Documente Academic
Documente Profesional
Documente Cultură
Siddique and Ismail 11 (1) 112-121 (2013) Review Article
Încărcat de
Noman FarookTitlu original
Drepturi de autor
Formate disponibile
Partajați acest document
Partajați sau inserați document
Vi se pare util acest document?
Este necorespunzător acest conținut?
Raportați acest documentDrepturi de autor:
Formate disponibile
Siddique and Ismail 11 (1) 112-121 (2013) Review Article
Încărcat de
Noman FarookDrepturi de autor:
Formate disponibile
The Agriculturists 11(1): 112-121 (2013) ISSN 2304-7321 (Online), ISSN 1729-5211 (Print) A Scientific Journal of Krishi Foundation
Indexed Journal
Review Article Rice Ecosystem, Allelopathy and Environment A Review
A. B. Siddique1 * and B. S. Ismail2
2
Senior Assistant Secretary, IMED, Ministry of Planning, Bangladesh. Professor, School of Environmental & Natural Resource Sciences, Universiti Kebangsaan Malaysia 43600 UKM, Bangi Selangor, Malaysia.
*
Corresponding author and Email: razia_abubakar@yahoo.com Received: 22 April 2013 Accepted: 18 May 2013
Abstract Allelopathy is an important factor which contributes in determining distribution of species and their abundance within communities. Plant-plant interference is the combined effect of allelopathy, resource competition, and many other factors. Weed infestation is a major problem limiting the growth and yield of rice. Synthetic herbicide has been used for over 50 years as the prime source of weed control. The repeated use of herbicides in rice has already led to the evolution of resistance in some weed species. The conventional synthetic herbicides are becoming less effective against the resistant weed biotypes. Due to increase in the number of herbicide-resistant weeds and environmental concerns in the use of synthetic herbicides, allelopathy has been gaining preference as one of the considerable efforts in designing alternative weed management strategies. Modern ecotoxicologists and allelopathy researchers have been trying to identify allelochemicals to use as biodegradable pesticide. Two allelochemicals have been discovered, namely hexanedioic acid dioctyl ester and di-n-octyl phthalate which can be used as biopesticide. However, still there is enough scope to conduct such research that will contribute to protect our environment as well as increase food safety. Key words: Allelopathy, rice eco-system, allelochemical, environment 1. Introduction Weed infestation is a major problem limiting the growth and yield of rice (Bhatt and Tewari, 2006). In rice, the loss of yield due to weed infestation is greater than the combined yield losses caused by insect pests and diseases (Isley, 1960). In Asia, yield losses due to uncontrolled weed growth in direct seeded lowland paddy was 45-75%, and for transplanted lowland paddy approximately 50% (Johnson, 1996). Weed infestation causes a 10-35% reduction in grain yield in Malaysia (Karim et al., 2004). Nearly 12% of the total loss of crop yields has been attributed to the weeds alone (Anaya, 1999). Weed plants may affect the growth of crop plants either by competition for nutrients, water, space and light (Zimdahl, 1980) or by allelopathy
Rice is the main food crop and primary source of food for more than half of the worlds population. More than 90% of the worlds rice is grown and consumed in Asia where 60% of the earths people live. Rice accounts for 30-75% of the calories consumed by more than 3 billion Asians. It is planted on about 154 million hectares annually or on about 11% of the worlds cultivated land. According to United Nations (UN) estimation, the world population will grow from 6 billion in 2000 to 8 billion in 2030. Therefore, 40% more rice is to be produced by 2030 to satisfy the growing demand without affecting the resource base adversely (Khush, 2005).
113 (Rice, 1984). In the field, both allelopathy and competition usually act simultaneously. In rice cultivation control of weeds is one of the important means of increasing crop yield. In all rice ecosystems, herbicides have become one of the most important components in weed control. There may be two reasons for increased use of herbicides: the first being the widespread adoption of high-yielding varieties which created economic incentives for farmers to reduce weed infestation; and the second is the availability of cheaper herbicides, indicating that the cost of weed control by herbicides in wet-seeded rice is less than one-fifth of the cost of a single handweeding (Moody, 1991). But the repeated use of herbicides in rice to control weed has already led to the evolution of resistance in some weed species to several herbicides in several countries (Watanabe et al., 1997). The conventional synthetic herbicides are becoming less and less effective against the resistant weed biotypes. Thirty weed species associated with rice have been evolved as resistance to herbicides globally, including the most widely used compounds such as propanil, 2, 4-D and some of the sulfonylureas (Valverde et al., 2000). Herbicide-resistant weeds pose the greatest threat to farmers when there are few or no other alternatives to control them (Heap, 2011). Due to increase in the number of herbicide-resistant weeds and environmental concerns in the use of synthetic herbicides, allelopathy has been gaining preference as one of the considerable efforts in designing alternative weed management strategies. 2. Allelopathy and agriculture
Rice allelopathy and environment chemically interfere with weed growth have also been identified, such as Secale cereale (rye) (Haramoto and Gallandt, 2004), Triticum aestivum (wheat) (Labbafi et al., 2010), Oryza sativa (rice) (Fang et al., 2013), Helianthus annus (sunflower) (Nikneshan et al., 2011) and Glycine max (Soyabean) (Mahmoodzadeh and Mahmoodzadeh, 2013). In addition to beneficial chemical interference of crops with weed growth, there is potential for the advantageous use of allelopathy for practices such as crop rotation, cover and smothers crops and retention of crop residues (Singh et al., 2003). According to Duke et al. (2002), two approaches for utilization of allelopathy in crops to increase weed suppression are possible: a) to enhance the existing allelopathic potential of a particular crop and b) to introduce allelopathic potential through the insertion of foreign genes encoding for allelochemicals. This can be achieved through employing conventional breeding as well as genetic modification techniques. With increased environmental awareness and public pressure, less detrimental means of weed control are continually being sought. One such approach is to consider allelochemicals as a new source of herbicides. This approach may be beneficial as natural plant products which have advantage over synthetic herbicides. The benefits are many: a) allelochemicals often possess complex structures and exhibit structural diversity, making them valuable lead compounds, b) the compounds have molecular weight with little or no halogens or heavy atoms, c) allelochemicals have little environmental impacts as they degrade rapidly in the environment, and d) allelochemicals have novel target sites very often different to those of synthetics (Duke et al., 2002; Singh et al., 2003). 3. Weed allelopathy
Weeds interfere crop growth and reduce yields, deteriorate crop quality, clog waterways and cause health problems; with eradication costs being massive (Singh et al., 2003). About 240 weeds have been reported to have allelopathic potential (Qasem and Foy, 2001), although many of these species have been tested with unrealistic bioassays (Inderjit and Keating, 1999). On the other hand, allelopathic crops that are able to
A total of 240 weeds have been reported to have allelopathic potential (Qasem and Foy, 2001). Since weeds are a major cause of yield losses, the aggressive growth habits of some of the most tenacious species have come in for scrutiny.
Siddique and Ismail /The Agriculturists 11(1): 112-121 (2013) Putnam and Weston (1986) listed 90 weed species that showed allelopathic potential. It has been reported that some of the world's worst weeds contain allelochemicals including parthenium (Parthenium hysterophorus) (Nath, 1988), quackgrass [Elytrigia repens (L.) (Putnam and Weston, 1986), Johnsongrass (Sorghum halepense (Abdul- Wahab and Rice, 1967), Canada thistle (Cirsium arvense) (Stachon and Zimdahl, 1980) and giant foxtail (Setaria faberi) (Bell and Koeppe, 1972). The most investigations on weeds have tried fieldbased evidence with a search for allelochemicals. Parthenium hysterophorus, a tropical weed endemic to America, has done great damage to human, livestock and crops since arriving as an exotic to the Indian landscape and other places.
