Documente Academic
Documente Profesional
Documente Cultură
InternalAuditSOP 012413
Încărcat de
Rony LesbtTitlu original
Drepturi de autor
Formate disponibile
Partajați acest document
Partajați sau inserați document
Vi se pare util acest document?
Este necorespunzător acest conținut?
Raportați acest documentDrepturi de autor:
Formate disponibile
InternalAuditSOP 012413
Încărcat de
Rony LesbtDrepturi de autor:
Formate disponibile
Laboratory Name and contact information
Title: Internal Audit Standard Operating Procedure and Simplified Quality System Chec list !oc" No" # $e%" No" # !ate: &'(#)('* Page: ' of #+
Approved by: Technical !irector ,,,,,,,,,,,,,,,,,,,,,,,,,,,,,, -Name. ,,,,,,,,,,,,,,,,,,,,,,,,,,,,,, -Signature. ,,,,,,,,,,,,,,, -Initials. Quality Assurance Officer ,,,,,,,,,,,,,,,,,,,,,,,,,,,,,, -Name. ,,,,,,,,,,,,,,,,,,,,,,,,,,,,,, -Signature. ,,,,,,,,,,,,,,, -Initials. ,,,,,,,,,,,,,,,,, -!ate. ,,,,,,,,,,,,,,,,, -!ate.
Controlled !ocument
Laboratory Name and contact information
Title: Internal Audit Standard Operating Procedure and Simplified Quality System Chec list !oc" No" # $e%" No" # !ate: &'(#)('* Page: # of #+
Revision History Rev ' # Date +(/(&0 &'('*('* Name Name Description of Change Initial $elease 1pdated all sections" $eformatted Appendi2 A"
Distribution List / Location This SOP is to be distributed to those indi%iduals in%ol%ed in the internal audit process of the lab"
Controlled !ocument
Laboratory Name and contact information
Title: Internal Audit Standard Operating Procedure and Simplified Quality System Chec list !oc" No" # $e%" No" # !ate: &'(#)('* Page: * of #+
Annual Review -The re%ie3 is to be documented if the document has not been re%ised in the past '# months. ,,,,,,,,,,,,,,,,,,,,,,, Signature ,,,,,,,,,,,,,,,,,,,,,, Signature ,,,,,,,,,,,,,,,,,,, Signature ,,,,,,,,,,,,,,,,,,,,,, Signature ,,,,,,,,,,,,,,,,,,, Signature ,,,,,,,,,,,,,,,,,,,, Title ,,,,,,,,,,,,,,,,,,,, Title ,,,,,,,,,,,,,,,,,,,, Title ,,,,,,,,,,,,,,,,,,,, Title ,,,,,,,,,,,,,,,,,,,, Title ,,,,,,, !ate ,,,,,,, !ate ,,,,,,, !ate ,,,,,,, !ate ,,,,,,, !ate
Training Record The follo3ing laboratory staff ha%e read and agree to follo3 the latest %ersion of the SOP" ,,,,,,,,,,,,,,,,,,,,, ,,,,,,,,,,,,,,,,,,,,,, ,,,,,,, ,,,,,,, Signature Name Initials !ate ,,,,,,,,,,,,,,,,,,,,, Signature ,,,,,,,,,,,,,,,,,,,,, Signature ,,,,,,,,,,,,,,,,,,,,, Signature ,,,,,,,,,,,,,,,,,,,,, Signature ,,,,,,,,,,,,,,,,,,,,,, Name ,,,,,,,,,,,,,,,,,,,,,, Name ,,,,,,,,,,,,,,,,,,,,,, Name ,,,,,,,,,,,,,,,,,,,,,, Name ,,,,,,, Initials ,,,,,,, Initials ,,,,,,, Initials ,,,,,,, Initials ,,,,,,, !ate ,,,,,,, !ate ,,,,,,, !ate ,,,,,,, !ate
Controlled !ocument
Laboratory Name and contact information
Title: Internal Audit Standard Operating Procedure and Simplified Quality System Chec list !oc" No" # $e%" No" # !ate: &'(#)('* Page: ) of #+
Table of Contents 1 !urpose # $cope % Responsibilities & !rocedure " Related Docu(entation and References ' Definitions A!!+,D-. A " " " ' ) * /
Controlled !ocument
Laboratory Name and contact information
Title: Internal Audit Standard Operating Procedure and Simplified Quality System Chec list !oc" No" # $e%" No" # !ate: &'(#)('* Page: / of #+
'" Purpose To ensure that the procedures in the 4uality manual5 related to 4uality systems5 and the lab6s method manual5 related to testing acti%ities5 are being follo3ed" To determine the effecti%eness of the lab6s procedures in controlling the 4uality of data reported To identify5 correct5 and implement any changes needed in any of the 4uality system and testing acti%ities procedures found to be deficient To ensure all deficiencies in the lab6s 4uality system and testing acti%ities are documented though its correcti%e action process
#" Scope The internal audit SOP and associated chec list is used to audit5 on an annual basis5 the lab6s 4uality system5 policies and procedures5 3or instructions5 analytical records5 and reports" In addition5 the lab audits its testing acti%ities -each method7technology. on an annual basis" *" $esponsibilities Quality Assurance -QA. 8anager or QA Officer -QAO.: is no3ledgeable and trained in 4uality system re4uirements5 including internal audits initiates all internal audits and ensures they are conducted in an efficient and timely manner delegates responsible5 trained staff5 if applicable5 to carry out specific audits of testing acti%ities notifies laboratory management 5 including the technical director5 of any deficiencies -findings. in the 4uality system or testing acti%ities documents and monitors correcti%e actions documents and trac s staff 3ho ha%e completed auditor training Auditor: has completed auditor training
Controlled !ocument
Laboratory Name and contact information
Title: Internal Audit Standard Operating Procedure and Simplified Quality System Chec list !oc" No" # $e%" No" # !ate: &'(#)('* Page: 9 of #+
has sufficient e2perience in performing audits performs audits in an efficient and timely manner reports all findings to the QAO
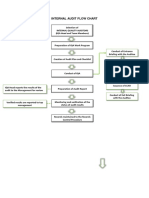
)" Procedure & 1 The Audit Tea( The Quality Assurance 8anager or QA Officer -QAO. selects trained staff5 if applicable5 to perform the audits defined in this procedure" If trained staff is limited5 the QAO and(or technical director may perform the audits" If trained staff is not limited5 the QAO 3ill designate one of the trained staff to ser%e as the lead auditor" :hen e%er possible5 auditors are selected from a function not directly in%ol%ed in the audit" & # Training Auditors are trained in auditing techni4ues" Training consists of reading and understanding this procedure and reference material related to internal auditing5 and 3here possible5 shado3ing a trained auditor or completing a formal5 e2ternal training course" The auditors are also pro%ided 3ith the applicable auditing guidelines and chec lists" ;oth 4uality system and method7specific chec lists are to be pro%ided to the auditor" <%idence of the training includes a signature that the auditor has read and understands this procedure -see page *." It may also include documentation of any e2ternal seminars or course 3or related to 4uality system auditing" All training records are to be ept for a minimum of fi%e years" & % Audit !lan The entire 4uality system5 including testing acti%ities5 is audited on an annual basis" The ma2imum inter%al bet3een audits is t3el%e months" The fre4uency may be ad=usted for ne3 procedures or deficiencies that resulted from complaints" The QAO creates the audit schedule" The audit schedule defines the follo3ing: timeframe of the audit scope of audit elements and( or areas to be audited & & !erfor(ing the Audit The QAO notifies the super%isors of the areas to be audited at least a month in ad%ance"
Controlled !ocument
Laboratory Name and contact information
Title: Internal Audit Standard Operating Procedure and Simplified Quality System Chec list !oc" No" # $e%" No" # !ate: &'(#)('* Page: 0 of #+
