Documente Academic
Documente Profesional
Documente Cultură
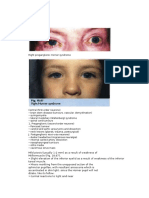
Horner's Syndrome
Încărcat de
Nastiti WidyariniDescriere originală:
Drepturi de autor
Formate disponibile
Partajați acest document
Partajați sau inserați document
Vi se pare util acest document?
Este necorespunzător acest conținut?
Raportați acest documentDrepturi de autor:
Formate disponibile
Horner's Syndrome
Încărcat de
Nastiti WidyariniDrepturi de autor:
Formate disponibile
Clinical Radiology (2008) 63, 499e505
REVIEW
Imaging of Horners syndrome
A. George, A.A. Haydar, W.M. Adams*
Derriford Hospital Plymouth, Derriford, Plymouth, UK
Received 25 September 2007; received in revised form 10 December 2007; accepted 14 December 2007
Horners syndrome, or oculosympathetic paresis, results from interruption of the sympathetic trunk innervation to the eye and presents typically with meiosis, ptosis and facial anhydrosis on the affected side.1 The pathological process ranges from benign, such as cluster headache, or life threatening, such as lung malignancy. Appropriate imaging requires an anatomical appreciation of the complex and circuitous route the neuronal pathway takes as it passes from the central nervous system to the eye. 2007 The Royal College of Radiologists. Published by Elsevier Ltd. All rights reserved.
Introduction
The rst description of the syndrome of oculosympathetic paresis occurred as early as 1727 by Francois Pourfour du Petit after experimental transection of the intercostal nerves of dogs. In 1852 Claude Bernard, vivisector and son of a French winegrower, gave a more complete description of the syndrome, which the French recognize as BernardeHorner syndrome. Johann Friedrich Horner, a Swiss ophthalmologist practising in Zurich and one of the rst to associate diseases of the eye with other disorders of the body, described the classical features of the syndrome in 1869.2 Horners syndrome results from interruption of the sympathetic innervation to the eye and presents typically with meiosis (a constricted pupil), blepharoptosis (a drooping eyelid), enophthalmos (a sunken globe), a narrow palpebral ssure and facial anhydrosis on the affected side.1 A number of pathologies can affect the oculosympathetic pathway ranging from the benign, such as cluster headache, or life threatening, such as an apical Pancoasts tumour. Appropriate imaging requires both close liaison with the physicians and an accurate
* Guarantor and correspondent: W.M. Adams, Derriford Hospital Plymouth, Derriford, Plymouth PL6 8DH, UK. Tel.: 44 1752 517917; fax: 44 1752 763277. E-mail address: william.adams@phnt.swest.nhs.uk (W.M. Adams).
anatomical appreciation of the complex and circuitous route the neuronal pathway takes as it passes from the central nervous system to the eye.
Anatomy
The three-neuron oculosympathetic pathway begins in the hypothalamus and ends in the eye (Fig. 1).
Central
First-order neurons descend caudally from the dorsolateral hypothalamus into the midbrain, passing lateral to the nucleus of the fourth nerve. The bres traverse anteriorly at the level of the pontomedullary junction from the region of the locus ceruleus in the oor of the fourth ventricle to lie anterior to the inferior olivary nucleus.1 The ipsilateral spinothalamic tract and vestibular structures are in close proximity at this point. On reaching the cervical spinal cord, the rst-order bres pass lateral to the dorsal grey matter to the rst synapse located at levels C8-T2 (also called the ciliospinal centre of Budge).
Preganglionic
Second-order neurons exit the spinal cord at the level of the C8 to T2 ventral roots. The bres
0009-9260/$ - see front matter 2007 The Royal College of Radiologists. Published by Elsevier Ltd. All rights reserved. doi:10.1016/j.crad.2007.12.006
