Documente Academic
Documente Profesional
Documente Cultură
Pediatric Head Injury
Încărcat de
Andy WijayaDrepturi de autor
Formate disponibile
Partajați acest document
Partajați sau inserați document
Vi se pare util acest document?
Este necorespunzător acest conținut?
Raportați acest documentDrepturi de autor:
Formate disponibile
Pediatric Head Injury
Încărcat de
Andy WijayaDrepturi de autor:
Formate disponibile
Paediatric Anaesthesia 1999
9: 377385
Review article
Paediatric head injury: incidence, aetiology and management
W. HIU LAM BM BS, MRCP, FRCA AND ANGELA MACKERSIE BSc, MB BS, FRCA
Department of Anaesthesia, Great Ormond Street Hospital for Children, NHS Trust, Great Ormond Street, London UK
Summary
Trauma is the commonest cause of hospital admission in children. Head injuries are present in 75% of children with trauma and 70% of all traumatic deaths are due to the head injury. The mechanism of brain injury is examined, resulting from the effects of the primary insult and secondary ischaemic damage. Therapeutic interventions will be discussed with specic emphasis on outcome studies. However, institution of adequate oxygen delivery and haemodynamic stability in the child at the earliest moment remains the most important aspect of the management plan. Keywords: head injury; management
Introduction
Worldwide, trauma is the commonest cause of admission to hospital in children, and it is also a major cause of morbidity and mortality. After the rst year of life, trauma is the most frequent cause of death and remains so until well into adult life, with the incidence increasing throughout childhood. In the rst year of life congenital anomalies, prematurity and sudden infant death syndrome (SIDS) are the major causes of mortality. However, following public campaigns concerning infant sleeping positions, the striking reduction in SIDS in some countries has resulted in trauma deaths relatively increasing in infants, to approximately 10% during the rst half of the 1990s. Three-quarters of all children admitted to hospital with trauma have an associated head injury and 70% of deaths result from the head injury. Overall, 15%
Correspondence to: Angela Mackersie, Department of Anaesthesia, Great Ormond Street Hospital for Children NHS Trust, Great Ormond Street, London WC1N 3JH, UK. 1999 Blackwell Science Ltd
of deaths under 15 years are due to head trauma and, between the ages of 5 and 15 years, the incidence is 25%. In the UK, road trafc accidents (RTAs) are the most common cause of head injuries, with the child pedestrian being the largest subgroup. In the USA, child car passengers have the highest incidence of cranial trauma. Falls and nonaccidental injury are the other major causes of head trauma in children. In the rst year, nonaccident injury is the commonest cause of major head injuries. Following the injury, there is a trimodal pattern to the time of death. Almost half paediatric trauma deaths occur within minutes of the impact where there is usually an overwhelming injury incompatible with life. In the second group (approximately 20%), death occurs within hours due to respiratory failure, circulatory insufciency or raised intracranial pressure (ICP). Death may be avoided if active treatment is timely and effective. The remaining onethird of deaths occur later, days or weeks after the event, and are not only due to raised intracranial pressure, but also to infection and organ failure. 377
378
W. HIU LAM AND A. MACKERSIE
Mechanism of brain injury
The resultant head injury is caused by the so called primary brain injury (PBI) and the effects of the secondary brain injury (SBI) caused by respiratory or circulatory insufciency or raised ICP.
Primary brain injury
The magnitude of the initial impact of the brain in trauma determines the severity of PBI. The injury results from contusions, lacerations, diffuse axonal injury and dural tears which are caused by abrupt acceleration and/or deceleration of the head. In children, the disproportionately larger and heavier head renders them more likely to head injury because the momentum on impact is increased. It is important to bear in mind that even in children, signs of hypovolaemic shock (e.g. hypotension and tachycardia) in head injury should alert the resuscitation team to look for other signs of occult bleeding. It is however, possible to lose signicant circulating blood volume from a scalp wound as the surface area of the head in an infant is 19% of the total body surface area, compared to 9% in adults. Intracranial haemorrhage may also contribute to hypovolaemic shock.
increased with the frequency of hypotensive episodes (5). Cerebral perfusion pressure (CPP) [mean arterial pressure (MAP) ICP]. A reduction in MAP causes a fall in CPP and exacerbates preexisting brain injury. Hypoxia, PaO2 of < 8 kPa (< 60 mmHg) and hypotension impair oxygen delivery to the brain (6). Hypoxia may be caused by a reduced respiratory drive secondary to the brain injury or from airway obstruction accompanying a reduction in conscious level in head injury. It may also be related to direct injury to the lung parenchyma in children with coexisting chest injury. Hypercapnia The arterioles in the brain respond to the local concentration of CO2 by autoregulation. An accumulation of CO2 (e.g. secondary to hypoventilation) increases hydrogen ion concentration which exerts a local vasodilatory effect on these vessels causing an increase in cerebral blood ow which in turn increases the ICP. The raised ICP further exacerbates SBI. Pyrexia For every 1C increase in body temperature, there is a 5% increase in the cerebral metabolic rate which precedes an increase in cerebral blood ow (and ICP) in response to this increased activity (7). In addition, pyrexia also causes an increase in insensible water loss by 12% per degree rise (8), and if not corrected, predisposes to hypovolaemia and a reduction in MAP and CPP. In an animal model, a core temperature rise of as little as 12C may worsen both the neurological signs and the histopathological evidence of ischaemia (9). Electrolyte abnormalities Disturbances in sodium homeostasis are the main abnormality. Hyponatraemia may be caused by inappropriate antidiuretic hormone secretion or cerebral salt wasting; both triggered by an insult to the brain. Although they both present with low serum sodium, the former with normo- or hypervolaemia; whereas the latter is usually with intravascular depletion. Hyponatraemia causes cerebral oedema with devastating morbidity and mortality (1012). Diabetes insipidus (caused by the disruption of antidiuretic hormone secreting cells) is recognized by the presence of serum hypernatraemia,
1999 Blackwell Science Ltd, Paediatric Anaesthesia, 9, 377385
Secondary brain injury
SBI is caused by the complications of PBI. Features of SBI were found in up to 90% of patients with head trauma in a study by Jones (1). Unlike PBI, SBI is largely preventable and its prevention plays a pivotal role in the management of head injury. Brain ischaemia (as a result of oxygen demand and supply mismatch) either locally or globally is the end result of SBI and has been demonstrated in over 80% of fatal head injury (2), but there is a whole range of insults that converge onto a nal common pathway to SBI. Therefore, understanding the mechanism of causation leading to SBI is imperative. Change in blood pressure and hypoxia Hypertension, hypotension and hypoxia have been independently shown to increase morbidity and mortality in severe head injury in both adults (3) and children (4). It has also been demonstrated that the mortality of patients with severe head injury is