The QAO briefs the auditors on the audit procedures and the areas to be audited" The auditors are to prepare prior to the audit by familiari>ing themsel%es 3ith the audit procedures" !uring the audit5 auditors use applicable chec lists" They record all findings on the chec lists" The findings are discussed 3ith the staff responsible for performing the function that 3as found to be deficient" & " Deficiency Report and Corrective Action Response A deficiency report is generated by the auditor for each legitimate finding" The QAO ma es the final decision as to 3hether the finding is legitimate if it can not be resol%ed bet3een the auditor and the staff audited" The QAO presents the final report to the audited staff5 as 3ell as5 laboratory management5 including the technical director" The audited staff responds to the deficiency report in a manner prescribed by the lab6s correcti%e action procedures5 3hich are included in the Quality 8anual" The correcti%e action must be completed 3ithin +& days of the date of the finding" :hen deficiencies cast doubt on the correctness or %alidity of the calibration or test results reported5 the lab needs to immediately notify its clients of the situation" A record of the client notification must be maintained" & ' Closing an Audit Audit findings are closed upon completion of an effecti%e correcti%e action for each of the findings" All documents related to the audit5 including chec lists5 deficiency reports5 correcti%e action responses5 are maintained by the QAO" & ) Review and +valuation The QAO %erifies successful implementation of the correcti%e action by obser%ing ob=ecti%e e%idence supplied by the audited staff as part of the correcti%e action process" ?ollo37up is performed by the QAO5 or designated staff5 as part of the ne2t scheduled audit to %erify the effecti%eness of the correcti%e actions that 3ere implemented" IN addition5 the QAO re%ie3s the audit report 3ith laboratory management5 including the technical director5 as part of the lab6s annual management re%ie3" /" Related Documentation and References Audit Plan
Controlled !ocument
Laboratory Name and contact information
Title: Internal Audit Standard Operating Procedure and Simplified Quality System Chec list !oc" No" # $e%" No" # !ate: &'(#)('* Page: @ of #+
Audit Chec lists5 including method7specific chec lists !eficiency -Audit. $eport Correcti%e Action $esponse -CA$. Correcti%e Action Procedures as noted in Quality 8anual National <n%ironmental Laboratory Accreditation Conference (NELAC), 2003 NELAC Standard, Approved une !, 2003, Effective ul" #, 2003, 32$ pp (E%A&'00&R(0$&003)) National <n%ironmental Laboratory Accreditation Conference (NELAC), 200* NELAC Standard, Approved Au+ust 2$, 200*, Effective ul" #, 20##) Ne3 Aor State !epartment of Bealth -NAS !OB. <n%ironmental Laboratory Appro%al Program -<LAP.5 method7specific chec lists5 http:((333"3ads3orth"org(labcert(elapcert(appforms"htm Ne3 Aor State !epartment of Bealth -NAS !OB.5 NAC$$ Subpart //7#5 Appro%al of Laboratories Performing <n%ironmental Analysis5 Sections //7#"' through //7#"'# effecti%e No%ember '05 #&&)5 and Section //7#"'* effecti%e October 95 #&&)" ' Definitions Audit ?inding 7 A conclusion of importance based on obser%ation-s." An undesirable de%iation or nonconformity" Correcti%e Action 7 Action ta en to eliminate the root cause-s. and the symptom-s. of an e2isting undesirable de%iation or nonconformity to pre%ent recurrence" Ob=ecti%e <%idence 7 Cerifiable 4ualitati%e or 4uantitati%e obser%ations5 information5 records5 or statements of fact pertaining to the 4uality of a product or ser%ice or to the e2istence and implementation of 4uality system element" Quality Audit 7 Systematic and independent e2amination to determine 3hether 4uality acti%ities and related results comply 3ith planned arrangements and 3hether these arrangements are implemented effecti%ely and are suitable to achie%e ob=ecti%es
Controlled !ocument
Laboratory Name and contact information
Title: Internal Audit Standard Operating Procedure and Simplified Quality System Chec list !oc" No" # $e%" No" # !ate: &'(#)('* Page: + of #+
A!!+,D-. A T0!-C 0rgani2ation 3 4anage(ent '". !oes the laboratory ha%e a policy to ensure its personnel are free from any commercial5 financial and other undue pressures5 3hich might ad%ersely affect the 4uality of the 3or D #". !oes the laboratory specify and document the responsibility5 authority5 and interrelation of all personnel 3ho manage5 perform or %erify 3or affecting the 4uality of calibrations and tests in =ob descriptions for all positions" *". !oes the laboratory ha%e documented certifications that personnel performing all tests for 3hich the laboratory is accredited ha%e the appropriate educational and(or technical bac groundsD )". !oes the laboratory nominate deputies in the case of absence of the technical director or QA officerD /". !oes the laboratory ha%e documented policies and procedures to ensure the protection of clientsE confidential information and proprietary rightsD 5uality $yste( '". Is the 4uality documentation a%ailable to5 understood by5 and implemented by all laboratory personnelD #". !oes the 4uality manual and related 4uality documentation include the ob=ecti%es and commitments by top managementD 1 , ,/A Co((ents
Controlled !ocument
Laboratory Name and contact information
Title: Internal Audit Standard Operating Procedure and Simplified Quality System Chec list !oc" No" # $e%" No" # !ate: &'(#)('* Page: '& of #+
*". !oes the 4uality manual and related 4uality documentation include the organi>ation and management structure of the laboratory5 its place in any parent organi>ation5 and rele%ant organi>ational chartsD )". !oes the 4uality manual and related 4uality documentation include procedures to ensure that all records re4uired under N<LAC are retainedD /". !oes the 4uality manual and related 4uality documentation include procedures for control and maintenance of documentation through a document control system 3hich ensures that all standard operating procedures5 manuals5 or documents clearly indicate the time period during 3hich the procedure or document 3as in forceD 9". !oes the 4uality manual and related 4uality documentation include procedures for achie%ing traceability of measurementsD 0". !oes the 4uality manual and related 4uality documentation include a list of all methods under 3hich the laboratory performs its accredited testingD @". !oes the 4uality manual and related 4uality documentation include mechanisms for ensuring that the laboratory re%ie3s all ne3 3or to ensure that it has the appropriate facilities and resources before commencing such 3or D +". !oes the 4uality manual and related 4uality documentation include reference to the calibration and(or %erification test procedures usedD '&". !oes the 4uality manual and related 4uality documentation include procedures for handling submitted samplesD
Controlled !ocument
Laboratory Name and contact information
Title: Internal Audit Standard Operating Procedure and Simplified Quality System Chec list !oc" No" # $e%" No" # !ate: &'(#)('* Page: '' of #+
''". !oes the 4uality manual and related 4uality documentation include reference to the ma=or e4uipment and reference measurement standards used as 3ell as the facilities and ser%ices used by the laboratory in conducting testsD '#". !oes the 4uality manual and related 4uality documentation include reference to procedures for calibration5 %erification and maintenance of e4uipmentD '*". !oes the 4uality manual and related 4uality documentation include reference to %erification practices including inter7 laboratory comparisons5 proficiency testing programs5 use of reference materials5 and internal 4uality control schemesD ')". !oes the 4uality manual and related 4uality documentation include procedures to be follo3ed for feedbac and correcti%e action for failed 4uality control samples5 or 3hen departures from documented policies5 procedures5 or N<LAC standards occurD '/". !oes the 4uality manual and related 4uality documentation include procedures for dealing 3ith complaintsD '9". !oes the 4uality manual and related 4uality documentation include processes(procedures for establishing that personnel are ade4uately e2perienced in the duties they are e2pected to carry out and(or recei%e any needed trainingD '0". !oes the 4uality manual and related 4uality documentation include processes and procedures for educating and training personnel in their ethical and legal responsibilities including the potential punishments and penalties for improper5 unethical5 or illegal actionsD
