Documente Academic
Documente Profesional
Documente Cultură
Synaptic Plasticity
Încărcat de
Rich ColeDrepturi de autor
Formate disponibile
Partajați acest document
Partajați sau inserați document
Vi se pare util acest document?
Este necorespunzător acest conținut?
Raportați acest documentDrepturi de autor:
Formate disponibile
Synaptic Plasticity
Încărcat de
Rich ColeDrepturi de autor:
Formate disponibile
23/04/2013
Synaptic Plasticity Get slides of Learning Central - A process in which the efficacy of synapses change over time - Region of the brain mainly studies is the hippocampus
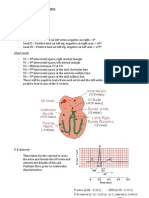
The Entorhinal/Hippocampal system Hippocampus is connected to Entorhinal cortex (part of cerebral cortex) ErC is the main input from cortex Entorhinal cortex feeds to dentate gyrus (granule cells) The mossy fibre pathway goes from DG to CA3 The two main pathways in hippo are mossy fibre pathway and the Schaffer Collaterals pathway which connects CA3 to ipsilateral CA1 CA1 then feed to ErC, subiculum, lateral septum and amygdala In CA1 we see the Glutamatergic pyramidal cells which connect with GABAergic interneurons o On top of this there are a number of neuromodulatory events: NE, Ach, DA and 5HT are all acting and exert a function on modulating activity on pyramidal cells
Recording Physiology Prepare hippocampal slices (coronal) so that we can see DG, CA1 and CA3. You can record from dorsal or ventral hippocampus (well mainly look at dorsal) Because input goes from DG to CA1 you need to stimulate border between CA1 and CA2 (give a stimulus in region of Schaffer Collaterals) You can have EC or IC recordings o EC recordings are done in dendritic region o IC recordings are done at the soma of a neuron You can record anaesthetised animals (Sleeping) or you can record from freely moving animals (you can measure behaviour and electrophysiological properties simultaneously) EC recordings can measure field potential; the overall behaviour of the region rather than activity of specific cells Or you can use sharp electrodes for IC recordings A third technique is Patch clamp technique o Glass electrodes with whole cell configurations. You can patch and then break a membrane and measure properties of whole cell o You can also do a Giga seal where you measure few ion channels o Whole cell configuration is used for measuring plasticity
You have a stimulating electrode and a recording electrode If we go back in history: Bliss and Lomos first Published LTP experiment In vivo work on synaptic plasticity precedes in vitro work In this work, it was showed that by repeatedly stimulating hippocampus of anaesthetised animals using strains of 100Hz you can see change in amplitude of PSEP This is the first example demonstrating the possibility of evoking LTP This change in efficacy of synapse was very long lasting and almost irreversible This time it was done in the Dentate Gyrus (typical of in vivo work; in vitro work normally involves dorsal striatum) If you apply small stimuli (low frequency) you have a transitory change in membrane potential (e.g. -70 to -60) which is reversible. But if you give repeat stimulation (high frequency stimulation) can almost irreversibly change the membrane potential up to the threshold
1
Recording Configuration and Typical responses in a Hippocampal slice recording experiment You can record in stratum pyramidale in area CA1 You can record in stratum radiatum I area C1 You can stimulate Schaffer Collaterals in Area CA3 At first you see a stimulus artefact peak and then you see a fiber volley and then you see an EPSP
LTP Triggered by Theta Burst Stimulation You can elicit different forms of LTP In the CA1 region, its possible to produce an early LTP (which you normal elicit with a single tetanic train and it lasts for an hour and then decays), but if you apply 3 trains of tetany you can have a very strong LTP (Late LTP This distinction between early and late LTP has a profound molecular reason Early LTP, similarly to the short term memory phase of learning, does not depend on protein synthesis; its due to relatively transient changes in synaptic efficiency (it doesnt require nuclear synthesis) and doesnt require the formation of new synapses (which is required for late LTP) If you inhibit protein synthesis you will block late LTP
Immediate, Early and Late LTP Immediate LTP can be caused within minute
Potential sites of synaptic modification in LTP Presynaptic = Altered o NT amount in vesicles o Number of vesicles released o Kinetics of release o Glutamate reuptake o Probability a vesicle fusion Postsynaptic = Altered o Number of AMPA receptors, insertion of AMPA receptors o Ion flow through AMPA channels o Membrane electrical properties Additional possibilities include changes in number of total synaptic connections between two cells
