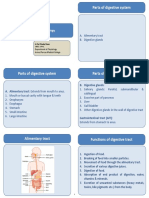
Documente Academic
Documente Profesional
Documente Cultură
Physiologicalaspectsofphysiquebuilding PDF
Încărcat de
PedroZ3Descriere originală:
Titlu original
Drepturi de autor
Formate disponibile
Partajați acest document
Partajați sau inserați document
Vi se pare util acest document?
Este necorespunzător acest conținut?
Raportați acest documentDrepturi de autor:
Formate disponibile
Physiologicalaspectsofphysiquebuilding PDF
Încărcat de
PedroZ3Drepturi de autor:
Formate disponibile
www.abcbodybuilding.
com
Muscle Physiology
Physiological Aspects of Physique Building Part 1
Researched and Composed by Jacob Wilson, BSc. (Hons), MSc. CSCS
Abstract
The purpose of this paper was to review the topic of maximized muscular hypertrophy within the bodybuilding regimen. Muscular hypertrophy is defined as an increase in diameter of muscular tissue.
Hypertrophy - Muscular Growth
Hypertrophy is the result of an increase in the contractile filaments, which comprise a muscle fiber. Of prime importance are the actin and myosin filaments within a myofibril. These allow for the expression of musculature contraction. Adaptations within these structures are a consequence of contractile demands on the system itself. The increase seen can be viewed grossly through two mechanisms. The first adaptation concerns the addition of myofilaments to the peripheral or outer region of the myofibril, resulting in greater overall fiber diameter. The second adaptation is accomplished through myofibrillar hyperplasia, in that the actual number of myofibrils within a muscle fiber increase (14, 15,). The majority of a muscle fiber is made up of these myofibrils. Therefore increasing their size and number will result in enhanced cross sectional area. It should be clearly understood that resistance training increases protein synthesis for up to 24 hours post exercise (16). Protein synthesis is the process by which DNA encodes for the production of amino acids and proteins. The process of anabolism in regards to contractile tissue is literally heightened, resulting in a super compensation effect (an effect which raises structural and contractile tissue to above pre training bout levels). Amino acids are the building blocks of the proteins, which comprise the musculature. An amino acid is characterized by a nitrogen containing group, an acid group, and a variable group. The latter of which can take on 20 different combinations. It is for this reason that a sufficient protein intake is vital to increasing muscular size. That is, a diet rich in amino acids must be supplied in order to augment the supercompensatory process previously described.
Why Moderate Reps Stimulate Optimal Hypertrophy
Evidence suggests that moderate repetition sets provide an optimal stimulus for growth in the fast twitch fibers, while high repetition sets may optimize the hypertrophy process in slower twitch fibers. Reasons follow. First, sets which fall within a 1 to 5 repetition continuum will most likely cause the participant to fail due to neurological signaling problems before an optimal stimulus for muscle growth can be induced (1, 2). Secondly moderate repetition sets (6-12) take full advantage of
www.abcbodybuilding.com
Muscle Physiology
human recruiting systems. In general, the nervous system will recruit lower threshold fibers first and enlist high threshold fibers progressively as the set continues on. By the end of a set all available muscle cells have been brought into play (3). Thirdly the release of anabolic hormones is highest after these types of sets (4, 5, 6, 7, 8). A greater circulation of anabolic hormones in the body, results in greater adaptations to imposed demands. Interestingly enough, much evidence supports the postulate that lactic acid production can be very conducive to the release of hormones such as testosterone and growth hormone ( 3, 4, 5 ). Lactic acid is a by-product of glycolysis (6). This is the energy system that is used most heavily during 30-90 seconds of work. Glycolysis is directed by a series of enzymes, which comprise what is known as a chemical pathway. The enzyme lactate dehydrogenase is more active than any enzyme in the pathway. Therefore the more the participant relies on this pathway, the greater the build up of lactate will be. Hakkinen et al. found that blood lactate concentration during exercise correlated significantly (P < 0.01) with the increase in serum GH concentration. While Lu et al. in the Journal of Sports medicine found that increased plasma testosterone levels in males during exercise is at least partially a result of a direct stimulatory effect of lactate on the secretion of testosterone by increasing testicular cAMP production. Consequently blood lactate levels rise highest in moderate sets, with moderate rest. As opposed to low repetition, relatively high rest sets. Power lifting type movements have a greater reliance on the creatine phosphate (PC) system, which is used for, extremely low repetition, high intensity sets. Note that