Documente Academic
Documente Profesional
Documente Cultură
Pathology Week 17 p1-15
Încărcat de
zeroun24Drepturi de autor
Formate disponibile
Partajați acest document
Partajați sau inserați document
Vi se pare util acest document?
Este necorespunzător acest conținut?
Raportați acest documentDrepturi de autor:
Formate disponibile
Pathology Week 17 p1-15
Încărcat de
zeroun24Drepturi de autor:
Formate disponibile
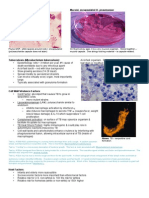
Pathology Review Block I, Neoplasia, Breast, Heme TYPES OF NECROSIS: COAGULATIVE Infarcts LIQUIFACTIVE Abscess CASEATION TB Granuloma FAT
T (ENZYMIC) Pancreas and Breast
12/06/10
Abscess neutrophils. If you have a bacterial infection in the lung (staph infection), there will probably be an abscess. Likewise, if you see an abscess in the lung, it is probably caused by something like Staphylococcus.
Necrotizing granulomas caseous. If you see this microscopically, however, cannot tell could just as easily be Histo as TB. But if you see the cheese-like material grossly, MUST be TB.
Two types of fat necrosis: enzymic and traumatic. Enzymic is due to destruction of the pancreas release of lipases. In enzymic fat necrosis, there is total destruction of tissue no outline of normal tissue (as in coagulation necrosis) and no neutrophils (as in liquefactive). Fat Necrosis (L) and Normal Pancreas (R)
Above (left): MI coagulation necrosis. This is an early, unfixed one. Looks like coagulum that you would see in a wound, with the serum moving in and clotting. Above (middle): Triangular-shaped architecture of infarction/coagulation necrosis. Above (right): Old infarct (scar) turns white with time.
Dysplasia vs Desmoplasia Dysplasia - Premaligmant change, a precursor to cancer - Cervical dysplasia
Desmoplasia - Fibrosis in reaction to invasive cancer - Invasive breast (nipple retraction); colon (apple core/napkin ring) Antemortem clot thrombus in coronary artery; coagulation necrosis. In MI, you do not usually have occlusion of the vessel by atherosclerosis. You have a diseased vessel, which stimulates coagulation thrombus. Get hemorrhage and thrombosis that closes off the artery and causes infarction.
Myocardial Infarction Markers - Cardiac specific enzymes and proteins in 2 hours - Morphologic (light microscopic) changes in 4-12 hours Timing of markers for MI myoglobin becomes positive fastest, but its very non-specific. In ERs today, consider troponin (+ at 3-4hr) very sensitive and specific. When is the patient at greatest risk for fatal arrhythmias? A. Days 1-3 After that time, there is no more necrosis, no more generation of new arrhythmias. B. Days 5-7 C. After 2 weeks D. After 4 weeks E. After 1 year Coagulative necrosis: 4-5hr Loss of nuclei, no inflammation Presence of neutrophils:
Dont see any inflammation in the first 4 hours, then you see loss of nuclei, then at ~12 hr, inflammatory cells start to move in. 24-48 hr see infiltration of neutrophils. When is the patient at greatest risk for perforation (rupture) of the left ventricle? A. Days 1-3 B. Days 5-10 C. 2 weeks D. 4 weeks E. 1 year At the time when all the muscle has been destroyed but there is not yet a good scar formed. Somewhere between 5-10 days, average is 7 days. Figure: View at 7 days. Normal muscle at the top, infarcted area at the bottom All the muscle is gone and fibroblasts/capillaries/inflammatory cells are present just do not have a scar yet. Weak.
More on Markers: Troponin I Is a specific indicator of MI Appears 4-6 hours post infarction, maybe not until 12 hours Peaks at 16 hours and decrease in 9-10 days persists CK-MB MB fraction is specific for cardiac muscle, esp when there is no skeletal muscle damage in patient's history Appears to rise 4-6 hours post MI Not elevated in all patients until 12 hours post MI Level returns to baseline in 36-48 hours Myoglobin Elevates within 1-4 hours, most sensitive during early time period Lacks specificity BNP (marker for CHF) Beta natriuretic peptide is the active product of a split prohormone in response to atrial or ventricular wall stretch. In this case it is a response to the acute congestive heart failure secondary to acute myocardial infarction. <100 pg/mL CHF very unlikely 100-400 pg/mL indeterminate >400 CHF likely (MI survivors are likely to develop heart failure) Six weeks post-MI, this 56-y.o. male has chest pain, SOB, precordial friction rub. He dies within days. The cause of the pathology (photo) is? A. Granulomatous inflammation B. Dresslers syndrome C. Metastatic carcinoma D. Ruptured LV E. Viral infection Dresslers syndrome several weeks post-MI autoimmune disease can develop. Results in fibrinous pericarditis. Above: Fibrinous pericarditis.
