Documente Academic
Documente Profesional
Documente Cultură
Cheat Sheet Chemistry Salt Analysis 12th CBSE
Încărcat de
TammanurRaviDrepturi de autor
Formate disponibile
Partajați acest document
Partajați sau inserați document
Vi se pare util acest document?
Este necorespunzător acest conținut?
Raportați acest documentDrepturi de autor:
Formate disponibile
Cheat Sheet Chemistry Salt Analysis 12th CBSE
Încărcat de
TammanurRaviDrepturi de autor:
Formate disponibile
1.
Preliminary Tests
a) Note the colour of the salt. Observations (i) Blue (ii) Bluish green (iii) Brown or yellowish brown (iv) Brown or deep pink or violet (v) Light green (vi) Light pink or flesh coloured (vii) White Inference Cu2+ Cu2+ or Ni2+ Fe3+ or Mn2+ Co2+ Fe2+ or Ni2+ Mn2+ Absence of Cu 2+, Ni2+ Fe3+, Fe2+, Mn2+ and Co2+ 1. Note the smell of the salt by rubbing the moistened salt between the fingers. (i) Ammoniacal smell (ii) Smell of rotten eggs (iii) Vinegar smell NH4+ S2CH3COO-
2. Take a little of the salt on a piece of paper and place this on the palm of your hand to find whether the salt is heavy or light.
(i) It is quite heavy salt (ii) It is a light salt and a fluffy powder
Ba2+ or Pb2+ Salt ZnCO3 or MgCO3 or CaCO3
2. Dil H2SO4 Test Take a little of the salt (about 0.5g) in a test tube and add dil. H2S04. Warm if no reaction takes place. (a) Gas Evolved (i) A colourless, odourless, gas evolves with effervescence which turns lime water milky (CO2 gas)
CO32-
(ii) A colourless gas having burning sulphur smell evolves which turns acidified potassium dichromate green (SO 2 gas)
SO32-
(iii) A colourless gas having rotten eggs smell evolves which turn lead acetate paper black H 2S gas (iv) A brown gas having pungent smell evolves which turns FeSO 4 solution black (NO2 gas)
S2-
NO2-
(v) No gas evolved
CO32- ,SO32- ,S2- ,NO2Absent Pb2+, Ba2+, Sr2+ or Ca2+ is present
(a) A white ppt is formed in the reaction mixture 2. Conc H2SO4 Test
Take a little of the salt in a test-tube. Add conc H2SO4 just to soak the salt and then heat.
(i) A colourless gas having pung-gentsmell evolves whichgives white dense fumes with a rod dipped in NH 4OH (HCI gas) (ii) A yellowish brown gas having irritating smell evolves which turns starch paper yellow (Br2 gas) (iii) A dark violet gas evolves which turns starch paper blue (l 2 gas) (iv) A reddish brown gas having pungent smell evolves which turns. FeSO 4 solution black. (NO2 gas) (v) A colourless gas having vinegar smell evolves. [Acetic acid (CH3COOH) vapours] (vi) A colourless, odourless gas which burns with a blue flame (CO gas) along with CO2 gas which turns lime water milky.
Cl-
BrINO3-
CH3COO-
Oxalate (C2O42-)
3.Confirmatory (or wet) Tests for Acid radicals
1. Confirmation of carbonate (a) Take a little of the salt in a test-tube and add distilled water. Shake (i) Soluble carbonate of NH4+ Insoluble CO 32-
(i) Clear solution (ii) Salt is insoluble in water
(b) Magnesium sulphate test : To the aqueous solution of soluble carbonate add MgSO4 solution (c) Phenolphthalein test: To another part of aqueous solution of soluble salt add phenolphthalein 2. Confirmation of sulphite
White precipitate
Soluble CO32-confirmed
Pink colour
Soluble CO32- confirmed
(a) Barium chloride test: To a portion of aqueous solution or soda extract add acetic acid till effervescence ceases. Then add BaCI2 solution.
