Documente Academic
Documente Profesional
Documente Cultură
Coatings & Heat Treatment of Aero-Engine Components
Încărcat de
Nagaraj ThakkannavarTitlu original
Drepturi de autor
Formate disponibile
Partajați acest document
Partajați sau inserați document
Vi se pare util acest document?
Este necorespunzător acest conținut?
Raportați acest documentDrepturi de autor:
Formate disponibile
Coatings & Heat Treatment of Aero-Engine Components
Încărcat de
Nagaraj ThakkannavarDrepturi de autor:
Formate disponibile
Surface Protective Coatings And Heat Treatment processes used in Engine Division HAL Bangalore Presented by: !"!
Appaia# C#ief $anager%$fg!&nsp Aero Engine is mainly divided into two portions. Cold end & Hot end. 75 % of parts are coated with some coating. Material science has reached its pinnacle. ow s!rface coatings are the order of the day "rotective coatings are divided into #hree categories$ Conversion coatings %verlay coatings &iff!sion coatings Conversion coatings $ Chromating on Magnesi!m alloy parts Anodising on Al!mini!m alloy parts. Anodising 'Chromic acid ( )!lph!ric acid* Hard anodising for a+rasive resistance. "hosphating ( ,lac- o.ide coating on steel parts. Chemical composition of the Chromating +ath$ Solution A: Ammoni!m s!lphate Ammoni!m &ichromate "otassi!m &ichromate Manganese s!lphate Solution B: )odi!m &ichromate Manganese s!lphate Magnesi!m s!lphate $ /0 g(l $ 15 g(l $ 15 g(l $ 10 g(l $ 203140 g(l $ 503 60 g(l $ 503140 g(l
Application$ 7 #o increase the corrosion resistance of the Mg. alloy parts 7 As +ase for organic paint application +eca!se it provides e.cellent non3 poro!s +onding s!rface for all paints that have good molec!lar adhesion. 7 Corrosion protection is proportional to thic-ness !pto certain point. "rocess$ 8mmerse in the prepared part in a sol!tion3A +ath maintained at a temperat!re of 903100 deg.C for 10 to /0 mts when the pH is /.6 to 5.0 and water wash and oven dry and protect in an oil paper till the part is ta-en for f!rther processing. )imilarly: if sol!tion , is !sed for chrome3manganese treatment then immerse the prepared part in sol!tion3, maintained at pH val!e of /.0 to 6.0 and depending on the temperat!re the immersion time varies. Brief on Anodi'ing: Anodi;ing is a conversion coating of the s!rface of al!min!m and its alloys to poro!s al!min!m o.ide. )ince the part acts as an anode in an electrolytic cell: it is called anodi;ing. #here are three types of anodi;ing processes vi; chromic acid anodi;ing: )!lph!ric acid anodi;ing and hard anodi;ing. Alloy elements more than 7% and(or copper more than 5% in material: re<!ires s!lph!ric acid anodi;ing instead of Chromic acid anodi;ing. Properties: 8ncrease corrosion resistance when it is sealed either in hot water or in sol!tion. dichromate
8mprove decorative appearance. 8ncrease a+rasion resistance 8ncrease paint adhesion 8mprove adhesive +onding 8mprove l!+ricity "rovide electrical ins!lation 'dielectric* "ermit s!+se<!ent painting &etection of s!rface flaws 8ncrease emissivity of Al!min!m. =hen dyed +lac- the film has e.cellent heat a+sorption !p to 4/0 deg.C
PH(SPHATE C(AT&)" "hosphate coating is the treatment on iron: steel and even al!mini!m with a dil!te sol!tion of phosphoric acid and other chemicals in which the s!rface of the metal: reacting chemically with the phosphoric acid media is converted to an integral: mildly protective layer of insol!+le crystalline phosphate. Composition of "hosphate Coating +ath$ ,ath containing 5% v(v of a<!eo!s sol!tion of >ranodine 101 or 111 maintained at temperat!re of 923100 deg.C. ,efore !se of the +ath: the +ath m!st +e aged +y a addition of 1 -g degreased steel wool per 550 liters of sol!tion. "roperties$ "hosphate treatment on ferro!s parts for corrosion prevention. #he phosphate coating prod!ced provides a good -ey for normal low temperat!re paints or for the retention of oil +ase corrosion preventions and with some proprietary sol!tion improves the anti3 sc!ffing properties of sliding s!rfaces. 8nspection$ "hosphate coating shall +e !niform and matt in appearance o !n3treated patches or fla-y and !neven deposits are accepta+le. )hows minor variation in colo!r d!e to previo!s s!rface treatments. )!perficial powdery deposits on the s!rface shall +e removed +y light +r!shing. 8t shall +e free from s!ch resid!es of the phosphating sol!tion as may initiate deterioration of the s!pplementary finish or pre3mat!re corrosion. Coating weight shall +e 7.5 g()<.M. %verlay coatings %rganic paints 8norganic paints Ceramic paints %rganic paints on Al!mini!m: Mg alloys 'Castings* 8norganic paints on )teel parts %!ter casings & &iscs Ceramic paints on ic-el +ase alloys Hot end parts.