500
A. George et al.
Sympathetic innervation of pupillary muscles
Mllers muscle Sudomotor and vasoconstrictor fibres to forehead Hypothalamus
Pons Pupil dilator Long ciliary nerve Ophthalmic artery
V
Medulla
Nasociliary nerve Carotid plexus Postganglionic neuron
Sudomotor and vasoconstrictor fibres to face Superior cervical ganglion Internal carotid artery External carotid artery Preganglionic neuron
Central neuron
Inferior cervical ganglion Subclavian artery
Lung
Ciliospinal centre of Budge
internal carotid nerve. The postganglionic branches inuence vasoconstriction and sweating in the face and neck; salivary gland secretion; pupillary dilatation; and the smooth muscles in the upper and lower eyelids. On entering the bony carotid canal, the internal carotid nerve divides into medial and lateral branches forming the carotid plexus. The medial branch lies medial to the internal carotid artery within the cavernous sinus. It supplies branches to the oculomotor, trochlear, ophthalmic, and abducens nerves, and also the ciliary ganglion. The lateral branch communicates with the trigeminal ganglion and the abducens nerve.3 Fibres continue without synapsing through the superior orbital ssure and ciliary ganglion into the orbit to supply the blood vessels of the eyeball. Within the orbit: (1) Sympathetic branches of the nasociliary nerve supply the dilator pupillae; (2) Fibres from the oculomotor nerve supply small smooth muscles in both the upper and lower eyelids responsible for a minor portion of upper lid elevation (Mu llers muscle) and retraction; and (3) Sympathetic bres innervate the lacrimal gland. Sudomotor bres responsible for vasoconstriction and sweating in the forehead follow the internal carotid plexus, those for the remainder of the face follow the external carotid plexus.
Figure 1 Anatomical pathway of the sympathetic supply to the pupil and eyelids. Courtesy: Medical Illustrations, Derriford Hospital, Plymouth, UK.
Clinical features
Disruption of the oculosympathetic pathway anywhere along its course results in mild drooping of the ipsilateral upper eyelid (ptosis), a contracted pupil (meiosis), a sunken globe (enophthalmos), a narrow palpebral ssure with or without loss of ipsilateral sweating (facial anhydrosis). The ptosis occurs as a result of denervation of Mullers muscle, which acts as an accessory elevator of the upper eyelid. Because it is responsible for about 2 mm of elevation, the resultant ptosis is subtle.4 Sympathetic denervation to the corresponding muscle of the inferior eyelid produces lower lid elevation. Pupillary meiosis is a consequence of loss of the balance between the sympathetic system controlling the iris dilator muscle and the parasympathetic system supplying the iris constrictor muscle. Anisocoria (the difference in size between the affected and non-affected pupil) is accentuated in dim light. If the oculosympathetic pathway is interrupted below the superior cervical ganglion (central or preganglionic), reduced ipsilateral sweating occurs. This is because bres supplying the sweat glands follow the course of the external carotid artery. If the disruption is
traverse the inferior (often fused with the rst thoracic ganglion to form the cervico-thoracic or stellate ganglion) and middle cervical ganglia to synapse in the superior cervical ganglion. These ganglia are in effect the continuation of the sympathetic trunk. The inferior cervical ganglion lies between the base of the transverse process of the seventh cervical vertebra and the neck of the rst rib. Anterior to it lie the vertebral artery and veins and inferiorly the lung apex. The middle cervical ganglion is connected to the inferior cervical ganglion by two or more cords, which can have a varied course. The posterior cord is often fenestrated to enclose the vertebral artery. The anterior cord (ansa subclavia) passes over the cervical pleura and then inferior to the rst part of the subclavian artery. The bres synapse in the superior cervical ganglion, located near the angle of the mandible and the bifurcation of the common carotid artery.
Postganglionic
Third-order neurons leave the superior cervical ganglion to form a sympathetic plexus, the
Imaging of Horners syndrome
501
postganglionic, then only the medial part of the upper forehead may be affected, this area being supplied by some third order bres. In practice, this distinction between preganglionic and postganglionic supply is not a reliable clinical feature.
of cases of Horners syndrome had an unknown diagnosis, presumed related to vascular disease. In the remaining 270 patients, 13% were related to a rst-order (central) lesion, 44% to a secondorder (preganglionic) lesion, and 43% to a thirdorder (postganglionic) lesion. In children causes of Horners syndrome are mainly related to either congenital or acquired/postsurgical lesions.
