Documente Academic
Documente Profesional
Documente Cultură
Invitro
Încărcat de
meforall84Drepturi de autor
Formate disponibile
Partajați acest document
Partajați sau inserați document
Vi se pare util acest document?
Este necorespunzător acest conținut?
Raportați acest documentDrepturi de autor:
Formate disponibile
Invitro
Încărcat de
meforall84Drepturi de autor:
Formate disponibile
Definition Flow cytometry is a powerful technique for the analysis of multiple parameters of individual cells within heterogeneous populations.
Flow cytometers are used in a range of applications from immunophenotyping, to ploidy analysis, to cell counting and GFP expression analysis. The flow cytometer performs this analysis by passing thousands of cells per second through a laser beam and capturing the light that emerges from each cell as it passes through. The data gathered can be analyzed statistically by flow cytometry software to report cellular characteristics such as size, complexity, phenotype, and health. In this tutorial, we will look at how a flow cytometer works, how scattered light and fluorescence are detected by a flow cytometer, and how the resulting data can be analyzed. Instrument Overview This view shows the primary systems of the flow cytometer schematically. These are: the fluidic system, which presents samples to the interrogation point and takes away the waste; the lasers, which are the light source for scatter and fluorescence; the optics, which gather and direct the light; the detectors, which receive the light; and, the electronics and the peripheral computer system, which convert the signals from the detectors into digital data and perform the necessary analyses. Interrogation point overview The interrogation point is the heart of the system. This is where the laser and the sample intersect and the optics collect the resulting scatter and fluorescence. First, lets talk about how the sample is delivered to the laser. Hydrodynamic Focusing Here we see how the sample is transported through the interrogation point. For accurate data collection, it is important that particles or cells are passed through the laser beam one at a time. Most flow cytometers accomplish this by injecting the sample stream containing the cells into a flowing stream of sheath fluid or saline solution. As you can see, the sample stream becomes
compressed to roughly one cell in diameter. This is called hydrodynamic focusing. SIZE COMPARISON In fact, flow cytometers can accommodate cells that span roughly three orders of magnitude in size. In most cases, cytometers will be detecting cells between one and 15 microns in diameter, although, through the use of specialized systems, it is possible to detect particles outside this range. Now lets see how laser light is used to detect individual cells in the stream. FORWARD SCATTER As a cell passes through the laser, it will refract or scatter light at all angles. Forward scatter, or low-angle light scatter, is the amount of light thats scattered in the forward direction as laser light strikes the cell. The magnitude of forward scatter is roughly proportional to the size of the cell, and this data can be used to quantify that parameter. FORWARD SCATTER DETECTOR But how can we record this scattered light? Light is quantified by a detector that converts intensity into voltage. In most cytometers, a blocking bar (called an obscuration bar) is placed in front of the forward scatter detector. The obscuration bar prevents any of the intense laser light from reaching the detector. As a cell crosses the laser, light is scattered around the obscuration bar and is collected by the detector. FORWARD SCATTER HISTOGRAM The scattered light received by the detector is translated into a voltage pulse. Because small cells produce a small amount of forward scatter and large cells produce a large amount of forward scatter, the magnitude of the voltage pulse recorded for each cell is proportional to the cell size. If we plot a histogram of these data, smaller cells appear toward the left and larger cells appear toward the right. A histogram of forward-scatter data is a graphical
representation of the size distribution within the population, but such a graph only presents one-dimensional data. SIDE SCATTER As we have already seen, a cell traveling through the laser beam will scatter light at all angles. Light scattering at larger angles, for example to the side, is caused by granularity and structural complexity inside the cell. This sidescattered light is focused through a lens system and is collected by a separate detector, usually located 90 degrees from the lasers path. The signals collected by the side-scatter detector can be plotted on one dimensional histograms like we saw for forward scatter. SCATTER PLOT The one-dimensional histograms we have seen so far do not necessarily show the complexity of the cell populations. For example, what appears to be a single population in the forward scatter histogram is in reality multiple populations that can only be discerned by looking at the data in a second dimension. This is done through the use of two-dimensional dot or scatter plots. You can see that the peaks from the forward and side-scatter histograms correlate with the colored dots in the scatter plot. 2D SCATTER PLOT OF BLOOD Now we can view the results obtained when we create a scatter plot using forward and side scatter data from a typical peripheral blood cell run. The populations that emerge include lymphocytes which are small cells possessing low internal complexity; monocytes which are medium-sized cells with slightly more internal complexity, and neutrophils and other granulocytes which are large cells that have a lot of internal complexity. This multiparametric analysis is the real power of flow cytometry. ENERGY STATE DIAGRAM