PAEDIATRIC HEAD INJURY
379
hyperosmolality and inappropriate production of hypotonic urine (urine osmolality < 200 mOsm.l1 and/or urine specicity < 1.002). This is associated with intravascular volume depletion and therefore haemodynamic instability. The depletion should initially be treated with volume replacement using 0.45% saline and 5% dextrose as there is a low sodium content in the urine. The large volume of uid required to restore the decit may cause hyperglycaemia, which can in turn lead to an osmotic diuresis and compound the problem of dehydration. Therefore, in practice, the early and judicial administration of vasopressin or its analogue, 1deamino-8-D-arginine (DDAVP) is usually effective (7,13,14). Hyperglycaemia induces lactic acidosis and subsequent oxygen free radical production which is detrimental to injured brain tissue and is related to poor outcome in head injury (15). Hypoglycaemia, which is more common in premature infants, should be actively sought and treated as glucose is the only substrate for brain metabolism. Careful assessment of the requirement for a glucose containing solution is therefore essential. Intracranial pathology Trauma induced intracranial haematomas such as extradural, subdural and intracerebral all cause disruption of the blood supply to the brain tissue and therefore induce ischaemia and local cerebral oedema. Subdural haemorrhages in infants deserve a special mention as a recent UK study conrms most haemorrhages of this kind are due to nonaccidental injury (16). The outcome in this type of injury is reported to have a poor outcome (17,18). As the brain is enclosed by a bony compartment, the expansion of brain tissue will eventually lead to a rise in ICP when all the compensatory mechanisms are exhausted (MonroKellie doctrine) and is thought to be a poor prognostic index in severe head injury (19). Cellular mechanism Excitatory amino acids (Eaas) are neurotransmitters that are widely distributed in the brain (20), but are neurotoxic in high concentration. It is now evident from animal studies that increased levels of glutamate (one of the excitatory amino acids) are detected in damaged brain (21,22) and in cerobrospinal uid
1999 Blackwell Science Ltd, Paediatric Anaesthesia, 9, 377385
Table 1 Treatment strategies for acute intracranial hypertension Treatment strategy Percentage of intensive care units in USA 83% 64% 57% 29% 44% 33% 28% Percentage of intensive care units in UK 100% 49% 44% 2.5% 5% 6% 6%
Osmotic diuretic Steroid administration Prophylactic hyperventilate CO2 2535 mmHg Prophylactic hyperventilate CO2 < 25 mmHg Cerebrospinal uid drainage Barbiturate administration ICP monitoring
(CSF) after head injury (23). The increase in glutamate appears to initiate an excitotoxic cascade by activating the inotropic and metabotropic glutamate receptors. The former include N-methyl D-aspartate (NMDA) receptors; the latter are associated with G-proteins and are involved in the modulation of intracellular secondary messengers. The net effect is an increase in intracellular calcium which initiates a cascade of intracellular destruction by activation of protein kinase, phospholipases, proteases causing proteolysis, lipid peroxidation, free radical formation and degeneration of the neurone (20).
Management
Almost half the paediatric trauma deaths occur with minutes from the primary injury, so the best way to deal with PBI is to reduce its occurrence by education of children and their families. Government policy and road safety campaigns, in conjunction with the help of schools, motoring organizations and parental education and cooperation, must be the key to success in preventing road accidents. The routine use of helmets for potentially dangerous activities such as cycling and skiing will also reduce the incidence of serious head injuries. In contrast, SBI can be treatable, and its management is the mainstream treatment of head injury, aiming to avoid, anticipate and actively treat the development of intracranial hypertension (ICH). The variability in ICH treatment strategy is well illustrated by surveys of intensive care units from the USA (24) and the UK (25). The variations in treatment strategies from these units are summarized in Table 1. The possible reasons for such different
380
W. HIU LAM AND A. MACKERSIE
approaches to treatment are partly due to scientic weakness, poor education and communication. The UK government is attempting to address the latter by introducing national guidelines for all treatment strategies from the National Institute of Clinical Excellence (NICE), a new statutory body (26,27). At present, there are no universally agreed guidelines for treatment of acute ICH. With this in mind, we set out to review the evidence currently available, looking especially for studies using human clinical outcome as endpoint. There is limited Class I evidence (information derived from large prospective randomized controlled trials) in the adult population of head injury, let alone paediatric subjects. Class II (prospective nonrandomized controlled trials or retrospective studies) and Class III (case series, reports and expert opinions) evidence will also be reviewed. The importance of immediate airway management with oxygen supplementation, cervical spine immobilization, assessment of the adequacy of breathing and optimal circulation at the scene of the accident through to the casualty department and PICU cannot be overemphasized (28). There is evidence for the inuence of hypoxia and hypertension on outcome to support the importance of immediate airway and circulation management (29). Airway management and mechanical ventilatory support have been shown to decrease mortality in various groups of patients with head injury, cerebral tumour, encephalitis and cerebrovascular accident (3033). Initial assessment of the severity of the head injury is needed and, for young children, the Glasgow Coma Scale has been modied to allow for the normal lack of verbal skills. Severe head injuries are dened as those with coma scores of 8 or less, moderate injuries with scores between 9 and 12, and minor between 13 and 15. Rapid assessment can be made whether the child is alert, responds to verbal command, responds to pain, or is unresponsive. Plain skull X-rays are required for initial assessment and the indications for computed tomography (CT) scanning are given in Table 2. Investigations are performed to exclude a surgically treatable lesion, to diagnose hyperaemia or oedema and to delineate fractures. Magnetic resonace imaging scans are not indicated routinely at the initial stage
Table 2 Indications for computed tomography scans in HI Glasgow Coma Scale 8 Skull fracture Onset of neurological signs Cushings response (bradycardia, hypertension, irregular respiration) Fall in Glasgow Coma Scale with normal blood gases Glasgow Coma Scale < 14 for more than 8 h
but may be useful as lesions develop or for planning surgery. Four to ve percent of paediatric head injuries have an associated cervical spinal fracture with or without dislocation., mostly at C1C3, also likely to be caused by the force of the injury on the relatively large head of the child. Unless dynamic images are seen it is impossible to exclude spinal damage or instability and therefore should be assumed to be present and appropriate care taken during intubation or positioning for other procedures.
