Documente Academic
Documente Profesional
Documente Cultură
Worksheet For Grade X - Copy 2008atp
Încărcat de
Khondokar TarakkyDescriere originală:
Titlu original
Drepturi de autor
Formate disponibile
Partajați acest document
Partajați sau inserați document
Vi se pare util acest document?
Este necorespunzător acest conținut?
Raportați acest documentDrepturi de autor:
Formate disponibile
Worksheet For Grade X - Copy 2008atp
Încărcat de
Khondokar TarakkyDrepturi de autor:
Formate disponibile
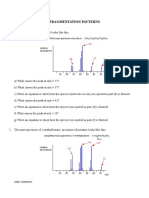
Name: Subject: Chemistry Grade:
Worksheet: Alternative to Practical
An experiment was done to find the concentration of an aqueous solution of ammonium chloride labelled P. Q was 0.0800 mol/dm3 hydrochloric acid. A 25.0 cm3 sample of P was measured into a flask followed by 25.0 cm3 of 2.00 mol/dm3 sodium hydroxide (an excess). Some of the sodium hydroxide reacted with the ammonium chloride to produce ammonia. The equation is given below. NaOH + NH4Cl NaCl + NH3 + H2O (a) What apparatus should be used to measure out 25.0 cm3 of a solution? ..................................................................................................................................... The flask was heated until no more ammonia was detected in the steam. (b) Suggest a test to detect ammonia in the steam leaving the flask. ..................................................................................................................................... After cooling, the mixture was transferred to a volumetric flask and made up to 250 cm3 with distilled water. This was solution R. 25.0 cm3 of R was transferred to a conical flask and a few drops of methyl orange indicator added. (c) What colour was the methyl orange in the flask? ............................................. A burette was filled with Q. Q was run into the conical flask until an end-point was reached. What was the colour of the methyl orange when the end-point was reached? .............................................
(d) Use the diagrams to complete the following table.
Summary: Tick () the best titration results. Using these results the average volume of Q was ..........................................cm3. (e) Q was 0.0800 mol/dm3 hydrochloric acid. Calculate the number of moles of hydrochloric acid in the average volume of Q calculated in (d). (f) Using the equation NaOH + HCl NaCl + H2O and your answer to (e), deduce the number of moles of sodium hydroxide in 25.0 cm3 of R. ............................................. (g) Using your answer to (f) calculate the number of moles of sodium hydroxide in 250 cm3 of R. ............................................. (h) Calculate the number of moles of sodium hydroxide present in 25.0 cm3 of 2.00 mol/dm3 aqueous sodium hydroxide which was added originally to 25.0 cm3 of P. ............................................. (i) Using your answers to (g) and (h), calculate the number of moles of sodium hydroxide that reacted with 25.0 cm3 of P. ............................................. (j) Using the equation NaOH + NH4Cl NH3 + NaCl + H2O and your answer to (i), (i) deduce the number of moles of ammonium chloride in 25.0 cm3 of P, ............................................. (ii) calculate the concentration, in mol/dm3, of P. .............................................
S-ar putea să vă placă și
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)De la EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Evaluare: 4.5 din 5 stele4.5/5 (119)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaDe la EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaEvaluare: 4.5 din 5 stele4.5/5 (265)
- The Little Book of Hygge: Danish Secrets to Happy LivingDe la EverandThe Little Book of Hygge: Danish Secrets to Happy LivingEvaluare: 3.5 din 5 stele3.5/5 (399)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryDe la EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryEvaluare: 3.5 din 5 stele3.5/5 (231)
- Grit: The Power of Passion and PerseveranceDe la EverandGrit: The Power of Passion and PerseveranceEvaluare: 4 din 5 stele4/5 (587)
- Never Split the Difference: Negotiating As If Your Life Depended On ItDe la EverandNever Split the Difference: Negotiating As If Your Life Depended On ItEvaluare: 4.5 din 5 stele4.5/5 (838)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeDe la EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeEvaluare: 4 din 5 stele4/5 (5794)
- Team of Rivals: The Political Genius of Abraham LincolnDe la EverandTeam of Rivals: The Political Genius of Abraham LincolnEvaluare: 4.5 din 5 stele4.5/5 (234)