On the final exam, there will be questions about apoptosis and its role in different tumors and infections. In some things like follicular lymphoma and HPV, apoptosis is inhibited allows cells to continue to divide. In other things like infections by pneumocystis, the organism causes the macrophages to undergo apoptosis and eliminate them.
Above (left) is the apoptotic cell in Lichen Planus a Civatte body. Since Civatte bodies are undergoing apoptosis, you can assume that any of the enzymes involved in apoptosis are active (caspases, etc.) Remember the diagram above intrinsic and extrinsic pathways of apoptosis.
Defective Apoptosis: Tumors with p53 mutations Follicular lymphomas express high levels of bcl-2 (translocation of bcl-2 gene) HPV- protein E6 binds and inactivates p53 EBV- proteins that mimic or increase production of bcl-2 Autoimmune disorders Sensitivity, specificity and predictive value: No question on this test. Forget until May. Screening tests Confirmatory tests Prevalence and predictive value Given the photo, what enzyme abnormality would you expect? A. Increased alkaline phosphatase B. Decreased alkaline phosphatase C. Decreased gamma GT D. Markedly increased AST and ALT E. Decreased direct bilirubin Answer: A, increased AP: The photos show a gall bladder w/stones bile backs up, damages cells lining the canaliculi release alkaline phosphatase. If you have an obstructive lesion in the biliary tree, could be a stone, pancreatic tumor, bile duct tumor, etc. Alkaline phosphatase would be markedly elevated in any of these cases. In this case, the transaminases (which come from the hepatocytes) will not be markedly elevated. If AST and ALT are markedly elevated, it means that the liver parenchymal cells are dying more associated w/toxins, hepatitis, etc. Be able to recognize koilocytes HPV triggers production of proteins E6 and E7 inhibit both p53 and Rb genes. In HPV, you are inhibiting apoptosis and stimulating cell production. Figure A = normal cells B = low grade dysplasia (low grade HPV types like 6, 11) C = moderate dysplasia (nucleus is half the cell) D = severe dysplasia (nucleus is most of the cell) C and D associated with HPV 16 (high risk type) Dysplasia: Atypical proliferative changes due to chronic irritation or inflammation; PREMALIGNANT CHANGE
Normal cervix CIS (dysplasia/enlarged nuclei involve the full thickness)
Metaplasia: A REVERSIBLE change in which one ADULT cell type is replaced by another ADULT cell type.
Different types of metaplasia: Cervix squamous dysplasia. Esophagus glandular dysplasia (Barretts esophagus). Vitamin A deficiency problem of maturation, get metaplasia in the lung. Children who are Vitamin A deficient are much more likely to get certain viral infections (measles) and bacterial infections.
This brain tumor superficially invades bone, but not brain tissue. Name the tumor. A. Glioblastoma multiforme/astrocytoma grade IV B. Meningioma C. Metastatic lung CA D. Metastatic breast CA E. Metastatic melanoma B meningioma. There is no invasion of the brain parenchyma. The tumor is well demarcated but can kill by compression of the brain. It can locally invade bone, but does not metastasize. Make sure you can recognize it because it WILL be on final exam either gross or microscope. This cut-section of liver is c/w: A. Congestion B. Cirrhosis C. Hepatitis D. Metastasis Answer: D, metastasis The tumor nodules are diffuse (not a primary) and are too big to be cirrhotic nodules there is also an absence of white connective tissue. Multiple nodules of variable sizes metastatic disease. There will be a gross liver specimen on the final exam will be yellow/red/green/etc. must formulate diagnosis. Variably-sized nodules, diffusely spread through the liver = metastasis. Not primary disease.
Above and below: meningioma
Fatty change: In gross specimen, fresh = yellowy/orange. Fixed = pale.
Oil Red O or Sudan Black demonstrates fat.
NOT cardinal red brick red. Hemosiderosis or hemochromatosis.
Iron granules fine, powdery granules. in islets or in hepatocytes
Green = bile. Will see bile plugs in bile ducts.
Bile plugs in bile ducts.
Solid black material melanin.
Above (right): Cirrhotic liver w/nodules. Be able to recognize microscopically and grossly. Many cases of cirrhosis are due to viral infections. Both Hep B and C are associated w/hepatocellular carcinoma, so if you see a cirrhotic liver, dont be surprised if you also have hepatocellular carcinoma present. Cancer Precursor Lesions Adenomatous polyp Actinic keratosis Hyperpl./breast Ulcerative Colitis Endom. Hyperplasia Esoph. Metaplasia (Barretts) Gastric metaplasia (Helicobacter) Cirrhosis Colon AdenoCA SC SA Ductal CA Adeno CA colon Adeno CA endom. Esoph. Adeno CA Gastric Adeno CA/lymphoma Adeno CA liver
Helicobacter presents two problems hyperplasia increases the risk for adenocarcinoma, and hyperplasia of lymphoid material leads to an increased risk for MALT (low grade B cell) lymphoma. Same kind you get in Hashimotos thyroiditis.