Now lets take a look at another parameter that can tell us more about cell structure and function: fluorescence. As a brief review, fluorescence is a term used to describe the excitation of a fluorophore to a higher energy level followed by the return of that fluorophore to its ground state with the emission of light. The energy in the emitted light is dependent on the energy level to which the fluorophore is excited, and that light has a specific wavelength, and, consequently, a specific color. USING FLOUROSCENCE IN FLOW CYTOMETRY One of the most common ways to study cellular characteristics using flow cytometry involves the use of fluorescent molecules such as fluorophorelabeled antibodies. In these experiments, the labeled antibody is added to the cell sample. The antibody then binds to a specific molecule on the cell surface or inside the cell. Finally, when laser light of the right wavelength strikes the fluorophore, a fluorescent signal is emitted and detected by the flow cytometer. FLOROSCENCE DETECTION How is this fluorescence information collected? The fluorescent light coming from labeled cells as they pass through the laser travels along the same path as the side scatter signal. As the light travels along this path, it is directed through a series of filters and mirrors, so that particular wavelength ranges are delivered to the appropriate detectors. FLOROSCENCE ONE COLOR HISTOGRAM Fluorescence data is collected in generally the same way as forward and side scatter data. In a population of labeled cells, some will be brighter than others. As each cell crosses the path of the laser, a fluorescence signal is generated. The fluorescent light is then directed to the appropriate detector where it is translated into a voltage pulse proportional to the amount of fluorescence emitted. All of the voltage pulses are recorded and can be presented graphically.
TWO COLOR EXPERIMENT, SPECTRA COMPATIBLE What if we want to do a two-color experiment? We need to look at the spectra of the two fluorophores to see if they are compatible. Alexa Fluor 488 and phycoerythrin (or R-PE) are a commonly used together. For these two fluorophores, 488 nanometer light is an efficient excitation source. When excited with 488 nanometer light, you can see that the emission peaks for these two dyes are far enough apart so that discrete emission data can be collected. Compatible dyes such as these allow scientists to easily detect two colors from a single laser. TWO COLOR DOT PLOT If we analyze data from the two-color experiment using a scatter plot, four distinct populations emerge. Looking at the dot plot in terms of quadrants, cells with only bright orange fluorescence appear in the upper left quadrant. Cells with only green fluorescence appear in the lower right quadrant. Cells with both bright green and bright orange fluorescence appear in the upper right quadrant and finally, cells with both low green and low orange fluorescence appear in the lower left quadrant. Multiple fluorescence parameters are necessary to dissect complex biological systems. FILTERS COLLECT TWO COLORS How does the flow cytometer collect discrete fluorescence data for the Alexa Fluor 488 and R-PE fluorophores? Filters are available that can capture the peak fluorescence from each of these molecules: a 530 nanometer bandpass filter will collect most of the Alexa Fluor 488 peak and a 585 nanometer bandpass filter will collect the bulk of the R-PE peak. Using these filters, the proportional amounts of Alexa Fluor 488 and R-PE fluorescence can be recorded for each cell. You can see that portions of each emission peak overlap one another. This is called spectral overlap. EMISSION FILTER TYPES Lets take a moment to talk about filter nomenclature. Filters are normally defined by one of two parameters: either the center point for a bandpass
filter, or the cutoff point for a long- or shortpass filter. This is a typical bandpass filter specific for R-PE. It has a center point of 585 nanometers and a width of 42 nanometers. So this filter optimally passes light in the wavelength range of 564 to 606 nanometers, which corresponds to the emission peak of R-PE. Other filters used to resolve this peak include a 550 nanometer longpass filter and a 610 nanometer shortpass filter. FORWARD SCATTER THRESHOLD One final important point regarding data collection is the use of a threshold. If every single particle passing through the laser caused the instrument to collect data, the data pool would be dominated by information coming from a very large number of minute particles, like platelets and debris. To prevent this, a threshold (or discriminator or trigger) is set such that a certain forward scatter pulse size must be exceeded for the instrument to collect the data. On the histogram, the blank area represents the small cells and debris that are excluded from analysis by the threshold. This means that the majority of events that the cytometer collects are the cells of interest. It is important to realize that the small particles are still passing through the instrument; they are just being ignored.