intensity in this light is in reference to percentage of the athletes one repetition maximum. The PC system does not result in lactic acid production and as a result is frequently referred to as the A Lactic anaerobic system. Further moderate repetition sets augment the blood pump phenomena. The benefits of which are numerous. For example, this phenomenon can facilitate myofibrillar hydration ( 9 ). A pump results through the collapsing of veins. These vessels carry blood away from the muscle tissue, while arteries act to deliver the blood. As veins begin to fail, the arteries continue to bring blood to the muscles. The resulting build up of fluid causes a flow of blood to go back into the musculature. Such an increase in fluid super hydrates the tissue. A process, which can lead to the inhibition of protein catabolism, and the augmentation of protein anabolism or synthetic rates (10, 11, 12). In a review on cellular hydration, Waldegger et al. (12) states that the paramount importance of cell volume for the regulation of cell function, including protein metabolism, has been recognized. The results of their research yielded the following results: cell swelling inhibits proteolysis cell swelling stimulates protein synthesis cell shrinkage stimulates proteolysis cell shrinkage inhibits protein synthesis
Haussinger suggests that the degree of cellular hydration is not only a major determinant of cellular protein and RNA turnover, but also that hormones and amino
www.abcbodybuilding.com
Muscle Physiology
acids can modify protein turnover by altering the hydration state of the cell. He concludes that cell swelling triggers an anabolic, proliferative pattern of metabolism, whereas cell shrinkage is a catabolic and antiproliferative signal. Further, blood is responsible for the delivery of nutrients needed for protein metabolism. Finally time under tension has been a proven factor in stimulating optimal growth (13). Contraction resulting from actin and myosin filamentous interaction, which will lead to damage of both the cytoskeleton and contractile units. The time under tension during lower repetition sets appears to be suboptimal for the hypertrophy sought stimulus.
Various Training Methodologies Between Various Regions of The Body
Knowing how to stimulate hypertrophy in type I, IIA and IIB fibers is half the battle. The other half is understanding how to apply these concepts to each individual body part. An understanding of the fact that each muscle group is made up of specific fiber compositions will augment this process. For example, if a muscle is 86 percent fast twitch, working the slow twitch fibers to a heightened extent would undermine the participants training efficiency. Further, if a muscle is predominantly slow twitch and the participant ignores this make up, then hypertrophy will be hindered. The following will provide a guide on how to target various musculature: 1. Always hit the fast twitch cells, even if the muscle group is pre-dominantly slow twitch. The reason is due to the relatively larger size of this particular fiber. What will not occur is an overly dominant fast twitch workout. 2. If a muscle group is dominated by a slow twitch makeup, the participant will need to dedicate significant time to stimulating growth in these cells. A recommendation would include a few medium range sets to target the small percentage of fast twitch fibers, followed by a regimen, which blasts the higher percentage cells to bits for the majority of the workout by using a higher repetition scheme. 3. If a muscle group has an even makeup, the majority of time should be spent working the fast twitch fibers due to their size. However, time should still be delineated to the slow twitch fibers. You should switch around the ratios as well. Lets say a muscle group is 50 / 50. Then Spend 60-70 percent of the time on fast twitch with the remainder of time on the remaining fibers. Occasionally for a burst of growth, emphasize the type I cells 50-80 percent of the time. This should be used sparingly, but certainly of prime importance. 4. If a muscle group such as the hamstrings are dominantly fast twitch (70-80 percent) then spend about 80-90 percent of the time on fast twitch fibers, and 10-20 percent of the time on the slower fibers. Perhaps just one burn out / high repetition set at the end of the workout. Note: Muscle Fiber Ratios are provided in the Anatomy Section of the Site
Muscular Density = Hyperplasia!
www.abcbodybuilding.com
Muscle Physiology
Muscular Hyperplasia is defined as the creation of new muscle fibers. Knowlden (2002) explains: There are two primary mechanisms in which new fibers can be formed. First large fibers can split into two or more smaller fibers and secondly satellite cells can be activated.
Satellite cells are myogenic stem cells, which are involved in skeletal muscle regeneration. When you stretch or intensely work a muscle fiber, satellite cells are activated. Satellite cells can undergo mitosis or cell division and give rise to new myoblastic cells.