Evidence to support treatment strategy for intracranial hypertension
Class I Only three aspects of management of acute intracranial hypertension have Class I evidence with human outcome as end point. Unfortunately, they all appear to be negative. Prophylactic hyperventilation. Prophylactic hyperventilation of any magnitude with no intracranial hypertension should be avoided as the vasoconstrictive effect of acute hyperventilation can produce decreases in cerebral blood ow to a potentially detrimental level (34). Most studies on hyperventilation deal with the effect of hypocapnia on cerebral blood ow, but it is demonstrated in one neurological outcome study at 3 and 6 months postinjury that prophylactic hyperventilation is deleterious in head injured patients without proven intracranial hypertension (35). In addition, this is supported by a human observational study that hypocapnia may have made the outcome worse (36). In a recent article by Dexter (37), over 980 articles in a 30-year-period were reviewed to evaluate the effect of hypocapnia and airway protection on cerebral
1999 Blackwell Science Ltd, Paediatric Anaesthesia, 9, 377385
PAEDIATRIC HEAD INJURY
381
outcome and there are no clinical data to support the hypothesis that hypocapnia improves outcome in patients with cerebral disease. Steroid administration. The use of steroids should not be advocated in the management of head injury. Recent studies have not shown a signicant change in mortality and morbidity 6 months after the initial head injured patients were treated with either low or high dose steroid (38,39). Furthermore, high dose steroid has been demonstrated to have no advantage on ICP trend (40). This, in conjunction with the well known complications of exogenous steroid administration have deterred its use. However, a recent systemic review of randomized controlled trials of steroid administration in acute traumatic brain injury shows uncertainty over its use and neither moderate harmful nor moderate benecial effects can be excluded (41). Anti-seizure prophylaxis. Studies dealing with the use of antiseizure drugs such as, phenytoin, carbamazepine or phenobarbitone show no statistically signicant effect in the prevention of late onset posttraumatic seizures (4245). Conversely, with respect to early posttraumatic seizures, data from the same prospective, randomized, controlled trials suggest that prophylactic treatment does have an effect on the incidence of early seizure; but what association this has with the outcome of head injury remains undened. The use of anticonvulsants to treat early posttraumatic seizures remains at the discretion of the clinician. Class II and III Intracranial pressure monitoring. There is no prospective randomized controlled trial examining the inuence of ICP on outcome. Such a study may never be performed due to ethical concerns. There are some data to suggest that denition of treatable intracranial hypertension should be set at 2025 mmHg (5,19). The difculty is the difference in normal ICP in adults (818 mmHg) and children (24 mmHg) (7). It may not be appropriate to extrapolate this data to the paediatric population, the ICP treatment threshold must therefore be assessed in conjunction with clinical status and CPP calculation. It is postulated that ICP monitoring should be initiated in those patients at risk of
1999 Blackwell Science Ltd, Paediatric Anaesthesia, 9, 377385
Table 3 Factors affecting patients at risk of developing intracranial hypertension with normal computed tomography scan Over 40 years old Systolic pressure below 90 mmHg Unilateral or bilateral motor posturing
developing ICH. Adult patients with abnormal CT on admission are associated with a 5363% incidence of ICH compared to 13% in the normal CT group. In those patients with normal CT, the groups at higher risk of developing ICH are summarized in Table 3. There is a 60% risk of developing ICH if two or more of these factors are present compared to 4% if only one factor is present (46). Whether this system can be applied to children with some modication remains to be evaluated. Cerebral perfusion pressure. There are no prospective randomized controlled studies to evaluate the critical CPP management threshold on clinical outcome. A minimal CPP of 50 mmHg is said to be adequate for adult brain perfusion (14) and a CPP of 70 mmHg has been shown retrospectively to be benecial in terms of clinical outcome (47). A reduction of CPP < 70 mmHg is associated with decreased jugular bulb venous oxygen saturation which suggests a failure of cerebral blood ow to meet metabolic demand (48). The minimal required CPP in children has not been dened. Presumably CPP would vary as children in different age groups have different MAP. Although CPP plays an important role in the treatment strategy of head injury, awareness of the class II evidence on which this treatment strategy is based is essential. It is therefore recommended that CPP should be maintained > 70 mmHg during the acute course of head injury (49). Ventricular drainage. Ventricular drainage is recommended by the Brain Trauma Foundation as one of the rst line treatments in acute intracranial hypertension (49). In older children, there is a limited application for this technique as it relies on the presence of surgical access, e.g. extraventricular drain or intraventricular ICP catheter; whereas in infancy, needle aspiration of CSF can be made through the anterior fontanelle. The reduction in cerebrospinal uid volume leads to a fall in ICP.
382
W. HIU LAM AND A. MACKERSIE
Mannitol. Mannitol has a denite place as a rst line treatment of intracranial hypertension provided the blood brain barrier is intact. It has been shown to reduce ICP and improve CPP (50,51). One study also demonstrated that it can simultaneously lower ICP and increase jugular venous oxygen saturation (52). It should not be given repeatedly as this may lead to the accumulation of mannitol in the brain. The mechanism of action is complex. In addition to its osmotic effect in increasing serum osmolality and drawing water from the brain, it also causes an immediate increase in systemic blood pressure and decrease in blood viscosity, both of which increase cerebral blood ow leading to cerebral vasoconstriction and subsequent reduction in ICP. The dosage is in the range of 0.250.5 gkg1 (14). Its use is limited by hyperosmolality, electrolyte disturbances and intravascular volume depletion. Barbiturates. Barbiturates are indicated in the treatment of raised ICP. Thiopentone 24 mgkg1 can be administered to treat an acute rise in ICP as it decreases cerebral metabolism and oxygen consumption Cerebral blood ow, cerebral blood volume and cerebrospinal uid pressure all fall, possibly due to a reduction in the carbon dioxide level (53). In spite of this, prophylactic use of barbiturates against the development of ICH is not recommended (54), nor as a substitute for mannitol in the early management of intracranial hypertension (55). However, the study of Eisenberg et al. (56) showed that barbiturates are highly effective in lowering a raised ICP that was previously refractory to conventional therapy such as CSF drainage, hyperventilation and mannitol administration. No formal association between the lowering of this ICP and clinical outcome has been established. As barbiturates commonly cause myocardial depression and peripheral vasodilatation, which may compromise CPP, the use of thiopentone has to be closely monitored; ideally with invasive arterial and venous pressure monitoring. In practice, these patients may require ionotropic support to maintain adequate circulation whilst in a barbiturate coma. Hypothermia. Pyrexia following acute head injury has a detrimental effect on the outcome (9). Conventional hypothermia (surface cooling to < 30C) decreases ICP, cerebral blood ow and the cerebral metabolic