114
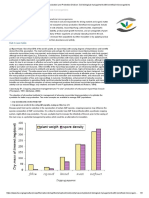
Numerous reports of the last two decades documented the phytotoxicity of its living and decomposing tissue, leachates, and root exudates (Forzwa and Karim, 2009). Recently, Ismail and Siddique (2012a, 2010(a), 2010(b); Siddique and Ismail (2010, 2009) reported that common rice field weeds i.e Fimbristylis miliacea, Cyperus iria, Echinochloa colona and Sphenoclea zeylanica showed growth inhibitory effects on the rice plants. Four common weeds grown in rice fields showed their allelopathic potential on paddy rice of Tarom variety in Pakistan (Alamdari and Deokule, 2009) (Table 1). Due to genetic variation, different rice verities responded differently (Table 2 and Figure 1) to the phytotoxic effect of Cyperus iria (Ismail and Siddique, 2011).
Table 1. Effect of whole plant leachates of studied weeds on growth parameters of paddy rice of Tarom variety (Alamdari and Deokule, 2009) Whole plant leachates of studied weeds Cyperus difformis Echinochloa crusgalli Paspalum paspaloides Sagittaria trifolia Control Mean Growth parameters Total number of tillers pot-1 17.11 1.57a 14.45 0.69a 15.56 1.17a 16.44 1.17a 16.44 1.17a 16.02 1.66
Plant height (cm) 103.76 13.67a 97.29 5.55a 99.80 9.20a 94.75 5.75a 100.72 3.02a 99.26 7.70
Elongation of flag leaf (cm) 21.89 3.04a 22.22 0.75a 25.41 2.58a 22.36 1.55a 24.23 2.85a 23.22 2.42
*Mean () Standard deviation and groups based on Duncan's Univariate comparison with 95% confidence intervals (n=3).
Table 2. Effect of the debris of C. iria, on plant height, seedling fresh and dry weight (% of control) of 5 rice varieties (Ismail and Siddique, 2011) Debris concentration Rice Control Quarter varieties Plant height MR211 100.0a 81.8b MRQ74 100.0a 85.0b a MR220 100.0 80.8b a MR84 100.0 68.1b MR232 100.0a 65.0b
Half 72.8b 71.7c 74.4b 66.8b 65.2b
Full 69.7b 67.0d 73.5b 66.8b 58.9c
Control Quarter Fresh weight 100.0a 74.7b 100.0a 73.1b 100.0a 72.9b 100.0a 70.4b 100.0a 59.1b
Half 73.5b 64.6b 66.0b 62.4bc 53.1b
Full 67.5b 66.9b 63.6b 57.1c 47.0c
Means within rows followed by same letter are not significantly different (p>0.05, LSD0.05)
115
Rice allelopathy and environment
120.0
Chlorophyll content (% of control)
100.0 80.0 Control 60.0 40.0 20.0 0.0 MR211 MRQ74 MR220
Rice varieties
Quarter Half Full
MR84
MR232
Fig. 1. Inhibitory effect of the debris of C. iria on leaf chlorophyll content (% of control) of five rice varieties (Ismail and Siddique, 2011)
4.
Allelopathy in rice
To establish an alternative strategy for weed management in rice, the phenomenon allelopathy has been a subject of continued research for a long time (Olofsdotter, .2001; Takeuchi et al., 2001) and already a large number of rice varieties have been found to suppress several weed species when grown together under field and laboratory condition (Dilday et al., 1998; Olofsdotter et al., 1999; Azmi et al., 2000). The first observation of rice was made in field examination in Arkansas, USA where about 191 of 5,000 rice accessions inhibited the growth of ducksalad weed (Heteranthera limosa) (Dilday et al., 1989) which led to a further large screening program. USA-ARS have screened more than 16,000 rice accessions from 99 countries and 412 rice accessions have shown the allelopathic potentiality against Ammonia
coceinea (Dilday et al., 1998). Dilday et al. (1998) evaluated the phytotoxic effects of 12,000 and 5000 rice accessions against ducksalad (Heteranthera limosa) and red stem (Spinacia oleracea), respectively. Several field screening was made in different countries such as Egypt, Korea, Japan, India, Thailand, Malaysia and United States (Dilday et al., 1998). In Egypt, more than 40 out of 1000 (were screened) rice varieties showed inhibitory activity with Echinochloa crusgalli and Cyperus difformis (Hassan et al., 1998) and suggested that genetic variations is the main factor to environment. Salam and Noguchi (2009) reported that the rice variety BR17 is the most allelopathic among 102 Bangladesh rice cultivars. Water extract of rice in different concentrations showed different degree of allelopathic inhibition on wheat, radish and lettuce (Tareak, 2010) (Table 3).
Siddique and Ismail /The Agriculturists 11(1): 112-121 (2013)
116
Table 3. Effect of six rice straw extract concentrations on seedling root growth of four plant species/cultivars over time (Tareak, 2010) Wheat (Triticum aestivum) Sakha 61 Sakha 94 72 h 51.9 51.9 37.9 23.8 14.4 100 15.1 96h 61.3 52.1 52.3 30.5 31.7 100 22.7 120h 67.5 56.5 59.1 59.7 47.3 100 21.1 72 h 35.2 31.5 25.4 21.9 19.8 100 7.5 96h 31.4 19.6 17.5 13.2 13.5 100 6.8 120h 23.9 9.2 12.5 7.1 7.0 100 7.1 Radish (Raphanus sativus) 72 h 45.6 36.8 0.0 0.0 0.0 100 8.8 96h 34.1 23.5 23.5 5.8 6.3 100 11.0 120h 33.9 32.7 35.6 11.0 11.1 100 10.6 Lettuce (Lactuca sativa) 120h 144h 23.2 0.0 0.0 0.0 0.0 100 1.8 5.4 0.0 0.0 0.0 0.0 100 1.8
Conc. (g 500 mL-1 H2O) 20 30 40 50 70 Control LSD 0.05
Values are expressed as a % of the control.
International Rice Research Institute (IRRI) developed a laboratory method for screening whole- plant-bioassay or allelopathic rice screening (Navarez and Olofsdotter, 1996) to eliminate the effects of competitive interference for resources between rice and test plants. Interestingly using this method, inhibitory activity of 111 rice varieties in Philippines has been shown to be inconsistent between laboratory and field trials (Olofsdotter et al., 1999). However, in both laboratory screening and field experiments revealed that rice allelopathy is active in both monocot and dicot weeds (Dilday et al., 1991; Fuji 1994; Olofsdotter and Navarez, 1996; Hasan et al., 1998; Kim and Shin, 1998). Laboratory screening for allelopathy study in rice have also been undertaken and it has been found that there was no mark difference among rice varieties for weed species inhibition activity (Fuji, 1994; Hasan et al., 1998; Azmi et al., 2000) suggesting that different rice cultivars are capable to supress particular weed species. 5. Rice allelochemicals
The exploiting research for isolating and identifying rice allelochemicals has started but yet not yielded chemicals that could exert
allelopathic effect (Rimando et al., 2001). Several studies have been conducted by various researchers to identify allelochemical(s) from rice and to find their allelopathic potential (Chung et al., 2001; Kim and Kim, 2002; Rimando et al., 2001; Kong et al., 2004; Seal et al., 2004a). Among them Seal et al. (2004a) found 200 different compounds in rice root exudates which fall under three main chemical classes such as phenolics, phenylalkanoic acids and indoles. Several classes of secondary metabolites determined from the root exudates of rice like phenolics (Jung et al., 2001; Kim and Kim, 2002; Rimando et al., 2001; Olofsdotter et al., 2002; Inderjit et al., 2002; Seal et al., 2004b), alkyl resocinols (Bouillant et al., 1994), momilactone B (Kato-Noguchi and Ino, 2005; Kong et al., 2004), amino acids (Bacillo_Jimenez et al., 2003) and flavones (Kong et al., 2004). Several studies focused that common putative allelochemicals found in rice are phenolic acid compounds (Chou et al., 1991; Inderjit 1996; Blum, 1998) such as p-coumaric acid, p-hydrobenzoic acid, ferulic acid and vanillic acid. Nontheless, it is belived that phenolics must play an important role for rice allelopathy. So, identifying allelochemical in rice is an important issue for understanding allelopathy mechanism.