These immature muscle cells can either fuse with a pre-existing muscle fiber causing that fiber to get bigger (hypertrophy), or these myoblastic cells can fuse with each other to form a new fiber. This is one of the ways to achieve hyperplasia! The application of this principle to bodybuilding is of extreme significance. You see it was long believed that an individual was born with a fixed number of muscle fibers. Density in bodybuilding has been defined as total muscle fibers per unit area. Potential in this sport is directly correlated to this muscle fiber number. Currently evidence from humans, rats, cats and birds suggests that hyperplasia does indeed occur (17, 18, 19, 20, 21, 22, 23)! Some of the more convincing of which has been found by comparing muscle biopsies with elite bodybuilders to that of normal human beings. One study compared the muscle size of strength athletes and normal individuals. The weight training athletes arm's were 27% greater in cross sectional area than the normal, sedentary individuals yet there was no significant difference in the size of their muscles fibers! Thus suggesting that a second mechanism was involved in the increase in overall size of the musculature. Some answer this question by saying that gifted bodybuilders simply were born with more muscle fibers than others. Dr. Antonio who is a leading expert on the subject answers this question, as follows(26): That is, they were born with more fibers. If that was true, then the intense training over years and decades performed by elite bodybuilders has produced at best average size fibers. That means, some bodybuilders were born with a bunch of below average size fibers and training enlarged them to average size. I don't know about you, but I'd find that explanation rather tenuous. It would seem more plausible (and scientifically defensible) that the larger muscle mass seen in bodybuilders is due primarily to muscle fiber hypertrophy but also to fiber hyperplasia....In my scientific opinion, this issue has already been settled. Muscle fiber hyperplasia contributes to whole muscle hypertrophy.
www.abcbodybuilding.com
Muscle Physiology
Further Nygaard and Nielsen (27) compared the deltoid size of competitive swimmers and normal individuals and found that the deltoid muscles of the swimmers were larger despite smaller muscle fibers! Once again, the swimmers superior size cannot be explained only by an increase in the size of each fibers since their fibers were actually smaller then the sedentary controls. Alway et al. found and concluded that this suggests that adaptations to resistance training may be complex and involve fiber hypertrophy and fiber number (e.g., proliferation). Larsson and Tesch (28) compared the muscle composition of elite bodybuilders with normal standards. Larger cross sectional size was found in the bodybuilders. However, they did not show a superior muscle fiber size compared to sedentary individuals. In fact Tesch concluded that muscle hyperplasia is one of the adaptation mechanisms of the muscle in the same way as muscle hypertrophy." Another example, is when Alway et al. (19) compared the biceps brachii muscle in elite male and female bodybuilders. A strong correlation in muscle fiber number and cross sectional area was found. It was concluded that the cross-sectional area of the biceps muscle was correlated to both fiber area and number. Hatfield Ph.D. in his book Power: A scientific approach is very adamant about the possibility of hyperplasia. Interestingly enough when a poll was taken by the National Strength and Conditioning Association, the majority believed that hyperplasia definitely did contribute to overall muscle growth (29). To comprehend the enormous growth in today's athletes as being purely based on hypertrophy would be a great leap of faith and evidence overwhelmingly points toward hyperplasia being a significant factor in bodybuilding.
The Stimulation of HYPERplasia
Unfortunately increasing muscular density is a very painful process during a workout and for many days to follow! Studies show that to increase the number of fibers, the participant will have to inflict significant damage to the muscle group (17, 18, 19, 20, 21, 22, 23, 26)! Literally to a point which pushes the envelope of over training. The best way to induce enough micro tears is through an emphasis on eccentric training. The eccentric portion of a repetition has been proven through countless studies to cause the most damage to the target muscle group. 5 Eccentric Techniques 1. Old School Negatives - These are without a doubt one of the best ways to increase muscular density! Click on the hyperlink to read about them! 2. Assisted Negatives - Knowlden suggests the utilization of assisted negatives for hyperplasic processes. These are also a favorite of Lee Priests, which would explain the absolutely insane mass that he has acquired on his quadriceps! Simply lift a weight and have your partner apply pressure on the negative portion of the rep. You need to perform these on machines and or exercises that don't risk trapping you under the weight. For example such a protocol would not be advised on a bench press. However, the technique would be useful on pull-ups and barbell curls. Machines are the safest way to go. If performing leg extensions the participant would lift the weight concentrically unassisted, and then on the eccentric portion have a partner apply excess pressure on the handle handle, while fighting the negative on the way down! This takes advantage of the fact that the athlete can lift more weight on the negative portion of a rep then the positive.