Causes and investigation of Horners syndrome
Testing the pupils with 4% cocaine eye drops can conrm a diagnosis of Horners syndrome. One percent hydroxyamphetamine can be used to distinguish central and preganglionic from postganglionic lesions4; however, in clinical practice pharmacological testing is rarely applied. Therefore, distinguishing between central, pre and postganglionic disease is reliant on recognizing the clinical clues available. For example, a patient presenting with a lesion in the cavernous sinus will often have accompanying decits affecting to varying degree the 3rd, 4th, 5th and 6th cranial nerves. In a patient with known lung malignancy presenting with shoulder and arm pain, Horners syndrome may be an additional feature of a Pancoast tumour. Most patients who present with Horners syndrome in isolation without additional clinical features will have a postganglionic lesion5 or may be idiopathic, i.e., no imaging abnormality can be demonstrated. In one large case series6 40%
Central (Table 1)
Because of the proximity of other structures within the hypothalamus, brainstem or spinal cord central Horners syndrome in isolation is unlikely.7 The most frequently identied central causes are infarction of the posterior inferior cerebellar artery (PICA) or distal vertebral artery occlusion producing lateral medullary syndrome (Fig. 2). These patients may also have vertigo, swallowing difculties, unilateral facial numbness, and loss of pain and temperature sensation in the opposite limbs.8 Crossed sensory or motor signs are highly suggestive of a brainstem lesion. Pathology within the midbrain may produce an accompanying, contralateral fourth nerve palsy.9,10 Tumour, trauma, stroke, and other vascular diseases, such as arteriovenous malformation (AVM), which can involve both the brain and spinal cord, have all been implicated. Rarer causes include acute disseminated encephalomyelitis11 and syrinx of the spinal cord.12
Table 1
Causes of Horners syndrome in adults Preganglionic (second order) Postganglionic (third order)
Central (rst order)
Hypothalamus Tumour Stroke Brainstem Demyelination Stroke Tumour Cervicothoracic spinal cord Myelitis Syringomyelia Arteriovenous malformation Demyelination Infarction Tumour Trauma
Pulmonary apical lesions Subclavian artery aneurysm Apical lung tumour (Pancoast tumour) Mediastinal tumours Cervical rib Iatrogenic Thyroid malignancy
Superior cervical ganglion Trauma Jugular venous ectasia Iatrogenic (surgical neck dissection) Internal carotid artery Dissection Aneurysm Trauma Arteritis Thrombosis Tumour Skull-base lesions (nasopharyngeal carcinoma, lymphoma) Cavernous sinus lesion Tumours Invasive pituitary tumour Inammation Thrombosis Carotid aneurysm
502
A. George et al.
artefact seen on computed tomography (CT). Imaging in at least two orthogonal planes is recommended. T1-weighted sequences with and without gadolinium enhancement may allow the detection of tumour or metastasis. Unenhanced CT, which is prone to beam hardening artefact in the posterior fossa, and conventional spin-echo (SE) T2-weighted or uid-attenuated inversion recovery sequences (FLAIR) sequences can fail to detect infarction occurring within the rst 12e24 h. Diffusion-weighted imaging (DWI) has more application for the early assessment of acute stroke.13 However, in the cervical cord the use of DWI to detect ischaemic lesions is limited because of the small size of the cord, cerebrospinal uid (CSF) ow and susceptibility artefact from adjacent structures.14 SE and fast SE (FSE) sequences suffer less from susceptibility artefact than gradient-echo techniques and are favoured for assessing anatomical detail within the cord.
Figure 2 Right posterior inferior cerebellar infarct. A 47-year-old man presented acutely with slurred speech, nystagmus, right-sided weakness, and an ipsilateral Horners syndrome. Axial T2-weighted image showing localized high signal change within the right hemi-medulla respecting the mid-line in keeping with recent infarction. A CT image taken 2 days prior to the MRI examination was normal.
Preganglionic (Table 1)
Preganglionic Horners syndrome is frequently caused by tumour or trauma.15 Nerve root avulsion disrupting the sympathetic pathway may produce symptoms referred to the brachial plexus distribution. In the newborn, iatrogenic causes such as forceps delivery can be responsible. Of tumours at the lung apex, Pancoast tumour and neurogenic tumours (Fig. 3)16 are most frequently associated. These are more likely to be malignant than benign. Other cited causes include paragangliomas of the sympathetic chain17;
Magnetic resonance imaging (MRI), which provides good soft-tissue contrast and spatial resolution, is the most appropriate method of imaging the central neuroaxis to include the hypothalamus, pons, and medulla and avoid the beam-hardening
Figure 3 Apical neuroma. A 37-year-old woman presented with gradual onset right-sided neck pain and ipsilateral Horners syndrome. (a) Chest radiograph shows a smoothly demarcated mass lesion at the medial aspect of the apex of the right upper lobe. (b) Multiplanar parasagittal oblique reformat contrast-enhanced CT image of the neck demonstrates both vascular detail and the relationship of the mass to the vertebral bodies at the level of the upper thoracic spine. (c) Parasagittal oblique T2-weighted MRI image demonstrates the 4 cm apical mass. It is of mixed signal and exhibits some intra-lesional necrosis. There was no communication with the exit foramina or spinal canal.