Controlled !ocument
Laboratory Name and contact information
Title: Internal Audit Standard Operating Procedure and Simplified Quality System Chec list !oc" No" # $e%" No" # !ate: &'(#)('* Page: '# of #+
'@". !oes the 4uality manual and related 4uality documentation include reference to procedures for reporting analytical resultsD '+". !oes the 4uality manual and related 4uality documentation include a Table of Contents5 and applicable lists of references and glossaries5 and appendicesD #&". !oes the QA officer eep the 4uality manual currentD #'". !oes the QA officer arrange for or conduct internal audits on the entire technical operation annually and notify laboratory management of deficiencies in the 4uality system and monitor correcti%e action ##". :here a complaint5 or any other circumstance5 raises doubt concerning the laboratoryEs compliance 3ith the laboratoryEs policies or procedures5 or 3ith the re4uirements of this Standard or other3ise concerning the 4uality of the laboratoryEs calibrations or tests5 does the laboratory ensure that those areas of acti%ity and responsibility in%ol%ed are promptly auditedD #*". !oes the laboratory ha%e a procedure for the annual management re%ie3 of the 4uality systemD #)". Is an annual re%ie3 of the 4uality system completed by management to e%aluate its continuing suitability and effecti%eness and ma e any necessary changes or impro%ementsD #/". !oes the annual re%ie3 ta e into account reports from managerial and super%isory personnel5 the outcome of recent internal audits5 assessments by e2ternal bodies5 the results of interlaboratory comparisons or proficiency tests5 any changes in the %olume and type of 3or underta en5 feedbac from clients5 correcti%e actions and other rele%ant factorsD
Controlled !ocument
Laboratory Name and contact information
Title: Internal Audit Standard Operating Procedure and Simplified Quality System Chec list !oc" No" # $e%" No" # !ate: &'(#)('* Page: '* of #+
#9". Are all audits and re%ie3 findings and any correcti%e actions that arise from them documentedD #0". !oes the laboratory management ensure that correcti%e actions are discharged 3ithin the agreed time frameD #@". !oes the laboratory implement chec s to monitor the 4uality of laboratory results using: ,a,, Internal 4uality control procedures -using statistical techni4ues 3hene%er possible.F ,b,, Participation in PT or other interlaboratory comparisonsF ,c,, $eference material and(or in7house 4uality control using secondary reference materialsF ,d,, $eplicate testingF ,e,, $e7testing of retained samplesF and(or ,f,, Correlation of results for different parameters of a sample" #+". !oes the laboratory ha%e general procedures to be follo3ed 3hen there are departures from documented policies5 procedures5 and QC ha%e occurredD *&". !o the procedures to be follo3ed 3hen there is a departure from documented policies5 procedures5 and QC include but not limited to: a,,, Identify the indi%iduals responsible for assessing each QC data typeF b,,, Identify the indi%iduals responsible for initiating and(or recommending correcti%e actionsF c,,, !efine ho3 the analyst should treat the data set if the associated QC measurements are unacceptableF d,,, Specify ho3 out7of7control situations and subse4uent correcti%e actions are to be documentedF and
Controlled !ocument
Laboratory Name and contact information
Title: Internal Audit Standard Operating Procedure and Simplified Quality System Chec list !oc" No" # $e%" No" # !ate: &'(#)('* Page: ') of #+
e,,, Specify procedures for management -including the QA officer. to re%ie3 correcti%e action reports" *'". Is a correcti%e action log maintained5 up7to7dateD *#". If a QC measure is out of control and the data is to be reported5 are data 4ualifiers reported 3ith samples associated 3ith failed QC measuresD **". Are all 4uality control measures assessed and e%aluated on an on7going basis5 and 4uality control acceptance limits used to determine the usability of the dataD *)". !oes the laboratory ha%e procedures for the de%elopment of acceptance(re=ection criteria 3here no method or regulatory criteria e2istD */". Are the 4uality control protocols specified by the laboratory6s method manual follo3edD Training '". Are training records a%ailable for all technical staff that include: a,,, <%idence that the employee has read5 understands5 and is using the latest %ersion of the lab6s in7house 4uality documentationF b,,, Training courses or 3or shops on specific e4uipment5 analytical techni4ues5 or lab proceduresF c,,, Training courses in ethical and legal responsibilities including the potential punishments G penalties for %iolations" d,,, <%idence that the employee has readF ac no3ledges5 and understands their personal G legal responsibilities including
Controlled !ocument
Laboratory Name and contact information
Title: Internal Audit Standard Operating Procedure and Simplified Quality System Chec list !oc" No" # $e%" No" # !ate: &'(#)('* Page: '/ of #+
potential punishments G penalties for %iolationsF and e,,, !ocumentation certifying that the employee has read5 understands5 and agrees to use the latest %ersion of a test method usedF and #"& Are initial demonstrations5 continuing demonstrations and method certification documented through the use of the forms in the latest appro%ed N<LAC document in Appendi2 CD *". !oes the laboratory use another approach5 documented in its Quality 8anual5 to demonstrate capability for analytes for 3hich spi ing is not an option and for 3hich 4uality control samples are not readily a%ailableD )". !oes the laboratory retain all associated supporting data necessary to reproduce the analytical results summari>ed in the I!C certification statementD /". Is a copy of the initial demonstration of Capability Certificate -I!C. in the personnel records for each employee performing a test methodD 9". !o the training records of each of the technical staff include documentation of continuing proficiency by at least one of the follo3ing: ,,, Acceptable performance of a blind sampleF ,,, Another demonstration of capabilityF ,,, Successful analysis of a blind performance sample on a similar test method using the same technologyF a ,,, Analysis of at least ) consecuti%e lab control samples 3ith acceptable le%els of precision and accuracyF or ,,, If one of the abo%e can be performed5 the analysis of authentic samples that ha%e been analy>ed by another trained
Controlled !ocument
Laboratory Name and contact information
Title: Internal Audit Standard Operating Procedure and Simplified Quality System Chec list !oc" No" # $e%" No" # !ate: &'(#)('* Page: '9 of #+
analyst 3ith statistically indistinguishable results" 0". !oes the laboratory complete a ne3 demonstration of capability 3hene%er there is a significant change in instrument type5 personnel5 or test methodD @". Bas the laboratory management de%eloped a proacti%e program for the detection of improper5 unethical5 or illegal actionsD +6uip(ent '". Are maintenance procedures documentedD #". Is each item of e4uipment including reference materials labeled5 mar ed or other3ise identified to indicate its calibration status5 3hen appropriateD *". Are maintenance records a%ailableD Support <4uipment Calibration and Traceability '". !oes the laboratory ha%e an established program for the calibration and %erification of its measuring and test e4uipment including balances5 thermometers and control standardsD #". Are measurements made by the labs traceable to national standards of measurement 3here a%ailableD *". !oes the laboratory maintain records of all certificates that indicate traceability to national standards of measurement and(or statements of compliance 3ith an identified metrological specificationD )". Is all support e4uipment calibrated annually5 using NIST traceable references 3hen a%ailable5 o%er the entire range in 3hich the e4uipment is usedD /". Are the results of support e4uipment calibration 3ithin the