www.abcbodybuilding.com
Muscle Physiology
3. Heavy Negatives - Here you would get a spotter and use a weight that you could not lift concentrically( positive portion of the rep ) but could use eccentrically( again you can lift heavier on this portion of a rep). I suggest only going 10-15 percent above what you normally can lift for 6 reps. Have your partner assist you on the positive portion of the rep, while you fight the negative! 4. Emphasizing the Negative - Again, the key is to literally focus an entire workout on the eccentric portion of a repetition! I would suggest taking 3-5 seconds to lower the weight to incur a maximum amount of damage. And if you attempt to take 10 seconds to lower the weight on a squat you will be destroyed! Normally athletes just take one second to lift a weight and one to two to lower it. In this case you would take much longer. By emphasizing the negative you will increase the micro tears in your muscles. This causes a higher release of satellite cells. 5. Forced Negatives: Forced Negatives are performed after you have reached concentric failure. Simply have your partner assist you with the positive rep (taking as much of the weight off as possible ) while you take the negative portion of the rep. Your partner may even apply a bit of pressure( careful, this is dangerous and I only recommend it for intermediate to advanced athletes! ) Stretch Overload - Hyperplasia has also been shown to be induced by exercises that enhance the stretch! Examples of these would be preacher curls, weighted sissy squats etc. The key is to employ the one and a half repetition method! If you were to perform a preacher curl, you would perform two reps on the lower half of the exercise. A perfect example of this is shown with Arnold Schwarzenegger's pectorals. He could touch the ground when performing dumbbell flys and I believe he is a clear case of hyperplasia success! Finally, just training insanely (The Austrian Blitz for example!! ) to the point where you are extremely sore the next day will induce an increase in muscular density. Just look at Tom Platz legs. The man trained his lower body harder than anyone in the history of the sport, and I know his legs are not simply the result of hypertrophy! Oliva's forearms are another example, he would perform endless sets of reverse curls with 135 pounds! To the point of exhaustion! To add I would not suggest training for hyperplasia every workout. Or at least not for everybody part. Perhaps pick one body part to destroy a week and train your others normally. Jacob Wilson jwilson@abcbodybuilding.com President Abcbodybuilding
References and Sources Cited
1. Zehr, E.P. Ballistic movement: Muscle activation and neuromuscular adaptation. Can. J. Appl. Physiol. 19:(4)363378. 1994. 3. Schoenfeld, Brad, 2000: Repetitions and Muscle Hypertrophy. Strength and Conditioning Journal: Vol. 22, No. 6, pp. 6769.
www.abcbodybuilding.com
Muscle Physiology
4. 8. Kraemer, W.J. Changes in hormonal concentrations after different heavyresistance exercise protocols in women. J. Appl. Physiol. 75:(2)594604. 1993. 5. Lu, S.S. Lactate and the effects of exercise on testosterone secretion: Evidence for the involvement of a cAMP-mediated mechanism. Med. Sci. Sports Exerc. 29:(8)10481054. 1997 6. 4.Dudley GA (1988) Metabolic consequences of resistive-type exercise. Med Sci Sports Exerc. 20(5 Suppl):S158-S161 7. Kraemer, W.J. Hormonal and growth factor responses to heavy resistance exercise protocols. J. Appl. Physiol. 69:(4)14421450. 1990. 8. 7. Kraemer, W.J. Endogenous anabolic hormonal and growth factor responses to heavy resistance exercise in males and females. Int. J. Sports Med. 12:(2)228235. 1991. 9. Wilmore, J.H. Physiology of Sport and Exercise. (2nd ed.). Champaign, IL: Human Kinetics, 1999. 10. 4. Hussinger, D. Cellular hydration state: An important determinant of protein catabolism in health and disease. Lancet. 341:(8856)13301332. 1993. 11. Millar, I.D. Mammary protein synthesis is acutely regulated by the cellular hydration state. Biochem. Biophys. Res. Commun. 230:(2)351355. 1997. 12. Waldegger, S. Effect of cellular hydration on protein metabolism. Miner. Electrolyte Metab. 23:(36)201205. 1997 13. Evans, W.J. The metabolic effects of exercise-induced muscle damage. Exerc. Sport Sci. Rev. 19: (-HD-). 99125. 1991. 14. Conroy, B.P. and R.W. EarleBone , muscle, and connective tissu adaptations to pjysical activity. In essentials of Strength Trianing and Conditioning. T. R. Baechle. ed. Champaign, Il; Human Kinetics, 1994. 15. Tesch, P.A. Skeletal muscle adaptations consequent to long term heavy resistance exercise Med. Sci. Sports Exerc. 20:S132-S134. 1988 16. Staribm R,S,, D.L. Karapondo, W.J. Kraemer, A.C. Fry, S.E. Gordon, J.E. Falkel, F.C. Hagerman, and R.S. Hikida. Skeletal muscle adaptations during early phase of heavy-resistance training in men and woman. J Appl. Physiol. 76:1247-1255. 1994 17. Alway, S. E., P. K. Winchester, M. E. Davis, and W. J. Gonyea. Regionalized adaptations and muscle fiber proliferation in stretch-induced enlargement. J. Appl. Physiol. 66(2): 771-781, 1989. 18. Alway, S. E., W. J. Gonyea, and M. E. Davis. Muscle fiber formation and fiber hypertrophy during the onset of stretch-overload. Am. J. Physiol. (Cell Physiol.). 259: C92-C102, 1990.
