Documente Academic
Documente Profesional
Documente Cultură
Isothermal Titration Calorimetry
Încărcat de
Lupu Andreea-CraitaDescriere originală:
Titlu original
Drepturi de autor
Formate disponibile
Partajați acest document
Partajați sau inserați document
Vi se pare util acest document?
Este necorespunzător acest conținut?
Raportați acest documentDrepturi de autor:
Formate disponibile
Isothermal Titration Calorimetry
Încărcat de
Lupu Andreea-CraitaDrepturi de autor:
Formate disponibile
Isothermal Titration Calorimetry (ITC)
Brief Overview: Isothermal titration calorimetry (ITC) is used to measure interactions
between ligands such as proteins and peptides, or proteins and other ligands (e.g. lipid heads groups). For ITC experiments, two binding partners (e.g., protein A and protein B, in the same buffer and at known concentrations) are placed in the injection syringe and the ITC cell. The ITC cell is kept at a tiny temperature difference compared to a reference cell filled with buffer.
When protein B is injected, the two proteins interact. If this interaction is exothermic, the ITC uses less energy to heat the cell; if the interaction is endothermic, the ITC uses more energy
to heat the cell. Precise measurement of the energy required to maintain temperature of the cell during the course of several injections leads to the calculation of free energy, enthalpy, entropy, Kd, and stoichiometry of binding from one single experiment. Furthermore the reaction can be measured without immobilization or labelling of the binding partners.
Tips and Tricks on how to set up an experiment
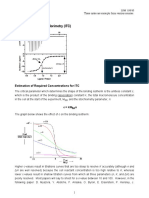
In order to obtain an optimal binding isotherm one needs to consider the following: 1. Relative concentrations of binding partners The binding partner in the sample cell (1.6 ml volume for a VP-ITC) should be at least at a 10 fold higher concentration than the expected dissociation constant (Kd) of the reactants. Ideally it should be 30 fold higher. If the concentration is more than 100 fold above the dissociation
constant the curve gets too steep and the fit is inaccurate. In this case the concentration should be lowered, if the signal is still be large enough. The ligand in the syringe should be titrated to a molar ratio of 4:1 compared to the binding partner in the sample cell. Since the total volume injected from the syringe is about 0.3 ml, the concentration of the ligand should be about 20 fold higher than the binding partner in the sample cell. The volume of the individual injections should be chosen such that the ligand and the binding partner reach a molar ratio of 1:1 after about 10-15 injections. Example: Dissociation constant 1 M Protein concentration in sample cell 30 M Ligand concentration in syringe 600 M Injection volume 6-8 ml For a protein with a molecular mass of 50 kDa, 2.4 mg are needed for a single experiment and 1.8 mg of a ligand of 10 kDa (e.g. a small protein domain). The actual values are even higher since about 2 ml are needed to fill the sample cell and 0.6 ml for the syringe. 2. Signal to noise ratio Although modern calorimeters have a fairly low thermal noise, it may sometimes be difficult to obtain reliable data, especially for weak interactions with a deltaH near zero. The value of deltaH alone does not tell much about the affinity. For the measurement exothermic and endothermic reactions are equally well suited. Small values of deltaH, however, can make the reaction unmeasurable although the binding might be tight if the reaction is purely driven by the entropy deltaS. In order to test whether a reaction is not happening or just not measurable, one can try to change the value of deltaH into either direction by changing: a. the concentrations (the higher the better) b. the temperature (since deltaH is temperature dependent)