117 A large number of rice varieties were found to inhibit the growth of several plant species when the rice varieties were grown together with the plants under field and/or laboratory conditions (Dilday et al., 1998; Hassan et al., 1998; Olofsdotter et al., 1999). These findings suggest that living rice may produce and release allelochemical(s) into the neighboring environment. A number of secondary metabolites, phenolic acids, phenylalkanoic acids, hydroxamic acids, fatty acids, terpenes and indoles were identified in rice extracts (Rimando and Duke, 2003). These compounds are ubiquitous in plants and some of these compounds have growth inhibitory activity against several plant species. It is not clear, however, whether these compounds are released from living rice plants into the neighboring environment, and act as allelochemicals in natural ecosystems. In addition, it was found that there was no significant correlation between the level of growth inhibitory substances in plants and their level in the root exudates (Wu et al., 2001). It was found that the growth of soybean callus was inhibited by rice callus when both callus tissues were incubated together (Yang and Futsuhara, 1991). Zhang et al. (2005) identified potent allelopathic rice germplasm for breeding new rice varieties with allelopathic potential. Yiqing et al. (2005) focussed on the evaluation of allelopathic wild rice species germplasm resources for barnyardgrass control. He et al. (2006) detected the diethyl ether extracts from the root exudates of two rice accessions, allelopathic rice (PI312777) and non-allelopathic rice (Lemont) seedlings. The individual compounds were identified by comparing their mass spectra with those from NIST and Wiley Library of mass spectral database. Sixty-three compounds were detected in root exudates of PI312777 and 77 compounds in Lemont. The substances were terpenoids, phenols (quinones), aldehydes (ketones), heterocyclic alcohols, ethers, hydrocarbons etc. (He et al., 2006). Hiroshi (2008) examined the effect of husk extracts of wild rice spp. on root and shoot growth of lettuce, barnyard grass and Eclipta thermalis (false daisy). The results suggested
Rice allelopathy and environment that the husk extracts of Oryza glumaepatula contain water soluble allelochemicals that inhibit root growth of lettuce but promote shoot growth of false daisy. Ethyl acetate soluble allelochemicals that inhibit root growth of barnyard grass. Olofsdotter et al. (1995) described the assessment of the allelopathic potential of root exudates from rice plants during their early developmental stage and allelopathic potential of rice leaching by using Petri dish bioassay under laboratory condition. 6. Conclusions
Despite the tremendous growth in allelopathy research in recent years there are lots of areas that have yet not been studied. Isolation and identification of rice allelochemicals are important to toxicological and eco-toxicological studies before crossing between present traits and commercial germplasm. Agronomic managements of rice like date of sowing, seeding depth, standing water depth, amount and type of fertilizers, duration of dry period, density and species of weeds are to be investigated for rice based allelopathy. Using allelopathic potential, rice cultivars in crop rotation and as companion crop need to be studied. Some rice cultivars are showing residual allelopathic effects on weed emergence as they contain acid during their decomposition. In paddy field, many weed species are either completely inhibited or significantly reduced by rice allelopathic potential. Eeveral studies have focused that the allelopathic materials on weed species are selective. Therefore, sensitive weeds are to be evaluated for their seed moisture content, growing habit, survival capability and extend of competitive ability against rice for natural weed management. So, to understand rice-weed chemical interaction, detail researches are needed with a long term vision. References Abdul-Wahab, A. S. and Rice, E. L. 1967. Plant inhibition by Johnson grass and its possible significance in old-field
Siddique and Ismail /The Agriculturists 11(1): 112-121 (2013) succession. Bull. Torrey Bot. Club, 94: 486-497. Alamdari, E. G and Deokule, S. S. 2009. Allelopathic effects of some weeds on growth and yield of paddy rice (Tarom variety) in northern Iran. Pak. J. Weed Sci. Res. 15 (2-3): 123-12. Anaya, A. L. 1999. Allelopathy as a tool in the management of biotic resource in agroecosystems. Crit. Rev. Plant Sci. 18: 697-739. Azmi M, Abdullah M. Z. and Fujii, Y. 2000. Exploratory study on allelopathic effect of selected Malaysian rice varieties and rice field weed species. J. Trop. Agri. Food Sci. 28: 39-54. Bacilio-Jimenez, M., Aguilar-Flores, S., Ventura-Zapata, E., Perez-Campos, E., Bouquelet, S. and Zenteno, E. 2003. Chemical characterization of root exudates from rice (Oryza sativa) and their effects on the chemotactic response of endophytic bacteria. Plant Soil, 249: 271-277. Bell, D. T. and Koeppe, D. E. 1972. Noncompetitive effects of giant foxtail on the growth of corn. Agron. J. 64: 321-325. Bhatt, M. D. and Tewari, A. 2006. Loss in growth and yield attributes due to weed composition in transplanted paddy in Terai region. Sci. World. 4(4): 99-101. Blum, U. 1998. Effects of microbial utilization of phenolic acids and their phenolic acid breakdown products on allelopathic interactions. J. Chem. Ecol. 24: 685-708. Bouillant, M. L., Jacoud, C., Zanella, I., FavreBonvin, J. and Vally, R. 1994. Identification of 5- (12-heptadecenyl)resorcinol in rice root exudates. Phytochemistry, 35: 769-771. Chou, C. H., Chang, F. J. and Oka, H. I. 1991. Allelopathic potential of wild rice Oryza perennis. Taiwania, 36 (3): 201-210. Chung, I. M., Ahn, J. K. and Yun, S. J. 2001. Identification of allelopathic compounds from rice (Oryza sativa L.) straw and
118
their biological activity. Can. J. Plant Sci. 81: 815-819. Dilday, R. H., Nastasi, P. and Smith, R. J. Jr. 1989. Allelopathic observations in rice (Orzya sativa L.) to ducksalad (Heteranthera limosa). Proceedings of the Arkansas Academy of Science, 43: 21 22. Dilday, R. H., Yan, W. G., Moldenhauer, K. A. K. and Gravois, K. A. 1998. Allelopathic activity in rice for controlling major aquatic weeds. In Olofsdotter, M., ed. Allelopathy in Rice. Manila, Philippines: Int. Rice Research Institute. 7-26 pp. Dilday, R. H., Nastasi, P., Lin, J. and Smith, R. J., Jr. 1991. Allelopathic activity in rice (Oryza sativa L.) against ducksalad (Heteranthera limosa (sw.) Willd.). In D. Hanson,M.J. Shaffer. D. A. Ball. and C.V. Cole., eds. Symposium Proc. on Sustainable Agriculture for the Great Plains. USDA, ARS-89. 193-201 pp. Duke, S. O., Dayan, F. E., Rimando, A. M., Scrader, K. K., Aliotta , G., Olivia, A. and Romangi, J. G. 2002. Chemicals from nature for weed management, Weed Sci. 50: 138-151. Fang, C., Zhuang, Y., Xu, T., Li, Y., Li, Y. and Lin, W. 2013. Changes in rice allelopathy and rhizosphere microflora by inhibiting rice phenylalanine ammonialyase gene expression. J. Chem. Ecol.. 39(2): 204-12. Forzwa, R. and Karim, S. M. R. 2009. Allelopathic effects of aqueous extracts of parthenium weed on the seed germination and growth of field crops. Abstracts, Annual conference of Bangladesh Botanical Society, held at Chittagong University, 16-17 pp. Fujii, Y. 1994. The potential biological control of paddy weeds with allelopathyallelopathic effect of some rice cultivars. Proceedings of the International Symposium on Biological Control and Integrated Management of Paddy and Aquatic Weeds, 1992. Tsukuba, Japan, 305-320 pp.