Controlled !ocument
Laboratory Name and contact information
Title: Internal Audit Standard Operating Procedure and Simplified Quality System Chec list !oc" No" # $e%" No" # !ate: &'(#)('* Page: '0 of #+
specifications re4uired of the application for 3hich it is usedD 9". Is support e4uipment remo%ed from ser%ice until repaired or is a de%iation cur%e prepared and all measurements corrected for the de%iation 3hen the calibration is not 3ithin acceptance limitsD 0". !oes the laboratory maintain records of established correction factors to correct measurementsD @". Are all ra3 data records retained to document e4uipment performanceD +". Prior to use on each 3or ing day5 are balances5 o%ens5 refrigerators5 free>ers5 incubators and 3ater baths chec ed 3ith NIST traceable references -3here possible. in the e2pected use rangeD '&". Are mechanical %olumetric de%ices chec ed for accuracy on a 4uarterly basisD ''". !emonstration of sterili>ation for biological tests pro%ided by use of a continuous temperature recording or 3ith the fre4uent use of spore stripsD $0!$ and Test 4ethods '". !oes the laboratory ha%e SOPs for all test methodsD #". Are all instructions5 standards5 manuals and reference data rele%ant to the 3or of the laboratory maintained up7to7date and readily a%ailable to the staffD *". Are copies of SOPs assessable to all personnelD )". !oes each SOP clearly indicate: a,,, <ffecti%e date of the SOP b,,, $e%ision number
Controlled !ocument
Laboratory Name and contact information
Title: Internal Audit Standard Operating Procedure and Simplified Quality System Chec list !oc" No" # $e%" No" # !ate: &'(#)('* Page: '@ of #+
c,,, Signature-s. of appro%ing authority /". !oes the laboratory ha%e an in7house method manual for each accredited analyte or test method that clearly describes the lab6s methodD 9". In cases 3here modifications are made to published methods or 3here the reference test method is ambiguous or pro%ides insufficient detail5 are any modifications5 changes5 or clarifications clearly indicatedD 0". Are the practices specified by the laboratory6s method manual follo3ed by all analystsD @". Are all essential 4uality control measures incorporated in the lab6s method manualD +". Are all 4uality control measures assessed and e%aluated on an on7going basisD '&". !oes the laboratory ha%e procedures for de%eloping acceptance(re=ection criteria for each test methodD ''". !o the SOPs or the test method SOP reference the details of the initial calibration procedures5 including calculations integrations5 and acceptance criteria associated statisticsD '#". Is the criteria for the acceptance of an initial calibration established -correlation coefficient or relati%e percent difference.D '*". Are the details of the continuing instrument calibration procedure5 calculations5 and associated statistics included or referenced in the test method SOPD ')". !oes the laboratory establish Standard Operating Procedures to ensure that the reported data is free from
Controlled !ocument
Laboratory Name and contact information
Title: Internal Audit Standard Operating Procedure and Simplified Quality System Chec list !oc" No" # $e%" No" # !ate: &'(#)('* Page: '+ of #+
transcription and calculation errorsD '/". !oes the laboratory establish Standard Operating Procedures to ensure that all 4uality control measures are re%ie3ed5 and e%aluated before data is reportedD '9". Are calculations and data transfers sub=ect to chec s as established in the laboratory6s SOPD '0". !o documented procedures e2ist for the purchase5 reception and storage of consumable materials used for the technical operations of the laboratoryD '@". !oes the laboratory retain records for all standards5 including manufacturer(%endor5 the manufacturer6s Certificate of Analysis or purity -if supplied.5 date of receipt5 recommended storage conditions5 and an e2piration date after 3hich the material shall not be used unless %erified by the laboratoryD '+". Are original reagent containers labeled 3ith the e2piration dateD #&". Are detailed records maintained on reagent and standard preparationD #'". !o the records of reagent and standard preparation indicate traceability to purchased stoc s or neat compounds5 and include the date of preparation and preparerEs initialsD ##". Are containers of prepared reagents and standards uni4uely identified and include an e2piration date and can it be lin ed to the documentation of its preparationD $a(ple Handling '". !oes the laboratory ha%e a documented system for uni4uely identifying the items to be tested5 to ensure that there can be no
Controlled !ocument
Laboratory Name and contact information
Title: Internal Audit Standard Operating Procedure and Simplified Quality System Chec list !oc" No" # $e%" No" # !ate: &'(#)('* Page: #& of #+
confusion regarding the identity of such items at any timeD #". !oes the laboratory assign a uni4ue identification -I!. code to each sample container recei%ed in the laboratoryD *". Is the laboratory I! code placed on the sample container as a durable labelD )". Is the laboratory I! code entered into the laboratory records -see /"''"*"d. and does the lin that associate the sample 3ith related laboratory acti%ities such as sample preparation or calibrationD /". !oes the laboratory ha%e a 3ritten sample acceptance policy that clearly outlines the circumstances under 3hich samples 3ill be acceptedD 9". Is data from any sample 3hich does not meet the policy criteria flagged in an unambiguous manner clearly defining the nature and substance of the %ariationD 0". Is the sample acceptance policy made a%ailable to sample collecting personnel and does it include at a minimum all the policy criteriaD @". 1pon receipt5 is the condition of the sample5 including any abnormalities or departures from standard condition as prescribed in the rele%ant test method5 recordedD +". Are all items specified in sample acceptance policy criteria chec edD '&". Are all samples5 3hich re4uire thermal preser%ation5 considered acceptable if the arri%al temperature is either 3ithin H(7#C of the re4uired temperature or in the method specified rangeD
Controlled !ocument
Laboratory Name and contact information
Title: Internal Audit Standard Operating Procedure and Simplified Quality System Chec list !oc" No" # $e%" No" # !ate: &'(#)('* Page: #' of #+
''". ?or samples 3ith a specified temperature of )C5 are samples maintained 3ithin a temperature of =ust abo%e free>ing to 9CD '#". In cases 3here samples are hand deli%ered to the laboratory immediately after collection and do not meet the temperature criteria considered acceptable5 is there e%idence that the chilling process has begun such as arri%al on iceD '*". !oes the laboratory ha%e procedures for chec ing chemical preser%ation using readily a%ailable techni4ues5 such as pB5 free chlorine or temperature5 prior to or during sample preparation or analysisD ')". !oes the laboratory implement procedures for chec ing chemical preser%ation using readily a%ailable techni4ues5 such as pB5 free chlorine or temperature5 prior to or during sample preparation or analysisD '/". Are the results of all chec s recordedD '9". If the sample does not meet the sample receipt acceptance criteria does the laboratory do any of the follo3ing: ,,, $etain correspondence and(or records of con%ersations concerning the final disposition of re=ected ,,, ?ully document any decision to proceed 3ith the analysis of samples not meeting acceptance criteria ,,, Is the condition of these samples5 at a minimum5 noted on the chain of custody or transmittal form and laboratory receipt documentsD ,,, Is the analysis data of these samples appropriately I4ualifiedI on the final reportD