rate of oxygen consumption (57). In the past decade, conventional hypothermia is less commonly used due to unconvincing clinical outcome as well as complications related to this technique, e.g. cardiac dysrhythmia, haemodynamic instability and coagulation abnormality. Mild to moderate hypothermia (surface cooling to 3234C) is not associated with these complications and may limit SBI by reducing cerebral metabolism, stabilizing cell membranes and suppressing high levels of extracellular excitotoxic amino acids present after severe head injury (58). Both Marion and Shiozakis studies found a signicant reduction of ICP and the latter also demonstrated signicantly improved survival rate in the hypothermic group (50% compared to 18% in the control group) (57,58). More data will become available on completion of the National Acute Brain Injury study on hypothermia. This multicentre trial aims to evaluate if hypothermia to 3234C improves Glasgow Outcome score at 6 months after head injury (49). Hypertonic saline. Recently, the introduction of hypertonic saline in the management of ICH and cerebral oedema has attracted attention (59,60). Effective use of hypertonic saline to treat cerebral oedema and resistant intracranial hypertension has been demonstrated (6163). It acts primarily by creating an osmotic gradient between intra-and extravascular spaces (similar to mannitol), hence drawing water from the brain. A rebound phenomenon occurs with other hypertonic uids, e.g. urea or mannitol as a result of accumulation of these molecules in the brain and hence reversing the osmotic gradient. A possible rebound phenomenon has recently been reported on two patients with cerebral oedema secondary to intracerebral haemorrhage who were treated with hypertonic saline solution (64). The use of this solution requires further evaluation. Surgical treatment. Surgical treatment is either aimed at reducing raised intracranial pressure or for skull and scalp wound repair. Decompressive craniotomy is again popular as part of an aggressive treatment of head injuries but whether this will show improvement in outcome is yet to be demonstrated. Where there is a severe contusion to a functionally
1999 Blackwell Science Ltd, Paediatric Anaesthesia, 9, 377385
PAEDIATRIC HEAD INJURY
383
unimportant area of the brain, it may be an indication for prompt decompression or excision of the contusion. Intracranial bleeds in paediatric head injury have half the incidence of adults, 2025% instead of 4050%, so that less than 1% of children admitted with a head injury require removal of a haematoma. Haematomas can be subdural, the most common and usually associated with a severe injury, extradural or intracerebral. Extradural haematomas, if producing a mass effect need rapid surgical drainage, usually performed through a small craniotomy. More than 85% of children have no history of alteration in consciousness at the time of injury, unlike adults where the classical lucid interval is common. The incidence of extradurals is also much lower in childhood, and accounts for only 25% of all intracranial bleeds in the paediatric age range. Subdural haematomas are associated with a severe head injury and can be caused by cerebral contusions or vessel damage, but are only rarely associated with fractures. It is thought that a bony fracture dissipates the force of the injury making intracranial damage less and reducing the likelihood of subdural haematoma formation. Intracerebral haematomas are also rare. Most develop from cortical contusions in a child with a tremendous head injury with a pathological progression from hyperaemia to a contusion and formation of a collection of blood which eventually progresses to atrophy and the development of cystic areas. If a haematoma is localized, e.g. frontally, excision may be considered. Fractures. Ninety percent of skull fractures in children are linear and are an indication of the force of injury. Most are uncomplicated and provided the child is asymptomatic, no further treatment is required. They tend to be more diastatic than in adults and therefore look more impressive on X-ray. It is important to see whether a large vessel, either middle meningeal artery or dural sinus, crosses the fracture site which could be damaged causing intracranial bleeding and subsequent pressure effect. There are two types of fracture unique to paediatric head injury. Firstly the Ping-Pong Ball fracture which occurs in neonates with a malleable skull and usually results from birth trauma, especially from obstetric forceps. Surgery is indicated for large lesions and for
1999 Blackwell Science Ltd, Paediatric Anaesthesia, 9, 377385
frontal fractures for cosmetic reasons. Because of the malleable skull, these fractures can usually be elevated easily through a small incision just lateral to the depressed area. They are not usually associated with any under lying brain injury, but great care needs to be taken to avoid causing secondary damage. The other fracture type that only occurs in children is the growing fracture. This occurs when torn dura or damaged brain is present between the edges of the fracture. A leptomeningeal cyst develops and there is absorption of bone from the edges of the fracture leading to a growing defect. This presents as a soft swelling, usually within weeks of the original injury. Surgical treatment involves removal of gliotic brain, dural closure and bone grafting. This usually done with split calvareal bone and therefore involves an area more than double the size of the original lesion. This increases blood and heat losses because of the large exposure. Compound fractures require wound debridement, dural repair and removal of bone fragments, but great care is taken to prevent additional damage either directly or from haemorrhage. Debridement usually is performed within 624 h. There is however, a surprisingly low infection rate with wounds due to the vascularity of the scalp. Anaesthesia and head injury. Anaesthesia may be required for the investigation and treatment of children with severe head injuries but also for some children who have had less severe cerebral insults. The most severely head injured children will already be receiving intensive care, be heavily sedated and ventilated which needs little supplementation for investigations, but they must be adequately anaesthetized for surgery, to avoid a stress response and its metabolic consequences. Standard neurosurgical anaesthetic technique is appropriate. Invasive monitoring is required if not already in use and vascular access should be appropriate for rapid transfusion. The hyperaemic response to injury may remain for weeks and increased losses can be expected even with delayed surgery.
Conclusions
The management of a head injured child clearly requires a multidisciplinary team approach (6567). This team should consist of paediatricians, surgeons,
384
W. HIU LAM AND A. MACKERSIE
anaesthetists, nurses, operating department assistants who treat children regularly. When the transfer of children to a specialist paediatric centre is necessary, it should be carried out by senior medical personnel or a specialist transport team (68). Undoubtedly, more paediatric trials need to be conducted before further guidelines in paediatric head injury management can be recommended. The institution of adequate oxygen delivery and haemodynamic stability in a child at the earliest possible moment after head injury remains the most important aspect of management strategy. In the presence of established intracranial hypertension, with the evidence currently available from literature, the recommendation for rst line treatment is cerebrospinal uid drainage, hyperventilation and mannitol administration. If ICH remains high, then barbiturate administration may be a useful option and moderate hypothermia may become standard treatment in the future.
References
1 Jones PA, Andrews PJD, Midgley S et al. Measuring the burden of secondary insults in head-injured patients during intensive care. J Neurosurg Anesthesiol 1994; 6: 414. 2 Graham DI, Ford I, Adams JH et al. Ischaemic brain damage is still common in fatal non-missile head injury. J Neurol Neurosurg Psychiatry 1989; 52: 346350. 3 Chestnut RM, Marshall LF, Klauber MR et al. The role of secondary brain injury in determining outcome from severe head injury. J Trauma 1993; 34: 216222. 4 Piugla FA, Wald SL, Shackford SR et al. The effect of hypotension and hypoxia on children with severe head injuries. J Pediatric Surg 1993; 28: 310316. 5 Marmarou A, Anderson RL, Ward JD et al. Impact of ICP instability and hypotension on outcome in patients with severe head trauma. J Neurosurg 1991; 75: S59S66. 6 Ganong WF. Gas transport between the lungs and the tissues. In: Ganong WF, ed. Review of Medical Physiology, 16th edn. New York: Prentice Hall, International Inc., 1993: 604610. 7 Bissonnette B. Anesthesia for neurosurgical procedures. In: Gregory GA, ed. Pediatric Anesthesia, 3rd edn. Edinburgh: Churchill Livingstone, 1994: 375419. 8 Lam WH. Fluids in paediatric patients. Care Crit Ill 1998; 14: 9396. 9 Wass CT, Lanier WL, Hofer RE et al. Temperature change of >1C alter functional neurologic outcome and histopathology in a canine model of complete cerebral ischaemia. Anesthesiology 1995; 83: 325335. 10 Arieff AI, Ayus JC, Fraser CL. Hyponatraemia and death or permanent brain damage in healthy children. BMJ 1992; 304: 12181222. 11 Arieff AI. Management of hyponatraemia. BMJ 1993; 307: 305308.