- Shoe Dog: A Memoir by the Creator of NikeDe la EverandShoe Dog: A Memoir by the Creator of NikeEvaluare: 4.5 din 5 stele4.5/5 (537)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyDe la EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyEvaluare: 3.5 din 5 stele3.5/5 (2219)
- The Emperor of All Maladies: A Biography of CancerDe la EverandThe Emperor of All Maladies: A Biography of CancerEvaluare: 4.5 din 5 stele4.5/5 (271)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreDe la EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreEvaluare: 4 din 5 stele4/5 (1090)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersDe la EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersEvaluare: 4.5 din 5 stele4.5/5 (344)
- Her Body and Other Parties: StoriesDe la EverandHer Body and Other Parties: StoriesEvaluare: 4 din 5 stele4/5 (821)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceDe la EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceEvaluare: 4 din 5 stele4/5 (894)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureDe la EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureEvaluare: 4.5 din 5 stele4.5/5 (474)
- The Unwinding: An Inner History of the New AmericaDe la EverandThe Unwinding: An Inner History of the New AmericaEvaluare: 4 din 5 stele4/5 (45)
- The Yellow House: A Memoir (2019 National Book Award Winner)De la EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Evaluare: 4 din 5 stele4/5 (98)
- On Fire: The (Burning) Case for a Green New DealDe la EverandOn Fire: The (Burning) Case for a Green New DealEvaluare: 4 din 5 stele4/5 (73)
- U04 Notes Part1 Ionic CovalentDocument52 paginiU04 Notes Part1 Ionic CovalentKhondokar TarakkyÎncă nu există evaluări
- Right To HealthDocument9 paginiRight To HealthPriya SharmaÎncă nu există evaluări
- Mufon Ufo JournalDocument21 paginiMufon Ufo JournalSAB78Încă nu există evaluări
- 10059-DC-K-01-A Design BasisDocument34 pagini10059-DC-K-01-A Design BasisAnonymous RvIgDUÎncă nu există evaluări
- Scaffolding Control & MeasuresDocument3 paginiScaffolding Control & Measuresviswamanoj100% (1)
- Benefits at Cognizant Technology SolutionsDocument5 paginiBenefits at Cognizant Technology Solutions8130089011Încă nu există evaluări
- HTM 2025 2 (New) Ventilation in HospitalsDocument123 paginiHTM 2025 2 (New) Ventilation in HospitalsArvish RamseebaluckÎncă nu există evaluări
- UMR Introduction 2023Document110 paginiUMR Introduction 2023tu reves mon filsÎncă nu există evaluări
- Nicenstripy Gardening Risk AssessmentDocument38 paginiNicenstripy Gardening Risk AssessmentVirta Nisa100% (1)
- Writing Ionic FormulaeDocument6 paginiWriting Ionic FormulaeKhondokar TarakkyÎncă nu există evaluări
- Hybridization TarakkyDocument36 paginiHybridization TarakkyKhondokar TarakkyÎncă nu există evaluări
- U05 Notes Part4 Entropy SpontaneityDocument47 paginiU05 Notes Part4 Entropy SpontaneityKhondokar TarakkyÎncă nu există evaluări
- U05 Notes Part1 Heat CalorimDocument32 paginiU05 Notes Part1 Heat CalorimKhondokar TarakkyÎncă nu există evaluări
- U04 Notes Part3 Sp3d2 DelocalizationDocument54 paginiU04 Notes Part3 Sp3d2 DelocalizationKhondokar TarakkyÎncă nu există evaluări
- Air and WaterDocument12 paginiAir and WatermirnaÎncă nu există evaluări
- U04 Notes Part2 Shapes PolarityDocument49 paginiU04 Notes Part2 Shapes PolarityKhondokar Tarakky100% (1)
- U04 Notes Part5 Metals Physical PropertiesDocument43 paginiU04 Notes Part5 Metals Physical PropertiesKhondokar TarakkyÎncă nu există evaluări
- Intermolecular Forces: The Key to Understanding PropertiesDocument66 paginiIntermolecular Forces: The Key to Understanding PropertiesKhondokar TarakkyÎncă nu există evaluări
- U05 Notes Part2 Bond Enthalpy HessDocument17 paginiU05 Notes Part2 Bond Enthalpy HessKhondokar TarakkyÎncă nu există evaluări
- U05 Notes Part3 Energy CyclesDocument29 paginiU05 Notes Part3 Energy CyclesKhondokar TarakkyÎncă nu există evaluări
- All A2 Level Terms and DefinationsDocument0 paginiAll A2 Level Terms and DefinationsHussain MustafaÎncă nu există evaluări
- 9701 m17 QP 12Document16 pagini9701 m17 QP 12Khondokar TarakkyÎncă nu există evaluări
- Naming Worksheet #1 Salts GuideDocument9 paginiNaming Worksheet #1 Salts GuideKhondokar TarakkyÎncă nu există evaluări
- Chapter 1 Kinetic Theory and DiffusionDocument4 paginiChapter 1 Kinetic Theory and DiffusionKhondokar TarakkyÎncă nu există evaluări
- Naming Worksheet #1 Salts GuideDocument9 paginiNaming Worksheet #1 Salts GuideKhondokar TarakkyÎncă nu există evaluări