Depotentiation and LTD You can revert potentiation You can depotentiate by applying a low frequency stimulus (1-2Hz for 10-15mins). You can depotentiate back to resting state If you apply this to a resting state, you will cause opposite process to LTP, which is long term depression o Low frequency stimulation and High frequency stimulation can cause depotentiation/LTD and LTP respectively The synapse is very flexible The cognitive process is not just about accumulating new memory but also getting rid of old memories At the molecular level there are major differences in engagement of signal transduction pathways in LTP and LDP Protein kinases are needed for LTP, phosphatases can block LTP. Changes at the post synaptic level can cause alteration in which protein kinases are more implicated in the formation of LTP and phosphatases for LTD (this is a very simple minded model)
Modulation of LTP induction You can induce it chemically, not just electrically E.g. isoproterenol (a beta agonist) You can apply low frequency stimulus (5Hz) along with isoproterenol you can chemically induce LTP
Learning occurs with the frequency of 1-10Hz
In normal synaptic transmission, low frequency, only non-NMDAr In tetanic stimulation there is also NMDA receptors (early LTP) NMDA receptors are crucial. If you give a NMDA receptor antagonist you block early LTP but dont block synaptic transmission. We think that in normal conditions, with a strong depolarisation there are conformational changes that experience the release the Mg2+ and influx of Ca, which brings about signal transduction mechanisms If you stimulate slices with 200Hz (the maximal theoretical frequency that you can apply to a slice) and give AP-5 you dont block LTP. If you used 100Hz the AP-5 would have blocked it. Therefore 200Hz is NMDAr independent LTP
When you move from early to late LTP, while early LTP is engaging AMPA in NMDAr (leads to robust signal transduction pathway which consequent effects are nuclear level), the late LTP requires de novo protein synthesis. This is at the basis of morphological changes at sites where the potentiation has occurred. This implies the formation of new synaptic boutons. The easiest interpretation to distinct between early and late LTP is the formation of new proteins and subsequently new synapses. In the hippocampus at the excitatory Glutamatergic synapse there is a structure, which is very important, and that post-synaptic density. We didnt know what this was for a long time but now we know that in excitatory synapses this post synaptic density is the morphological manifestation of the existence of large multi-protein complexes which are associated with AMPA and glutamate receptors.
NMDAr and AMPAr cannot any more only be seen as ion channels, but rather the combination of an ion channel and the core of a multi-complex signal transduction machinery needed to convey the proper signal to the dendrite and nucleus of post-synaptic cells. You can elicit LTP in the basolateral amygdala too You can elicit it in the dorsal portion of the striatum. LTP and LTD is elicited differently in the dorsal striatum. If you apply high frequency stimulation, in normal physiological concentration, you would see LTP in the hippocampus, but it caused LTD in the dorsal portion of the striatum because physiological Mg concentrations are sufficient to keep the NMDAr blocked. To elicit LTP in the dorsal striatum you must remove magnesium (this is an artificial situation of course) and then apply the high frequency stimulation and you get LTP. Striatal LTP depends on ERK signally, but LTD isnt
S-ar putea să vă placă și
- Shoe Dog: A Memoir by the Creator of NikeDe la EverandShoe Dog: A Memoir by the Creator of NikeEvaluare: 4.5 din 5 stele4.5/5 (537)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeDe la EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeEvaluare: 4 din 5 stele4/5 (5794)
- Sepsis During PregnancyDocument45 paginiSepsis During PregnancyRich ColeÎncă nu există evaluări
- ECG Practical NotesDocument2 paginiECG Practical NotesRich ColeÎncă nu există evaluări
- Amygdala FunctionDocument5 paginiAmygdala FunctionRich Cole100% (1)
- Brain, Vision, Memory - History of Neuroscience PDFDocument261 paginiBrain, Vision, Memory - History of Neuroscience PDFjuan90Încă nu există evaluări
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceDe la EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceEvaluare: 4 din 5 stele4/5 (895)
- The Yellow House: A Memoir (2019 National Book Award Winner)De la EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Evaluare: 4 din 5 stele4/5 (98)
- Grit: The Power of Passion and PerseveranceDe la EverandGrit: The Power of Passion and PerseveranceEvaluare: 4 din 5 stele4/5 (588)
- The Little Book of Hygge: Danish Secrets to Happy LivingDe la EverandThe Little Book of Hygge: Danish Secrets to Happy LivingEvaluare: 3.5 din 5 stele3.5/5 (400)
- The Emperor of All Maladies: A Biography of CancerDe la EverandThe Emperor of All Maladies: A Biography of CancerEvaluare: 4.5 din 5 stele4.5/5 (271)
- Never Split the Difference: Negotiating As If Your Life Depended On ItDe la EverandNever Split the Difference: Negotiating As If Your Life Depended On ItEvaluare: 4.5 din 5 stele4.5/5 (838)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyDe la EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyEvaluare: 3.5 din 5 stele3.5/5 (2259)