12 Arieff AI. Postoperative hyponatraemic encephalopathy following elective surgery in children. Paed Anaesth 1998; 8: 14. 13 Ganong WF. Central Regulation of Visceral Function. In: Ganong WF, ed. Review of Medical Physiology, 16th edn. New York: Prentice Hall, International Inc, 1993: 208230. 14 Pascucci RC. Pediatrc Intensive Care. In: Gregory GA, ed. Pediatric Anesthesia, 3rd edn. Edinburgh: Churchill Livingstone, 1994: 837 899. 15 Lam AM, Winn HR, Cullen BF et al. Hyperglycaemia and neurological outcome in patients with head injury. J Neurosurg 1991; 75: 545551. 16 Jayawant S, Rawlinson A, Gibbon F et al. Subdural haemorrhages in infants: population based study. BMJ 1998; 317: 15581561. 17 Ewing-Cobbs L, Kramer L, Prasad M et al. Neuroimaging, physical and developmental ndings after inicted and noninicted traumatic brain injury in young children. Pediatrics 1998; 102: 300307. 18 Haviland J, Ross Russell RI. Outcome after severe nonaccidental head injury. Arch Dis Child 1997; 77: 504507. 19 Saul TG, Ducker TB. Effect of intracranial pressure monitoring and aggressive treatment on mortality in severe head injury. J Neurosurg 1982; 56: 498503. 20 Hudspith MJ. Glutamate: a role in normal brain function, anaesthesia, analgesia and CNS injury. Br J Anaesth 1997; 78: 731747. 21 Beneveniste H, Drejer J, Schousboe A et al. Elevation of the extracellular concentrations of glutamate and aspartate in rat hippocampus during transient cerebral ischaemia monitored by intracerebral microdialysis. J Neurochem 1984; 43: 13681374. 22 Katayama Y, Becker DP, Tamura T et al. Massive increase in extracellular potassium and the indiscriminate release of glutamate following concussive brain injury. J Neurosurg 1990; 73: 889900. 23 Baker AJ, Moulton RJ, MacMillan VH et al. Excitatory amino acids in cerebral spinal uid following traumatic brain injury in humans. J Neurosurg 1993; 79: 369372. 24 Ghajar J, Hariri RJ, Narayan RK et al. Survey of critical care management of comatose, head-injured patients in the United States. Crit Care Med 1995; 23: 560567. 25 Jeevaratnam DR, Menon DK. Survey of intensive care of severely head injured patients in the United Kingdom. BMJ 1996; 312: 944947. 26 Scally G, Donaldson LJ. Clinical governance and the drive for quality improvement in the new NHS in England. BMJ 1998; 317: 6165. 27 Donaldson LJ. Clinical governance: a statutory duty for quality improvement. J Epidemiol Commun Health 1998; 52: 7374. 28 Waldmann CS, Thyveetil D. Management of head injury in a district general hospital. Care Crit Ill 1998; 14: 6570. 29 Fearnside MR, Cook RJ, McDougall P et al. The Westmead Head Injury Project outcome in severe head injury. A comparative analysis of pre-hospital, clinical and CT variables. Br J Neurosurg 1993; 7: 267279. 30 Rossanda M, Di Giugno G, Corona S et al. Oxygen supply to the brain and respirator treatment in severe comatose states. Acta Anaesthesiol Scand Suppl 1966; 23: 766774. 31 Gordon E. Controlled respiration in the management of patients with traumatic brain injuries. Acta Anaesthesiol Scand 1971; 15: 193208. 31 Bricolo A, Formenton A, Turella G et al. Clinical and EEG effects of mechnical hyperventilation in acute traumatic coma. Eur Neurol 1972; 8: 219224. 1999 Blackwell Science Ltd, Paediatric Anaesthesia, 9, 377385
PAEDIATRIC HEAD INJURY
385
33 Klauber MR, Marshall LF, Barrett-Connor E et al. Prospective study of patients hospitalized with head injury in San Diego County 1978. Neurosurgery 1981; 9: 236241. 34 Obrist WD, Langtt TW, Jaggi JL et al. Cerebral blood ow and metabolism in comatose patients with acute head injury. Relationship to intracranial hypertension. J Neurosurg 1984; 61: 241253. 35 Muizelaar JP, Marmarou A, Ward JD et al. Adverse effects of prolonged hyperventilation in patients with severe head injury: a randomized clinical trial. J Neurosurg 1991; 75: 731739. 36 Elias-Jones AC, Punt JA, Turnbull AE et al. Management and outcome of severe head injuries in the Trent region 198590. Arch Dis Child 1992; 67: 14301435. 37 Dexter F. Research synthesis of controlled studies evaluating the effect of hypocapnia and airway protection on cerebral outcome. J Neurosurg Anesthesiol 1997; 9: 217222. 38 Cooper PR, Moody S, Clark WK et al. Dexamethasone and severe head injury. J Neurosurg 1979; 51: 307316. 39 Braakman R, Schouten HJA, Dishoeck MBV et al. Megadose steroids in severe head injury. J Neurosurg 1983; 58: 326330. 40 Dearden NM, Gibson JS, McDowall DG et al. Effect of high dose dexamethasone on outcome from severe head injury. J Neurosurg 1986; 64: 8188. 41 Alderson P, Roberts I. Corticosteroids in acute traumatic brain injury: systematic review of randomised controlled trials. BMJ 1997; 314: 18551859. 42 Penry JK, White BG, Brackett CE. A controlled prospective study of the pharmacologic prophylaxis of posttraumatic epilepsy. Neurology 1979; 29: 600A. 43 Young B, Rapp RP, Norton JA et al. Failure of prophylactically administered phenytoin to prevent late posttraumatic seizures. J Neurosurg 1983; 58: 236241. 44 McQueen JK, Blackwood DH, Harris P et al. Low risk of late post-traumatic seizures following severe head injury: implications for clinical trials of prophylacxis. J Neurol Neurosurg Psych 1983; 46: 899904. 45 Temkin NR, Dikmen SS, Winn HR. Management of head injury. Posttraumatic seizures. Neurosurg Clin N Am 1991; 2: 425435. 46 Narayan RK, Kishore PR, Becker DP et al. Intracranial pressure: to monitor or not to monitor? A review of our experience with head injury. J Neurosurg 1982; 56: 650659. 47 Rosner MJ, Rosner SD, Johnson AH. Cerebral perfusion pressure: management protocol and clinical results. J Neurosurg 1995; 83: 949962. 48 Chan KH, Miller JD, Dearden NM et al. The effect of changes in cerebral perfusion pressure upon middle cerebral artery blood ow velocity and jugular bulb venous oxygen saturation after severe brain injury. J Neurosurg 1992; 77: 5561. 49 Bullock R, Chestnut R, Clifton G et al. Guidelines for Management of Severe Head Injury. New York: Brain Trauma Foundation, 1996. 50 Kirkpatrick PJ, Smielewski P, Piechnik S et al. Early effects of mannitol in patients with head injuries assessed using bedside multimodality monitoring. Neurosurgery 1996; 39: 714720. 51 Mendelow AD, Teasdale GM, Russell T et al. Effect of mannitol on cerebral blood ow and cerebral perfusion pressure in human head injury. J Neurosurg 1985; 63: 4348.
