDocument52 paginiBondingArian CoenÎncă nu există evaluări
- Science - 2nd Quarter Periodical Exam ReviewerDocument3 paginiScience - 2nd Quarter Periodical Exam ReviewerJann Nicole BautistaÎncă nu există evaluări
- 03 Organic Chemistry Introduction 2Document126 pagini03 Organic Chemistry Introduction 2Hamid Hussain HamidÎncă nu există evaluări
- Answer Key HMWK - 1 CHPT 9 - 10Document11 paginiAnswer Key HMWK - 1 CHPT 9 - 10jts399Încă nu există evaluări
- Chemical Bonding File4 PDFDocument11 paginiChemical Bonding File4 PDFyaswanthÎncă nu există evaluări
- Chemical Bond & ReactionDocument39 paginiChemical Bond & ReactionPratik MekheÎncă nu există evaluări
- INTRODUCTION TO CHEMICAL BONDINGDocument96 paginiINTRODUCTION TO CHEMICAL BONDINGgsharkzÎncă nu există evaluări
- Chemical Bonding and Periodic Trends Part 1Document9 paginiChemical Bonding and Periodic Trends Part 1danielmahsaÎncă nu există evaluări
- Chapter 10Document18 paginiChapter 10bi_hpu2Încă nu există evaluări
- The Periodic LawDocument33 paginiThe Periodic Lawviolaplayer09Încă nu există evaluări
- Pentalene Example 0Document7 paginiPentalene Example 0Omar SantiagoÎncă nu există evaluări
- Topic 14 Bonding HL NotesDocument26 paginiTopic 14 Bonding HL NotesaabbccÎncă nu există evaluări
- Section A Notes - Periodic PropertiesDocument10 paginiSection A Notes - Periodic PropertiesBhavesh GargÎncă nu există evaluări
- Social Interaction ConceptDocument3 paginiSocial Interaction ConceptGIDEON, JR. INESÎncă nu există evaluări
- Drawing Lewis Structures Notes HandoutDocument3 paginiDrawing Lewis Structures Notes HandoutNICOLE HILLÎncă nu există evaluări
- 1456897461CHE P1 M1 E-Text PDFDocument14 pagini1456897461CHE P1 M1 E-Text PDFanu MalikÎncă nu există evaluări
- Chapter 9 Questions and AnswersDocument8 paginiChapter 9 Questions and AnswersFausto SalazarÎncă nu există evaluări
- CHE 2511 - 003 - Electron-Dot Formulae, Lewis Structures and Formal ChargesDocument25 paginiCHE 2511 - 003 - Electron-Dot Formulae, Lewis Structures and Formal ChargesWebster KafungaÎncă nu există evaluări
- Hybridisation and Bonding in Carbon CompoundsDocument29 paginiHybridisation and Bonding in Carbon Compoundsdela2Încă nu există evaluări
- Chemical Bonding and StructureDocument199 paginiChemical Bonding and StructureRichard NestorÎncă nu există evaluări
- Chapter 3 Classification of Elements and Periodicity in PropertiesDocument9 paginiChapter 3 Classification of Elements and Periodicity in PropertiesNitish Mehra100% (1)