A white ppt which dissolves in dil HCI with the evolution of SO 2 gas
SO32- confirmed
(b) Ferric chloride test: To a portion of aqueous extract add FeCI 3solution. 3. Confirmation of Chloride (a) Silver nitrate test:To aqueous solution or soda extract, add dil HNO3 till acidic. Then add AgNO 3 solution.
Dark red colour which changes into reddish brown ppt on boiling
SO32- confirmed
Curdy white ppt soluble in NH4OH
Cl- confirmed
(b) MnO2 test: Mix salt with MnO2 in a test tube. Then add conc. H 2SO4 and heat.
Yellowish green gas evolves which bleaches moist blue litmus paper
Cl- confirmed
(c)Chromyl Chloride test: Mix salt with solid K2Cr2O7 in a test tube. Then add conc H 2SO4 and heat. 4. Confirmation of bromide (a )Silver nitrate test:To aqueous solution or soda extract add HN0 3till acidic.Then add AgN03 solution. 5. Confirmation of iodide (a) Silver nitrate test:To aqueous solution or soda extract add HN0 3 till acidic. Then add AgN03 solution. 6. Confirmation of nitrate
Red vapours of chromyl chloride evolve which on passing through water gives yellow solution which on adding lead acetate solution gives yellow ppt
Cl- confirmed
Pale yellow ppt sparingly soluble in NH4OH
Br- confirmed
Yellow ppt insoluble in NH4OH
I- confirmed
(a) Ring test: To the aqueous solution of the salt, add freshly prepared FeS0 4 solution. Shake and then add conc. H 2S04 along the sides of test-tube.
Dark brown or black ring is formed at the junction of the layers of the acid and the solution
NO3- confirmed
(b) Copper chips test: To a little of the salt in a test tube add copper chips (or paper pellet) and then add conc.H 2S04. Heat.
Dark brown fumes evolves
NO3- confirmed
7. Confirmation of acetate (a) Oxalic acid test: Mix a little of the salt with solid oxalic acid. Moisten it with a drop of water. Rub this paste between the fingers.
Vinegar smell
Acetate confirmed
(b) Ester test: To a little of the salt taken in a test tube add conc. H2SO4 & few drops of ethyl alcohol. Heat
Pleasant fruity smell of ester
Acetate confirmed
(c) FeCI3 test: To the aqueous solution of the salt add neutral FeCI 3 solution
Blood red colour
Acetate confirmed
8. Confirmation of sulphate
(a) BaCI2 test: To a little of the solution add HCI. Heat to expel if any gas is formed. Then add BaCI2 solution.
White ppt insoluble in HCI
S042- confirmed
4.Wet Tests for Basic radicals
1. Preparation of original solution (O.S.)
Take a little of the salt and try to dissolve in the following solvents one by one (i) (ii) (iii) (iv) Distilled water - cold or hot Dil. HCI - cold or hot Conc. HCI - cold or hot HNO3 - cold or hot
Once the solvent is found out then prepare O.S. with 0.5 to 1g. of the salt Note : (i) If solvent is conc. HCI please dilute the solution with distilled water. (ii) If solvent is HNO3, evaporate the solution to dryness and dissolve in water 2. Analysis of Group Zero (NH4+ ) Sodium hydroxide test :To a little of salt add conc, solution of NaOH and Heat. 3. Analysis of Group 1 (Pb2+) (i) To the original salt solution in water add HCl (a) White ppt (b) No ppt (ii) Hot water test: Filter the white ppt, wash with water and add 10 ml of water. Heat ppt dissolves Pb2+ is present 1st group is present 1st group is absent A colourless gas having ammonical smell is evolved which turns Nesselers solution reddish brown
NH4+ is present
(iii) Potassium chromate test : To one part of hot aqueous solution of the salt add potassium chromate solution. Note: If on adding HCI to aqueous solution no ppt is formed or original solution is prepared in HCI, then Pb 2+ is absent.
yellow ppt
Pb2+ is confirmed
4. Analysis of Group II [Pb2+, Cu2+ and As34] This group salts are not in labs To the original solution add dil HCL and then pass H2S gas through a portion of this solution. If a coloured ppt is formed then pass H2S gas through the whole of the solution. 5. Analysis of Group III (Fe3+ & Al3+) (i) To the original solution, add conc. HNO 3 heat cool. Then add NH4CI solid. Again heat and cool. Then add NaOH till it gives smell of NH3.