52 Cruz J, Miner ME, Allen SJ et al. Continuous monitoring of cerebral oxygenation in acute brain injury: injection of mannitol during hyperventilation. J Neurosurg 1990; 73: 725730. 53 Calvey TN, Williams NE. Intravenous Anaesthetic Agents. In: Calvey TN, Williams NE, ed. Principles and Practice of Pharmacology for Anaesthetists, 2nd edn. Oxford: Blackwell Scientic Publications, 1991: 154185. 54 Ward JD, Becker DB, Miller JD et al. Failure of prophylactic barbiturate coma in the treatment of severe head injury. J Neurosurg 1985; 62: 383388. 55 Schwartz ML, Tator CH, Rowed DW et al. The University of Toronto head injury treatment study: a prospective, randomised comparison of pentobarbital and mannitol. Can J Neurol Sci 1984; 11: 434440. 56 Eisenberg HM, Frankowski RF, Contant CF et al. for the comprehensive central nervous system trauma centres. High dose barbiturate control of elevated intracranial pressure in patients with severe head injury. J Neurosurg 1988; 69: 1523. 57 Shiozaki T, Sugimoto H, Taneda M et al. Effect of mild hypothermia on uncontrollable intracranial hypertension after severe head injury. J Neurosurg 1993; 79: 363368. 58 Marion DW, Obrist WD, Carlier PM et al. The use of moderate therapeutic hypothermia for patients with severe head injuries: a preliminary report. J Neurosurg 1993; 79: 354362. 59 Zornow MH. Hypertonic saline as a safe and efcacious treatment of intracranial hypertension. J Neurosurg Anesthesiol 1996; 8: 175177. 60 Petrozza PH. Hypertonic saline: is it time? J Neurosurg Anesthesiol 1996; 8: 174. 61 Qureshi AI, Suarez JI, Bhardwaj A et al. Use of hypertonic (3%) saline/acetate infusion in the treatment of cerebral edema: effect on intracranial pressure and lateral displacement of the brain. Crit Care Med 1998; 26: 440446. 62 Worthley LI, Cooper DJ, Jones N. Treatment of resistant intracranial hypertension with hypertonic saline. Report of two cases. J Neurosurg 1988; 68: 478481. 63 Weinstabl C, Mayer N, Germann P et al. Hypertonic, hyperoncotic hydroxyethyl starch decreases intracranial pressure following neurotrauma. Anesthesiology 1991; 75: A201. 64 Qureshi AI, Suarez JI, Bhardwaj A. Malignant cerebral edema in patients with hypertensive intracerebral hemorrhage associated with hypertonic saline infusion. A rebound phenomenon? J Neurosurg Anesthesiol 1998; 10: 188192. 65 Nicholl J, Willatts S. Paediatric intensive care the way ahead? Anaesthesia 1998; 53: 11411143. 66 Rollin AM. Paediatric anaesthesia who should do it? The view from the district general hospital. Anaesthesia 1997; 52: 513516. 67 Ratcliffe J. Provision for intensive care for children: a geographically integrated service may now be achieved. BMJ 1998; 316: 15471548. 68 Recommendations for the Transfer of Patients with Acute Head Injuries to Neurosurgical Units. The Neuroanaesthesia Society of Great Britain and Ireland and The Association of Anaesthetists of Great Britain and Ireland, 1996.
Accepted 26 March 1999
1999 Blackwell Science Ltd, Paediatric Anaesthesia, 9, 377385
S-ar putea să vă placă și
- Key Answer Toefl PBT Bruce RogersDocument89 paginiKey Answer Toefl PBT Bruce RogersAndy Wijaya73% (11)
- BLS Skills Lab For SimulationDocument116 paginiBLS Skills Lab For Simulationczeremar chan100% (1)
- Head Trauma: Yoon Seung-Hwan, M.DDocument53 paginiHead Trauma: Yoon Seung-Hwan, M.DAhmed ZahranÎncă nu există evaluări
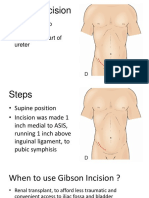
- Gibson IncisionDocument5 paginiGibson IncisionAndy Wijaya100% (1)
- Kansas College Immunization WaiverDocument1 paginăKansas College Immunization WaiverDonnaÎncă nu există evaluări
- 10th Monthly Compliance Report On Parkland Memorial HospitalDocument92 pagini10th Monthly Compliance Report On Parkland Memorial HospitalmilesmoffeitÎncă nu există evaluări
- Head TraumaDocument15 paginiHead TraumaDede Yusuf FÎncă nu există evaluări
- Managing Anaphylactic Shock Journal of Modern Pharmacy 2006Document3 paginiManaging Anaphylactic Shock Journal of Modern Pharmacy 2006Saputro AbdiÎncă nu există evaluări
- Anaphylaxis Treatment ProcedureDocument4 paginiAnaphylaxis Treatment ProcedureIvy Jorene Roman RodriguezÎncă nu există evaluări
- BIMC Adult DKA Protocol 2012Document5 paginiBIMC Adult DKA Protocol 2012djizhieeÎncă nu există evaluări
- Atls SpineDocument11 paginiAtls SpineRonald David EvansÎncă nu există evaluări
- Causes Obstruction Causes CSF To Build Up in The Brain. If The Cause Is Congenital, Symptoms Such As AnDocument22 paginiCauses Obstruction Causes CSF To Build Up in The Brain. If The Cause Is Congenital, Symptoms Such As Anmhelandie100% (1)
- Snake Bite Medical ManagementDocument35 paginiSnake Bite Medical Managementsyarifah nurlailaÎncă nu există evaluări
- Basic Emergency CareDocument96 paginiBasic Emergency CareAnukriti MamgainÎncă nu există evaluări
- GEA ProtocolsDocument101 paginiGEA Protocolsffbrians100% (1)
- Brain InjuryDocument35 paginiBrain InjuryAkhil Adhithyan RamÎncă nu există evaluări
- Traumatic Brain InjuryDocument40 paginiTraumatic Brain InjuryRed DevilÎncă nu există evaluări
- The Neuro Exam: Yes, You Really Do Have To Wake Them Up and Do ThisDocument12 paginiThe Neuro Exam: Yes, You Really Do Have To Wake Them Up and Do ThisDrGasnasÎncă nu există evaluări