c. the buffering system (since they have different heat of protonation which might be measurable) In order to keep the noise low, one has to make sure that the buffer of the binding partner in the sample cell and of the ligand are as similar as possible. Otherwise there will be large peaks due to the mixing and dilution of the buffers, especially if they have a different pH. We found it useful to either dialyze or gel-filter both binding partners in the same buffer and also to keep some of that same buffer frozen for later repetitions. For ligands which may contain a high concentration of acid or base, such as lyophilized peptides from chemical synthesis, a high concentration of the buffering agent should be used to keep the pH constant when dissolving the ligand. High noise in the experiment can also be caused by a bent injection syringe. Replacing the syringe is the best solution for this problem. Increasing noise during an experiment can be a sign of protein precipitation. This will become obvious when emptying the sample cell afterwards. Finally, air bubbles in the sample cell also cause a large amount of noise. 3. Stoichiometry and multiple sites One of the powerful features of microcalorimetry is the fact that the stoichiometry (N) of binding can be determined directly and simultaneously. On the other hand any error in determining the concentration of the binding partner or the ligand becomes apparent after fitting traces as one expects the molar ratio of the reaction to be an integral multiple of 1. In case N deviates by more that 20 % of the expected value, one should carefully repeat the measurement of the concentration, ideally with an independent assay (Bradford, OD280, BCA, ). If both concentrations are correct, one can start to think about the fraction of active protein in the sample or about alternative binding models. If only 80 % of a protein are active and take part in the reaction, the concentrations can be adjusted before fitting the isotherm to the data. For 2:1 complexes it is advisable to have the binding partner with two binding sites in the sample cell. If the two ligands bind with different affinities, this will be much easier to
recognize than in the opposite scenario where the N is 0.5:1. The fitting algorithms can be adjusted in either way (Ligand in syringe vs. protein in syringe) and will always give the correct values. Example of the power of ITC measurements from our own work on the interactions of repeated peptide motifs, found in motif domains of endocytic proteins, which bind to the appendage domains of adaptor complexes:
Click on picture below for a Powerpoint tutorial given to students on the use of Calorimetry for measuring various ligand interactions in clathrin-mediated endocytosis
S-ar putea să vă placă și
- Pharmaceutics: Basic Principles and FormulationsDe la EverandPharmaceutics: Basic Principles and FormulationsEvaluare: 3.5 din 5 stele3.5/5 (6)
- Instruction Manual Twin Lobe CompressorDocument10 paginiInstruction Manual Twin Lobe Compressorvsaagar100% (1)
- Chemistry Ia SampleDocument15 paginiChemistry Ia SampleDanisa Irianto100% (2)
- Abstract For CSTR Lab ReportDocument4 paginiAbstract For CSTR Lab ReportNabilah SyaheeraÎncă nu există evaluări
- Cash Budget Sharpe Corporation S Projected Sales First 8 Month oDocument1 paginăCash Budget Sharpe Corporation S Projected Sales First 8 Month oAmit PandeyÎncă nu există evaluări