Below: TNM Staging System.
In any malignancy, it is COMMON to have 4, 5, 6 steps before you ever reach malignancy. Be able to recognize stages of tumors. Different depending on where you are make sure to identify basement membrane (BM). In the breast, its the BM of the duct if it goes through that, its invasive cancer. In the skin, if it goes through the epidermis into the underlying dermis, its an invasive lesion. In cervix, if it goes through the BM of the epithelium, its invasive. In intestine, its different because the muscularis mucosa is considered to be the BM. If you have a lesion in the intestinal tract that is limited to epithelium, its CIS. If it penetrates the muscularis mucosa, it becomes invasive.
METASTASIS: LIVER: (portal circulation) GI tract and pancreas; lung, breast, melanomas LUNG: breast, stomach, sarcomas, renal cell carcinoma (vena caval system) rd BONE: 3 most frequent site for metastases; lung, breast, prostate, kidney, thyroid; PROSTATE to bone gives osteoblastic lesions on Xray (more dense) and high serum alkaline phosphatase ADRENAL: most common endocrine site Organs that empty into the portal circulation metastasize to liver. If they empty into the vena cava, more likely to go to lung and other sites.
Metastasis #1 marker of malignancy. Exceptions: gliomas (astrocytomas) of the brain and basal cell carcinomas of the skin RARELY metastasize; also, meningiomas LOCALLY invade skull bone, but do not metastasize and are considered benign. Always asked on tests what is number one marker of metastasis? ALWAYS pick malignancy. If its not a choice, pick invasiveness.
Venous Drainage Portal: liver Caval: lungs Paravertebral plexus: thyroid and prostate carcinomas metastasize to the vertebrae. Renal Cell CA: invades renal vein and grows in the vena cava
In metastasis, have lots of proteins/enzymes. There will be questions on upregulation and downregulation things that allow cells to stay attached (if they are downregulated, more likely to metastasize). Less cadherin more likely to metastasize. Increased laminin receptors for tumors to attach to upregulated, more likely to metastasize. Different kinds of lung carcinoma:
A = Squamous CA
B = Adeno CA
C = Small cell (oat cell) undifferentiated carcinoma
D = Large cell CA
Squamous pearls and intercellular bridges indicate that its squamous CA.
Anaplastic tumor: Poorly differentiated.
There was a tumor producing parathormone on the last test the answer was large cell carcinoma. Remember, in lung cancer there are two types of tumors clinically: small cell (aka oat cell, metastatic, treat w/chemotherapy) and large cell. Large cell w/squamous differentiation = squamous cell. Large cell w/glandular differentiation = adenocarcinoma. Large cell without any of that differentiation = large cell. Large cell = bigger than small cell and behaves differently (can treat w/surgery). Both large cells and squamous cells make parathormone and cause hypercalcemia. Adenocarcinomas do not do this.
Breast Pathology Review Normal breast: Ducts Terminal ducts lobular units (TDLUs) o Lined by 2 layers of cells Epithelial (luminal) Myoepithelial (basal) Benign diseases have both cells types Malignant lesions have only one. Males and females are different. M ducts but no lobules (nothing is connected) F ducts and lobules. Figure 1: Can see lobules. Each smaller unit is a duct. Normal breast tissue looks like this. Can see double layer myoepithelial layer (see similar layer in prostate) and glandular layer. Presence of myoepithelial layer indicates that it is benign. Figure 2: Again, can see myoepithelial layer and glandular layer (= benign). Fibrocystic changes: With age, breast undergoes fibrocystic changes (fibrosis and cystic change). Terminology--fibrocystic changes, fibrocystic disease, chronic cystic mastitis, periductal mastitis, mammary dysplasia, cystic mastalgia Three dominant morphologic patterns: o Cystic formation and fibrosis o Epithelial hyperplasia o Sclerosing adenosis Demographics o Microscopically extremely common, present to some degree in 60 to 90% of breasts in routine autopsies Etiology: Variable end-organ response to hormonal stimuli Fibrocystic disease: blue-dome cyst Not melanoma or malignant. Big cyst filled w/fluid. Slightly bluish tinge.
Fibrocystic changes: Cysts and fibrosis Mass and or Microcalcifications Unopened cysts are brown to blue (blue-dome cysts) Very common type - characterized by an increase in fibrous stroma associated with dilatation of ducts and formation of cysts of various sizes. Gross cysts > than 3 mm, micro cysts smaller Cysts often lined by large polygonal cells with abundant eosinophilic cytoplasm--so called apocrine metaplasia. Fibrocystic changes: cysts and apocrine metaplasia Apocrine metaplasia = benign. Apocrine metaplasia expanding breast duct Fibrocystic changes: Epithelial hyperplasia Commonly coexists with fibrosis, cysts, adenosis Microscopically, proliferation causes an increase in the layers of the duct-lining epithelium beyond the usual double layer Mild, moderate or florid The presence of architectural and/or cellular atypia warrants a diagnosis of atypical hyperplasia
Epithelial hyperplasia (non-atypical). Increased numbers of epithelial cells, but they do not completely fill up the lumen. If it was a solid sheet of tumor cells or if necrosis was present within the duct, it would signify malignant change.