a) No ppt
Group II is absent
(a) Reddish Brown ppt
Fe3+ is present
(b) Gelatinous White ppt (c) No ppt (a) Potassium sulphocyanide test : To one part add potassium sulphocyanide solution Blood red colour
Al3+ is present Fe3+ and Al3+ absent Fe3+ confirmed
(b) Potassium ferrocyanide test: To the second part add potassium ferrocyanide solution Lake test: To one part add HCI till the solution turns acidic. Then add 2 drops of blue litmus followed by NH4OH till it gives smell of ammonia 6. Analysis of Group IV (Ni2+, Co2+, Mn2+, Zn2+) This group salts are not in labs (i) To the solution of 5(ii), which gives smell of ammonia, pass H2S gas through a test portion 7. Analysis of Group V( Ba2+,Sr2+ & Ca2+) (i) To the rest of solution from 6(i) through which H2S gas has not been passed, add saturated ammonium carbonate solution and warm.
Deep blue solution
Fe3+ confirmed
Blue ppt floating in colourless solution
Al3+ confirmed
No ppt
IV group is absent
(a) A white ppt (b) No ppt
Ba2+, Sr2+ or Ca2+ is present V group is absent
Potassium chromate test : To the white ppt of 7(i) add acetic acid and heat. To the warm solution add potassium chromate solution. Wash the yellow ppt with hot water & perform flame test with it.
A yellow ppt
Ba2+ present
Grassy green flame through naked eye and bluish green through blue glass Ammonium oxalate test: To the filtrate of 7(iii) add ammonium oxalate solution Flame test: Perform flame test with the white ppt of 7(v) 8. Analysis of GroupVI (Mg2+) (i) Ammonium phosphate test: To the Oriqinal solution add NH 4Cl,NH4OH inslight excess and then ammonium phosphate. (ii) Cobalt nitrate test: Perform cobalt nitrate test with the salt A white ppt appears on scratching the sides of the test tube A white ppt Brick red through naked eye
Ba2+ confirmed
Ca2+ present Ca2+ confirmed
Mg2+ present
Pink mass
Mg2+ confirmed
S-ar putea să vă placă și
- How To Predict The Outcome of A DashaDocument25 paginiHow To Predict The Outcome of A DashaKALSHUBH100% (4)
- SAT Writing Essentials (LearningExpress)Document170 paginiSAT Writing Essentials (LearningExpress)Shafik96100% (1)
- Skincare Routine Order Cheat SheetDocument10 paginiSkincare Routine Order Cheat SheetYel Salenga100% (3)
- 501 Critical Reading QuestionsDocument283 pagini501 Critical Reading Questionsapi-3813392100% (9)
- Pusheen With Donut: Light Grey, Dark Grey, Brown, RoséDocument13 paginiPusheen With Donut: Light Grey, Dark Grey, Brown, RosémafaldasÎncă nu există evaluări
- Arco Sat 2Document334 paginiArco Sat 2TammanurRaviÎncă nu există evaluări
- Physics Grade 10Document228 paginiPhysics Grade 10Jan92% (26)
- Multi Pressure Refrigeration CyclesDocument41 paginiMulti Pressure Refrigeration CyclesSyed Wajih Ul Hassan80% (10)
- Amount of Acetic Acid in VinegerDocument15 paginiAmount of Acetic Acid in VinegerSurajMandal100% (1)
- Salt Analysis - Barium NitrateDocument2 paginiSalt Analysis - Barium NitrateSwarnabha Bhattacharyya100% (2)
- Chemistry Investigatory ProjectDocument13 paginiChemistry Investigatory ProjectAshrayee Wasnik100% (1)
- Basic Viva Questions With AnswersDocument5 paginiBasic Viva Questions With AnswersGovind Singh KhatiyanÎncă nu există evaluări