Imaging of Horners syndrome
503
hydatid cyst18; intercostal drain insertion19; regional anaesthetic blocks,20 sympathectomy,21 subclavian artery aneurysm,22 thyroid malignancy,23 and even disc prolapse.24 Digre et al.5 provide a suggested protocol for the MRI investigation of preganglionic Horners syndrome. Coverage of the entire cervical spine in three orthogonal planes is recommended, to include the upper thoracic spine and thoracic inlet. The sagittal images can be offset to the side of interest. Coronal sequences with and without fat suppression can also provide detail of any coexistent brachial plexus injury. If an apical lung lesion or a tumour within the upper mediastinum or anterior neck is considered likely, axial contrast-enhanced CT may be required.
Postganglionic (Table 1)
Postganglionic Horners syndrome can be caused by conditions ranging from the relatively trivial to life threatening. Pain is often an accompanying feature. The anatomical distribution extends from the internal carotid artery to the skull base, the cavernous sinus and the orbital apex. By far the most common cause is spontaneous or traumatic carotid artery dissection (Fig. 4), often accompanied by carotydynia (pain of face and neck). Thrombosis within the vessel may induce contralateral hemiplegia. Patients with connective tissue disorders, such as bromuscular dysplasia or Ehlerse Danlos syndrome, may be more susceptible. Both tumour and trauma can disrupt third-order neurons within the skull base and cavernous sinus (Fig. 5). The presence of 3rd, 4th, 5th or 6th cranial nerve palsies points to a lesion within the cavernous sinus or superior orbital ssure.25 An orbital apex lesion might also be expected to produce visual loss in addition. Cluster headaches are thought to be due to injury of the sympathetic bres within the bony carotid canal. In affected patients the history is often typical; severe unilateral, short-lived headaches localized to the orbital, temporal and mid-face areas in which imaging is not helpful.5 Generally, imaging has a greater positive yield in postganglionic disease if symptoms are acute rather than chronic. Formal catheter digital subtraction angiography is a valuable technique for the detection of internal carotid artery dissection, but is being superseded by both MR and CT angiography.26 An axial MRI of the neck with T1-weighted, fatsuppressed sequences and magnetic resonance angiography (MRA) will detect most internal carotid artery dissections.27 On a T1-weighted sequence thrombosis within the false lumen of a carotid
Figure 4 Internal carotid artery dissection. A 50-yearold woman presented with carotydynia and right-sided Horners syndrome. (a) Axial T2-weighted MRI image at the level of the skull base shows high signal change replacing the normal ow void within the right extracranial internal carotid artery (white arrow). (b) Axial T1-weighted enhanced MRI image demonstrates the dissection ap and high signal within the narrowed true lumen (white arrow). Courtesy: Royal Cornwall Hospitals NHS Trust, Imaging Department.
504
A. George et al.
dissection may appear hyperintense due to the presence of methaemoglobin. Time-of-ight (TOF) MRA, which is a T1-weighted technique, may register this as normal ow unless supplemented with other sequences. Skull base fractures are readily identied using high-resolution, multidetector CT using a bone algorithm allowing reconstruction in multiple planes. Imaging of the parasellar region and skull base can be performed with both MRI and CT, although CT can be compromised by beam hardening artefact and the presence of dental amalgam.28 To view the cavernous sinus the coronal and axial planes are most useful. Unenhanced and enhanced T1-weighted sequences are often necessary for the identication of subtle lesions.4,25 The appropriate investigation of Horners syndrome is as heavily reliant on history and examination as imaging and requires close liaison between the physician and radiologist. Accompanying physical signs may allow precise localization but occasionally imaging of the entire oculosympathetic pathway may be required.