SUMMARY Weve come to the end of this tutorial. Our journey began with a look at the systems that make up the flow cytometer and how those systems work together to collect information on cells as they pass through a laser. We went on to examine in more detail how cytometers detect light scatter and fluorescence, and how that information can be viewed on various plots. In summary, flow cytometry is a unique tool, providing scientists with a way to gather statistical data on large numbers of cells and use that information to correlate multiple parameters within a cell population. Four- to six-color experiments are becoming easier; a few labs in the world are able to distinguish up to 18 colors simultaneously. In the next tutorial, we will talk in more detail about some of the trickier aspects of data analysis that are employed in flow cytometry, especially when two or more fluorescent colors are used.
S-ar putea să vă placă și
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeDe la EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeEvaluare: 4 din 5 stele4/5 (5794)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreDe la EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreEvaluare: 4 din 5 stele4/5 (1090)
- Never Split the Difference: Negotiating As If Your Life Depended On ItDe la EverandNever Split the Difference: Negotiating As If Your Life Depended On ItEvaluare: 4.5 din 5 stele4.5/5 (838)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceDe la EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceEvaluare: 4 din 5 stele4/5 (895)
- Grit: The Power of Passion and PerseveranceDe la EverandGrit: The Power of Passion and PerseveranceEvaluare: 4 din 5 stele4/5 (588)
- Shoe Dog: A Memoir by the Creator of NikeDe la EverandShoe Dog: A Memoir by the Creator of NikeEvaluare: 4.5 din 5 stele4.5/5 (537)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersDe la EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersEvaluare: 4.5 din 5 stele4.5/5 (345)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureDe la EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureEvaluare: 4.5 din 5 stele4.5/5 (474)
- Her Body and Other Parties: StoriesDe la EverandHer Body and Other Parties: StoriesEvaluare: 4 din 5 stele4/5 (821)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)De la EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Evaluare: 4.5 din 5 stele4.5/5 (121)
- The Emperor of All Maladies: A Biography of CancerDe la EverandThe Emperor of All Maladies: A Biography of CancerEvaluare: 4.5 din 5 stele4.5/5 (271)
- The Little Book of Hygge: Danish Secrets to Happy LivingDe la EverandThe Little Book of Hygge: Danish Secrets to Happy LivingEvaluare: 3.5 din 5 stele3.5/5 (400)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyDe la EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyEvaluare: 3.5 din 5 stele3.5/5 (2259)
- Biodanza by Rolando Toro in EnglishDocument90 paginiBiodanza by Rolando Toro in EnglishJohn Jamieson100% (3)
- The Yellow House: A Memoir (2019 National Book Award Winner)De la EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Evaluare: 4 din 5 stele4/5 (98)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaDe la EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaEvaluare: 4.5 din 5 stele4.5/5 (266)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryDe la EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryEvaluare: 3.5 din 5 stele3.5/5 (231)