www.abcbodybuilding.com
Muscle Physiology
19. Alway, S.E., W.H. Grumbt, W.J. Gonyea, and J. Stray-Gundersen. Contrasts in muscle and myofibers of elite male and female bodybuilders. J. Appl. Physiol. 67(1): 24-31, 1989. 20. Antonio, J. and W. J. Gonyea. The role of fiber hypertrophy and hyperplasia in intermittently stretched avian muscle. J. Appl. Physiol. 74(4): 1893-1898, 1993. 21. Antonio, J. and W.J. Gonyea. Progressive stretch overload of avian muscle results in muscle fiber hypertrophy prior to fiber hyperplasia. J. Appl. Physiol., 75(3): 1263-1271, 1993. 22. Antonio, J. and W. J. Gonyea. Muscle fiber splitting in stretch-enlarged avian muscle. Med. Sci. Sports Exerc. 26(8): 973-977, 1994. 23. Antonio, J. and W.J. Gonyea. Skeletal muscle fiber hyperplasia. Med. Sci Sports. Exerc. 25(12): 1333-1345, 1993. 24. Yamada, S., N. Buffinger, J. Dimario, and R. C. Strohman. Fibroblast growth factor is stored in fiber extracellular matrix and plays a role in regulating muscle hypertrophy. Med. Sci. Sports Exerc. 21(5): S173-S180, 1989 25. Schantz, P., E. Randall Fox, P. Norgen, and A. Tyden. The relationship between mean muscle fiber area and the muscle cross-sectional area of the thigh in subjects with large differences in thigh girth. Acta Physiol. Scand. 113: 537-539, 1981. 26. Antonio, J., Muscle fiber hypertrophy vs. hyperplasia: Has the debate been settled? 27. Nygaard E., and Nielsen E., Skeletal muscle fiber capilarisation with extreme endurance training in man. In Eriksson B, Furberg B. Swimming Medicine IV (vol. 6 pp 282-293). University Park Press, Baltimore 1978. 28. MacDougall, J.D., D.G. Sale, J.R. Moroz, G.C.B. Elder, J.R. Sutton, and H. Howard. Muscle ultra-structural characteristics of elite power-lifters and bodybuilders. Eur. J. Appl. Physiol. 48:117126. 1982. 29. Craig, Bruce W., 2001: BRIDGING THE GAP: Hyperplasia: Scientific Fact or Fiction?. Strength and Conditioning Journal: Vol. 23, No. 5, pp. 4244. 30. Target Bodybuilding 31. Barnett, C., V. Kippers, and P. Turner. Effects of variations of the bench press exercise on the EMG activity of five shoulder muscles. J. Strength Cond. Res. 9:222 227. 1995. 32. Brown, J.M.M., C. Solomon, and M. Paton. Further evidence of functional differentiation within biceps brachii. Electromyogr. Clin. Neurophysiol. 33:301309. 1993. 33. 29. Glass, S.C., and T. Armstrong. Electromyographical activity of the pectoralis muscle during decline bench presses. J. Strength Cond. Res. 11:163167. 1997.
www.abcbodybuilding.com
Muscle Physiology
34. Sarti, M.A., M. Monfort, M.A. Fuster, and L.A. Villaplana. Muscle activity in upper and lower rectus abdominus during abdominal exercises. Arch. Phys. Med. Rehabil. 77:12931297. 1996. 35. Lipetz, S., and B. Gutin. An electromyographic study of four abdominal exercises. Med. Sci. Sports 2:35-38. 1970. 37. Kawakami, Y., T. Abe, S.-Y. Kuno, and T. Fukunaga. Training-induced changes in muscle architecture and specific tension. Eur. J. Appl. Physiol. 72:37-43 1995. 38. 4.Kawakami, Y., T. Abe, S.-Y. Kuno, and T. Fukunaga. Training-induced changes in muscle architecture and specific tension. Eur. J. Appl. Physiol. 72:37-43 1995. 39. ANTONIO, JOSE, 2000: Nonuniform Response of Skeletal Muscle to Heavy
Resistance Training: Can Bodybuilders Induce Regional Muscle Hypertrophy?. The Journal of Strength and Conditioning Research: Vol. 14, No. 1, pp. 102113.