Controlled !ocument
Laboratory Name and contact information
Title: Internal Audit Standard Operating Procedure and Simplified Quality System Chec list !oc" No" # $e%" No" # !ate: &'(#)('* Page: ## of #+
'0". !oes the laboratory utili>e a permanent5 se4uential log5 such as a logboo or electronic record5 to document receipt of all sample containersD '@". Is the follo3ing information recorded in the laboratory chronological logD a,,, Client(Pro=ect Name b,,, !ate and time of laboratory receipt of sample c,,, 1ni4ue laboratory I! code -see /"''"'. d,,, Signature or initials of the person ma ing the entries '+". Are samples stored a3ay from all standards5 reagents5 food and other potentially contaminating sources in such a manner as to pre%ent cross contaminationD #&". Are samples5 sample fractions5 e2tracts5 leachates or other sample preparation fractions stored according to the conditions specified by preser%ation protocols or according to the test methodD #'". !oes the laboratory ha%e standard operating procedures for the disposal of samples5 digestates5 leachates and e2tracts or other sample preparation productsD Records '". !oes the laboratory retain on record all original obser%ations5 calculations and deri%ed data5 calibration records and a copy of the test report for fi%e yearsD #". !oes the record eeping system allo3 historical reconstruction of all laboratory acti%ities that produced the resultant sample analytical dataD *". Is the history of the sample readily understood through the
Controlled !ocument
Laboratory Name and contact information
Title: Internal Audit Standard Operating Procedure and Simplified Quality System Chec list !oc" No" # $e%" No" # !ate: &'(#)('* Page: #* of #+
documentation including inter7laboratory transfers of samples and(or e2tractsD )". !o the records include the identity of personnel in%ol%ed in sampling5 preparation5 calibration or testingD /". Are all documentation entries signed or initialed by responsible staff 3ith the reason for the signature or initial clearly indicated in the recordsD -<2" Jsampled byK5 Jprepared byK5 re%ie3ed byK. 9". Are all generated data5 e2cept those that are generated by automated data collection systems5 recorded directly5 promptly and legibly in permanent in D 0". Are entries in records not obliterated by methods such as erasures5 o%er3ritten files or mar ingsD @". Are all corrections to record7 eeping errors made by one line mar ed through the error and the indi%idual ma ing the correction signing -or initialing. and dating the correctionD +". !o records that are stored or generated by computers or personal computers -PCS. ha%e hard copy or 3rite7protected bac up copiesD '&". !oes the laboratory ha%e a record management system for control of laboratory noteboo sF instrument logboo sF standards logboo sF and records for data reduction5 %alidation storage and reportingD ''". Is access to archi%ed information documented 3ith an access logD '#". Is archi%ed information protected against fire5 theft5 loss5 en%ironmental deterioration5 and %ermin and5 in the case of
Controlled !ocument
Laboratory Name and contact information
Title: Internal Audit Standard Operating Procedure and Simplified Quality System Chec list !oc" No" # $e%" No" # !ate: &'(#)('* Page: #) of #+
electronic records5 electronic or magnetic sourcesD Reports '". !oes the test report contain all information necessary for the interpretation of the test results and all information re4uired by the method usedD #". !oes the facility management ensure that the appropriate report items are in the report to the regulatory authority if the report is prepared by another indi%idual 3ithin the organi>ation" *". :here the certificate or report contains results of tests performed by sub7contractors5 are these results clearly identified by subcontractor name or applicable accreditation numberD )". !oes the laboratory certify that the test results meet all re4uirements of N<LAC or pro%ide reasons and(or =ustification if they do notD Che(istry 5uality Control (To be used with the method checklist) '". Is a method blan performed ' per batch5 per matri2 type per sample e2traction or preparation methodD #". Is the analysis stopped5 corrected and the problem eliminated if the blan contamination is greater than '('&th of the measured sample contamination or '('&th of the regulatory limitF or are the results reported 3ith appropriate data 4ualifying codesD *". Is an LCS -a sample matri2 free of analytes of interest spi ed 3ith a %erified no3n amount of analyte. analy>ed at a minimum of ' per batch of #& or less samples per matri25 per sample e2traction or preparation method e2cept for analytes for 3hich spi ing solutions are not a%ailableD
Controlled !ocument
Laboratory Name and contact information
Title: Internal Audit Standard Operating Procedure and Simplified Quality System Chec list !oc" No" # $e%" No" # !ate: &'(#)('* Page: #/ of #+
)". Is a matri2 spi e -sample prepared by adding a no3n mass of target analyte to a specific amount of matri2 sample. performed at a fre4uency of ' in #& samples per matri25 per sample e2traction or preparation methodD /". Is a matri2 spi e duplicate -8S!. or laboratory duplicate performed at a fre4uency of ' in #& samples per matri25 per sample e2traction or preparation methodD 9". Is the initial instrument calibration used directly for 4uantitationD 0". Is the continuing instrument calibration %erification used to confirm the continued %alidity of the initial calibrationD @". Are all initial calibrations %erified 3ith a standard obtained from a second sourceD +". If the results of samples are not brac eted by the initial calibration5 are the results reported as ha%ing less certainty -defined 4ualifiers5 flags5 or e2planation in the case narrati%e.D '&". Is the lo3est calibration standard of the initial calibration abo%e the detection limitD ''". :hen an initial calibration is not performed on the day of analysis5 does the laboratory %erify the %alidity of the initial calibration prior to the analysis of samples by analy>ing a continuing instrument calibration %erification sampleD '#". Is continuing instrument calibration %erification repeated at the beginning and end of each analytical batchD -If an internal standard is used5 only one continuing calibration %erification must be analy>ed per analytical batch. '*". Are the concentrations of the continuing calibration
Controlled !ocument
Laboratory Name and contact information
Title: Internal Audit Standard Operating Procedure and Simplified Quality System Chec list !oc" No" # $e%" No" # !ate: &'(#)('* Page: #9 of #+
standard %aried 3ithin the established calibration rangeD ')". Are sufficient ra3 data records retained to permit reconstruction of the initial calibration including: a,,, Calibration date b,,, Test method c,,, Instrument d,,, Analysis date e,,, <ach analyte name f,,, Concentration g,,, $esponse h,,, Calibration cur%e or response factor '/". !oes the laboratory use detection limits that are determined by the protocol in the mandated test method or applicable regulationD '9". Is the 4uality of 3ater sources monitored and documented to meet method specified re4uirementsD 5uality Control for 7acteriology (To be used with the method checklist) '". Are temperatures of incubators and 3ater baths recorded t3ice daily -morning G afternoon. as re4uired by the methods as indicated belo3: a" ,,,, b" ,,,, Total Coliform bacteria incubation at */"& H(7 &"/ degrees Celsius -S8+##';5 S8+##'!5 S8+###; G <PA79&&(@70@7&'0. ?ecal Coliform bacteria incubation at ))"/ H(7 &"# degrees Celsius -S8+##'<5 S8+###!5 G <PA7 9&&(@70@7&'0.