- Experiment - Salt Analysis Calcium Chloride 2223Document2 paginiExperiment - Salt Analysis Calcium Chloride 2223ARYAN GOELÎncă nu există evaluări
- Chemistry Project - ConductivityDocument19 paginiChemistry Project - ConductivityPankaj Gill67% (3)
- Zinc AcetateDocument4 paginiZinc AcetateAbinaya chettiappanÎncă nu există evaluări
- Preparation of CrystalsDocument2 paginiPreparation of Crystalsjanu kandwalÎncă nu există evaluări
- Chemistry Project On Preparation of Potash AlumDocument16 paginiChemistry Project On Preparation of Potash AlumAbhinav Singh100% (1)
- 4.SALT ANALYSIS Ferric NitrateDocument3 pagini4.SALT ANALYSIS Ferric Nitratemohnish100% (1)
- Calcium Acetate-1Document3 paginiCalcium Acetate-1Bimal Krishna BiswasÎncă nu există evaluări
- Analysis of Simple Salt-II-magnesium Nitrate For Record and ReferenceDocument6 paginiAnalysis of Simple Salt-II-magnesium Nitrate For Record and Referencenikil saibaba100% (1)
- Salt Analysis With EquationsDocument12 paginiSalt Analysis With Equationsabhikhya aryaÎncă nu există evaluări
- Aluminium Bromide Salt AnalysisDocument3 paginiAluminium Bromide Salt AnalysisShanmuganathan100% (1)
- Chemistry Project On Measuring Solubility of Saturated SolutionsDocument14 paginiChemistry Project On Measuring Solubility of Saturated SolutionsNaven Bansal75% (4)
- 6 CaCO3Document3 pagini6 CaCO3Abhi Suresh100% (3)
- Copper SulphateDocument4 paginiCopper Sulphatesumathi siva50% (2)
- Chemistry Investigatory Report On "Adulteration of Food-Stuffs"Document16 paginiChemistry Investigatory Report On "Adulteration of Food-Stuffs"Samyak Sau78% (40)
- Salt AnalysisDocument23 paginiSalt AnalysisflippodynamicsÎncă nu există evaluări
- Analysis of Simple Salt - (Copper Nitrate) : Dry TestsDocument3 paginiAnalysis of Simple Salt - (Copper Nitrate) : Dry TestsSarvan SankaranÎncă nu există evaluări
- Chemistry ProjectDocument20 paginiChemistry ProjectManash BorahÎncă nu există evaluări
- Scheme of Salt AnalysisDocument8 paginiScheme of Salt AnalysisAz Ahmed100% (1)
- XII Chemistry Practical-22Document23 paginiXII Chemistry Practical-22Anbuchelvan VKÎncă nu există evaluări
- Chemistry ProjectDocument15 paginiChemistry ProjectArjun Chauhan50% (2)
- Chemistry PracticalDocument12 paginiChemistry PracticalSuperdudeGauravÎncă nu există evaluări
- Lead Nitrate Salt AnalysisDocument2 paginiLead Nitrate Salt AnalysisSantosh Kumar Sahu0% (1)
- Chemistry Practical Procedure Systematic Analysis of Salt STD: Xi & XiiDocument9 paginiChemistry Practical Procedure Systematic Analysis of Salt STD: Xi & XiivarshiniÎncă nu există evaluări
- Zinc SulphateDocument2 paginiZinc Sulphategumtamm100% (1)
- Salt Analysis - Xii PDFDocument9 paginiSalt Analysis - Xii PDFहर्ष सैनी. कक्षा::बारहवीं 'द'Încă nu există evaluări
- Chemical Test To Distinguish Between Pair of Organic CompoundDocument11 paginiChemical Test To Distinguish Between Pair of Organic CompoundHishq Dhiman100% (1)
- Viva Questions Class 12 ChemistryDocument17 paginiViva Questions Class 12 ChemistrymrinalinimalavigaÎncă nu există evaluări
- Chemistry Investigatory Project 1Document23 paginiChemistry Investigatory Project 1Rishabh Pawani 11 B100% (1)
- Chemistry Project For Class 12 Topic: Saturated Solution: Measuring SolubilityDocument13 paginiChemistry Project For Class 12 Topic: Saturated Solution: Measuring SolubilityNishith Naik100% (1)