40. Elder, G.C.B., K. Bradbury, and R. Roberts. Variability of fiber type distributions within human muscles. J. Appl. Physiol. 53:14731480. 1982. 41. Punkt, K., H. Mehlhorn, and H. Hilbig. Region- and age-dependent variations of muscle fiber properties. Acta Histochem. 100:3758. 1998. 42. Sola, O.M., S. Herring, G. Zhang, X. Huang, N. Hayashida, L.C. Haines, R. Thomas, B.A. Kakulas, and L.R. Sauvage. Significance of the biopsy site of the latissimus dorsi muscle for fiber typing. J. Heart Transplant. 11:S315S319. 1992. 44. Johnson, M.A., Polgar, J., Weightman, D. & Appleton, D. (1973). Data on the distribution of fibre types in thirty-six human muscles: An autopsy study. Journal of the Neurological Sciences, 18, 111 - 129. 45. ('Muscle Function after Exercise-Induced Muscle Damage and Rapid Adaptation,' Medicine and Science in Sports and Exercise, vol. 24(5), pp. 512-520, 1992) 46. Differences in Skeletal Muscles,' Journal of Applied Physiology, vol. 85(1), pp. 98-104, 1998) 47. Antonio, J., and W.J. Gonyea. Ring fibres express ventricular myosin in stretch overloaded quail muscle. Acta Physiol. Scand. 152:429430. 1994. 48. Andersen, J.L., H. Klitgaard, and B. Saltin. Myosin heavy chain isoforms in single fibres from m. vastus lateralis of sprinters: Influence of training. Acta Physiol. Scand. 151:135142. 1994. 49. Klitgaard, H., M. Zhou, and E.A. Richter. Myosin heavy chain composition of single fibers from m. biceps brachii of male body builders. Acta Physiol. Scand. 140:175180. 1990. 50. Sakuma, K., A. Yamaguchi, and S. Katsuta. Are region-specific changes in fibre types attributable to nonuniform muscle hypertrophy by overloading?. Eur. J. Appl. Physiol. 71:499504. 1995.
www.abcbodybuilding.com
Muscle Physiology 10
51. Alway, S.E. Stretch induces non-uniform isomyosin expression in the quail anterior latissimus dorsi muscle. Anat. Rec. 237:17. 1993 52. Alway, S.E., W.H. Grumbt, J. Stray-Gundersen, and W.J. Gonyea. Effects of resistance training on elbow flexors of highly competitive bodybuilders. J. Appl. Physiol. 72:15121521. 1992 53. Alway, S.E., P.K. Winchester, M.E. Davis, and W.J. Gonyea. Regionalized adaptations and muscle fiber proliferation in stretch-induced enlargement. J. Appl. Physiol. 66:771781. 1989 54. Antonio, J., and W.J. Gonyea. Skeletal muscle fiber hyperplasia. Med. Sci. Sports Exerc. 25:13331345. 1993. 55. Antonio, J., and W.J. Gonyea. Muscle fiber splitting in stretch-enlarged avian muscle. Med. Sci. Sports Exerc. 26:973977. 1994.. 56. Gardiner, P.F., B.J. Jasmin, and P. Corriveau. Rostrocaudal pattern of fiber-type changes in an overloaded rat ankle extensor. J. Appl. Physiol. 71:558564. 1991. 57. Brown, J.M.M., C. Solomon, and M. Paton. Further evidence of functional differentiation within biceps brachii. Electromyogr. Clin. Neurophysiol. 33:301309. 1993 58. English, A.W., S.L. Wolf, and R.L. Segal. Compartmentalization of muscles and their motor nuclei: The partitioning hypothesis. Phys. Ther. 73:857867. 1993. 59. Wickiewicz, T.L., R.R. Roy, P.L. Powell, and V.R. Edgerton. Muscle architecture of the human lower limb. Clin. Orthop. Related Res. 179:275283. 1983 60. Heron, M.I., and F.J.R. Richmond. In-series fiber architecture in long human muscles. J. Morphol. 216:3545. 1993. 61. Lindman, R., A. Eriksson, and L.-E. Thornell. Fiber type composition of the human male trapezius muscle: Enzymehistochemical characteristics. Am. J. Anat. 189:236244. 1990 62. Lindman, R., A. Eriksson, and L.-E. Thornell. Fiber type composition of the human female trapezius muscle: Enzymehistochemical characteristics. Am. J. Anat. 190:385392. 1991. 63. Haussinger D. Control of protein turnover by the cellular hydratation state. Ital J Gastroenterol. 1993 Jan;25(1):42-8.