- On Fire: The (Burning) Case for a Green New DealDe la EverandOn Fire: The (Burning) Case for a Green New DealEvaluare: 4 din 5 stele4/5 (74)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureDe la EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureEvaluare: 4.5 din 5 stele4.5/5 (474)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryDe la EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryEvaluare: 3.5 din 5 stele3.5/5 (231)
- Team of Rivals: The Political Genius of Abraham LincolnDe la EverandTeam of Rivals: The Political Genius of Abraham LincolnEvaluare: 4.5 din 5 stele4.5/5 (234)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaDe la EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaEvaluare: 4.5 din 5 stele4.5/5 (266)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersDe la EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersEvaluare: 4.5 din 5 stele4.5/5 (345)
- The Unwinding: An Inner History of the New AmericaDe la EverandThe Unwinding: An Inner History of the New AmericaEvaluare: 4 din 5 stele4/5 (45)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreDe la EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreEvaluare: 4 din 5 stele4/5 (1090)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)De la EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Evaluare: 4.5 din 5 stele4.5/5 (121)
- Her Body and Other Parties: StoriesDe la EverandHer Body and Other Parties: StoriesEvaluare: 4 din 5 stele4/5 (821)
- The Endocrine System The Endocrine System: © 2018 Pearson Education, Ltd. 1Document12 paginiThe Endocrine System The Endocrine System: © 2018 Pearson Education, Ltd. 1lourd nabÎncă nu există evaluări
- List of ContributorsDocument485 paginiList of ContributorsRock BandÎncă nu există evaluări
- Extension Worksheet - Option HDocument2 paginiExtension Worksheet - Option HHellö PëachÎncă nu există evaluări
- Рецептор ба түүний төрлүүдDocument9 paginiРецептор ба түүний төрлүүдАРСАЙÎncă nu există evaluări
- TCM Student Study GuideDocument40 paginiTCM Student Study GuideHomework PingÎncă nu există evaluări
- Jaw RelationDocument48 paginiJaw RelationBharanija100% (1)
- 10 Interesting Facts About CatsDocument8 pagini10 Interesting Facts About CatsAndreea DanaÎncă nu există evaluări
- Leukodystrophies and MucopolysacridosisDocument78 paginiLeukodystrophies and MucopolysacridosisMobin Ur Rehman KhanÎncă nu există evaluări
- Receptors Ion ChannelsDocument42 paginiReceptors Ion ChannelsMd. Ahsan-Ul BariÎncă nu există evaluări
- Specialization of Cerebral Hemispheres in Humans: December 2013Document9 paginiSpecialization of Cerebral Hemispheres in Humans: December 2013Huy NgoÎncă nu există evaluări
- Med Tech Manual 2014Document103 paginiMed Tech Manual 2014mapleleaf4evr100% (3)
- Concise Anatomy For Anaesthesia PDFDocument1 paginăConcise Anatomy For Anaesthesia PDFAnca SfrÎncă nu există evaluări
- Argaud Et Al. - 2018 - Facial Emotion Recognition in Parkinson's Disease A Review and New HypothesesDocument14 paginiArgaud Et Al. - 2018 - Facial Emotion Recognition in Parkinson's Disease A Review and New HypothesesAleja ToPaÎncă nu există evaluări
- Pediatrics Protocols - Pediatric Handbook 3rd EditionDocument25 paginiPediatrics Protocols - Pediatric Handbook 3rd Editionklteng85Încă nu există evaluări
- Tear DynamicsDocument9 paginiTear DynamicsAshwin ThakaliÎncă nu există evaluări
- Circulatory System AssessmentDocument4 paginiCirculatory System Assessmentapi-98991203Încă nu există evaluări
- Egyptian Healing RODSDocument67 paginiEgyptian Healing RODSŽarko Dačević100% (8)
- Cardiology I WorkbookDocument68 paginiCardiology I WorkbookPharmacist DinaÎncă nu există evaluări
- Cell and Its Structure and Functions: Q1. Write Down The Main Differences Between The Animal Cell and The Plant CellDocument5 paginiCell and Its Structure and Functions: Q1. Write Down The Main Differences Between The Animal Cell and The Plant CellMuhhammed AliÎncă nu există evaluări
- Yuvdz3wu 13jDocument2 paginiYuvdz3wu 13jNael LacerdaÎncă nu există evaluări
- BISC403 Sample Exam 4 W - Answers 20SpDocument5 paginiBISC403 Sample Exam 4 W - Answers 20SpGrace MillsÎncă nu există evaluări
- Medind - Nic.in Icb t05 I5 Icbt05i5p445Document4 paginiMedind - Nic.in Icb t05 I5 Icbt05i5p445Ester SibaraniÎncă nu există evaluări
- DXN Is The Perfect BusinessDocument45 paginiDXN Is The Perfect Businesssomasekharvasudevan83% (6)
- Pharmacology of Antipyretic DrugsDocument19 paginiPharmacology of Antipyretic DrugsPretty HiaÎncă nu există evaluări
- An Are Rob I C DegradationDocument18 paginiAn Are Rob I C DegradationMartuchis EstradaÎncă nu există evaluări
- Autopsy ReportDocument9 paginiAutopsy ReportHank LeeÎncă nu există evaluări
- Diabetes Mellitus and Laboratory Tests of DiabetesDocument24 paginiDiabetes Mellitus and Laboratory Tests of DiabetesturkiÎncă nu există evaluări
- In Brief: Inguinal HerniaDocument4 paginiIn Brief: Inguinal HerniaSaf DicamÎncă nu există evaluări
- AsthmaDocument55 paginiAsthmaAlessandra CruzÎncă nu există evaluări
- Biomechanics in Applications PDFDocument424 paginiBiomechanics in Applications PDFRAUL EDUARDO GUTIERREZ COITIÑO100% (1)