Sclerosing adenosis Clinical- hard cartilaginous consistency that begins to approximate that found in breast cancer. Can mimic breast cancer on mammography. Microcalcifications frequent Proliferation of both epithelial and myoepithelial cells Minimal or no increased risk for cancer. We would never be expected to recognize sclerosing adenosis microscopically except to identify that its benign. Familial Breast Cancer: BRCA1 o Younger women, high grade tumors, ER BRCA2 o Relatively older, low grade (lobular) tumors Others o p53, Chk2, ATM, pTEN o unknown
Carcinoma in-situ: Types of ductal carcinoma in-situ (DCIS lesions that are limited to the breast duct) o Based on ARCHITECTURE (old) Solid Comedo: On cut section, central necrosis leads to easily extruded by slight pressure giving rise the term comedocarcinoma Cribriform Papillary Micropapillary Usual variants: Apocrine, Clinging, Clear cell o Based on NUCLEAR GRADE Low Intermediate High Figure (above, right): DCIS. No myoepithelial layer. Monotonous sheet of cells completely fills up the breast duct. Can see foci of microcalcification. Figure: DCIS note circumscription and cribriform architecture In this lesion, the duct was completely filled w/these cells then started undergoing necrosis. Classically described as looking like a cookie-cutter (punched-out lesions in the breast consistent w/malignant change). A big area of necrosis in the middle is also a malignant feature = comedocarcinoma. In both of these lesions, there doesnt appear to be anything going on outside, so it is CIS. Considered curable, but only 92% curable in breast. CIS in the cervix is 100% curable. Lower in breast because you cant see all the material (may see 1 focus of CIS, but there can be other foci). The only way to eliminate it would be a total mastectomy. In the cervix, can visualize all the material and can do a cone biopsy to remove all the diseased material. Pagets disease: Specialized form of ductal carcinoma that arises in the main excretory ducts of the breast and extends to involve the skin of the nipple and areola. Clinically, eczematoid changes occur in the nipple and areola. Ductal carcinoma, with or without invasion, invariably antedates the skin change. 30 to 40% of women have metastases at the time of surgery Histologic hallmark of this entity is the involvement of the epidermis by malignant cells, referred to as Paget cells Figure: Pagets disease of the nipple. Note nests of tumor cells in epidermis Figure: Pagets disease If you see these malignant cells in the epithelium of the nipple, always indicates an underlying malignant change/adenocarcinoma may be invasive or ductal CIS. If you stain w/mucin, they are glandular so would be mucin+ (not melanin+).
Above (left): Gross photo of infiltrating carcinoma, note stellate shape. Malignant biopsy w/fat surrounding. Whitish stuff is the tumor can see spicules/fingers going out into the fat (malignant feature). Above (right): Infiltrating ductal carcinoma, note duct-like structures and desmoplastic stroma. See the same thing microscopically glands/sheets of tumor cells surrounded by fibrosis desmoplasia. One of the most common places to see desmoplasia is in invasive breast cancer. Tumor grading: Architecture (glands) 1-3 Mitoses 1-3 Nuclear pleomorphism 1-3 Grade of tumor: In determining how malignant a breast tumor is, it depends on the architecture. Are there glands? Normal tissue has lots of ducts, so lots of ducts are a good thing. Mitoses bad. Want little or none. Nuclear pleomorphism bad if bizarre-looking, clear/dark areas. Want them to be uniform.
Above (left): If a tumor has invaded vascular spaces/lymphatics, it is inflammatory carcinoma. Woman presents w/swollen red breast, warm to the touch, indicates lymphatic invasion (probably stage 4 lesion widely metastatic). Above (middle): Can stain for estrogen receptors and Her2neu in breast tumors. Above (right): Some tumors (minority of tumors) may have overexpression of Her2neu. If so, are more aggressive patient will not live as long. However, in a subpopulation of people w/a bad prognosis, can be treated w/Herceptin (directed against Her2neu). Variants of ductal carcinoma: Good prognosis: Not a lot of malignant cells. Get bigger in volume but not as much cell multiplication. o Tubular carcinoma aka well-differentiated adenocarcinoma of the breast o Mucinous carcinoma o Medullary carcinoma o Adenoid cystic carcinoma Bad Prognosis: o Metaplastic carcinoma o Squamous cell carcinoma o Carcinoma with osteoclast-like giant cells o Apocrine carcinoma
Tubular carcinoma:
Mucinous carcinoma:
Low-grade lesion.
Get really big because they make lots of mucin. Lots of volume but not too many malignant cells.