64. Tesch, P.A., and L. Larsons. Muscle hypertrophy in bodybuilders. Eur. J. Appl. Physiol. 49:301306. 1982
ABC Bodybuilding Company. All rights reserved. Disclaimer
S-ar putea să vă placă și
- Notes On Reactive Training Manual: 1 The Basic TemplateDocument2 paginiNotes On Reactive Training Manual: 1 The Basic TemplatePedroZ3Încă nu există evaluări
- The Adaptations To Strength Training - Morphological - Neurological Contributions - Increased - Strength PDFDocument24 paginiThe Adaptations To Strength Training - Morphological - Neurological Contributions - Increased - Strength PDFPedroZ3Încă nu există evaluări
- Ericsson - Deliberate PracticeDocument44 paginiEricsson - Deliberate PracticeKenny CuiÎncă nu există evaluări
- CFJ Devor CrossFit Publication 1Document17 paginiCFJ Devor CrossFit Publication 1j_j_m_tÎncă nu există evaluări
- Mighty Men of OldDocument25 paginiMighty Men of OldadiseifÎncă nu există evaluări
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeDe la EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeEvaluare: 4 din 5 stele4/5 (5784)
- The Little Book of Hygge: Danish Secrets to Happy LivingDe la EverandThe Little Book of Hygge: Danish Secrets to Happy LivingEvaluare: 3.5 din 5 stele3.5/5 (399)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceDe la EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceEvaluare: 4 din 5 stele4/5 (890)
- Shoe Dog: A Memoir by the Creator of NikeDe la EverandShoe Dog: A Memoir by the Creator of NikeEvaluare: 4.5 din 5 stele4.5/5 (537)
- Grit: The Power of Passion and PerseveranceDe la EverandGrit: The Power of Passion and PerseveranceEvaluare: 4 din 5 stele4/5 (587)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureDe la EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureEvaluare: 4.5 din 5 stele4.5/5 (474)
- The Yellow House: A Memoir (2019 National Book Award Winner)De la EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Evaluare: 4 din 5 stele4/5 (98)
- Team of Rivals: The Political Genius of Abraham LincolnDe la EverandTeam of Rivals: The Political Genius of Abraham LincolnEvaluare: 4.5 din 5 stele4.5/5 (234)
- Never Split the Difference: Negotiating As If Your Life Depended On ItDe la EverandNever Split the Difference: Negotiating As If Your Life Depended On ItEvaluare: 4.5 din 5 stele4.5/5 (838)
- The Emperor of All Maladies: A Biography of CancerDe la EverandThe Emperor of All Maladies: A Biography of CancerEvaluare: 4.5 din 5 stele4.5/5 (271)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryDe la EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryEvaluare: 3.5 din 5 stele3.5/5 (231)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaDe la EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaEvaluare: 4.5 din 5 stele4.5/5 (265)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersDe la EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersEvaluare: 4.5 din 5 stele4.5/5 (344)
- On Fire: The (Burning) Case for a Green New DealDe la EverandOn Fire: The (Burning) Case for a Green New DealEvaluare: 4 din 5 stele4/5 (72)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyDe la EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyEvaluare: 3.5 din 5 stele3.5/5 (2219)
- The Unwinding: An Inner History of the New AmericaDe la EverandThe Unwinding: An Inner History of the New AmericaEvaluare: 4 din 5 stele4/5 (45)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreDe la EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreEvaluare: 4 din 5 stele4/5 (1090)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)De la EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Evaluare: 4.5 din 5 stele4.5/5 (119)