Controlled !ocument
Laboratory Name and contact information
Title: Internal Audit Standard Operating Procedure and Simplified Quality System Chec list !oc" No" # $e%" No" # !ate: &'(#)('* Page: #0 of #+
c" ,,,,
Total Coliform G <scherichia coli -<" coli. incubation at */"& H(7 &"/ degrees Celsius -S8+##* H 1CF Colilert5 Ide227'@5 G Colisure. d" ,,,, <" coli incubation at ))"/ H(7 &"# degrees Celsius -<C 3ith 81L or Nutrient Agar 3ith 81L. #". Is the follo3ing support e4uipment associated 3ith microbiological testing chec ed 3ith NIST traceable materials -3here possible. a" ,,,, pB meter b" ,,,, ;alance-s. c" ,,,, Conducti%ity meter d" ,,,, $efrigerator-s. for sample storage and(or media storage e" ,,,, Incubators f" ,,,, :ater baths *". Is a minimum of one uninoculated control prepared and analy>edD )". :hen the same e4uipment is used to prepare multiple samples does the laboratory prepare at least one blan at the beginning5 one at the end5 3ith additional blan s inserted after e%ery '& samplesD /". Is a no3n negati%e culture analy>ed 3ith each set of samples" 9". Is each lot of media tested on a monthly basis 3ith at least one pure culture of a no3n positi%e reaction -positi%e control.D -Not re4uired if the laboratory has at least one no3n positi%e result of the appropriate organism during the month."
Controlled !ocument
Laboratory Name and contact information
Title: Internal Audit Standard Operating Procedure and Simplified Quality System Chec list !oc" No" # $e%" No" # !ate: &'(#)('* Page: #@ of #+
0". Is the positi%e control test tested 3ith a sample test batchD @". Are at least /M of the suspected positi%e samples analy>ed in duplicateD +". In laboratories 3ith more than one analyst performs the testing does each analyst ma e parallel analyses on at least one positi%e sample per monthD '&". Are the calculations5 data reduction and statistical interpretations specified by each method follo3edD ''". :here the method specifies colony counts5 such as membrane filter or colony counting5 is the ability of indi%idual analysts to count colonies %erified at least once per month5 by ha%ing t3o or more analysts count colonies from the same plateD '#". In order to demonstrate traceability and selecti%ity5 does the laboratory use reference cultures of microorganisms obtained from a recogni>ed national collection or an organi>ation recogni>ed by the assessor bodyD '*". Are the graduations of the temperature measuring de%ices appropriate for the re4uired accuracy of measurementD ')". Are records maintained on all laboratory reagent 3ater monitoring acti%ities as belo3 3hen dilution 3ater and(or media are prepared in house: a, $esidual Chlorine N '"& mg(L" b, Conducti%ity N #"& umho(cm at #/ degrees Celsius c, Beterotrophic Plate Count N '&&& cfu per mL" d, ;acteriological ratio &"@ O *"&" e, Cd5 Cr5 Cu5 Ni5 Pb5 Pn each N &"&/ mg(L5 collecti%ely
Controlled !ocument
Laboratory Name and contact information
Title: Internal Audit Standard Operating Procedure and Simplified Quality System Chec list !oc" No" # $e%" No" # !ate: &'(#)('* Page: #+ of #+
N &"' mg(L" f, $ecords maintained for the past fi%e yearsD
Controlled !ocument
S-ar putea să vă placă și
- 3.9 SOP Internal Audit v1Document11 pagini3.9 SOP Internal Audit v1Pramod AthiyarathuÎncă nu există evaluări
- AM-QMS-05-Internal Quality Audit Procedure - Ver 1Document6 paginiAM-QMS-05-Internal Quality Audit Procedure - Ver 1Deepan TravellerÎncă nu există evaluări
- E Internal Audit Procedure Section 5Document3 paginiE Internal Audit Procedure Section 5Ngonidzashe Zvarevashe100% (1)
- InternalAuditSOP 012413Document8 paginiInternalAuditSOP 012413zubair90Încă nu există evaluări
- Example of An Internal Audit SOPDocument3 paginiExample of An Internal Audit SOPVeronica Sebald100% (1)
- QSP 02 - Record Control ProcedureDocument5 paginiQSP 02 - Record Control ProcedureVivek V100% (1)
- Internal Audit Planning and Scheduling Sample FormatDocument3 paginiInternal Audit Planning and Scheduling Sample Formatsameh100% (2)
- Internal Audit SOPDocument4 paginiInternal Audit SOPIftikhar Khan100% (1)
- ISO27k Nonconformity Corrective Preventive Action FormDocument3 paginiISO27k Nonconformity Corrective Preventive Action FormJasim's BhaignaÎncă nu există evaluări
- COMP-OPP-02 Procedure For Control and Validation of Service ProvisionDocument6 paginiCOMP-OPP-02 Procedure For Control and Validation of Service ProvisionISODCC DSPIÎncă nu există evaluări
- Internal Audit SOPDocument2 paginiInternal Audit SOPNazmun100% (3)
- QP-004 Management Review ProcessDocument3 paginiQP-004 Management Review Processesraa asemÎncă nu există evaluări
- Internal Quality Audit ProcedureDocument3 paginiInternal Quality Audit ProcedureAcholonu Emeka Jp100% (2)
- GDP Write Up PDFDocument23 paginiGDP Write Up PDFSaicharan Reddy100% (1)
- 014 IITS Internal AuditDocument5 pagini014 IITS Internal AuditSirajul IslamÎncă nu există evaluări
- Sop (Internal Audit)Document6 paginiSop (Internal Audit)Arijit Pattanayak100% (1)
- Steps of An Internal AuditDocument3 paginiSteps of An Internal AuditDownload100% (1)
- Audit Report Template 13Document5 paginiAudit Report Template 13Ahmed HosneyÎncă nu există evaluări
- Internal Audit ScheduleDocument4 paginiInternal Audit ScheduleCQMS 5S DivisionÎncă nu există evaluări
- ESCL-SOP-018, Inspection and Test Procedure For Egba Split-ClampsDocument6 paginiESCL-SOP-018, Inspection and Test Procedure For Egba Split-ClampsadiqualityconsultÎncă nu există evaluări
- FDA Volume II - Audits Ora-Lab.4.14Document7 paginiFDA Volume II - Audits Ora-Lab.4.14nilayÎncă nu există evaluări
- Handling of Out of Specification ResultsDocument39 paginiHandling of Out of Specification ResultsDevang GondaliyaÎncă nu există evaluări
- Control of Records: Organization Details JustificationDocument1 paginăControl of Records: Organization Details JustificationaezacsÎncă nu există evaluări
- Guide For Internal Audit & Management Review For LaboratoriesDocument24 paginiGuide For Internal Audit & Management Review For LaboratoriesRaman GolaÎncă nu există evaluări