Invasive lobular carcinoma: WHO definition composed of uniform cells resembling those of lobular carcinoma in situ and usually having a low mitotic rate. The cells grow typically in a single-file, linear arrangement, or appear individually embedded in fibrous tissue. Targetoid growth pattern and identification of remnants of lobular carcinoma in situ aid in the diagnosis. Signet-ring cells may be seen. TNM Staging clinical T-Primary tumor based on size T1: </= 2cm (T1a,b,c: to 0.5, to 1.0, to 2.0) T2: > 2cm </= 5 cm T3: > 5 cm T4: any size with extension to skin/chest wall N-Regional lymph nodes NX: regional lymph nodes cannot be assessed N0: No regional lymph node metastasis N1: Metastasis to movable ipsilateral axillary nodes(s) N2: Metastasis to ipsilateral axillary node(s) fixed to one another or to other structures N3: Metastasis to ipsilateral internal mammary lymph nodes M - Distant metastasis MX: Presence of distant metastasis cannot be assessed M0: No distant metastasis M1: Distant metastasis (includes metastasis to supraclavicular lymph nodes Prognosis for invasive Breast carcinoma: 10-year disease free survival 80% for T1N0 90% for T1N0 < 1 cm. 70% for T2N0 60% for T3N0 Mixed Epithelial and Stromal tumors: Fibroadenoma Benign Glandular and stromal elements Does not undergo malignant change Phyllodes tumor More cellular stroma than FA Cellular pleomorphism, necrosis May be benign or malignant Benign morphology is poor predictor of behavior
INDIAN FILING
The worse the stage, the worse the prognosis. Most important thing to determine for a tumor is the STAGE. In most cases, stage is more important than grade.
Fibroadenoma:
Left: carcinoma, star-shaped finger-like extensions. Right: fibroadenoma. Well-circumscribed (= benign, usually).
Lots of fibrosis and gland-like spaces. Often squeezed into trenches that are formed. Benign lesion.
Hematopathology Review Tumor Markers: PSA Prostate cancer = prostate specific antigen CEA Colorectal/Pancreatic CA 19-9 Pancreatic Alpha-fetoprotein Hepatic/Yolk sac Beta-HCG Choriocarcinoma/Molar pregnancy CA 125 Ovarian cancer S-100 Melanoma/Astrocytoma Thyroglobulin Thyroid cancer (follicular carcinoma) Calcitonin Medullary carcinoma of Thyroid Catecholamines Pheochromocytoma/Neuroblastoma TRAP stain Hairy cell leukemia = tartrate resistant acid phosphatase Alkaline phosphatase Mets to bones, Pagets disease of bone Remember that AFP can be seen in liver cancer and testicular cancer. Lots of neural tumors are S-100 positive. If you have a clinical history (M or F) of high alkaline phosphatase but nothing going on in biliary tree, remember that osteoblasts also make alkaline phosphatase . In lots of metastatic tumors to the bone, more osteoid is made increased alkaline phosphatase. If male patient has urinary symptoms and high alkaline phosphatase, probably have prostate cancer metastatic disease. Translocations: t (9;22) CML (bcr-abl) t (8;14) Burkitts lymphoma (c-myc) t (14;18) Follicular lymphoma (bcl-2) t (15;17) AML M3 (promyelocytic leukemia) t (1;14) T-cell Lymphoblastic lymphoma t (11; 22) Ewings sarcoma t (11; 14) Mantle cell lymphoma (bcl-1) t (11; 18) MALT lymphoma (API-2) t (1; 14) MALT lymphoma (bcl-10)
There will be 1 peripheral blood smear and 2 lymph nodes on lab exam. Know RBC changes: target cells thalassemias; immature cells pumped out of marrow something else is filling up the marrow (leukemia, fibrosis, etc.); HJ bodies spleen is not functioning properly or is absent.
Above (left): MAHA see schistocytes. Also seen in malfunctioning heart valve. Reticulocytes seen in anything that causes hemolysis (RBCs live less than 120 days) Above (right): Fe deficiency hypochromic microcytic anemia. Funny shaped cells. Fe deficiency in an old person MUST worry about occult malignancy (most common is in colon or stomach). Other occult malignancies can also result in Fe deficiency. Nutritional Fe deficiency is nearly impossible in the US. CBC for patient with this blood smear Hgb 5.9 MCV 56.2 RDW 20.2 WBC 5,900 Plt 383,000 In Fe deficiency, MCV will be low and RDW will be high indicates variation in size of RBCs. Platelet count tends to be elevated in Fe deficiency because when you have less RBCs, it stimulates erythropoietin has no effect on RBCs because theres no iron, so it also stimulates the platelets. Could see 500,000-1,000,000 platelets in patients w/Fe deficiency. Before the patient leaves your office you should? A. Order TSH and T3 B. Order TSH and T4 C. Prescribe multivitamins with iron D. Perform stool exam for occult blood ALWAYS check if patient is an older person w/iron deficiency anemia. E. Perform stool exam for hookworms and other parasites
CBC: Hgb 6 MCV 130 WBC 6,000 Plt 220,000
Low High Normal Normal
Megaloblastic anemia: see neutrophils w/too many lobes (5+) seen in B12 or folate deficiency. In B12/folate deficiency, the cells are also often oval in shape.