- Designing Itc v7 ExptsDocument5 paginiDesigning Itc v7 ExptsRigel_TÎncă nu există evaluări
- Itc Current ProtocolsDocument24 paginiItc Current ProtocolsRigel_TÎncă nu există evaluări
- M2 Formulation of Optimal Conditions For An Immunonephelometric AssayDocument8 paginiM2 Formulation of Optimal Conditions For An Immunonephelometric AssayELISAÎncă nu există evaluări
- StoichiometryDocument4 paginiStoichiometryCourtney JenningsÎncă nu există evaluări
- Raciloimmunoassay: Principle and TechniqueDocument6 paginiRaciloimmunoassay: Principle and Techniqueandi novrianiÎncă nu există evaluări
- Protein Assay STD ProtocolDocument3 paginiProtein Assay STD ProtocolMahidul IslamÎncă nu există evaluări
- Indian Institute of Technology, Bombay. Lecture-8. Protein-Protein Interaction Study: Binding AnalysisDocument26 paginiIndian Institute of Technology, Bombay. Lecture-8. Protein-Protein Interaction Study: Binding AnalysisAl AmeenÎncă nu există evaluări
- Lab 3 Standard Protein Assay LabDocument5 paginiLab 3 Standard Protein Assay LabMark WayneÎncă nu există evaluări
- New Microsoft Office Word DocumentDocument3 paginiNew Microsoft Office Word DocumentVooy RajÎncă nu există evaluări
- Bchem Report1Document7 paginiBchem Report1lenny lemoogeÎncă nu există evaluări
- BIO 103 Lab 4 Bradford AssayDocument4 paginiBIO 103 Lab 4 Bradford AssayMaheshMeenaÎncă nu există evaluări
- Kinetics of Protein BindingDocument37 paginiKinetics of Protein BindingNoorulhuda AbbasiÎncă nu există evaluări
- DSP Lab ManualDocument27 paginiDSP Lab ManualRaghav SureshÎncă nu există evaluări
- Detailed Notes Topic 5 Formulae Equations and Amounts of Substance Edexcel Chemistry A LevelDocument7 paginiDetailed Notes Topic 5 Formulae Equations and Amounts of Substance Edexcel Chemistry A LevelttjjjÎncă nu există evaluări
- TSH Accubind Elisa Rev 3Document0 paginiTSH Accubind Elisa Rev 3Rafael ZevallosÎncă nu există evaluări
- Bio312 Final Exam Spring 2020Document5 paginiBio312 Final Exam Spring 2020Lejla MillerÎncă nu există evaluări
- Determination of The Equivalent Weight of An Unknown AcidDocument4 paginiDetermination of The Equivalent Weight of An Unknown AcidAlphonse SambranoÎncă nu există evaluări
- Guidelines For Performance Testing of Packed ColumnsDocument3 paginiGuidelines For Performance Testing of Packed ColumnsseetharamÎncă nu există evaluări
- Indian Institute of Technology, Bombay. Lecture-9. Protein-Protein Interaction Study: Kinetic AnalysisDocument25 paginiIndian Institute of Technology, Bombay. Lecture-9. Protein-Protein Interaction Study: Kinetic AnalysisAl AmeenÎncă nu există evaluări
- Laboratory Methods - BAM Appendix 2 - Most Probable Number From Serial Dilutions PDFDocument25 paginiLaboratory Methods - BAM Appendix 2 - Most Probable Number From Serial Dilutions PDFPhuong LeÎncă nu există evaluări
- Analytical NotesDocument25 paginiAnalytical NotesRyan BoodramlallÎncă nu există evaluări
- BAM Appendix 2 MPNDocument7 paginiBAM Appendix 2 MPNrozaÎncă nu există evaluări
- Collected Coursework Problems in Biochemical Engineering BlanchDocument6 paginiCollected Coursework Problems in Biochemical Engineering Blanchbcqy65mx100% (2)
- Lab No 4 - Affinity ChromatographyDocument8 paginiLab No 4 - Affinity Chromatographydead_knightÎncă nu există evaluări
- Lab2 BCA Assay HandoutDocument6 paginiLab2 BCA Assay HandoutsandalailaÎncă nu există evaluări
- Affinity ChromatographyDocument3 paginiAffinity ChromatographyNithya Ram100% (1)
- 7 Mixing StudiesDocument16 pagini7 Mixing StudiesMohamedSElzaharna0% (1)