- Internal AuditsDocument9 paginiInternal Audits李哲祥100% (1)
- Internal Quality Audit Procedure ExampleDocument3 paginiInternal Quality Audit Procedure ExampleRaj Kumar Ahmed100% (1)
- ISODocument11 paginiISOaiswaryacdas9853Încă nu există evaluări
- Internal Audit Planning and Scheduling Sample FormatDocument3 paginiInternal Audit Planning and Scheduling Sample FormatAkasha KhanÎncă nu există evaluări
- Internal Audit ProcedureDocument1 paginăInternal Audit ProcedureXi MoÎncă nu există evaluări
- F4E-QA-102 Supplier Audit Implementation 296E7T v2 3Document18 paginiF4E-QA-102 Supplier Audit Implementation 296E7T v2 3Jai BhandariÎncă nu există evaluări
- Form IA-010NC (Audit Notification Letter) (3!28!2012)Document1 paginăForm IA-010NC (Audit Notification Letter) (3!28!2012)granburyjohnstevensÎncă nu există evaluări
- List of Documents ISO 13485 Documentation ToolkitDocument4 paginiList of Documents ISO 13485 Documentation ToolkitGreeshma Certvalue100% (3)
- Iso15189 2022checklistDocument42 paginiIso15189 2022checklistReema Md100% (1)
- New Supplier Survey FormDocument14 paginiNew Supplier Survey Formsutharitessh100% (1)
- Internal Audit Annual Report and 2017 Audit Plan (PDFDrive)Document29 paginiInternal Audit Annual Report and 2017 Audit Plan (PDFDrive)samirÎncă nu există evaluări
- 17 - Procedure - Control of Records ProcessDocument4 pagini17 - Procedure - Control of Records ProcessSaAhRa100% (1)
- Annual Audit Plan Template - Revised Version 2011Document36 paginiAnnual Audit Plan Template - Revised Version 2011Jasim BalochÎncă nu există evaluări
- PR018 Internal Audit Procedure: ISO 9001:2008 Clause 8.2.2Document9 paginiPR018 Internal Audit Procedure: ISO 9001:2008 Clause 8.2.2uks444100% (1)
- SOP of Internal AuditDocument1 paginăSOP of Internal AuditZiaul HuqÎncă nu există evaluări
- Performance EvaluationDocument3 paginiPerformance Evaluationmool raj100% (1)
- Internal Audit ChecklistDocument5 paginiInternal Audit ChecklistBalkan Bpromo KosovëÎncă nu există evaluări
- SOP ThermometerDocument4 paginiSOP Thermometersuko winartiÎncă nu există evaluări
- Internal Audit 2020Document3 paginiInternal Audit 2020anupriya mittalhy6Încă nu există evaluări
- Internal Audit NC ReportDocument1 paginăInternal Audit NC Reportmorshed_mahamud7055Încă nu există evaluări
- Internal Audit SOPDocument3 paginiInternal Audit SOPImran Alam ChowdhuryÎncă nu există evaluări
- Procedure On Internal AuditDocument16 paginiProcedure On Internal Auditndayiragije JMVÎncă nu există evaluări
- Internal Audit Procedure ExampleDocument4 paginiInternal Audit Procedure Examplestephenb4uÎncă nu există evaluări
- CLAUSE 8.5 Production and Service ProvisionDocument10 paginiCLAUSE 8.5 Production and Service ProvisionNavnath TamhaneÎncă nu există evaluări
- Preventive Maintenance PlanDocument7 paginiPreventive Maintenance PlanCuyapo Infirmary Lying-In HospitalÎncă nu există evaluări
- SOP of Glassware HandlingDocument1 paginăSOP of Glassware HandlingPrince Moni100% (2)
- Minutes of Management Review Meeting OkDocument3 paginiMinutes of Management Review Meeting Okdidar100% (1)
- Internal Quality Audit Plan Dilg Region 10Document8 paginiInternal Quality Audit Plan Dilg Region 10Cess AyomaÎncă nu există evaluări
- 6.4 Incident ReportDocument5 pagini6.4 Incident ReportBALAJI0% (1)
- Management System Manual Iso 90014 fEB-11Document23 paginiManagement System Manual Iso 90014 fEB-11ilgarÎncă nu există evaluări
- 5S Audit Checklist: Sort Set in Order Shine Standardize Sustain Total Total Score No. of Questions Average ScoreDocument4 pagini5S Audit Checklist: Sort Set in Order Shine Standardize Sustain Total Total Score No. of Questions Average ScoreCaroline Eliza MendesÎncă nu există evaluări
- Audit Schedule - Department WiseDocument10 paginiAudit Schedule - Department WiseashishvaidÎncă nu există evaluări
- SOP - 0111 - 10 - Vendor Audit SOPDocument9 paginiSOP - 0111 - 10 - Vendor Audit SOPrana_ehsan1163100% (2)
- E 178 Iso17025 ChecklistDocument19 paginiE 178 Iso17025 ChecklistShweta Rawal VijÎncă nu există evaluări
- Auditing Gas Analysis LaboratoriesDocument14 paginiAuditing Gas Analysis LaboratorieshentadwyÎncă nu există evaluări
- Tabular Summary of Selected Relevant Points From ISO/IEC 17025:2005Document11 paginiTabular Summary of Selected Relevant Points From ISO/IEC 17025:2005ahkiaenaaaa100% (1)
- NQA ISO 9001 Implementation GuideDocument32 paginiNQA ISO 9001 Implementation Guiderifki75% (4)
- Form JO Foundation ChecklistDocument1 paginăForm JO Foundation ChecklistRony LesbtÎncă nu există evaluări
- Steel Pipe Pile ASTM A252 PDFDocument4 paginiSteel Pipe Pile ASTM A252 PDFfikriy_civil86Încă nu există evaluări
- NQA Aerospace Workshop Webinar 02.10.2020Document53 paginiNQA Aerospace Workshop Webinar 02.10.2020Rony LesbtÎncă nu există evaluări
- NQA Webinar - Cyber Security Risk and Assurance in The Supply Chain (WMCRC & Tuned To RISK) 21.04.21Document36 paginiNQA Webinar - Cyber Security Risk and Assurance in The Supply Chain (WMCRC & Tuned To RISK) 21.04.21Rony LesbtÎncă nu există evaluări
- NQA Webinar PAS 2060 Carbon Neutrality 30-04-2021Document38 paginiNQA Webinar PAS 2060 Carbon Neutrality 30-04-2021Rony LesbtÎncă nu există evaluări
- NQA ISO 45001 Implementation GuideDocument36 paginiNQA ISO 45001 Implementation GuideSubhi El Haj Saleh100% (5)
- 43 - hs-78013 - Earthing High Voltage ApparatusDocument6 pagini43 - hs-78013 - Earthing High Voltage ApparatusRony Lesbt100% (1)
- 6 RABQSA - OH Examination Guide Final Rev2Document16 pagini6 RABQSA - OH Examination Guide Final Rev2Rony LesbtÎncă nu există evaluări
- NQA Webinar - Demystifying ISO 27001 30.06.2021Document45 paginiNQA Webinar - Demystifying ISO 27001 30.06.2021Rony LesbtÎncă nu există evaluări
- TBM FormDocument1 paginăTBM FormRony LesbtÎncă nu există evaluări
- Cause Analysis For Spun Pile Crack and BrokenDocument11 paginiCause Analysis For Spun Pile Crack and BrokenRony LesbtÎncă nu există evaluări