- Brain InjuriesDocument46 paginiBrain Injuriesbasic100% (5)
- Snake Bite: DR Ugi Sugiri SP EM Emergency Dept. Fatmawati General HospitalDocument59 paginiSnake Bite: DR Ugi Sugiri SP EM Emergency Dept. Fatmawati General HospitalAdrianus AdrianusÎncă nu există evaluări
- 8.the Atls ProtocolDocument57 pagini8.the Atls ProtocolReuben DutiÎncă nu există evaluări
- Initial Evaluation VertigoDocument8 paginiInitial Evaluation VertigoTanri Hadinata WiranegaraÎncă nu există evaluări
- Early Cardiopulmonary Resuscitation in Out-of-Hospital Cardiac ArrestDocument9 paginiEarly Cardiopulmonary Resuscitation in Out-of-Hospital Cardiac ArrestHammad AjaÎncă nu există evaluări
- Chest Pain ProtocolDocument4 paginiChest Pain ProtocolArul ShanmugamÎncă nu există evaluări
- Turner SyndromeDocument10 paginiTurner SyndromeAlexandraÎncă nu există evaluări
- 2 - Airway and Ventilatory ManagementDocument5 pagini2 - Airway and Ventilatory ManagementJessie E. GeeÎncă nu există evaluări
- PICU Common ProblemDocument49 paginiPICU Common ProblemRawabi rawabi1997Încă nu există evaluări
- ILS Case Studies COMP 2020.ppsxDocument39 paginiILS Case Studies COMP 2020.ppsxKim Orven KhoÎncă nu există evaluări
- Brain AbscessDocument25 paginiBrain AbscessprembarnabasÎncă nu există evaluări
- Cerebrovascular Disorders: PathophysiologyDocument4 paginiCerebrovascular Disorders: PathophysiologyMhae De GuzmanÎncă nu există evaluări
- k4 - Disorders of Cranial NervesDocument67 paginik4 - Disorders of Cranial Nerveswlmhfp100% (1)
- StrokeDocument12 paginiStrokeSam JamesÎncă nu există evaluări
- Cerebrospinal CSFDocument31 paginiCerebrospinal CSFRashid MohamedÎncă nu există evaluări
- Peadiatric Brain Tumour: Wong Ann Cheng MD (Ukm) MRCPCH (Uk)Document48 paginiPeadiatric Brain Tumour: Wong Ann Cheng MD (Ukm) MRCPCH (Uk)An Zheng100% (4)
- Subdural Hematomas in The ElderlyDocument19 paginiSubdural Hematomas in The ElderlyRegina PhilyriaÎncă nu există evaluări
- Advanced Life Support Training Manual Final 2017Document87 paginiAdvanced Life Support Training Manual Final 2017Azhar MohamedÎncă nu există evaluări
- Febrile Seizure: Satanun Charoencholvanich, MDDocument10 paginiFebrile Seizure: Satanun Charoencholvanich, MDAPETT WichaiyoÎncă nu există evaluări
- Common Medical Emergencies: Jude D. Positos, RNDocument27 paginiCommon Medical Emergencies: Jude D. Positos, RNNenen PositosÎncă nu există evaluări
- Health-Related Quality of LifeDocument23 paginiHealth-Related Quality of LifeHope GemidaÎncă nu există evaluări
- Head Trauma: Khamim Thohari Rsud DR Muhammad Soewandhie SurabayaDocument33 paginiHead Trauma: Khamim Thohari Rsud DR Muhammad Soewandhie SurabayaJessica Alexandria WuÎncă nu există evaluări
- Cad ....Document94 paginiCad ....AnanthibalaÎncă nu există evaluări
- NIH Public Access: Author ManuscriptDocument12 paginiNIH Public Access: Author ManuscriptMohammad Kholil SidikÎncă nu există evaluări
- Fascia Iliaca GuidelineDocument12 paginiFascia Iliaca GuidelineSyahrul Mubarak Danar SumantriÎncă nu există evaluări
- RECMOD6 Neurological EmergenciesDocument45 paginiRECMOD6 Neurological Emergenciesdragon66Încă nu există evaluări
- Head Injury Final TurelDocument52 paginiHead Injury Final TurelkarahmanÎncă nu există evaluări
- Basic Concept of BLS: Muhammad SaleemDocument27 paginiBasic Concept of BLS: Muhammad Saleemms khanÎncă nu există evaluări
- Presented By:: Ali Jaber Al-Faifi Salman NasserDocument23 paginiPresented By:: Ali Jaber Al-Faifi Salman NasserCalvin PrasetioÎncă nu există evaluări
- LA Union: PDRRM ODocument32 paginiLA Union: PDRRM OEnash RidÎncă nu există evaluări
- Brain AbscessDocument13 paginiBrain Abscesskashim123Încă nu există evaluări
- Cerebro Vascular AccidentDocument82 paginiCerebro Vascular AccidentJayvee Novenario Casaljay100% (1)
- Acute Stroke Management by Carlos L Chua PDFDocument61 paginiAcute Stroke Management by Carlos L Chua PDFHynne Jhea EchavezÎncă nu există evaluări
- For Best Viewing:: Open in Slide Show Mode Click On IconDocument32 paginiFor Best Viewing:: Open in Slide Show Mode Click On IconSutapa PawarÎncă nu există evaluări
- Anaphylactic ShockDocument11 paginiAnaphylactic ShockBushra NaeemÎncă nu există evaluări
- Spinal InjuriesDocument22 paginiSpinal InjuriesPak Budi warsonoÎncă nu există evaluări
- Oculocardiac ReflexDocument12 paginiOculocardiac ReflexTeshome AbebeÎncă nu există evaluări
- Management of Shock in Emergency Room by DR Prannoy George, Department of Emergency Medicine, Amrita Institute of Medical Sceinces Kochi, KeralaDocument77 paginiManagement of Shock in Emergency Room by DR Prannoy George, Department of Emergency Medicine, Amrita Institute of Medical Sceinces Kochi, KeralaAETCM Emergency medicineÎncă nu există evaluări
- Chapter 08 ITLS - 04-2020Document36 paginiChapter 08 ITLS - 04-2020Ahyar Moh100% (1)
- Hemorrhagic StrokeDocument57 paginiHemorrhagic StrokeAnonymous oEbVOEÎncă nu există evaluări
- Thoracic TraumaDocument24 paginiThoracic TraumaOmar MohammedÎncă nu există evaluări
- Tumours of The Central Nervous System: FM Brett MD., FrcpathDocument57 paginiTumours of The Central Nervous System: FM Brett MD., FrcpathDrGasnasÎncă nu există evaluări