119 Haramoto, E. R. and Gallandt, E. R. 2004. Brassica cover cropping for weed management: A review. Renewable Agriculture and Food Systems 19:187198. Hassan, S. M., Aidy, I. R., Bastawisi, A. O. and Draz, A. E. 1998. Weed management using allelopathic rice varieties in Egypt. In Allelopathy in Rice. (Ed. M Olofsdotter, International Rice Research Institute: Manila, 27-37 pp.). Heap, I. M. 2011. International Survey of Herbicide Resistant Weeds, http://www.weedscience.org/in.asp (15 January 2011). He, H. B., Lin, W. X., Wang, H. B., Fang, C. X. and Liang, Y. Y. 2006. Analysis of metabolites in root exudates from allelopathic and non allelopathic rice seedlings. Allelopathy J. 18: 247254. Hiroshi, N. 2008. Effects of husk extracts of wild rice spp. on seedling growth of lettuce, barnyard grass and Eclipta thermalis. Allelopathy J. 22: 391-396. Inderjit and Keating K. I. 1999. Allelopathy: principles, procedures, processes, and promises for biological control. Adv. Agron. 67: 141-231. Inderjit., Streibig, J. C. and Olofsdotter, M. 2002. Joint action of phenolic acid mixtures and its significance in allelopathy research. Physiol. Plant. 114: 422-428. Inderjit. 1996. Plant phenolics in allelopathy. Bot. Rev. 62: 186202. Isley, D. 1960. Weed Identification and Control in North Central States. Iowa State Univ. Press. Ames. Iowa, USA. 28-39 pp. Ismail, B. S. and Siddique, A. B. 2010(a). Allelopathic Effects of Echinochloa colona On The Growth Of The Rice Plant. Proc. of The 16th Asian Agricultural Symposium (AAS) and 1st International Symposium on Agriculture Technology (ISAT), 25-27
Rice allelopathy and environment August 2010, Bangkok, Thailand, 429-431 pp. Ismail, B. S. and Siddique, A.B. 2010(b). Phytotoxic Effects of the Aqueous Extracts of Ludwigia hysopifolia on the Growth of Rice Seedlings. Proceedings of The International Seminar on Weed Management in Indonesia, November, 2010. Bandung, Inonesia, 9-11 pp. Ismail, B. S. and Siddique, A. B. 2011. The Inhibitory Effect of Grasshoppers Cyperus (Cyperus iria L.) on the Seedling Growth of Five Malaysian Rice Varieties. Trop. Life Sci. Res. 22(1): 81 89. Ismail, B. S. and Siddique, A. B. 2012a. Allelopathic Inhibition by Fimbristylis miliacea on the Growth of the Rice Plants. Adv. Envr. Biol. 6(8): 2423-2427. Ismail, B. S. and Siddique, A. B. 2012b. Identification of allelochemicals from Fimbristylis miliacea and their allelopathic potential against weed species. Allelopathy J, 30(2): 311-318. . Johnson, D. E. 1996. Weed Management in Small Holder Paddy Production in the Tropics. Available: http://ipmworld.umn.edu/hapters/Johnson. htlm. 20 July 2010. Jung, I. M., Ahn, J. K. and Yun, S. J. 2001. Assessment of allelopathic compounds from rice (Oryza sativa L.) straw and their biological activities. Can. J. Plant Sci.81: 815819. Karim, S. M. R., Azmi, M. and Ismail, B.vS. 2004. Weed problems and their management in rice fields of Malaysia: An overview. Weed Biol. Manag. 4:177-186. Kato-Noguchi, H. and Ino, T. 2005. Possible involvement of momilactone B in rice allelopathy. J. Plant Physiol. 162: 718721. Khush, G.S. 2005. What it will take to feed 5.0 Billion consumers in 2030. Plant Mol. Biol. 56: 1-6.
Siddique and Ismail /The Agriculturists 11(1): 112-121 (2013) Kim, J. T. and Kim, S. H. 2002. Screening of allelochemicals on barnyard grass (Echinochloa crus-galli) and identification of potentially allelopathic compounds from rice (Oryza sativa) variety hull extracts. Crop Prot. 21: 913920. Kim, K. U. and Shin, D. H. 1998. Rice allelopathy research in Korea. p. 39-44. In M. Olofsdotter (ed.) Proc. of the Workshop on Allelopathy in Rice.Manila, Philippines. 2527 Nov. 1996. Int. Rice Res. Inst., Makati City, Philippines. Kong, C., Liang, Xu, W., Xu, X. and Hu, F. 2004. Release and activity of allelochemicals from allelopthic rice seedlings. J. Agril. Food Chem. 52: 2861-2865. Labbafi, M. R., Hejazi, A., Maighany, F., 2, Khalaj, H. and Ali Mehrafarin, A. 2010. Evaluation of allelopathic potential of Iranian wheat (Triticum aestivum L.) cultivars against weeds. Agri. Bio. J. North Amer. 1(3): 355-361. Mahmoodzadeh, H. and Mahmoodzadeh, M. 2013. Allelopathic potential of soybean (Glycine max L.) on the germination and root growth of weed species. Life Sci. J. 10(5): 63-69. Moody, K. 1991. Weed management in rice. In Handbook of Pest Management in Agriculture.2nd edition. Pimenteal, D. ed. CRC Press Boca Raton, Florida, USA. 301-328 pp. Nath, R. 1988. Parthenium hysterophorus review. Agric Review. 9: 171-179. a
120
Navarez, D. and Olofsdotter, M. 1996. Relay seeding procedure as screening method in allelopathy research. Proc. 2nd Int. Weed Control Conf. 4: 285-1290. Nikneshan, P., Karimmojeni, H., Moghanibashi, M. and Nayereh al sadat Hosseini. 2011. Allelopathic potential of sunflower on weed management in safflower and wheat Australian J. Crop Sci. 5(11): 1434-1440.