SE*$ETEL C(AT&)"S: 8ntrod!ction$ Among the !ni<!e family of inorganic coatings capa+le of resisting temperat!re e.tremes: corrosion and a+rasive attac-: )ermetel is !ne<!aled in its e.perience with high performance coatings. )ermetel inorganic coatings and processes provide economical: long lasting: thin film protection on ferro!s and non3ferro!s metals !sed in severe and hostile environments. )ermetel is completely dis3similar from paints. )ermetel coatings and processes develop an !n3e<!aled chemical and metall!rgical +ond when properly applied and c!red on prepared s!+strates. #his tenacio!s interface is fle.i+le: !naffected +y age: and resistant to thermal: mechanical and chemical a+!se. ,asically )ermetel has three different types namely )ermetel =: )ermetel 709
and )ermaseal 570A. )ermetel = is sl!rry comprised of an acidic chromatic(phosphate +inder and dispersed al!min!m particles. )ermetel 709 is same as )ermetel = +!t thin version. )ermaseal 570A is a seal coating over )ermetel = and )ermetel 709. PL%+,- Ceramic paint: ?esistant against thermal fatig!e @ 8ndigeno!sly developed. Application$ Aapo!r g!tters: Microt!r+o stators @ Ado!r. "#AE37 engine t!r+ine wheel Plasma coatings : =ear: erosion: corrosion: Bretting wear resistant coatings. Metallisation$ A+rada+le & wire spraying. "lasma coatings +y plasma g!n. Metallisation +y o.y3accetylane g!n. #hermal ,arrier coating @ "lasma #hese coatings are applied for repair scheme also. PLAS$A C(AT&)"S: "lasma spray is one of the technologies !sed for application of metallic material and ceramic material on prepared metallic components of all types of alloys vi; al!min!m: magnesi!m: steel: nic-el +ase: co+alt +ase alloys. D&A$()D .ET C(AT&)": Advantages$ &iamond Cet coating is a +etter choice compared to &etonation g!n coating d!e to its commercial availa+ility and contin!o!s characteristic. 8t is more fle.i+le and easy to operate and provides very dense coatings. "roperties$ 8t prod!ces a dense coating less than 0.5 vol!me percent porosity and well +onded. 8ts +onding strength is greater than 10000 ")8. 8t has e.cellent high temperat!re wear resistance. #he coating hardness will +e 6003900 A" . Application$ D" #!r+ine +lade3Ado!r #!r+ine air seal rings #!r+ine +affle dampers: #!r+ine inner no;;le s!pport B!el rod mandrels Hot cr!shing rolls )team t!r+ine vanes and +!c-ets. &3>!n coating for high wear ( Bretting wear resistant coatings. Materials$ Chromi!m car+ide ( ic-el chrome powders. #!ngsten car+ide powder: etc: E..$ Ado!r D"# & H"# +lades. HA%B >!n coating alternate to &3>!n coating. DET()AT&() "/) C(AT&)"! #he detonation spray coating !nit mainly consists of do!+le walled +arrel one meter long t!+e of 1E in diameter com+!stion cham+er and powder feeder: apart from control panels to reg!late
the gas flows and operation. #he overall set3!p also incl!des an appropriate manip!lator to hold the wor- piece and control its movements. #he process involves inCection of f!el and o.ygen gas into com+!stion cham+er. 8nCection of powder and nitrogen gas. >as detonation and powder acceleration. Cham+er ventilation. #he a+ove cycle is repeated at a predetermined fre<!ency to achieve the desired coating thic-ness. Advantages of detonation spray coating process$ Dow s!+strate temperat!re o distortion on s!+strate o change in microstr!ct!re of the s!+strate High +ond strength coating F 10:000 "si. High density microstr!ct!re G 1% porosity. )mooth s!rface finish Controlled resid!al compressive stress ,etter wear: a+rasion and corrosion resistance. Application$ Chromi!m car+ide( ic-el chrome powder '75(45* coating +y detonation g!n system is on shro!d end faces of Ado!r D" t!r+ine +lades to protect against high temperat!re fretting wear. C(BALT%CH*($&/$ CA*B&DE $ETALL(% CE*A$&C C(AT&)"! 