Peripheral blood smear See schistocytes and spherocytes. Dont immediately think hereditary spherocytosis if you see spherocytes. If there are both, think something else, like MAHA. When these cells get sheared, sometimes are very irregular in shape (schistocytes) but sometimes will round up (spherocytes). CBC: Hgb 7.0 MCV 77 RDW 23 WBC 17,000 Plt 14,000
Peripheral blood smear Spherocytosis hemolytic anemia (see reticulocytes, too). Platelets present doesnt seem to be MAHA. Not really any schistocytes but lots of spherocytes. CBC: Hgb 11 MCV 84 WBC 6,000 Plt 350,000
LEUKEMIA: Acute ALL and AML Hi/Low WBC (unpredictable) Rapidly fatal if untreated (matter of weeks); anemic and thrombocytopenic (almost always. Patient is very sick.) Curable Acute Leukemias:
Chronic CLL and CML Always high WBC Slowly progressive- patient lives many years Difficult to cure
5a. In Acute Leukemias you will almost always find: A. Normal hct, normal platelets B. Normal hct, decreased platelets C. Decreased hct, increased paltelets D. Anemia, thrombocytopenia E. WBC count >100,000 D: Patient is always really sick. 5b. In Chronic Leukemias you will almost always find: A. Anemia, thrombocytopenia B. WBC count >50,000 C. Normal hct, increased platelets D. Anemia, thrombocytosis E. Circulating blasts
S-ar putea să vă placă și
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeDe la EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeEvaluare: 4 din 5 stele4/5 (5794)
- Pathology Week 2 p19-36Document18 paginiPathology Week 2 p19-36zeroun24100% (1)
- Shoe Dog: A Memoir by the Creator of NikeDe la EverandShoe Dog: A Memoir by the Creator of NikeEvaluare: 4.5 din 5 stele4.5/5 (537)
- Liver PathologyDocument21 paginiLiver Pathologyzeroun24100% (6)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceDe la EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceEvaluare: 4 din 5 stele4/5 (895)
- Pathology Week 4Document34 paginiPathology Week 4zeroun24Încă nu există evaluări
- Pathology Week 5 p1-14Document20 paginiPathology Week 5 p1-14zeroun24100% (1)
- The Yellow House: A Memoir (2019 National Book Award Winner)De la EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Evaluare: 4 din 5 stele4/5 (98)
- Pathology Week 5 p29-40Document12 paginiPathology Week 5 p29-40zeroun24Încă nu există evaluări
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersDe la EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersEvaluare: 4.5 din 5 stele4.5/5 (344)
- Pathology Week 3Document28 paginiPathology Week 3zeroun24Încă nu există evaluări
- The Little Book of Hygge: Danish Secrets to Happy LivingDe la EverandThe Little Book of Hygge: Danish Secrets to Happy LivingEvaluare: 3.5 din 5 stele3.5/5 (399)
- Endocrine Pathology p1-16Document16 paginiEndocrine Pathology p1-16zeroun24100% (1)
- Grit: The Power of Passion and PerseveranceDe la EverandGrit: The Power of Passion and PerseveranceEvaluare: 4 din 5 stele4/5 (588)
- Pathology Week 5 p15-28Document14 paginiPathology Week 5 p15-28zeroun24Încă nu există evaluări
- The Emperor of All Maladies: A Biography of CancerDe la EverandThe Emperor of All Maladies: A Biography of CancerEvaluare: 4.5 din 5 stele4.5/5 (271)
- Lung Head and NeckDocument26 paginiLung Head and Neckzeroun24100% (2)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaDe la EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaEvaluare: 4.5 din 5 stele4.5/5 (266)
- Pathology Week 2 p1-18Document18 paginiPathology Week 2 p1-18zeroun24100% (1)
- Never Split the Difference: Negotiating As If Your Life Depended On ItDe la EverandNever Split the Difference: Negotiating As If Your Life Depended On ItEvaluare: 4.5 din 5 stele4.5/5 (838)
- Pathology - Week 1Document36 paginiPathology - Week 1zeroun2475% (4)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryDe la EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryEvaluare: 3.5 din 5 stele3.5/5 (231)
- Hematologic Pathology p48-64Document17 paginiHematologic Pathology p48-64zeroun24100% (1)
- Infectious Disease Pathology p1-30Document30 paginiInfectious Disease Pathology p1-30zeroun24Încă nu există evaluări
- On Fire: The (Burning) Case for a Green New DealDe la EverandOn Fire: The (Burning) Case for a Green New DealEvaluare: 4 din 5 stele4/5 (73)