Olofsdotter M., Navarez D., Rebulanan, M. and Streibig, J. C. 1999. Weed-suppressing rice cultivars does allelopathy play a role? Weed Res. 39: 441-454. Olofsdotter, M., Rebulanan, M., Madrid, A., Dali, W., Navarez, D. and Olk, D. C. 2002. Why phenolic acids are unlikely primary allelochemicals in rice. J. Chem. Ecol. 28: 229-242. Olofsdotter, M. 2001. Getting closer to breeding for competitive ability and the role of allelopathy-an example from rice (Oryza sativa). Weed Tech. 15: 798-806. Olofsdotter, M. and Navarez, D. 1996. Allelopathic rice in Echinochloa crus-galli control. p. 1175-1182. In H. Brown, et al. (ed.) Proc. of the 2nd Int. Weed Control Congress, Copenhagen, Denmark. 2528 June 1996. DJF, Flakkebjerg, Denmark. Olofsdotter, M., Navarez, D. and Moody, K. 1995. Allelopathic potential in rice (Oryza sativa L.) germplasm. Ann. Applied Biol. 127: 543-560. Putnam, A. R. and Weston, L. A. 1986. Adverse impacts of allelopathy in agricultural systems. In The Science of Allelopathy (ed. A. R. Putnam and C. S. Tang), pp. 43-65. Wiley, New York. Qasem, J. R. and Foy, C. l. 2001. Weed allelopathy, its ecological impact and future prospects: a review. In: Allelopathy in Agroecosystems. R. K. Kohli, H.P. Singh and D. R. Batish. New York: Haworth Press, pp, 43-119. Rice, E. L. 1984. Allelopathy. 2nd Edn. New York: Academic Press. Rice, E. L. 1987. Allelopathy: an overview. In: G.R. Waller (Ed.), Allelochemical: Role in Agriculture and Forestry. American Chemical Society Symposium. 330: 8-22. Rimando, A. M and Duke, S. O. 2003. Studies on rice allelochemicals. In Rice; Origin, History, Technology and Production. (Eds. CW Smith, RH Dilday) pp. 221244, (John Wiley & Sons, Inc.: Hoboken, New Jersey).
121 Rimando, A. M., Olofsdotter, M., Dayan, F. E. and Duke, S. O. 2001. Searching for rice allelochemicals: An example of bioassayguided isolation. Agron J. 93: 16-20. Salam, M. A. and Kato-Noguchi, H. 2009. Screening of Allelopathic Potential Bangladesh Rice Cultivars by DonorReceiver Bioassay. Asian J. Plant Sci. 8: 20-27. Seal, A. N., Haig, T., and Pratley, J. E. 2004(b). Evaluation of putative allelochemicals in rice roots exudates for their role in the suppression of arrowhead root growth. J. Chem. Ecol. 30: 1663-1678. Seal, A. N., Pratley, J. E., Haig, T., and An, M. 2004(a). Identification and quantitation of compounds in a series of allelopathic and non-allelopathic rice root exudates. J. Chem. Ecol. 30: 1647-1662. Siddique, A. B. and Ismail, B. S. 2009. Assessment of Allelopathic Potential of Goose Weed (Sphenoclea eylanica) on Selected commonly used rice varieties in Malaysia. Prosiding Kolokium Siswazah Ke-9, Fakulti Sains dan teknologi, Ukm, Bangi. pp: 150- 152. Siddique, A. B. and Ismail, B. S. 2010. Allelopathic Effects of Fimbristylis miliaceae on Rice Plants. . Proceedings of The 16th Asian Agricultural Symposium (AAS) and 1st International Symposium on Agriculture Technology (ISAT) Bangkok, Thailand, on 2527 August. 72-75 pp. Singh, H. P., Batish, D. R. and Kohli, R. K. 2003. Allelopathic interactions and allelochemicals; New possibilities for sustainable weed management. Crit. Rev. Plant Sci. 22: 239-311. Stachon, W. J. and Zimdahl, R. L. 1980. Allelopathic activity of Canada thistle (Cirsium arvense) in Colorado. Weed Sci. 28: 83-86. Takeuchi, Y., Kawaguchi, S. and Yoneyama, K. 2001. Inhibitory and promotive allelopathy in rice (Oryza sativa L.). Weed Biol. Manag. 1: 147-156.
Rice allelopathy and environment Tarek A, E. S. 2010. Rice straw as an allelopathic agent for controlling weeds. Seventeenth Australasian Weeds Conference. 26-30 September, 2010. 143146 pp. Valverde, B. E., Riches, C. R. and Caseley, J. C. 2000. Prevention and management of herbicide resistant weeds in rice. Published by Grafos, S. A., Cartago, Costa Rica. 25-30 pp. Watanabe, H., Azmi, M. & Ismail, M. Z. 1997. Emergence of major weeds and their population change in wet-seeded rice fields of the MUDA area, Peninsular Malaysia. In: Rajan, A. (ed.), Proc. 16th Asian Pacific Weed Science Society Conf. pp: 246250. Kuala Lumpur, Malaysia. Weston, L. A. 1996. Utilization of allelopathy for weed management in agroecosystems. Agron. J. 88: 860866. Wu, H., Haig, T., Pratley, J., Lemerle, D. and An, M. 2001. Allelochemicals in wheat (Triticum aestivum L.): Production and exudation of 2,4-dihydroxy-7methoxy-1,4benzoxazin-3- one. J. Chem. Ecol. 27: 1691-1700. Yang Y. S and Futsuhara, Y. 1991. Inhibitory effects of volatile compounds released from rice callus on soybean callus growth: allelopathic evidence observed using in vitro culture. Plant Sci. 77: 103-110. Yiqing, G., Fudou, Z., Dayun, T., Liuqing, Y. and David, G. 2005. Preliminary studies on the allelopathic potential of wild Rice (Oryza) germplasm. Allelopathy J. 15: 13-20. Zhang, Z., Zhou,Y., Lu, D. and Yu, L. 2005. Identification of allelopathic potential of Chinese rice (Oryza sativa L.) germplasm. Allelopathy J. 15: 111-118. Zimdahl, R. L. (1980). Weed Crop Competition: A Review. International Plant Protection Centre; Oregon State University. (3rd March, 1990).