- Team of Rivals: The Political Genius of Abraham LincolnDe la EverandTeam of Rivals: The Political Genius of Abraham LincolnEvaluare: 4.5 din 5 stele4.5/5 (234)
- On Fire: The (Burning) Case for a Green New DealDe la EverandOn Fire: The (Burning) Case for a Green New DealEvaluare: 4 din 5 stele4/5 (74)
- Shielding Tips of Robert DuncanDocument12 paginiShielding Tips of Robert Duncancruchot777100% (9)
- Art of Male MasturbationDocument4 paginiArt of Male Masturbationmeforall8450% (4)
- The Unwinding: An Inner History of the New AmericaDe la EverandThe Unwinding: An Inner History of the New AmericaEvaluare: 4 din 5 stele4/5 (45)
- A. Waterman - The Triple O Guide To Female OrgasmsDocument22 paginiA. Waterman - The Triple O Guide To Female OrgasmsJill Camacho50% (18)
- Bashar All Years To March 2023Document262 paginiBashar All Years To March 20232lmqibmsÎncă nu există evaluări
- Covid 19Document51 paginiCovid 19Engr AhmedÎncă nu există evaluări
- The80approach 091101063727 Phpapp01Document52 paginiThe80approach 091101063727 Phpapp01meforall84Încă nu există evaluări
- Taiwan Flow Chart of Visitor Visa ApplicationDocument3 paginiTaiwan Flow Chart of Visitor Visa Applicationmeforall84Încă nu există evaluări
- Mahabharat 1Document14 paginiMahabharat 1meforall84Încă nu există evaluări
- Gabon Business Visa ApplicationDocument3 paginiGabon Business Visa Applicationmeforall84Încă nu există evaluări
- Precision T7500 T5500 Technical GuideDocument37 paginiPrecision T7500 T5500 Technical GuideRonan CurranÎncă nu există evaluări
- MEH Jan Feb 2013Document196 paginiMEH Jan Feb 2013meforall84Încă nu există evaluări
- Conflict Resolution Skills - Turning Conflicts Into OpportunitiesDocument5 paginiConflict Resolution Skills - Turning Conflicts Into Opportunitiesmeforall84Încă nu există evaluări
- Baraha Telugu Tips 1Document1 paginăBaraha Telugu Tips 1meforall84Încă nu există evaluări
- Public Speaking Skills: WWW - Hct.ac - AeDocument2 paginiPublic Speaking Skills: WWW - Hct.ac - Aemeforall84Încă nu există evaluări
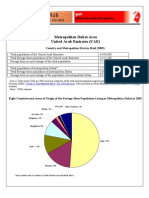
- Metropolitan Dubai Area United Arab Emirates (UAE) : Country and Metropolitan Stats in Brief (2005)Document2 paginiMetropolitan Dubai Area United Arab Emirates (UAE) : Country and Metropolitan Stats in Brief (2005)meforall84Încă nu există evaluări
- Conflict Resolution Skills - Turning Conflicts Into OpportunitiesDocument5 paginiConflict Resolution Skills - Turning Conflicts Into Opportunitiesmeforall84Încă nu există evaluări
- Hadef & Partners Welcome To DubaiDocument11 paginiHadef & Partners Welcome To Dubaimeforall84Încă nu există evaluări
- Bank Branches 2012Document1 paginăBank Branches 2012meforall84Încă nu există evaluări
- A525915100074284348 RLS PDFDocument13 paginiA525915100074284348 RLS PDFHhh HhhÎncă nu există evaluări
- Application of Molecular Markers in Forensic BotanyDocument35 paginiApplication of Molecular Markers in Forensic BotanyMostafaÎncă nu există evaluări
- Bang-Bang and Singular in BiologyDocument45 paginiBang-Bang and Singular in Biologyva3ttnÎncă nu există evaluări