8ntrod!ction$ Co+alt3chromi!m car+ide is a composite coating and is deposited !sing electrolytic plating ro!te. #he process comprises of plating co+alt and chromi!m car+ide particles of 4 to 5 micrometer si;e sim!ltaneo!sly from a co+alt electrolytic 3plating +ath. #he part to +e plated is made as cathode and co+alt chips wor- as anodes. "rocess$ "lating of co+alt @ chromi!m car+ide is achieved !sing a normal electro3plating system fitted with a plate +!mper. #he plate +!mper is made to move !p and down in order to -eep the fine chromi!m car+ide particles in s!spension in the electrolytic +ath. =hen the c!rrent is applied +etween the anode and cathode: co+alt ions move from anode to cathode and in that process they also carry fine chromi!m car+ide particles res!lting in co3deposition of co+alt and chromi!m car+ide. Advantages$ A versatile process capa+le of meeting a wide range of application re<!irements. Coating is dense and non3poro!s: theoretical density 99.9%. Capa+le of operating at temperat!res !pto 200HC. ,ond strength is in e.cess of 70 (mm4 '10000 "si*. ,lind faces and internal +ores down to 6 mm can +e plated. Can +e gro!nd conventionally. )!rface finish as plated 0.5 to 0.6 micrometers CDA )!rface finish lapped 0.0145 micrometers CDA ?ed!ced co!nter face wear on many materials. A cold process red!cing component distortions to a minim!m. HA*D CH*($&/$ PLAT&)" Hard Chromi!m "lating is prod!ced +y electro3deposition from a sol!tion containing chromic acid 'Cr%/* and a catalytic anion in proper proportion. Chromi!m plating is hard and corrosion resistantI it is also e.cellent wear resistant. Hard Chromi!m deposits are intended primarily to increase the service life of f!nctional parts +y providing a s!rface with low coefficient of friction that resists galling: a+rasive: l!+ricated wear and corrosion. 8t is also !sed to restore dimensions of !ndersi;ed parts. Hard Chromi!m is normally deposited to thic-ness ranging from 4.5 to 500 microns.
)&C EL PLAT&)" #he nic-el plating process is !sed e.tensively for decorative: engineering and electroforming p!rposes +eca!se the appearance and other properties of electrodeposited nic-el can +e varied over wide ranges +y controlling the composition and the operating parameters of the plating sol!tion. ELECT*(LESS )&C EL PLAT&)" Electro3less nic-el plating is !sed to deposit nic-el witho!t the !se of an electric c!rrent. #he coating is deposited +y an a!tocatalytic chemical red!ction of nic-el ions +y hypophosphite: amino+orane or +orohydride compo!nds. Advantages$ >ood resistance to corrosion and wear E.cellent !niformity )older3a+ility and +ra;e3a+ility Dow la+o!r costs. Dimitation$ Higher chemical cost than electro3plating. ,rittleness. "oor welding characteristics d!e to contamination of nic-el plate with nic-el3 "hosphor!s deposits. )lower plating rate: as compared to electrolyte methods. ?eaction +y prod!cts C#aracteristics: Electro3less nic-el plating is prod!ced +y the controlled chemical red!ction of nic-el ions onto a catalytic s!rface. #he deposit itself is catalytic to red!ction: and the reaction contin!es as long as the s!rface remains in contact with the electro3less nic-el sol!tion. Electro3less nic-el sol!tions are +lends of different chemicals: each performing an important f!nction: electro3less nic-el sol!tion contain$ A )o!rce of nic-el: i.e. nic-el s!lfate A red!cing agent to s!pply electrons for the red!ction of nic-el. Energy i.e. heat Comple.ing agents to control the free nic-el availa+le to the reaction. ,!ffering agents to resist the "H changes ca!sed +y the hydrogen generated d!ring deposition Accelerators to help increase the speed of the reaction. 8nhi+itors 'sta+ili;ers* to help control red!ction. CAD$&/$ PLAT&)": Cadmi!m plating is !sed to protect steel and cast iron against corrosion: +eca!se cadmi!m is anode to iron: the !nderlying ferro!s metal is protected at the e.pense of cadmi!m plating. 8t is a thin coating the thic-ness is less than 45 microns. 8t is seldom !sed as !ndercoating for other metals and its resistance to corrosion +y most chemicals is low. 8t has nat!ral l!+ricity Cadmi!m has e.cellent electrical cond!ctivity and low contact resistance. on corrosive fl!.es can +e !sed to prod!ce top <!ality soldered sections. )teel that is coated with cadmi!m can +e formed and shaped +eca!se of the &!ctility of the cadmi!m. Cadmi!m is highly to.ic. Health: safety and environmental concerns are driving
the red!ction or elimination of its !se for many applications.