- Study of Oxalate Ion Content in Guava FruitDocument18 paginiStudy of Oxalate Ion Content in Guava FruitRamuKaka12344321Încă nu există evaluări
- Experiment - Salt Analysis Ammonium AcetateDocument1 paginăExperiment - Salt Analysis Ammonium AcetateprafullÎncă nu există evaluări
- Salt Analysis-Calcium ChlorideDocument3 paginiSalt Analysis-Calcium ChlorideAmythÎncă nu există evaluări
- Presence of Oxalate Ions in Guava Chemistry Investigatory ProjectDocument11 paginiPresence of Oxalate Ions in Guava Chemistry Investigatory ProjectRonit GauravÎncă nu există evaluări
- 18.salt Zinc Carbonate 4Document3 pagini18.salt Zinc Carbonate 4Sarthika Gaulkar0% (1)
- Calcium CarbonateDocument1 paginăCalcium CarbonateShreeÎncă nu există evaluări
- Dry Test For Basic Radical: Experiment Observation InferenceDocument6 paginiDry Test For Basic Radical: Experiment Observation InferenceJoy DeyÎncă nu există evaluări
- Chemistry Investigatory ProjectDocument27 paginiChemistry Investigatory ProjectDeepak Kumar MoudÎncă nu există evaluări
- Measuring The Amount of Acetic Acid in VinegarDocument16 paginiMeasuring The Amount of Acetic Acid in VinegarMansi Jain100% (1)
- Maharishi Vidya Mandir: Project FileDocument4 paginiMaharishi Vidya Mandir: Project FileAyush YadavÎncă nu există evaluări
- Study of Constituents of An AlloyDocument14 paginiStudy of Constituents of An AlloySahildeep Singh Kohli75% (8)
- Strontium ChlorideDocument5 paginiStrontium ChlorideAbinov Kumar KTÎncă nu există evaluări
- (Zinc Acetate) Systematic Analysis of Simple Salt No 8Document3 pagini(Zinc Acetate) Systematic Analysis of Simple Salt No 8Jo RajÎncă nu există evaluări
- Class 12 ChemistryDocument16 paginiClass 12 ChemistrysipherbizÎncă nu există evaluări
- Chem Project HiteshDocument16 paginiChem Project HiteshSahil Sharma64% (14)
- Preparation of Potash AlumDocument14 paginiPreparation of Potash AlumXI-A Vishal BishnoiÎncă nu există evaluări
- Chemistry Project: Removal of Alcohol From The Body Through EsterificationDocument5 paginiChemistry Project: Removal of Alcohol From The Body Through Esterificationharshit chhikaraÎncă nu există evaluări
- 1 Salt Analysis Lead AcetateDocument2 pagini1 Salt Analysis Lead AcetateSuman PandeyÎncă nu există evaluări
- Paper Chromatography Project Class 12Document23 paginiPaper Chromatography Project Class 12Shruti Garje100% (1)
- Chemistry Investigatory ProjectDocument20 paginiChemistry Investigatory Projectyatharth joshiÎncă nu există evaluări
- Chemistry Project: Adverse Effects of Contentsof Soft DrinksDocument20 paginiChemistry Project: Adverse Effects of Contentsof Soft DrinksAkash Achu100% (3)
- Chemistry Sahodaya PaperDocument10 paginiChemistry Sahodaya PaperflippodynamicsÎncă nu există evaluări
- Investigatory Project Class 12Document12 paginiInvestigatory Project Class 12kartik auluck100% (4)
- Quantitative Analysis Salt AnalysisDocument7 paginiQuantitative Analysis Salt AnalysisVishal RaghavendranÎncă nu există evaluări
- Systematic Semi-Micro Qualitative Analysis of An Inorganic SaltDocument11 paginiSystematic Semi-Micro Qualitative Analysis of An Inorganic SaltNidhi PrasadÎncă nu există evaluări