S-ar putea să vă placă și
- Family: Palmae Scientific Name: Elaeis GuineensisDocument124 paginiFamily: Palmae Scientific Name: Elaeis GuineensisRosita MatsaidinÎncă nu există evaluări
- Beetroot Production Guideline 2014Document4 paginiBeetroot Production Guideline 2014Diana Maletzky-GeingobÎncă nu există evaluări
- Agronomy Crop Production (CH)Document45 paginiAgronomy Crop Production (CH)Parvathi M LÎncă nu există evaluări
- Attra Cut Flower ProductionDocument24 paginiAttra Cut Flower ProductionTomi Risley AnumoÎncă nu există evaluări
- (1899007X - Journal of Plant Protection Research) Management of Soil-Borne Diseases of Organic VegetablesDocument10 pagini(1899007X - Journal of Plant Protection Research) Management of Soil-Borne Diseases of Organic VegetablesJesus OcampoÎncă nu există evaluări
- Phaseolina (Jalaluddin, 2008) - Charcoal Rot Disease Is Considered Most Prevalent Than TheDocument10 paginiPhaseolina (Jalaluddin, 2008) - Charcoal Rot Disease Is Considered Most Prevalent Than TheAsad AliÎncă nu există evaluări
- Weeds Are One of The Major Constraints in Sustainable Agricultural ProductionDocument2 paginiWeeds Are One of The Major Constraints in Sustainable Agricultural ProductionIsnaini Mela KurniaÎncă nu există evaluări
- Allelopathic weed suppression in agroecosystemsDocument10 paginiAllelopathic weed suppression in agroecosystemsArarsa LetaÎncă nu există evaluări
- Review On The Role of Allelopathy in Pest Management and Crop Production By: Serkie Habtamu IDNO - DBUR/1994/09Document19 paginiReview On The Role of Allelopathy in Pest Management and Crop Production By: Serkie Habtamu IDNO - DBUR/1994/09wedajeÎncă nu există evaluări
- Allelopathy For Weed ControlDocument9 paginiAllelopathy For Weed ControlAnn LicaÎncă nu există evaluări
- 2 Ijasrjun20182Document26 pagini2 Ijasrjun20182TJPRC PublicationsÎncă nu există evaluări
- A Review On Various Management Method of Rice BlasDocument5 paginiA Review On Various Management Method of Rice BlasroseÎncă nu există evaluări
- Challenges and Opportunities in Implementing Allelopathy For Natural Weed ManagementDocument32 paginiChallenges and Opportunities in Implementing Allelopathy For Natural Weed Managementbrkica2011Încă nu există evaluări
- Agriculture Is The Backbone of Nepalese EconomyDocument4 paginiAgriculture Is The Backbone of Nepalese EconomySmriti pantÎncă nu există evaluări
- Crop Protection: Virender Sardana, Gulshan Mahajan, Khawar Jabran, Bhagirath S. ChauhanDocument7 paginiCrop Protection: Virender Sardana, Gulshan Mahajan, Khawar Jabran, Bhagirath S. ChauhanEdilsonGiraldoÎncă nu există evaluări
- 5 PaperDocument6 pagini5 PaperDr. Nilesh Baburao JawalkarÎncă nu există evaluări
- 13 - Organic Farming For Sustainable Development - Final Draft - 021221Document20 pagini13 - Organic Farming For Sustainable Development - Final Draft - 021221khaliduthmaniÎncă nu există evaluări
- Thesis Outline FinalDocument11 paginiThesis Outline FinalLiana Rose Alviar PingolÎncă nu există evaluări
- Management of Bacterial Wilt (Ralstonia Solanacearum) of Potato: Focus On Natural Bioactive CompoundsDocument20 paginiManagement of Bacterial Wilt (Ralstonia Solanacearum) of Potato: Focus On Natural Bioactive Compoundsdawit gÎncă nu există evaluări
- Allelopathy for weed managementDocument6 paginiAllelopathy for weed managementArarsa LetaÎncă nu există evaluări
- 6weed Control in Clean Agriculture A ReviewDocument16 pagini6weed Control in Clean Agriculture A ReviewJOSUEÎncă nu există evaluări
- Risks of Herbicide-Resistant Rice in India: A Review: V. Kumar, R.R. Bellinder, D.C. Brainard, R.K. Malik, R.K. GuptaDocument10 paginiRisks of Herbicide-Resistant Rice in India: A Review: V. Kumar, R.R. Bellinder, D.C. Brainard, R.K. Malik, R.K. GuptaaniketsinghaniaÎncă nu există evaluări
- 2003 - BHOWMICKnINDERJIT - Challenges and Opportunities in Implementing Allelopathy For Natural Weed ManagementDocument11 pagini2003 - BHOWMICKnINDERJIT - Challenges and Opportunities in Implementing Allelopathy For Natural Weed ManagementujjalkrpatiÎncă nu există evaluări
- Aromatic PlantsDocument8 paginiAromatic PlantspankkichanÎncă nu există evaluări
- Applications of Radioisotopes in Agriculture: Balwinder Singh, Jaspreet Singh and Amritpal KaurDocument8 paginiApplications of Radioisotopes in Agriculture: Balwinder Singh, Jaspreet Singh and Amritpal KaurfransescatomasilaÎncă nu există evaluări
- Plant Production and Protection Division - Soil Biological Management With Beneficial MicroorganismsDocument3 paginiPlant Production and Protection Division - Soil Biological Management With Beneficial MicroorganismsCyberSquare IndiaÎncă nu există evaluări
- 2020 - Book - EntomovectoringForPrecisionBio (1) - 27-59Document33 pagini2020 - Book - EntomovectoringForPrecisionBio (1) - 27-59jefferson.juniorÎncă nu există evaluări
- Bajwa 2016Document17 paginiBajwa 2016Sandra Carolina Daza GachaÎncă nu există evaluări
- Sustainable Weed Management in Conservation AgricultureDocument9 paginiSustainable Weed Management in Conservation AgricultureAlmirÎncă nu există evaluări
- 9.weed Control Practices of Organic Farmers in Malaysia (Mohamad Amir Shah Yusop) PP 57-62Document6 pagini9.weed Control Practices of Organic Farmers in Malaysia (Mohamad Amir Shah Yusop) PP 57-62upenapahangÎncă nu există evaluări
- Conserving Agrobiodiversity For Sustainable Food Systems in The Hindu Kush HimalayaDocument20 paginiConserving Agrobiodiversity For Sustainable Food Systems in The Hindu Kush HimalayaCarolina CarrascoÎncă nu există evaluări
- Microbial Antagonists as Biocontrol AgentsDocument8 paginiMicrobial Antagonists as Biocontrol AgentskhairunnisaÎncă nu există evaluări
- Biofumigation: A Potential Aspect For Suppression of Plant-Parasitic NematodesDocument7 paginiBiofumigation: A Potential Aspect For Suppression of Plant-Parasitic NematodesIJEAB JournalÎncă nu există evaluări
- Fruit FliesDocument20 paginiFruit FliesSimone BM MinduimÎncă nu există evaluări
- Impact of soil health management practices on soilborne pathogensDocument11 paginiImpact of soil health management practices on soilborne pathogensFranky zÎncă nu există evaluări
- 4 Paper AllellopathyDocument6 pagini4 Paper AllellopathyGhislayne VacaÎncă nu există evaluări
- Effect of Foliar Application of Urea Fertilizer and Post Emergence Herbicide OnDocument27 paginiEffect of Foliar Application of Urea Fertilizer and Post Emergence Herbicide OnArarsa LetaÎncă nu există evaluări
- Review of Related LiteratureDocument7 paginiReview of Related LiteratureMark Henry50% (4)