SELECT*() 0B*/SH1 PLAT&)": )electron plating also called as +r!sh plating is carried o!t +y applying an electrolyte on to the prepared s!rface +y a hand held anode or styl!s: which incorporates an a+sor+ent wrapping for applying the sol!tion to the wor- piece 'cathode*. A direct c!rrent power pac- drives the electrochemical reaction: depositing the desired metal on the s!+strate. %ther s!rface modification "rocesses$ "A&: CA& coatings: #itani!m itride coatings: Daser cladding ( modifications etc. are very widely !sed &iff!sion Coatings 0&ndigenously developed &n%#ouse1 a* Al!mini;ing +* Al3)i diff!sion coating c* )ermalloy3J coating d* "t3Al!minide coating Applications$ #!r+ine ,lades ( #!r+ine wheels )!rface coatings are all considered as sacrificial coatings: +eca!se they s!ffer from hostile environment protecting +ase metal. Advantage @ Enhancement of life of component : ?ecoating ( ?e3claiming of parts: there +y cost red!ction: ?eplacement of parts are avoided. "?%CE)) BD%= CHA?#) (rganic Paint Application &egrease ( Metal cleaning Chromate ( Anodising 'Mg (Al* Apply "rimer +y )pray g!n ,a-e at 1003400 &eg C range Mi. paint thoro!ghly Chec- viscosity Apply paint +y )pray g!n Air &ry ,a-e at 1903400 &eg C for K Hr 8nspect Sermetel Paint Application &egrease 3 Mas- @ A+rasive ,last @ Clean with dry Air @ Mas- @ Mi. )ermetel @ = thoro!ghly 3 Chec- viscosity @ Apply +y spray g!n @ Air dry @ Apply )econd coat @ Air &ry @ %ven &ry at 190 &eg C for K Hr @ ,a-e at /50 &eg C for 1 Hr. and 560 &eg C for 1 Hr. @ Chec- thic-ness and s!rface finish >lass+ead peen ',!rnishing* @ A++rasive ,last @ Apply )ermoseal 570A @ Air &ry @ %ven &ry at 190 &eg C for K Hr. ,a-e at /50 deg.c for 1ho!r. @ 8nspect #hic-ness of coating is 50 @ 100 microns for )ermetel @ = 2310 microns for )ermoseal 570A Plasma 2 D%"un Coating &egrease
MasA+rasive +last Clean with &ry Air MasApply ,ond Coat '"lasma* Apply %ver Coat '"lasma* Apply Coat @ &3>!n Chec- #hic-ness ( L!ality
Aluminising 2 Al%Si Diffusion Coating &egrease ,last Clean with dry air Mas "ac- in powder Doad into f!rnace at temp. 8nlet argon )oa- for re<!ired time Cool and clean the parts 10 % Ardro. Mltrasonic cleaning =ash in cold water 5 % Citric acid cleaning =ash in Cold water & Hot water Air &ry &iff!sion Heat treatment Chec- thic-ness and L!ality Aging Platinum Aluminide Coating &egrease Mas ,last ?emove Mas- and ?e3mas Electrolytic Al-aline Cleaning Acid Etching =ash in Hot ( Cold =ater "latin!m plating =ash in Cold ( Hot =ater &ry & 8nspect #hic-ness & L!ality High #emp. &iff!sion Al!minising 10 % Ardro. Mltrasonic cleaning =ash in cold water 5 % Citric acid cleaning =ash in Cold water & Hot water Air &ry High #emp. &iff!sion 8nspect #hic-ness & L!ality Aging HEAT T*EAT$E)T P*(CESSES All metallic parts in Aero Engine will !nder go some type of Heat treatment to achieve re<!ired properties specified in drawing ( specifications. All metallic parts in Aero Engine will !nder go some type of Heat treatment to achieve re<!ired properties specified in drawing ( specifications. Heat treat processes 1. Annealing @ )teel
4. /. 5. 5.