References
1. Standing S. Grays anatomy. 39th ed. Edinburgh: Churchill Livingstone; 2005. 2. <http://www.whonamedit.com/doctor.cfm/1044.html>, <http://www.whonamedit.com/synd.cfm/1056.html> [accessed 25.9.2007] 3. Williams PL, Warwick R. Grays anatomy. 36th ed. Edinburgh: Churchill Livingstone; 1980. 4. Walton KA, Buono LM. Horner syndrome. Curr Opin Ophthalmol 2003;14:357e63. 5. Digre KB, Smoker WR, Johnston P, et al. Selective MR imaging approach for patients with Horners syndrome. AJNR Am J Neuroradiol 1992;13:223e7. 6. Maloney WF, Younge BR, Moyer NJ. Evaluation of the causes and accuracy of pharmacologic localization in Horners syndrome. Am J Ophthalmol 1980;90:394. 7. Sacco RL, Freddo L, Bello JA, et al. Wallenbergs lateral medullary syndrome e clinicalemagnetic resonance imaging correlations. Arch Neurol 1993;50:609e14. 8. Pearce JM. Wallenbergs syndrome. J Neurol Neurosurg Psychiatry 2000;68:570. 9. Nagy AN, Hayman LA, Diaz-Marchan PJ, et al. Horners syndrome due to rst-order neuron lesions of the oculosympathetic pathway. AJR Am J Roentgenol 1997;169: 581e4. 10. Guy J, Day AL, Mickle JP, et al. Contralateral trochlear nerve paresis and ipsilateral Horners syndrome. Am J Ophthalmol 1989;107:73e6. 11. Muramoto T, Kira J, Yoshimura T, et al. Acute disseminated encephalomyelitis affecting medulla and upper cervical cord. Rinsho Shinkeigaku 1989;29:1013e6. 12. Pomeranz H. Isolated Horner syndrome and syrinx of the spinal cord. Am J Ophthalmol 2002;133:702e4. 13. Yoshiura T, Wu O, Sorensen G. Advanced MR techniques. Neuroimaging Clin N Am 1999;9:439e53.
Figure 5 Cavernous sinus metastasis. A 55-year-old man with a known history of nasopharyngeal carcinoma developed Horners syndrome on the right side associated with ophthalmoplegia. (a) Axial T1-weighted MRI image of the head through the level of skull base shows a soft-tissue mass isointense to brain lling the right cavernous sinus and extending along the oor of the middle cranial fossa. The intracavernous internal carotid artery is partly compressed. (b) Axial T1-weighted MRI image after intravenous gadolinium demonstrates avid enhancement of the cavernous sinus mass.
Imaging of Horners syndrome
505
14. Vertinsky AT, Krasnokutsky MV, Augustin M, et al. Spinal imaging: overview and update. Neuroimaging Clin N Am 2007;17:117e36. 15. Lee JH, Lee HK, Lee DH, et al. Neuroimaging strategies for three types of Horner syndrome with emphasis on anatomic location. AJR Am J Roentgenol 2007;188:W74e81. 16. Miura J, Doita M, Miyata K, et al. Horners syndrome caused by a thoracic dumbbell-shaped schwannoma, sympathetic chain reconstruction after a one-stage removal of the tumor. Spine 2003;28(2):E33e6. 17. Moyer JS, Bradford CR. Sympathetic paraganglioma as an unusual cause of Horners syndrome. Head Neck 2001;23: 338e42. 18. Sirmali M, Gezer S, Aydin E, et al. Giant primary mediastinal hydatid cyst causing Horners syndrome: report of a case. Acta Chir Belg 2005;105:221e3. 19. Campbell P, Neil T, Wake PN. Horners syndrome caused by an intercostal chest drain. Thorax 1989;44:305e6. 20. Segura P, Speeg-Schatz C, Wagner JM. Claude Bernarde Horner syndrome and its opposite Pourfour du petit syndrome in anaesthesia and intensive care. Ann Fr Anesth Reanim 1998;17:707e24.
21. Magnaes B. Extensive or partial microsurgical sympathectomy of the arm by supraclavicular route for primary or secondary Raynaud symptoms. Acta Chir Scand 1987;153:353e9. 22. Levent E, Posacioglu H. A rare cause of Horners syndrome: subclavian artery aneurysm. Respiration 2001;68:620. 23. Harding JL, Sywak MS, Sidhu S, et al. Horner syndrome in association with thyroid and parathyroid disease. ANZ J Surg 2004;74:442e5. 24. Simper LB, Jorgensen AK. Intervertebral disc prolapse as an unusual cause of Horners Syndrome. Ugeskr Laeger 1989; 151:949e50. 25. Bone I, Hadley DM. Syndromes of the orbital ssure, cavernous sinus, cerebello-pontine angle, and skull base. J Neurol Neurosurg Psychiatry 2005;76(Suppl. 3):iii29eiii38. 26. Oelerich M, Sto gbauer F, Kurlemann G, et al. Craniocervical artery dissection: MR imaging and MR angiographic ndings. Eur Radiol 1999;9:1385e91. 27. Gelal FM, Kitis O, Calli C, et al. Craniocervical artery dissection: diagnosis and follow-up with MR imaging and MR angiography. Med Sci Monit 2004;10:MT109. 28. Simonetta AB. Imaging of suprasellar and parasellar tumours. Neuroimaging Clin N Am 1999;9:717e32.