- Evaluating the Effectiveness of Piper guineense Seed Powder for Controlling Callosobruchus maculatus Infestation in Stored Cowpea SeedsDocument21 paginiEvaluating the Effectiveness of Piper guineense Seed Powder for Controlling Callosobruchus maculatus Infestation in Stored Cowpea SeedsMajestyÎncă nu există evaluări
- Chapter One 1.1 Background of The StudyDocument21 paginiChapter One 1.1 Background of The StudyMajestyÎncă nu există evaluări
- 2014 - Garibaldi - From Research To Action Enhancing Crop Yield Through Wild PollinatorsDocument10 pagini2014 - Garibaldi - From Research To Action Enhancing Crop Yield Through Wild PollinatorsDanielaÎncă nu există evaluări
- Some Commonly Used PesticidesDocument8 paginiSome Commonly Used PesticidesumarmuhammadwaliÎncă nu există evaluări
- Journal of Agriculture and Applied BiologyDocument18 paginiJournal of Agriculture and Applied BiologyDikshya NiraulaÎncă nu există evaluări
- Constraints and Solutions of Country Bean (Lablab: Purpureus L.) Production: A ReviewDocument9 paginiConstraints and Solutions of Country Bean (Lablab: Purpureus L.) Production: A ReviewAhasan Ullah KhanÎncă nu există evaluări
- CB 1. Fonseca Et Al.Document6 paginiCB 1. Fonseca Et Al.Maria NaudeauÎncă nu există evaluări
- Biocontrol Potential of Trichoderma Sp. and Rhizosphere Bacteria From Infected Garlic (Allium Sativum L.) : A Promising StudyDocument24 paginiBiocontrol Potential of Trichoderma Sp. and Rhizosphere Bacteria From Infected Garlic (Allium Sativum L.) : A Promising StudyInternational Journal of Innovative Science and Research TechnologyÎncă nu există evaluări
- Tasawar Abbas - Soil Sci - 2017 - UAF - Main PT With RefDocument218 paginiTasawar Abbas - Soil Sci - 2017 - UAF - Main PT With RefKing KhanÎncă nu există evaluări
- Effects of Neem Insecticides on Cauliflower PestDocument7 paginiEffects of Neem Insecticides on Cauliflower PestDuvánE.DueñasLópezÎncă nu există evaluări
- 20.App-Effect of Weeds and Their Management in Transplanted Rice - A ReviewDocument16 pagini20.App-Effect of Weeds and Their Management in Transplanted Rice - A ReviewImpact JournalsÎncă nu există evaluări
- Nodulation, Growth and Yield Response of Five Cowpea (VignaDocument12 paginiNodulation, Growth and Yield Response of Five Cowpea (VignaPremier PublishersÎncă nu există evaluări
- Combined Control of Striga Hermonthica and Stemborers by Maize-Desmodium Spp. IntercropsDocument7 paginiCombined Control of Striga Hermonthica and Stemborers by Maize-Desmodium Spp. IntercropsCleaver BrightÎncă nu există evaluări
- Applied Soil Ecology: Walid Ellouze, Chantal Hamel, Sadok Bouzid, Marc St-ArnaudDocument10 paginiApplied Soil Ecology: Walid Ellouze, Chantal Hamel, Sadok Bouzid, Marc St-ArnaudJorge CorderoÎncă nu există evaluări
- Fatholahi Phenol CompoundsDocument10 paginiFatholahi Phenol Compoundsmasoud mnÎncă nu există evaluări
- 297S 1Document5 pagini297S 1aneelayasminÎncă nu există evaluări
- Assessment of Pests Control Methods and Its Perceived Effect On Agricultural Production Among Farmers in Kwara State, NigeriaDocument6 paginiAssessment of Pests Control Methods and Its Perceived Effect On Agricultural Production Among Farmers in Kwara State, NigeriaAlbert LiwagÎncă nu există evaluări
- The - Evolution - of - Cultivated - Plant - Species - Classical Plant Breeding Vs Genetic Engineering PDFDocument11 paginiThe - Evolution - of - Cultivated - Plant - Species - Classical Plant Breeding Vs Genetic Engineering PDFsanduni edirisingheÎncă nu există evaluări
- IntercroppingincitrusDocument11 paginiIntercroppingincitrusmanishdgÎncă nu există evaluări
- 13-033. My Syahrawati - AJAB 2018Document11 pagini13-033. My Syahrawati - AJAB 2018Mysyahra WatiÎncă nu există evaluări
- Biological Control of Weeds by Using Allelopathic PotentialDocument9 paginiBiological Control of Weeds by Using Allelopathic PotentialRoxana CupinÎncă nu există evaluări
- Natural Enemy Enhancement and Botanical Insecticide Source - A Review of Dual Use Companion PlantsDocument19 paginiNatural Enemy Enhancement and Botanical Insecticide Source - A Review of Dual Use Companion PlantsbeneÎncă nu există evaluări
- 119786-Article Text-330335-1-10-20150722Document14 pagini119786-Article Text-330335-1-10-20150722Luis GarciaÎncă nu există evaluări
- Evaluation of Rice Genotypes For Resistance To The Stalk-Eyed Fly (Diopsis Longicornis) in Rice in UgandaDocument13 paginiEvaluation of Rice Genotypes For Resistance To The Stalk-Eyed Fly (Diopsis Longicornis) in Rice in UgandaMd Ashikur RahmanÎncă nu există evaluări
- 641-Article Text-1162-1-10-20171110Document19 pagini641-Article Text-1162-1-10-20171110Krishan Kumar sharmaÎncă nu există evaluări
- Islam Et Al. 11 - 2 - 104-108 - 2013 - Short ComDocument5 paginiIslam Et Al. 11 - 2 - 104-108 - 2013 - Short ComNoman FarookÎncă nu există evaluări
- Islam Et Al. 11 - 2 - 109-113 - 2013 - Short ComDocument5 paginiIslam Et Al. 11 - 2 - 109-113 - 2013 - Short ComNoman FarookÎncă nu există evaluări
- Sarker Et Al. 11 - 2 - 96-103 - 2013Document8 paginiSarker Et Al. 11 - 2 - 96-103 - 2013Noman FarookÎncă nu există evaluări
- Instructions To Authors Vol 11 (1) June 2013Document3 paginiInstructions To Authors Vol 11 (1) June 2013Noman FarookÎncă nu există evaluări
- Khalil Et Al. 11 - 2 - 79-86 - 2013Document8 paginiKhalil Et Al. 11 - 2 - 79-86 - 2013Noman FarookÎncă nu există evaluări
- Islam Et Al. 11 - 2 - 44-51 - 2013Document8 paginiIslam Et Al. 11 - 2 - 44-51 - 2013Noman FarookÎncă nu există evaluări
- Uddin Et Al. 11 - 2 - 66-73 - 2013Document8 paginiUddin Et Al. 11 - 2 - 66-73 - 2013Noman FarookÎncă nu există evaluări
- Emdad Et Al. 11 - 2 - 74-78 - 2013Document5 paginiEmdad Et Al. 11 - 2 - 74-78 - 2013Noman FarookÎncă nu există evaluări
- Rahman Et Al. 11 - 2 - 38-43 - 2013Document6 paginiRahman Et Al. 11 - 2 - 38-43 - 2013Noman FarookÎncă nu există evaluări
- Islam Et Al. 11 - 2 - 87-95 - 2013Document9 paginiIslam Et Al. 11 - 2 - 87-95 - 2013Noman FarookÎncă nu există evaluări
- Patwary Et Al. 11 - 2 - 52-57 - 2013Document6 paginiPatwary Et Al. 11 - 2 - 52-57 - 2013Noman FarookÎncă nu există evaluări
- Talukder Et Al. 11 - 2 - 58-65 - 2013Document8 paginiTalukder Et Al. 11 - 2 - 58-65 - 2013Noman FarookÎncă nu există evaluări
- Ali Et Al. 11 - 2 - 28-37 - 2013Document10 paginiAli Et Al. 11 - 2 - 28-37 - 2013Noman FarookÎncă nu există evaluări
- Rahman Et Al. 11 - 2 - 10-13 - 2013Document4 paginiRahman Et Al. 11 - 2 - 10-13 - 2013Noman FarookÎncă nu există evaluări