- SD A1cCare User GuideDocument61 paginiSD A1cCare User GuideHabibat TurabiaÎncă nu există evaluări
- BIO 31A Exercise 4Document6 paginiBIO 31A Exercise 4Genevieve Gayoso50% (2)
- Detailed CV JYOTI VERMA 2016Document6 paginiDetailed CV JYOTI VERMA 2016Arvind NegiÎncă nu există evaluări
- Eclipse MaidDocument38 paginiEclipse MaidAnonymous 8vH1ERCwÎncă nu există evaluări
- Conflict of Interest List CIRM Directors Quest Awards June 2017Document2 paginiConflict of Interest List CIRM Directors Quest Awards June 2017California Stem Cell ReportÎncă nu există evaluări
- PASS Tutoring Active Link SP 21Document1 paginăPASS Tutoring Active Link SP 21wwooowwmanÎncă nu există evaluări
- Evolution of Nakedness Among Homo SapiensDocument7 paginiEvolution of Nakedness Among Homo SapiensSherry SalazarÎncă nu există evaluări
- Sexual Vs Asexual Reproduction and Plant Reproduction: IGCSE Biology (O610) Workbook Chapter 16 - Part ADocument8 paginiSexual Vs Asexual Reproduction and Plant Reproduction: IGCSE Biology (O610) Workbook Chapter 16 - Part Awafa elias100% (1)
- Profile Summary: Nadiah28@um - Edu.myDocument10 paginiProfile Summary: Nadiah28@um - Edu.myAnonymous CQ36UukOTÎncă nu există evaluări
- Radiation Safety Manual (Duke Univ Laboratory, 2001)Document54 paginiRadiation Safety Manual (Duke Univ Laboratory, 2001)AndréRochaÎncă nu există evaluări
- The Big-Chart of Acupuncture Points: Source PointDocument1 paginăThe Big-Chart of Acupuncture Points: Source PointSelvakumarÎncă nu există evaluări
- Beetroot Core Practical Writing FrameDocument5 paginiBeetroot Core Practical Writing FrameJett0% (1)
- Introduction To Fish MicrobiologyDocument6 paginiIntroduction To Fish MicrobiologysimasÎncă nu există evaluări
- Example of Declaration of Biological Shipments FormDocument2 paginiExample of Declaration of Biological Shipments Formrubana reazÎncă nu există evaluări
- Shark Research Paper TopicsDocument6 paginiShark Research Paper Topicsqptwukrif100% (1)
- Evaluation of Piscicidal Activity of Plant Asclepias Curassavica Linn (Fam. Asclepiadaceae) .Document4 paginiEvaluation of Piscicidal Activity of Plant Asclepias Curassavica Linn (Fam. Asclepiadaceae) .Dr. Jawale Chetan S.Încă nu există evaluări
- Haploid CultureDocument35 paginiHaploid CultureSpark is within me89% (18)
- Molecular Oral Microbiology - 2023 - D Amico - Efficacy of Cetylpyridinium Chloride Mouthwash Against SARS CoV 2 ADocument10 paginiMolecular Oral Microbiology - 2023 - D Amico - Efficacy of Cetylpyridinium Chloride Mouthwash Against SARS CoV 2 Akatcharles.persoÎncă nu există evaluări
- Cell Disruption MethodsDocument19 paginiCell Disruption MethodsMalek Marry AnneÎncă nu există evaluări
- A) Purpose of The Examination:: Sop No. Imm /Qm/12 Issue No.: 04 ISSUE DATE: 15april 2015 REV. NO.: 00 Rev. Date: 00Document7 paginiA) Purpose of The Examination:: Sop No. Imm /Qm/12 Issue No.: 04 ISSUE DATE: 15april 2015 REV. NO.: 00 Rev. Date: 00prityÎncă nu există evaluări
- 02 Group 3 Chapter 1-5Document37 pagini02 Group 3 Chapter 1-5maryann ruedaÎncă nu există evaluări
- Anatomy Chapters 6&7Document17 paginiAnatomy Chapters 6&7Tamara IdalyÎncă nu există evaluări
- Environmental Monitoring Report-Oktober 2016Document31 paginiEnvironmental Monitoring Report-Oktober 2016azuraidaÎncă nu există evaluări