- Scheme For Systematic Analysis of A Mixture Containing Two SaltsDocument10 paginiScheme For Systematic Analysis of A Mixture Containing Two SaltsMuhammad Shaheer JavedÎncă nu există evaluări
- Higher Algebra - Hall & KnightDocument593 paginiHigher Algebra - Hall & KnightRam Gollamudi100% (2)
- Normality Study AddaDocument26 paginiNormality Study AddaTammanurRaviÎncă nu există evaluări
- Polar NyquistDocument2 paginiPolar NyquistTammanurRaviÎncă nu există evaluări
- Clark's TablesDocument54 paginiClark's TablesTammanurRaviÎncă nu există evaluări
- Chemical Equilibrium Theory 1Document20 paginiChemical Equilibrium Theory 1TammanurRaviÎncă nu există evaluări
- Root Locus TechniqueDocument18 paginiRoot Locus TechniqueTammanurRaviÎncă nu există evaluări
- Control Systems - ClassicalDocument130 paginiControl Systems - ClassicalTammanurRaviÎncă nu există evaluări
- Fourier TransformDocument6 paginiFourier TransformTammanurRaviÎncă nu există evaluări
- Nagkan L Linear System DesignDocument26 paginiNagkan L Linear System DesignTammanurRaviÎncă nu există evaluări
- Virginia Tech Admission RequirementsDocument2 paginiVirginia Tech Admission RequirementsTammanurRaviÎncă nu există evaluări
- Lecture 3Document106 paginiLecture 3abhilashaÎncă nu există evaluări
- DC MachineDocument20 paginiDC MachineTammanurRaviÎncă nu există evaluări
- ELG3311 Lab 6Document5 paginiELG3311 Lab 6Nabeel MosawedÎncă nu există evaluări
- NUS Application Guide Cat DDocument10 paginiNUS Application Guide Cat DTammanurRaviÎncă nu există evaluări
- Sem Charts SingleDocument39 paginiSem Charts SingleAbdul Quadeer100% (1)
- DR C A R - Article - 3 - Paean in The EmpyreanDocument2 paginiDR C A R - Article - 3 - Paean in The EmpyreanTammanurRaviÎncă nu există evaluări
- Electric Circuits Lab ManualDocument33 paginiElectric Circuits Lab Manualsheikmd80% (5)
- SCBA RefillingDocument8 paginiSCBA RefillingTammanurRaviÎncă nu există evaluări
- A Guide To Selecting Appropriate Tools To Improve HSE CultureDocument24 paginiA Guide To Selecting Appropriate Tools To Improve HSE CultureSAYED100% (3)
- 2015 0005 Ar TheloveofmasterDocument6 pagini2015 0005 Ar TheloveofmasterTammanurRaviÎncă nu există evaluări
- 2015 0002 Ar PralayaDocument4 pagini2015 0002 Ar PralayaTammanurRaviÎncă nu există evaluări
- DR C A R Article 2 DedicationDocument4 paginiDR C A R Article 2 DedicationTammanurRaviÎncă nu există evaluări
- DR C A R Article 2 DedicationDocument4 paginiDR C A R Article 2 DedicationTammanurRaviÎncă nu există evaluări
- 2015 0004 Ar Harmonising Worldly LifeDocument5 pagini2015 0004 Ar Harmonising Worldly LifeTammanurRaviÎncă nu există evaluări
- 2014-0041-AR-Special Characteristics of Sahaj Marg SadhanaDocument12 pagini2014-0041-AR-Special Characteristics of Sahaj Marg SadhanaTammanurRaviÎncă nu există evaluări
- Diagnostic Imaging of The Pharynx and Esophagus: Key PointsDocument33 paginiDiagnostic Imaging of The Pharynx and Esophagus: Key PointsChutcharwan JintasoponÎncă nu există evaluări
- 3M Novec 1230 Fire Protection Fluid FAQDocument8 pagini3M Novec 1230 Fire Protection Fluid FAQEden CansonÎncă nu există evaluări
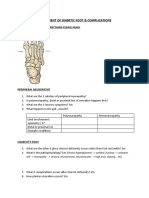
- Assessment of Diabetic FootDocument7 paginiAssessment of Diabetic FootChathiya Banu KrishenanÎncă nu există evaluări