- Jahan Et Al. 11 - 2 - 14-20 - 2013Document7 paginiJahan Et Al. 11 - 2 - 14-20 - 2013Noman FarookÎncă nu există evaluări
- Rashid Et Al. 11 - 2 - 1-9 - 2013Document9 paginiRashid Et Al. 11 - 2 - 1-9 - 2013Noman FarookÎncă nu există evaluări
- An Evaluation of Some Japanese Lisianthus (Eustoma Grandiflorum) Varieties Grown in BangladeshDocument5 paginiAn Evaluation of Some Japanese Lisianthus (Eustoma Grandiflorum) Varieties Grown in BangladeshNoman FarookÎncă nu există evaluări
- 5.rahman Et Al. 11 - 2 - 21-27 - 2013Document7 pagini5.rahman Et Al. 11 - 2 - 21-27 - 2013Noman FarookÎncă nu există evaluări
- Efficacy of Herbicides On The Yield of Lentil (Lens Culinaris Medik.)Document6 paginiEfficacy of Herbicides On The Yield of Lentil (Lens Culinaris Medik.)Noman FarookÎncă nu există evaluări
- Ahmad Et Al. 11 - 1 - 103-111 - 2013Document9 paginiAhmad Et Al. 11 - 1 - 103-111 - 2013Noman FarookÎncă nu există evaluări
- Instructions To Authors Vol 11 (1) June 2013Document3 paginiInstructions To Authors Vol 11 (1) June 2013Noman FarookÎncă nu există evaluări
- Comparative Study of Mustard Oil Cake and Soybean Meal Based Artificial Diet For Rohu, Labeo Rohita (Ham.) FingerlingsDocument6 paginiComparative Study of Mustard Oil Cake and Soybean Meal Based Artificial Diet For Rohu, Labeo Rohita (Ham.) FingerlingsNoman FarookÎncă nu există evaluări
- Bacteriological and Pathological Investigation of Nasal Passage Infections of Chickens (Gallus Gallus)Document9 paginiBacteriological and Pathological Investigation of Nasal Passage Infections of Chickens (Gallus Gallus)Noman FarookÎncă nu există evaluări
- Screening of Salt Tolerant CIP Potato Germplasm For Saline AreasDocument8 paginiScreening of Salt Tolerant CIP Potato Germplasm For Saline AreasNoman FarookÎncă nu există evaluări
- Genetic Divergence in Stem Amaranth (Amaranthus Tricolor L.) Genotypes For Yield and Its Component CharactersDocument7 paginiGenetic Divergence in Stem Amaranth (Amaranthus Tricolor L.) Genotypes For Yield and Its Component CharactersNoman FarookÎncă nu există evaluări
- Effect of Sowing Date On Germination and Vigour of Soybean (Glycine Max (L.) Merr) SeedsDocument9 paginiEffect of Sowing Date On Germination and Vigour of Soybean (Glycine Max (L.) Merr) SeedsNoman FarookÎncă nu există evaluări
- Effect of Nitrogen On The Growth and Yield of Carrot (Daucus Carota L.)Document6 paginiEffect of Nitrogen On The Growth and Yield of Carrot (Daucus Carota L.)Noman FarookÎncă nu există evaluări
- Pathogenesis of Infectious Coryza in Chickens (Gallus Gallus) by AvibacteriumDocument8 paginiPathogenesis of Infectious Coryza in Chickens (Gallus Gallus) by AvibacteriumNoman FarookÎncă nu există evaluări
- Effect of Row Spacing and Cultivar On The Growth and Seed Yield of Soybean (Glycine Max (L.) Merrill) in Kharif-II SeasonDocument6 paginiEffect of Row Spacing and Cultivar On The Growth and Seed Yield of Soybean (Glycine Max (L.) Merrill) in Kharif-II SeasonNoman FarookÎncă nu există evaluări
- Internship Report on UPBVN's Seed Production ProcessDocument36 paginiInternship Report on UPBVN's Seed Production ProcessAmit Kumar0% (1)
- The Biology of SoybeanDocument11 paginiThe Biology of SoybeanRenato SergioÎncă nu există evaluări
- Yellow Shatavari Cultivation GuideDocument5 paginiYellow Shatavari Cultivation GuideClick-N-Grow Agroventures Pvt LtdÎncă nu există evaluări
- Growing Watermelon Commercially in Nigeria Training ManualDocument14 paginiGrowing Watermelon Commercially in Nigeria Training ManualGeorge IkpeÎncă nu există evaluări
- Cabbage ProductionDocument5 paginiCabbage Productionaa2951Încă nu există evaluări
- CBSE Class 9 Science Chapter 15 Food Resources Revision NotesDocument6 paginiCBSE Class 9 Science Chapter 15 Food Resources Revision NotesRavinderÎncă nu există evaluări
- Direct Drilling IS 2.3.2: Crop Establishment To Protect Soils Best Practice Information SheetDocument3 paginiDirect Drilling IS 2.3.2: Crop Establishment To Protect Soils Best Practice Information SheetZuhari lumambasÎncă nu există evaluări
- SST 201 After Mid-Sem NotesDocument120 paginiSST 201 After Mid-Sem NotesAnanda PreethiÎncă nu există evaluări
- Organic FarmingDocument60 paginiOrganic FarmingJP Taccad RomeroÎncă nu există evaluări
- Assessments of Herbicides Resistant Weeds in Metahara Sugar EstateDocument7 paginiAssessments of Herbicides Resistant Weeds in Metahara Sugar EstatePremier PublishersÎncă nu există evaluări
- Organic Farmers and Farms of GujaratDocument29 paginiOrganic Farmers and Farms of GujaratHD SapphireÎncă nu există evaluări
- Farm Consulting Proposal for Agro FarmDocument20 paginiFarm Consulting Proposal for Agro FarmMadan PoudelÎncă nu există evaluări
- Potential Weeds in AustraliaDocument202 paginiPotential Weeds in AustraliaAnne CalyxÎncă nu există evaluări
- KCSE Past Papers on Agriculture TopicsDocument98 paginiKCSE Past Papers on Agriculture TopicsMambo SawaÎncă nu există evaluări
- A Guide To Rotational GrazingDocument43 paginiA Guide To Rotational GrazingAmit RoyÎncă nu există evaluări
- Haricot BeanDocument42 paginiHaricot Beanbodana bokaÎncă nu există evaluări
- 09 Science Notes Ch15 Improvement in Food Resources-1Document7 pagini09 Science Notes Ch15 Improvement in Food Resources-1Nirmal SharmaÎncă nu există evaluări
- Integrated Pest Management Lab TechniquesDocument5 paginiIntegrated Pest Management Lab TechniquesTrisha MalaluanÎncă nu există evaluări
- CROP PRODUCTION AND MANAGEMENT TheoryDocument20 paginiCROP PRODUCTION AND MANAGEMENT TheoryCOE19B040 VIDYA SRI GUMMADIÎncă nu există evaluări
- Crop Production 160216131603Document22 paginiCrop Production 160216131603C VDÎncă nu există evaluări
- Agric JS 3 Revision T2Document11 paginiAgric JS 3 Revision T2Toluwani FilaniÎncă nu există evaluări
- 2015 NCO-National Classification of Occupations - Vol II-BDocument581 pagini2015 NCO-National Classification of Occupations - Vol II-BVaishnavi JayakumarÎncă nu există evaluări
- Edge EffectsDocument2 paginiEdge EffectsVeteran Tree Group AustraliaÎncă nu există evaluări
- Weed ID and Management WorksheetDocument4 paginiWeed ID and Management WorksheetMichelleAdanteMorong100% (1)
- Project Report: Establishment and Determining The Yields of Beans in A 3M by 4M Piece of Land at Eldoret National PolytechnicDocument12 paginiProject Report: Establishment and Determining The Yields of Beans in A 3M by 4M Piece of Land at Eldoret National PolytechnicvinalÎncă nu există evaluări
- Sinochem ReportDocument46 paginiSinochem Reportabhishek singh100% (1)