S-ar putea să vă placă și
- AnisokoriaDocument5 paginiAnisokoriaNurunnisa IsnyÎncă nu există evaluări
- SX HornerDocument5 paginiSX HornermariferÎncă nu există evaluări
- Slide MusculoDocument19 paginiSlide MusculoStefan CandraÎncă nu există evaluări
- 3RD CN - SeminarDocument49 pagini3RD CN - Seminarabhishek tÎncă nu există evaluări
- Bahan CPADocument13 paginiBahan CPAfadhilsyafeiÎncă nu există evaluări
- Ruptured Cerebral Aneurysms: PerspectiveDocument4 paginiRuptured Cerebral Aneurysms: PerspectiveDini NanamiÎncă nu există evaluări
- HornerDocument7 paginiHornerpriyaÎncă nu există evaluări
- 2 OverviewIII Iaat12i5p621Document4 pagini2 OverviewIII Iaat12i5p621FadhilAfifÎncă nu există evaluări
- Horner Syndrome - StatPearls - NCBI BookshelfDocument9 paginiHorner Syndrome - StatPearls - NCBI BookshelfShweh Fern LooÎncă nu există evaluări
- Facial PalsyDocument12 paginiFacial PalsyIqbal HabibieÎncă nu există evaluări
- Atlas of Ocular Anatomy PDFDocument112 paginiAtlas of Ocular Anatomy PDFAnonymous 3RS7obGÎncă nu există evaluări
- Ptosis (Emedicine)Document18 paginiPtosis (Emedicine)lochnezÎncă nu există evaluări
- Холестериновая гранулемаDocument13 paginiХолестериновая гранулемаНатальяÎncă nu există evaluări
- CP TumorsDocument21 paginiCP TumorsDr. T. Balasubramanian100% (4)
- AnisocoriaDocument11 paginiAnisocoriaChaturangaNSenerathÎncă nu există evaluări
- Sindrom HornerDocument15 paginiSindrom HornervivinÎncă nu există evaluări
- Draw It To Know It NotesDocument13 paginiDraw It To Know It Noteskat9210Încă nu există evaluări
- Ref 02Document6 paginiRef 02Mohamad FachryÎncă nu există evaluări
- Seram2012 S-1544Document101 paginiSeram2012 S-1544Cindy AlvarezÎncă nu există evaluări
- Cavernous Sinus SyndromeDocument8 paginiCavernous Sinus SyndromeMuresan Ioana Catalina100% (1)
- Abducens Nerve Palsy - AAODocument6 paginiAbducens Nerve Palsy - AAOnurcahayaantikaÎncă nu există evaluări
- Clinical Approach To Brainstem LesionsDocument10 paginiClinical Approach To Brainstem LesionsJosé SánchezÎncă nu există evaluări
- Gurwood 1990604Document2 paginiGurwood 1990604Warisa NuhurridhaÎncă nu există evaluări
- Cavernous Sinus AnatomyDocument8 paginiCavernous Sinus AnatomyPrashant TiwariÎncă nu există evaluări
- Horner SYndromeDocument3 paginiHorner SYndromeHendri Wijaya WangÎncă nu există evaluări
- Neuroanatomy Through Clinical Cases, 2E PDFDocument3 paginiNeuroanatomy Through Clinical Cases, 2E PDFjwongggg55140% (6)
- Brainstem II: Eye Movements and Pupillary ControlDocument34 paginiBrainstem II: Eye Movements and Pupillary ControlhemendreÎncă nu există evaluări
- Right Preganglionic Horner Syndrome Dfagmn Akjdg Amg 'Lakg'lakg'lakg'alkg'akg'ag'akg Akg A Kgakg'agkaDocument5 paginiRight Preganglionic Horner Syndrome Dfagmn Akjdg Amg 'Lakg'lakg'lakg'alkg'akg'ag'akg Akg A Kgakg'agkaHendri Wijaya WangÎncă nu există evaluări
- Brainstem and Cerebellum Lesions in Adults: Pearls To The Diagnosis With MRIDocument48 paginiBrainstem and Cerebellum Lesions in Adults: Pearls To The Diagnosis With MRIradiologirsckÎncă nu există evaluări
- Jugular Foramen Syndrome - StatPearls - NCBI BookshelfDocument9 paginiJugular Foramen Syndrome - StatPearls - NCBI BookshelfJose ColinaÎncă nu există evaluări
- Anatomy Pupillary Pathways AND Abnormal Pupils: Dr. Ravula Hasika M.S.Ophthalmology (1 YR)Document100 paginiAnatomy Pupillary Pathways AND Abnormal Pupils: Dr. Ravula Hasika M.S.Ophthalmology (1 YR)Sonia Afika Aziza100% (1)