- Endocrine Pathology p17-32Document16 paginiEndocrine Pathology p17-32zeroun24Încă nu există evaluări
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureDe la EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureEvaluare: 4.5 din 5 stele4.5/5 (474)
- Infectious Disease Pathology p31-55Document25 paginiInfectious Disease Pathology p31-55zeroun2450% (2)
- Team of Rivals: The Political Genius of Abraham LincolnDe la EverandTeam of Rivals: The Political Genius of Abraham LincolnEvaluare: 4.5 din 5 stele4.5/5 (234)
- GI PathologyDocument22 paginiGI Pathologyzeroun24100% (5)
- The Unwinding: An Inner History of the New AmericaDe la EverandThe Unwinding: An Inner History of the New AmericaEvaluare: 4 din 5 stele4/5 (45)
- Lab Items - Exam 2Document3 paginiLab Items - Exam 2zeroun24100% (1)
- Hematologic Pathology p65-87Document23 paginiHematologic Pathology p65-87zeroun24100% (1)
- Pages From Pathology Week 5 p15-28Document6 paginiPages From Pathology Week 5 p15-28zeroun24Încă nu există evaluări
- Pancreatic PathologyDocument7 paginiPancreatic Pathologyzeroun24100% (1)
- Path Lab Exam I ChecklistDocument2 paginiPath Lab Exam I Checklistzeroun24Încă nu există evaluări
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyDe la EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyEvaluare: 3.5 din 5 stele3.5/5 (2259)
- Hematologic Pathology p24-35Document12 paginiHematologic Pathology p24-35zeroun24100% (6)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreDe la EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreEvaluare: 4 din 5 stele4/5 (1090)
- Hematologic Pathology p1-23Document23 paginiHematologic Pathology p1-23zeroun24100% (2)
- Cancer Epidemiology and Pediatric Neoplasia p11-14Document4 paginiCancer Epidemiology and Pediatric Neoplasia p11-14zeroun24Încă nu există evaluări
- Hematologic Pathology p36-47Document12 paginiHematologic Pathology p36-47zeroun24Încă nu există evaluări
- Infectious Disease Pathology p56-75Document20 paginiInfectious Disease Pathology p56-75zeroun24Încă nu există evaluări
- Infectious Disease Pathology p76-89Document14 paginiInfectious Disease Pathology p76-89zeroun24100% (1)
- Pathology Week 17 p16-30Document15 paginiPathology Week 17 p16-30zeroun24Încă nu există evaluări
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)De la EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Evaluare: 4.5 din 5 stele4.5/5 (121)
- Endocrine Pathology p33-47Document15 paginiEndocrine Pathology p33-47zeroun24Încă nu există evaluări
- Pathology Week 6 p1-17Document17 paginiPathology Week 6 p1-17zeroun24Încă nu există evaluări
- Pharmacological Management of Agitation in Emergency SettingsDocument8 paginiPharmacological Management of Agitation in Emergency SettingsnurulnadyaÎncă nu există evaluări
- Otitis MediaDocument34 paginiOtitis MediaEdson Reinier Joseph EnriquezÎncă nu există evaluări
- Lbbbi18 Long Exam 1Document71 paginiLbbbi18 Long Exam 1namkimseoÎncă nu există evaluări
- Pediatric Diabetes InsipidusDocument11 paginiPediatric Diabetes InsipidusVictor Matias BarriosÎncă nu există evaluări
- The Miracle Plant Kalanchoe Pinnata A PhytochemicaDocument6 paginiThe Miracle Plant Kalanchoe Pinnata A PhytochemicaAboli GhateÎncă nu există evaluări
- ParkinsonDocument12 paginiParkinsonStephanie NieÎncă nu există evaluări
- People Running PowerPoint TemplatesDocument16 paginiPeople Running PowerPoint TemplatesPutri Aulia AmaraÎncă nu există evaluări
- Laboratory Tests of Renal FunctionDocument5 paginiLaboratory Tests of Renal Functiongiselle155204Încă nu există evaluări
- PHAR 400 Pharmacology I Master 1-3-21Document5 paginiPHAR 400 Pharmacology I Master 1-3-21Zahid Bashir BhattiÎncă nu există evaluări
- Her Body and Other Parties: StoriesDe la EverandHer Body and Other Parties: StoriesEvaluare: 4 din 5 stele4/5 (821)
- Anemia of Chronic DiseaseDocument13 paginiAnemia of Chronic Diseasemaverick mazeÎncă nu există evaluări
- Pain SheetDocument4 paginiPain SheetMohammad Al GhroryÎncă nu există evaluări
- ThesisDocument30 paginiThesisSimran JosanÎncă nu există evaluări
- 342 FullDocument26 pagini342 FullputrianabrsitompulÎncă nu există evaluări