- Clocks (New) PDFDocument5 paginiClocks (New) PDFAbhay DabhadeÎncă nu există evaluări
- Kami Export - Subject Complements-1 PDFDocument3 paginiKami Export - Subject Complements-1 PDFkcv kfdsaÎncă nu există evaluări
- Sap Ewm OverviewDocument11 paginiSap Ewm OverviewsachinÎncă nu există evaluări
- Test7 PointersDocument16 paginiTest7 PointersPratibha DwivediÎncă nu există evaluări
- Technical Data Sheet: LPI HVSC PlusDocument2 paginiTechnical Data Sheet: LPI HVSC PlusNguyễn TấnÎncă nu există evaluări
- Diagnosis of TrypanosomiasisDocument82 paginiDiagnosis of TrypanosomiasisDrVijayata Choudhary100% (1)
- Birla MEEP Op ManualDocument43 paginiBirla MEEP Op ManualAshok ChettiyarÎncă nu există evaluări
- Lima Indiana Oil FieldDocument32 paginiLima Indiana Oil FieldCHARLES PATULAYÎncă nu există evaluări
- Solar Charge Controller: Solar Car Solar Home Solar Backpack Solar Boat Solar Street Light Solar Power GeneratorDocument4 paginiSolar Charge Controller: Solar Car Solar Home Solar Backpack Solar Boat Solar Street Light Solar Power Generatorluis fernandoÎncă nu există evaluări
- Scholomance 1 GravitonDocument18 paginiScholomance 1 GravitonFabiano SaccolÎncă nu există evaluări
- Manuscript FsDocument76 paginiManuscript FsRalph HumpaÎncă nu există evaluări
- Human Wildlife Conflict Resolution PDFDocument9 paginiHuman Wildlife Conflict Resolution PDFdemiÎncă nu există evaluări
- SAT Practice Test 10 - College BoardDocument34 paginiSAT Practice Test 10 - College BoardAdissaya BEAM S.Încă nu există evaluări
- ARK - Intel® Core™ I3-370m Processor (3M Cache, 2Document3 paginiARK - Intel® Core™ I3-370m Processor (3M Cache, 2Delzi Guindra AdriÎncă nu există evaluări
- Harmonic Analysis of Separately Excited DC Motor Drives Fed by Single Phase Controlled Rectifier and PWM RectifierDocument112 paginiHarmonic Analysis of Separately Excited DC Motor Drives Fed by Single Phase Controlled Rectifier and PWM RectifierGautam Umapathy0% (1)
- ETR Series: A Full Spectrum of Products To Solve Your Application NeedsDocument106 paginiETR Series: A Full Spectrum of Products To Solve Your Application Needs周小安Încă nu există evaluări
- The Manufacture and Uses of Expanded Clay Aggregate: Thursday 15 November 2012 SCI HQ, LondonDocument36 paginiThe Manufacture and Uses of Expanded Clay Aggregate: Thursday 15 November 2012 SCI HQ, LondonVibhuti JainÎncă nu există evaluări
- Blue Modern Company Profile PresentationDocument15 paginiBlue Modern Company Profile PresentationjaneÎncă nu există evaluări
- 988611457NK448908 Vehicle Scan ReportDocument5 pagini988611457NK448908 Vehicle Scan ReportVictor Daniel Piñeros ZubietaÎncă nu există evaluări
- English Class Vii PDFDocument101 paginiEnglish Class Vii PDFpannapurohitÎncă nu există evaluări
- Conceptual Artist in Nigeria UNILAGDocument13 paginiConceptual Artist in Nigeria UNILAGAdelekan FortuneÎncă nu există evaluări
- Precision CatalogDocument256 paginiPrecision CatalogImad AghilaÎncă nu există evaluări
- Pharmalytica Exhibitor List 2023Document3 paginiPharmalytica Exhibitor List 2023Suchita PoojaryÎncă nu există evaluări
- RD Sharma Class8 SolutionsDocument2 paginiRD Sharma Class8 Solutionsncertsoluitons100% (2)