- Tumors of The Pituitary Gland: John W. Gittinger, JRDocument4 paginiTumors of The Pituitary Gland: John W. Gittinger, JRBilly UntuÎncă nu există evaluări
- Acute Dizziness, Vertigo, and Unsteadiness 2021 NCDocument17 paginiAcute Dizziness, Vertigo, and Unsteadiness 2021 NCLaura A M MÎncă nu există evaluări
- Neurology Multiple Choice Questions With Explanations: Volume IDe la EverandNeurology Multiple Choice Questions With Explanations: Volume IEvaluare: 4 din 5 stele4/5 (7)
- Neuro-Ophthalmology: DR Jusuf Wijaya, SPM FK - Uki CawangDocument65 paginiNeuro-Ophthalmology: DR Jusuf Wijaya, SPM FK - Uki CawanggeorgyÎncă nu există evaluări
- Bell's Palsy: Pathogenesis, Clinical Features, and Diagnosis in AdultsDocument16 paginiBell's Palsy: Pathogenesis, Clinical Features, and Diagnosis in AdultsAmada Angel VillanuevaÎncă nu există evaluări
- PRADEEP'S - Brain TumorDocument32 paginiPRADEEP'S - Brain TumorPRADEEPÎncă nu există evaluări
- The FACIAL NERVE Current Trends in Diagnosis, Treatment, and RehabilitationDocument17 paginiThe FACIAL NERVE Current Trends in Diagnosis, Treatment, and RehabilitationkrazeedoctorÎncă nu există evaluări
- Bells PalacyDocument6 paginiBells PalacyBhushan VichareÎncă nu există evaluări
- Subdural Hematoma Case PresentationDocument20 paginiSubdural Hematoma Case PresentationDarwin Arockia RajÎncă nu există evaluări
- Horner, S Syndrome &Document39 paginiHorner, S Syndrome &Shahzada KhanÎncă nu există evaluări
- GlandDocument52 paginiGlandAya EyadÎncă nu există evaluări
- Suprachoroidal Space InterventionsDe la EverandSuprachoroidal Space InterventionsShohista SaidkasimovaÎncă nu există evaluări
- TRANSCRANIALDocument52 paginiTRANSCRANIALayman shomanÎncă nu există evaluări
- The Myth of Autism: How a Misunderstood Epidemic Is Destroying Our ChildrenDe la EverandThe Myth of Autism: How a Misunderstood Epidemic Is Destroying Our ChildrenEvaluare: 2.5 din 5 stele2.5/5 (8)
- Neuro SBA3Document3 paginiNeuro SBA3Mehdi Hasan MazumderÎncă nu există evaluări
- Neurology Multiple Choice Questions With Explanations: Volume IIDe la EverandNeurology Multiple Choice Questions With Explanations: Volume IIEvaluare: 5 din 5 stele5/5 (2)
- From The Bone By3Document20 paginiFrom The Bone By3Igo RiskoÎncă nu există evaluări
- Birthinjuries Dr.M.maniDocument65 paginiBirthinjuries Dr.M.manimaniÎncă nu există evaluări
- CNS PBL 2Document6 paginiCNS PBL 2Hugh JacobsÎncă nu există evaluări
- CNS Congenital AnomaliesDocument74 paginiCNS Congenital AnomaliesMoh DrhusseinyÎncă nu există evaluări
- Cavernous Sinus ThrombosisDocument6 paginiCavernous Sinus ThrombosisSulabh ShresthaÎncă nu există evaluări
- Articulo PDFDocument6 paginiArticulo PDFLuisa Vanessa CueroÎncă nu există evaluări
- Pleural EffusionDocument10 paginiPleural EffusionShane PangilinanÎncă nu există evaluări
- Facial Nerve Disroders and OtalgiaDocument39 paginiFacial Nerve Disroders and Otalgiaياسر كوثر هانيÎncă nu există evaluări
- Exophthalmos Is Defined in DorlandDocument15 paginiExophthalmos Is Defined in DorlandAmin Kamaril Wahyudi ArrdianÎncă nu există evaluări
- 28-Cranial Nerves and MusclesDocument26 pagini28-Cranial Nerves and Musclesrodrigocorcino899959Încă nu există evaluări
- Practice EssentialsDocument10 paginiPractice EssentialsAnonymous gMLTpER9IUÎncă nu există evaluări
- Frey Syndrome Complicating Parotidectomy: A Case Report and Review of LiteratureDocument2 paginiFrey Syndrome Complicating Parotidectomy: A Case Report and Review of LiteratureAngga Witra NandaÎncă nu există evaluări