- TR0006 Extinction CoefficientsDocument3 paginiTR0006 Extinction CoefficientsRizmahardian Ashari KurniawanÎncă nu există evaluări
- Atom Base Titration NotesDocument4 paginiAtom Base Titration NotesVooy RajÎncă nu există evaluări
- Analytical Chemistry: University of TechnologyDocument25 paginiAnalytical Chemistry: University of TechnologyAbdullah IyadÎncă nu există evaluări
- CN 2Document24 paginiCN 2Meg MaxilomÎncă nu există evaluări
- Activity 12Document4 paginiActivity 12James Carbonell Dela PeñaÎncă nu există evaluări
- BAM Appendix 2 - MPNDocument37 paginiBAM Appendix 2 - MPNTiti Lasmini100% (1)
- Jobses 1Document10 paginiJobses 1Mindy MunozÎncă nu există evaluări
- Paper 3 - Analysis, Conclusions and EvaluationDocument5 paginiPaper 3 - Analysis, Conclusions and EvaluationKhadija KaziÎncă nu există evaluări
- BIOASSAYDocument78 paginiBIOASSAYAshly chackoÎncă nu există evaluări
- Bioassay of DrugsDocument45 paginiBioassay of DrugssivaÎncă nu există evaluări
- 27.02 Detecting Antigen-Antibody Complexes in VitroDocument13 pagini27.02 Detecting Antigen-Antibody Complexes in VitroveerdedajiÎncă nu există evaluări
- Bradford AssayDocument7 paginiBradford AssayTiara CahyadiÎncă nu există evaluări
- BAM Appendix 2Document21 paginiBAM Appendix 2winaÎncă nu există evaluări
- Analysis AssignmentDocument7 paginiAnalysis AssignmentUmama WarrichÎncă nu există evaluări
- ProteinsDocument69 paginiProteinsRodger12Încă nu există evaluări
- Immobilized Enzyme Reactor 1 PDFDocument5 paginiImmobilized Enzyme Reactor 1 PDFPrajakta NakateÎncă nu există evaluări
- Physical Science Week 11-12-1Document4 paginiPhysical Science Week 11-12-1Drasszen SombilonÎncă nu există evaluări
- Integrating Computers Into The First-Year Chemistry Laboratory: Application of Raoult's Law To A Two-Component SystemDocument2 paginiIntegrating Computers Into The First-Year Chemistry Laboratory: Application of Raoult's Law To A Two-Component SystemjohnÎncă nu există evaluări
- Paper 3 - Analysis, Conclusions and EvaluationDocument5 paginiPaper 3 - Analysis, Conclusions and EvaluationUrvah TauseefÎncă nu există evaluări
- Experiment 4: Direct Haemagglutination Test Objective: To Learn The Agglutination Theory by Using The Lectin and Red BloodDocument5 paginiExperiment 4: Direct Haemagglutination Test Objective: To Learn The Agglutination Theory by Using The Lectin and Red BloodSkyoda SoonÎncă nu există evaluări
- A Guide To Protein Blotting: Sds-PageDocument11 paginiA Guide To Protein Blotting: Sds-PageMehedi HossainÎncă nu există evaluări
- TS - How To Use A Protein Assay Standard CurveDocument4 paginiTS - How To Use A Protein Assay Standard CurveJLÎncă nu există evaluări
- C STR Kinetics 2012Document12 paginiC STR Kinetics 2012JpojÎncă nu există evaluări
- MB C Technical Perspective A Guide To Simple and Informative Binding AssaysDocument7 paginiMB C Technical Perspective A Guide To Simple and Informative Binding AssaysJulia MachajÎncă nu există evaluări
- At Enolo LolDocument5 paginiAt Enolo Loldrever2010Încă nu există evaluări
- Biochem Lab ReportDocument4 paginiBiochem Lab ReportAdenagr TeklegiorgisÎncă nu există evaluări
- The Bradford Method For Protein Quantitation. Nicholas Kruger PDFDocument7 paginiThe Bradford Method For Protein Quantitation. Nicholas Kruger PDFGise Clara HernandezÎncă nu există evaluări
- Binding Affinity of Candesartan, Losartan, Telmisartan and Valsartan With Angiotensin II Receptor 1 SubtypeDocument4 paginiBinding Affinity of Candesartan, Losartan, Telmisartan and Valsartan With Angiotensin II Receptor 1 SubtypeRicardo RodriguezÎncă nu există evaluări