- NQA Webinar - Keeing Your Management System Simple (AvISO) - 11.06.21Document21 paginiNQA Webinar - Keeing Your Management System Simple (AvISO) - 11.06.21Rony LesbtÎncă nu există evaluări
- Summary RFI of FDT UG PipeDocument2 paginiSummary RFI of FDT UG PipeRony LesbtÎncă nu există evaluări
- Hand Out Shop Inspection - ISBL - OSBL 210527Document3 paginiHand Out Shop Inspection - ISBL - OSBL 210527Rony LesbtÎncă nu există evaluări
- 63 - hs-78033 - Application of Safety Rule General ProvisionsDocument5 pagini63 - hs-78033 - Application of Safety Rule General ProvisionsRony LesbtÎncă nu există evaluări
- 35 - HS-78005 - Isolation ProcedureDocument3 pagini35 - HS-78005 - Isolation ProcedureRony LesbtÎncă nu există evaluări
- Name Target # of Abnormality Finding (# of Mudawalk/month) Week 1 Week 2 Week 3Document3 paginiName Target # of Abnormality Finding (# of Mudawalk/month) Week 1 Week 2 Week 3Rony LesbtÎncă nu există evaluări
- Course Assessment Form Date .. . Course Title: . Facilitator: Location: .. 1Document1 paginăCourse Assessment Form Date .. . Course Title: . Facilitator: Location: .. 1Rony LesbtÎncă nu există evaluări
- Module 1 OH (Book)Document38 paginiModule 1 OH (Book)Rony LesbtÎncă nu există evaluări
- Register of Operation On ProcessDocument18 paginiRegister of Operation On ProcessRony LesbtÎncă nu există evaluări
- 6 RABQSA - OH Examination Guide Final Rev2Document16 pagini6 RABQSA - OH Examination Guide Final Rev2Rony LesbtÎncă nu există evaluări
- 8 Module 1 RABQSA-OH InstructionsDocument1 pagină8 Module 1 RABQSA-OH InstructionsRony LesbtÎncă nu există evaluări
- B0 HS 78047 Material Transfer ProcedureDocument5 paginiB0 HS 78047 Material Transfer ProcedureRony LesbtÎncă nu există evaluări
- TgBin TTB B0 HS 78045 Handling SRCC Documentation and DistributionDocument15 paginiTgBin TTB B0 HS 78045 Handling SRCC Documentation and DistributionRony LesbtÎncă nu există evaluări
- 70 - HS-78040 - Access To Confined SpacesDocument8 pagini70 - HS-78040 - Access To Confined SpacesRony LesbtÎncă nu există evaluări
- 35 - HS-78005 - Isolation ProcedureDocument3 pagini35 - HS-78005 - Isolation ProcedureRony LesbtÎncă nu există evaluări
- 73 - hs-78043 - Handling of Vaporising LiquidsDocument9 pagini73 - hs-78043 - Handling of Vaporising LiquidsRony LesbtÎncă nu există evaluări
- 74 - hs-78044 - Ionising Radiation's For NDTDocument13 pagini74 - hs-78044 - Ionising Radiation's For NDTRony LesbtÎncă nu există evaluări
- TgBin TTB B0 HS 78046 Tagging Procedure LatestDocument16 paginiTgBin TTB B0 HS 78046 Tagging Procedure LatestRony Lesbt100% (1)
- Schedule Q Based QuestionsDocument11 paginiSchedule Q Based Questionschandu666creator100% (7)
- Internship Report AitasamDocument39 paginiInternship Report AitasamQareena ChÎncă nu există evaluări
- MS -37-Proposal For Diversion of Existing 200Φ D.I. Sewer PiDocument13 paginiMS -37-Proposal For Diversion of Existing 200Φ D.I. Sewer PiSyed Umair HashmiÎncă nu există evaluări
- Quality Control ProcedureDocument12 paginiQuality Control ProcedureZiya Ahmed100% (6)
- 4 SQAandSDLCDocument17 pagini4 SQAandSDLCSandy CyrusÎncă nu există evaluări
- 9020 Quality Assurance-Quality Control (1997)Document13 pagini9020 Quality Assurance-Quality Control (1997)Helena RubinatoÎncă nu există evaluări
- Smoke Test Sanity TestDocument7 paginiSmoke Test Sanity TestantoniealbertoÎncă nu există evaluări
- SOP For Stability Studies of Finished Goods - Pharmaceutical Guidelines PDFDocument2 paginiSOP For Stability Studies of Finished Goods - Pharmaceutical Guidelines PDFsivabioteckÎncă nu există evaluări
- Balaji Scope 17-19 PDFDocument7 paginiBalaji Scope 17-19 PDFSwapnil MahajanÎncă nu există evaluări
- Amusement Rides and Leisure Parks PDFDocument4 paginiAmusement Rides and Leisure Parks PDFMohd Aidil OsmanÎncă nu există evaluări
- Advanced Gas Analysis - Kept SimpleDocument24 paginiAdvanced Gas Analysis - Kept SimplePaulo VidasÎncă nu există evaluări
- Introduction To QualityDocument13 paginiIntroduction To QualityDipak ShindeÎncă nu există evaluări
- Navair 01 1a 17 (With Change 2)Document218 paginiNavair 01 1a 17 (With Change 2)Ozkhar AFÎncă nu există evaluări
- PV QCDocument1 paginăPV QCrameshqcÎncă nu există evaluări
- SQMP ManualDocument81 paginiSQMP ManualAnkita DwivediÎncă nu există evaluări
- RPT 148Document37 paginiRPT 148Christopher Scott RoseÎncă nu există evaluări
- Work Execution Plan (Swamp)Document93 paginiWork Execution Plan (Swamp)Victor Egharevba100% (1)
- EOI Empl-Engg ConsultantsDocument54 paginiEOI Empl-Engg ConsultantsvenspalÎncă nu există evaluări
- Integrated Offshore Constr Work-Instruction To Tenderers (Final)Document74 paginiIntegrated Offshore Constr Work-Instruction To Tenderers (Final)Idara Okopido0% (1)
- Astm F2972 - 15 - SGQDocument3 paginiAstm F2972 - 15 - SGQrafaelengemecanicoÎncă nu există evaluări
- Bechtel QAQC AdvisorDocument5 paginiBechtel QAQC Advisoryoonchankim0911100% (1)
- Circulation of Draft Specification Central Road Lab PDFDocument688 paginiCirculation of Draft Specification Central Road Lab PDFsuman subediÎncă nu există evaluări
- Qa and QCDocument4 paginiQa and QCEmmanuel IsiayinekifeÎncă nu există evaluări
- BFD81 Relief ValvesDocument26 paginiBFD81 Relief ValvesNilesh MistryÎncă nu există evaluări
- Masood Textile Mills ReportDocument42 paginiMasood Textile Mills ReportZeeshan Haider Awan100% (4)
- Anil Kumar PatnaikbantupalliDocument4 paginiAnil Kumar PatnaikbantupalliShiva KumarÎncă nu există evaluări
- ITP Procedure & FormsDocument18 paginiITP Procedure & FormsResearcherÎncă nu există evaluări
- Lessons Learned in Startup and Commissioning of Simple Cycle and Combined Cycle Combustion Turbine PlantsDocument114 paginiLessons Learned in Startup and Commissioning of Simple Cycle and Combined Cycle Combustion Turbine Plantsphoenix609100% (1)
- Pressure Vessel Quality Manual 2Document51 paginiPressure Vessel Quality Manual 2Nguyen Duc Dung75% (4)
- SSPC CS 36Document5 paginiSSPC CS 36Gaapchu0% (1)