- Gangguan Saraf KranialDocument30 paginiGangguan Saraf KranialWisnu Surya WardhanaÎncă nu există evaluări
- Girls and Puberty BookletDocument9 paginiGirls and Puberty Bookletఫణీంద్ర నర్సెట్టి100% (1)
- Enfermedades EmergentesDocument216 paginiEnfermedades EmergentesCaaarolÎncă nu există evaluări
- Spoof TextDocument9 paginiSpoof TextHeather LeeÎncă nu există evaluări
- Keyword Ideas On Hair Loss For SEODocument18 paginiKeyword Ideas On Hair Loss For SEOChitrakarudu KalabhimaniÎncă nu există evaluări
- Health Assessment Form For LearnersDocument2 paginiHealth Assessment Form For LearnersIvy Jessa CandilanzaÎncă nu există evaluări
- Chapter 19 Microbial Diseases of The Skin and WoundsDocument8 paginiChapter 19 Microbial Diseases of The Skin and WoundsGRACE MAR CABAHUG100% (1)
- Campbell Chapter 18: The Genetic of Viruses and BacteriaDocument69 paginiCampbell Chapter 18: The Genetic of Viruses and BacteriaRafika Nur Handayani100% (1)
- Rabies SeminarDocument73 paginiRabies SeminarQazi Muhammad IqbalÎncă nu există evaluări
- Congenital Heart Disease NursingDocument21 paginiCongenital Heart Disease NursingAshiqAhleBaytÎncă nu există evaluări
- Reflexology On FootDocument16 paginiReflexology On FootManish Anand100% (1)
- Anatomy and Physiology PDFDocument51 paginiAnatomy and Physiology PDFalexenneth canilaÎncă nu există evaluări
- Case Study DengueDocument11 paginiCase Study DengueCarl Julienne MasangcayÎncă nu există evaluări
- Biomentors (MCDB 1B) - Immunology Quiz (ANSWERS)Document2 paginiBiomentors (MCDB 1B) - Immunology Quiz (ANSWERS)Tiff VoÎncă nu există evaluări
- Ear InfectionDocument7 paginiEar Infectionbhatti19Încă nu există evaluări
- Biology PPT 11Document11 paginiBiology PPT 11agarwalabhinav050Încă nu există evaluări
- Resolving Negative Life ExperiencesDocument19 paginiResolving Negative Life ExperiencessoriboÎncă nu există evaluări
- Bites and Stings - Dermatology - MKSAP 17Document4 paginiBites and Stings - Dermatology - MKSAP 17Ciara Marjorie HannaÎncă nu există evaluări
- 2804 Central Concepts January 2006 Mark SchemeDocument0 pagini2804 Central Concepts January 2006 Mark SchemeWK SoohgÎncă nu există evaluări
- Equine Dental BrochureDocument2 paginiEquine Dental BrochurevetthamilÎncă nu există evaluări
- Yr 8 Complete Answers PDFDocument111 paginiYr 8 Complete Answers PDFstudent_4_eva61% (98)
- The Natural History of Human Poliomyelitis IDocument23 paginiThe Natural History of Human Poliomyelitis IFitria Ayu LestariÎncă nu există evaluări
- First Aid - Ready ReckonerDocument2 paginiFirst Aid - Ready ReckonerDr VJ GeorgeÎncă nu există evaluări
- Head To Toe Assessment 1Document2 paginiHead To Toe Assessment 1Monica Ugarte Treta100% (4)
- Syndrome of Inappropriate Vasopressin Sexretion (Siadh)Document22 paginiSyndrome of Inappropriate Vasopressin Sexretion (Siadh)Moni RethÎncă nu există evaluări
- PBL Report Traumatology: Group 6Document42 paginiPBL Report Traumatology: Group 6anthy putrisriyantiÎncă nu există evaluări
- Development of VeinsDocument37 paginiDevelopment of Veinsokolodivine334Încă nu există evaluări
- Skema Pemarkahan: Tingkatan 5 Bahasa Inggeris 1119 Kertas 1 Dan 2Document12 paginiSkema Pemarkahan: Tingkatan 5 Bahasa Inggeris 1119 Kertas 1 Dan 2nurmalajamaludinÎncă nu există evaluări
- Artritis Gout in Aviation MedicineDocument22 paginiArtritis Gout in Aviation MedicineBuyungÎncă nu există evaluări
- Spinal Cord and Spinal NervesDocument46 paginiSpinal Cord and Spinal Nerveskombat13_708353334Încă nu există evaluări