- Bells PalsyDocument18 paginiBells PalsyDr AnandÎncă nu există evaluări
- Headache, CithaDocument20 paginiHeadache, CithaCitha TallesangÎncă nu există evaluări
- Assessment of the Politraumatized PatientDe la EverandAssessment of the Politraumatized PatientÎncă nu există evaluări
- ICCS Standardization Report On Urodynamic StudiesDocument8 paginiICCS Standardization Report On Urodynamic StudiesAndy WijayaÎncă nu există evaluări
- Salvagability Giant HydronephrosisDocument14 paginiSalvagability Giant HydronephrosisAndy WijayaÎncă nu există evaluări
- UrethralDocument7 paginiUrethralAndy WijayaÎncă nu există evaluări
- EffectDocument3 paginiEffectAndy WijayaÎncă nu există evaluări
- Surgical Hand AntisepsisDocument19 paginiSurgical Hand AntisepsisAndy WijayaÎncă nu există evaluări
- Stricture UrethraDocument3 paginiStricture UrethraAndy WijayaÎncă nu există evaluări
- 2011 Article 262Document11 pagini2011 Article 262Andy WijayaÎncă nu există evaluări
- Egan 2016Document9 paginiEgan 2016Andy WijayaÎncă nu există evaluări
- What Is Microsurgical Tubal Ligation Reversal?Document2 paginiWhat Is Microsurgical Tubal Ligation Reversal?Andy WijayaÎncă nu există evaluări
- Head Injuries AnaesthesiaDocument14 paginiHead Injuries AnaesthesiaAndy WijayaÎncă nu există evaluări
- Online Radiography Continuing EducationDocument39 paginiOnline Radiography Continuing EducationAndy WijayaÎncă nu există evaluări
- PAMPHLETDocument2 paginiPAMPHLETMarshin Thea CelociaÎncă nu există evaluări
- Acute Pancreatitis Maria Coats v2Document35 paginiAcute Pancreatitis Maria Coats v2Henry MauricioÎncă nu există evaluări
- Des Fibril Ad or Welch Allyn Pic 30Document109 paginiDes Fibril Ad or Welch Allyn Pic 30Nadia RiveraÎncă nu există evaluări
- Terapia Clark Referencias Cientificas OzonoDocument17 paginiTerapia Clark Referencias Cientificas OzonoGomez Gomez100% (2)
- Nursing Care of The ElderlyDocument11 paginiNursing Care of The ElderlySpislgal PhilipÎncă nu există evaluări
- Topical Steroids DermatologyDocument23 paginiTopical Steroids DermatologyRitika Agarwal100% (1)
- 65 Interview Questions For Nurses.13Document3 pagini65 Interview Questions For Nurses.13natalieshirleyÎncă nu există evaluări
- Form 2 Reporting Form Revision 1Document1 paginăForm 2 Reporting Form Revision 1Cha Tuban DianaÎncă nu există evaluări
- Gambaran Penggunaan Antibiotik Tanpa Resep Di Apotek X Kabupaten SragenDocument8 paginiGambaran Penggunaan Antibiotik Tanpa Resep Di Apotek X Kabupaten Srageneka handayaniÎncă nu există evaluări
- Thoracic Radiofrequency AblationDocument2 paginiThoracic Radiofrequency AblationIevgen DanylchukÎncă nu există evaluări
- Injectable Anesthesia and Analgesia of Birds 5-Aug PDFDocument15 paginiInjectable Anesthesia and Analgesia of Birds 5-Aug PDFYaserAbbasiÎncă nu există evaluări
- ABCDEFGHI Systematic Approach To Wound Assessment and ManagementDocument36 paginiABCDEFGHI Systematic Approach To Wound Assessment and ManagementsaerodinÎncă nu există evaluări
- Mefenamic Acid Public Assessment Report For Pediatric StudiesDocument17 paginiMefenamic Acid Public Assessment Report For Pediatric StudiesNyoman SuryadinataÎncă nu există evaluări
- Centers For Disease Control and Prevention's Sexually Transmitted Diseases Infection GuidelinesDocument6 paginiCenters For Disease Control and Prevention's Sexually Transmitted Diseases Infection GuidelinesabhinavrautÎncă nu există evaluări
- Cycle BeadsDocument2 paginiCycle Beadsgihan200100% (2)
- Doh Admin. 2010-0018Document29 paginiDoh Admin. 2010-0018Mar OrdanzaÎncă nu există evaluări
- Diagram of Pathophysiology CancerDocument5 paginiDiagram of Pathophysiology CancerKristaMaeC.Lazo0% (3)
- MCQ April 2015Document15 paginiMCQ April 2015Sylphana Astharica LawalataÎncă nu există evaluări
- Glucocorticoids in Systemic Lupus Erythematosus. Ten Questions and Some IssuesDocument13 paginiGlucocorticoids in Systemic Lupus Erythematosus. Ten Questions and Some IssuesSundas EjazÎncă nu există evaluări
- Management of A Case of Ventricular Bigeminy UsingDocument2 paginiManagement of A Case of Ventricular Bigeminy UsingAlfian AlfianÎncă nu există evaluări
- MK 2866Document3 paginiMK 2866haydunn55Încă nu există evaluări
- Debra Hall Fisher ResumeDocument4 paginiDebra Hall Fisher Resumeapi-347999772Încă nu există evaluări
- Understanding Independent Medical Assessments - A Multi-Jurisdictional Analysis Iggy Kosny ACHRF 2013Document15 paginiUnderstanding Independent Medical Assessments - A Multi-Jurisdictional Analysis Iggy Kosny ACHRF 2013ISCRRÎncă nu există evaluări
- Mission Indradhanush: Submitted By-Jayesh Agrawal Mba-Rural Development Semester-IstDocument20 paginiMission Indradhanush: Submitted By-Jayesh Agrawal Mba-Rural Development Semester-IstJayeshAgrawalÎncă nu există evaluări
- Irda Non Payable ListDocument21 paginiIrda Non Payable ListMUKESH SINGHÎncă nu există evaluări
- Eeg, PSG, Sleep DisordersDocument48 paginiEeg, PSG, Sleep DisordersIoana MunteanuÎncă nu există evaluări
- AgonistsDocument25 paginiAgonistsOfficially RandomÎncă nu există evaluări
- LaporanDocument214 paginiLaporankadek sariÎncă nu există evaluări