- Her Body and Other Parties: StoriesDe la EverandHer Body and Other Parties: StoriesEvaluare: 4 din 5 stele4/5 (821)
- Occlusal Relationships in RPDsDocument22 paginiOcclusal Relationships in RPDssherylreyesÎncă nu există evaluări
- Medical PhysicsDocument24 paginiMedical PhysicsgeethaÎncă nu există evaluări
- Youtube Videos NBDE1Document12 paginiYoutube Videos NBDE1rajanOrajanÎncă nu există evaluări
- Trauma Tulang Belakang-Ppgd - Dr. YoyosDocument57 paginiTrauma Tulang Belakang-Ppgd - Dr. YoyoszaroziÎncă nu există evaluări
- Care of Shoulder Pain in The Overhead AthleteDocument8 paginiCare of Shoulder Pain in The Overhead Athlete110313Încă nu există evaluări
- 4 Cotofana 2016Document8 pagini4 Cotofana 2016Thamyres Branco100% (1)
- Grossing Protocol of Laryngectomy Specimen DR Noreen PDFDocument26 paginiGrossing Protocol of Laryngectomy Specimen DR Noreen PDFsam umerÎncă nu există evaluări
- Ex - Neuro LLDocument3 paginiEx - Neuro LLssÎncă nu există evaluări
- Scan Report PDFDocument1 paginăScan Report PDFsrikanth PosaÎncă nu există evaluări
- Thoracic Outlet Syndrome Treated With Acupuncture, Manual Techniques and Self-Stretching ExercisesDocument3 paginiThoracic Outlet Syndrome Treated With Acupuncture, Manual Techniques and Self-Stretching Exercisesdc6463Încă nu există evaluări
- Lab Activity 2 HeartDocument5 paginiLab Activity 2 HeartRaraÎncă nu există evaluări
- Deviated Nasal SeptumDocument6 paginiDeviated Nasal SeptumHetvi ParmarÎncă nu există evaluări
- The Skeletal System HDocument23 paginiThe Skeletal System HRajorshi MishraÎncă nu există evaluări
- FLEXIBILITY and STRETCHING EXERCISES PDFDocument3 paginiFLEXIBILITY and STRETCHING EXERCISES PDFRubamoÎncă nu există evaluări
- Diploma Lab manual-HB-IIDocument103 paginiDiploma Lab manual-HB-IIvanshÎncă nu există evaluări
- Snakes AnatomyDocument26 paginiSnakes AnatomyRoxana Nae PuccaÎncă nu există evaluări
- Active Anti Shear Device For Knee RehabiDocument94 paginiActive Anti Shear Device For Knee Rehabiаримотома аримотомаÎncă nu există evaluări
- Woodward, 1896 On Two Mesozoic Crocodilians From The Red Sandstone of The Territory of Neuquen Argentine RepublicDocument18 paginiWoodward, 1896 On Two Mesozoic Crocodilians From The Red Sandstone of The Territory of Neuquen Argentine RepublicRodrigo Giesta FigueiredoÎncă nu există evaluări
- Medisound 3000: Ultrasound TherapyDocument2 paginiMedisound 3000: Ultrasound TherapyAsdf GhjklÎncă nu există evaluări
- Pages From Physio Term 2 BKLTDocument76 paginiPages From Physio Term 2 BKLTDeshi SportsÎncă nu există evaluări
- Development - of - CVS-5Document20 paginiDevelopment - of - CVS-5Ahsan IslamÎncă nu există evaluări
- Yoga Za StarijeDocument89 paginiYoga Za StarijeMiroslav MatošÎncă nu există evaluări
- 6.5 Neurons & SynapsesDocument1 pagină6.5 Neurons & SynapsesNaomi BoesonoÎncă nu există evaluări
- Stroke Rehab ExercisesDocument14 paginiStroke Rehab ExercisesEdna Mineva50% (2)
- DLP Science 9 61119Document4 paginiDLP Science 9 61119IRISÎncă nu există evaluări
- Exam 2 ReviewDocument31 paginiExam 2 ReviewAhsan Tebha50% (2)
- 5-3 IOM-repair or ReplaceDocument37 pagini5-3 IOM-repair or ReplaceProfesseur Christian DumontierÎncă nu există evaluări
- Physiology Lecture 9 Q-Bank (Smooth Muscle Structure, Contraction, and Relationships)Document5 paginiPhysiology Lecture 9 Q-Bank (Smooth Muscle Structure, Contraction, and Relationships)ChrisOrtÎncă nu există evaluări
- CBSE Class 8 Science, Reaching The Age of AdolescenceDocument5 paginiCBSE Class 8 Science, Reaching The Age of AdolescenceR.Shruti 1040-12Încă nu există evaluări
- Orthodontics AssignmentDocument4 paginiOrthodontics AssignmentTasneem SaleemÎncă nu există evaluări