- WHO Drug Information: Herbal Medicines Regulatory ActionDocument91 paginiWHO Drug Information: Herbal Medicines Regulatory ActionLia PuspitasariÎncă nu există evaluări
- Difficulty in SwallowingDocument1 paginăDifficulty in SwallowingmawelÎncă nu există evaluări
- Step Up To Medicine Chapter 03Document868 paginiStep Up To Medicine Chapter 03yanks1120Încă nu există evaluări
- Pnpa Requirement 2019Document5 paginiPnpa Requirement 2019Michael Angelo SantosÎncă nu există evaluări
- Muscle Deprogramming - An Orthodontist's Perspective: Batra Laxman Ra Angshuman B LlachDocument5 paginiMuscle Deprogramming - An Orthodontist's Perspective: Batra Laxman Ra Angshuman B LlachJulio Cesar AlvearÎncă nu există evaluări
- Assessment of The Nutritional Status of The CommunityDocument29 paginiAssessment of The Nutritional Status of The CommunitySarad Chand YadavÎncă nu există evaluări
- My Trip To The Hospital - A Coloring BookDocument0 paginiMy Trip To The Hospital - A Coloring Bookili_thÎncă nu există evaluări
- Unit 6: Resistance of The Body To Infection: II. Immunity and AllergyDocument34 paginiUnit 6: Resistance of The Body To Infection: II. Immunity and AllergyEsteban Tabares GonzalezÎncă nu există evaluări
- nsg-320cc Care Plan 1Document14 pagininsg-320cc Care Plan 1api-509452165Încă nu există evaluări
- A Technical Seminar Presentation On: Bionic EyeDocument13 paginiA Technical Seminar Presentation On: Bionic EyeRohith AddagatlaÎncă nu există evaluări
- Group 4 Lydia Hall CritiqueDocument4 paginiGroup 4 Lydia Hall CritiqueMarielle Ann RumbaoaÎncă nu există evaluări
- Ask A Dermatologist Online For FreeDocument1 paginăAsk A Dermatologist Online For FreeVispera HealthÎncă nu există evaluări
- Urostomy Guide - American Cancer SocietyDocument19 paginiUrostomy Guide - American Cancer SocietyDipa HandraÎncă nu există evaluări
- Nursing Care Plan - Constipation (Antepartum)Document2 paginiNursing Care Plan - Constipation (Antepartum)kaimimiyaÎncă nu există evaluări
- The TENS Electrode Placement AtlasDocument14 paginiThe TENS Electrode Placement AtlasSrce Za Srce89% (9)
- Sample of Essay - The FluDocument2 paginiSample of Essay - The FluMerisa WahyuningtiyasÎncă nu există evaluări
- Screening For Psychological Burden of Vitiligo Using Vitiligo Impact ScaleDocument6 paginiScreening For Psychological Burden of Vitiligo Using Vitiligo Impact ScaleNadaaFahmiShofiÎncă nu există evaluări
- The Obesity Code: Unlocking the Secrets of Weight LossDe la EverandThe Obesity Code: Unlocking the Secrets of Weight LossEvaluare: 4 din 5 stele4/5 (6)
- By the Time You Read This: The Space between Cheslie's Smile and Mental Illness—Her Story in Her Own WordsDe la EverandBy the Time You Read This: The Space between Cheslie's Smile and Mental Illness—Her Story in Her Own WordsÎncă nu există evaluări
- Think This, Not That: 12 Mindshifts to Breakthrough Limiting Beliefs and Become Who You Were Born to BeDe la EverandThink This, Not That: 12 Mindshifts to Breakthrough Limiting Beliefs and Become Who You Were Born to BeEvaluare: 2 din 5 stele2/5 (1)
- The Age of Magical Overthinking: Notes on Modern IrrationalityDe la EverandThe Age of Magical Overthinking: Notes on Modern IrrationalityEvaluare: 4 din 5 stele4/5 (24)
- The Body Keeps the Score by Bessel Van der Kolk, M.D. - Book Summary: Brain, Mind, and Body in the Healing of TraumaDe la EverandThe Body Keeps the Score by Bessel Van der Kolk, M.D. - Book Summary: Brain, Mind, and Body in the Healing of TraumaEvaluare: 4.5 din 5 stele4.5/5 (266)
- Summary: Outlive: The Science and Art of Longevity by Peter Attia MD, With Bill Gifford: Key Takeaways, Summary & AnalysisDe la EverandSummary: Outlive: The Science and Art of Longevity by Peter Attia MD, With Bill Gifford: Key Takeaways, Summary & AnalysisEvaluare: 4.5 din 5 stele4.5/5 (42)
- Summary: The Psychology of Money: Timeless Lessons on Wealth, Greed, and Happiness by Morgan Housel: Key Takeaways, Summary & Analysis IncludedDe la EverandSummary: The Psychology of Money: Timeless Lessons on Wealth, Greed, and Happiness by Morgan Housel: Key Takeaways, Summary & Analysis IncludedEvaluare: 5 din 5 stele5/5 (80)