- Drying Agent and Dehydrating AgentDocument1 paginăDrying Agent and Dehydrating AgentKhondokar TarakkyÎncă nu există evaluări
- CT On at STR For VII SeptDocument4 paginiCT On at STR For VII SeptKhondokar TarakkyÎncă nu există evaluări
- Answer All The Questions in This Section in The Spaces Provided. The Total Mark For This Section Is 45Document20 paginiAnswer All The Questions in This Section in The Spaces Provided. The Total Mark For This Section Is 45Khondokar TarakkyÎncă nu există evaluări
- H-1 NMR Introduction GuideDocument2 paginiH-1 NMR Introduction GuideKhondokar TarakkyÎncă nu există evaluări
- 7038 02BangladeshStudiesDocument8 pagini7038 02BangladeshStudiesKhondokar TarakkyÎncă nu există evaluări
- The Mass Spectrometer: Kms TarakkyDocument2 paginiThe Mass Spectrometer: Kms TarakkyKhondokar TarakkyÎncă nu există evaluări
- Q RateexptsDocument3 paginiQ RateexptsKhondokar TarakkyÎncă nu există evaluări
- Q NmrH1highresDocument5 paginiQ NmrH1highresKhondokar TarakkyÎncă nu există evaluări
- Finding Orders of Reaction ExperimentallyDocument2 paginiFinding Orders of Reaction ExperimentallyKhondokar TarakkyÎncă nu există evaluări
- H-1 NMR: Low Resolution: Chemical ShiftsDocument1 paginăH-1 NMR: Low Resolution: Chemical ShiftsKhondokar TarakkyÎncă nu există evaluări
- Q MsmplusDocument1 paginăQ MsmplusKhondokar TarakkyÎncă nu există evaluări
- Mass Spectrometry Fragmentation PatternsDocument2 paginiMass Spectrometry Fragmentation PatternsKhondokar TarakkyÎncă nu există evaluări
- Mass Spectra of Elements: Kms TarakkyDocument1 paginăMass Spectra of Elements: Kms TarakkyKhondokar TarakkyÎncă nu există evaluări
- Analisis Dampak Reklamasi Teluk Banten Terhadap Kondisi Lingkungan Dan Sosial EkonomiDocument10 paginiAnalisis Dampak Reklamasi Teluk Banten Terhadap Kondisi Lingkungan Dan Sosial EkonomiSYIFA ABIYU SAGITA 08211840000099Încă nu există evaluări
- Hinduism Today April May June 2015Document43 paginiHinduism Today April May June 2015jpmahadevÎncă nu există evaluări
- SM RSJ 420 800Document77 paginiSM RSJ 420 800elshan_asgarovÎncă nu există evaluări
- December - Cost of Goods Sold (Journal)Document14 paginiDecember - Cost of Goods Sold (Journal)kuro hanabusaÎncă nu există evaluări
- Ucg200 12Document3 paginiUcg200 12ArielÎncă nu există evaluări
- Soal UTS Bahasa Inggris SMP Semester Genap Tahun Ajaran 2020Document5 paginiSoal UTS Bahasa Inggris SMP Semester Genap Tahun Ajaran 2020awan MustofaÎncă nu există evaluări
- Supply Chain Management of VodafoneDocument8 paginiSupply Chain Management of VodafoneAnamika MisraÎncă nu există evaluări
- Pulsar2 User Manual - ENDocument83 paginiPulsar2 User Manual - ENJanette SouzaÎncă nu există evaluări
- BOF, LF & CasterDocument14 paginiBOF, LF & CastermaklesurrahmanÎncă nu există evaluări
- English III Module 2 Simple Present Job and Job VerbsDocument4 paginiEnglish III Module 2 Simple Present Job and Job VerbsAdrian CortesÎncă nu există evaluări
- Ensure Even Preload with Proper Tightening Tools and SequenceDocument2 paginiEnsure Even Preload with Proper Tightening Tools and SequenceMachineryengÎncă nu există evaluări
- Bs8161 - Chemistry Laboratory Syllabus: Course ObjectivesDocument47 paginiBs8161 - Chemistry Laboratory Syllabus: Course ObjectiveslevisÎncă nu există evaluări
- Intake Sheet SampleDocument1 paginăIntake Sheet SampleRochelleÎncă nu există evaluări
- BIRADS Lexicon and Its Histopathological Corroboration in The Diagnosis of Breast LesionsDocument7 paginiBIRADS Lexicon and Its Histopathological Corroboration in The Diagnosis of Breast LesionsInternational Journal of Innovative Science and Research TechnologyÎncă nu există evaluări
- Heat Exchanger Sodium SilicateDocument2 paginiHeat Exchanger Sodium SilicateChristopher BrownÎncă nu există evaluări
- Quality Control Plan Static EquipmentDocument1 paginăQuality Control Plan Static EquipmentdhasdjÎncă nu există evaluări
- Moral Character ViolationsDocument2 paginiMoral Character ViolationsAnne SchindlerÎncă nu există evaluări
- 2 English Course BDocument8 pagini2 English Course BAnjana27Încă nu există evaluări
- Mabuhay Wedding Package2006Document3 paginiMabuhay Wedding Package2006Darwin Dionisio ClementeÎncă nu există evaluări
- Solcon Catalog WebDocument12 paginiSolcon Catalog Webquocviet612Încă nu există evaluări
- RA8485 Animal Welfare Act (Carabao Slaughter)Document2 paginiRA8485 Animal Welfare Act (Carabao Slaughter)Jazreth Gaile100% (1)
- FileDocument284 paginiFileJesse GarciaÎncă nu există evaluări