- AP Chemistry - Finding The Ratio of Moles of Reactants in A Chemical ReactionDocument4 paginiAP Chemistry - Finding The Ratio of Moles of Reactants in A Chemical ReactionJonathan Chen88% (8)
- Applied Biophysics for Drug DiscoveryDe la EverandApplied Biophysics for Drug DiscoveryDonald HuddlerÎncă nu există evaluări
- The Fluency Course Teacher Instructions PDFDocument9 paginiThe Fluency Course Teacher Instructions PDFGabriel da RochaÎncă nu există evaluări
- Singer 900 Series Service ManualDocument188 paginiSinger 900 Series Service ManualGinny RossÎncă nu există evaluări
- Sample Valuation ReportDocument15 paginiSample Valuation Reportayush singlaÎncă nu există evaluări
- Accounting System (Compatibility Mode) PDFDocument10 paginiAccounting System (Compatibility Mode) PDFAftab AlamÎncă nu există evaluări
- Bofa Turkish Banks-Back On The RadarDocument15 paginiBofa Turkish Banks-Back On The RadarexperhtmÎncă nu există evaluări
- Zkp8006 Posperu Inc SacDocument2 paginiZkp8006 Posperu Inc SacANDREA BRUNO SOLANOÎncă nu există evaluări
- Gothic ArchitectureDocument6 paginiGothic ArchitectureleeÎncă nu există evaluări
- 24 Inch MonitorDocument10 pagini24 Inch MonitorMihir SaveÎncă nu există evaluări
- CSCU Module 08 Securing Online Transactions PDFDocument29 paginiCSCU Module 08 Securing Online Transactions PDFdkdkaÎncă nu există evaluări
- Space Saving, Tight AccessibilityDocument4 paginiSpace Saving, Tight AccessibilityTran HuyÎncă nu există evaluări
- Korea Times - Korean-EnglishDocument313 paginiKorea Times - Korean-EnglishgyeryongÎncă nu există evaluări
- Line Integrals in The Plane: 4. 4A. Plane Vector FieldsDocument7 paginiLine Integrals in The Plane: 4. 4A. Plane Vector FieldsShaip DautiÎncă nu există evaluări
- Practical Applications of Electrical ConductorsDocument12 paginiPractical Applications of Electrical ConductorsHans De Keulenaer100% (5)
- PDFDocument3 paginiPDFvaliÎncă nu există evaluări
- 12 Constructor and DistructorDocument15 pagini12 Constructor and DistructorJatin BhasinÎncă nu există evaluări
- Beer Lambert'S Law: Dr. Swastika Das Professor of ChemistryDocument19 paginiBeer Lambert'S Law: Dr. Swastika Das Professor of ChemistryShabanaÎncă nu există evaluări
- Javascript NotesDocument5 paginiJavascript NotesRajashekar PrasadÎncă nu există evaluări
- A Short History of Denim: (C) Lynn Downey, Levi Strauss & Co. HistorianDocument11 paginiA Short History of Denim: (C) Lynn Downey, Levi Strauss & Co. HistorianBoier Sesh PataÎncă nu există evaluări
- Tanque: Equipment Data SheetDocument1 paginăTanque: Equipment Data SheetAlonso DIAZÎncă nu există evaluări
- Corporate Valuation WhartonDocument6 paginiCorporate Valuation Whartonebrahimnejad64Încă nu există evaluări
- Image Hosting SitesDocument16 paginiImage Hosting SitesstudentÎncă nu există evaluări
- Thesis Topics in Medicine in Delhi UniversityDocument8 paginiThesis Topics in Medicine in Delhi UniversityBecky Goins100% (2)
- Game ApiDocument16 paginiGame ApiIsidora Núñez PavezÎncă nu există evaluări
- Intergard 475HS - Part B - EVA046 - GBR - ENG PDFDocument10 paginiIntergard 475HS - Part B - EVA046 - GBR - ENG PDFMohamed NouzerÎncă nu există evaluări
- Human Capital PlanningDocument27 paginiHuman Capital Planningalokshri25Încă nu există evaluări
- APRStt Implementation Notes PDFDocument36 paginiAPRStt Implementation Notes PDFCT2IWWÎncă nu există evaluări
- Cs205-E S3dec18 KtuwebDocument2 paginiCs205-E S3dec18 KtuwebVighnesh MuralyÎncă nu există evaluări
- Growing Onion Management and Water NeedsDocument25 paginiGrowing Onion Management and Water NeedsKATE NAVAJAÎncă nu există evaluări