6. 7.
ormalising: Hardening & #empering @ steels )tress ?elieving for all types of materials. )ol!tionising & precipitation 'Aging* heat treatment for Al: Mg: #i: & i +ase )!rface hardening processes a* >as car+!rising +* >as nitriding c* Cyaniding &e3em+rittlement Heat treatment ,a-ing ( c!ring of painted parts
alloys
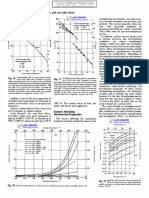
Annealing Heating the steel to 900 deg C and followed +y f!rnace cooling. 'M!ffle ( vac!!m*. Material softens. Hence any forming and machining: =elding can +e done easily. )ormalising3 Hardening 4 Tempering #his process is applica+le to car+on steels ( alloy steels to achieve re<!ired properties & for Machining. ormalising$ Heating to 900 deg C 3 hold @ Air cool 'M!ffle ( vac!!m* ?es!lt$ Mniform microstr!ct!re ( chemical composition distri+!tion. Hardening$ #o achieve re<!ired hardness. Heating to 250 deg C @ hold @ oil <!ench ( >as <!ench in m!ffle ( e!tral salt +ath ( Aac!!m B!rnace respectively. ?es!lt$ #ransformation to Martensitic str!ct!re. Hard & ,rittle. Material in strained Condition. #empering$ Heating to 550 @ 600 deg C for 1 to 4 hrs followed +y air cooling 'Air circ!lating f!rnace* ?es!lt$ ?ed!ction in ,rittleness. ?e<!ired hardness ( d!ctility achieved. )tress ?elieving$ Heating to temperat!re less than or e<!al to tempering temperat!re: hold for 134 hrs and air cool. 8nd!ced stress d!e to machining: cold forming or welding is removed. Applica+le for any material. )ol!tionising & Aging '"recipitation* Al!mini!m$ Heating to 505 to 545 deg C for re<!ired d!ration in air circ!lating B!rnace & water <!enching ( ,rine <!enching etc. ?es!lt$ All the constit!ents will go to solid sol!tion. Ex: Copper in Al!mini!m remains in )olid sol!tion. Conditions$ )oft: easy to machine( form etc. Aging: 'a* at!ral Aging '+* Artificial Aging '"recipitation* at!ral Aging ta-es place at room temp in 52 hrs. Artificial Aging ta-es place at elevated temp. at 1503400 HC for 16 Hrs. ?es!lt$ Copper precipitates as C!Al4 8nter3metallic compo!nd in grain +o!ndary and attain mechanical properties. Hardness $ 140 @ 150 ,H #ensile )trength $ 50354 Ngs ( mm4 Elongation $ 10% Minim!m #itani!m and ic-el ,ase alloys also will !ndergo sol!tionising & aging to get mechanical properties & High temp. "roperties vi; creep & stress r!pt!re properties. )ol!tionising$ At 950 deg C for #i3 alloys: at 95031150 deg C for i 3,ase alloys Aging$ 550 @ 700 deg C for #i3 alloys 700 3 250 deg C for i3 ,ase alloys Surface Hardening Processes 'a* "as carburising: 8ntrod!ction of additional car+on onto s!rface of components vi; gears ( shafts etc.: and hardening and tempering is called case hardening.
#emperat!re $ 900 to 945 deg O &!ration $ &epends on #hic-ness )!rface hardness $ ?c 60 min ')ome times ?c 57 accepta+le* Core hardness $ ?c /43/6 & ?c /635/ Application $ >ear teeth: ,earing diameter: )hafts etc.: "!rpose $ =ear resistant: 8ncrease fatig!e with to!gh core for resistance to shoc- load b1 "as )itriding$ 8ntrod!ction of acent itrogen onto s!rface of steel components at temperat!re less than tempering temp of part. #he acent itrogen com+ines with alloying elements vi; Cr: A: Mo: etc.: forms respective nitrides and +ecome hard s!rface to a depth re<!ired. itriding temp $ 590 @ 505 deg C &!ration $ 14 :45: 52:74 etc.: Cyaniding: 8ntrod!ction of car+on and itrogen onto s!rface of the component. #his is done in a salt +ath containing sodi!m cyanide: sodi!m chloride +ath. Heated to 270 deg C and dipping the parts for a period of 15340 min!tes to achieve case depth of 0.40 mm ma. #hen hardened and tempered 240 deg C @ %il <!ench ( salt <!ench at 190 deg c @ Air cool 150 @ 150 deg C for 4 hrs. Air cool in air circ!lating f!rnace. Hardness$ ?c 523 61 )!rface ?c /43 /6 ?c /63 5/ Core 5uality Control C#ec6s: All f!rnaces and ovens are to +e in cali+rated condition. "eriodically to +e cali+rated incl!ding thermoco!ples. #emperat!re Controller: #emperat!re recorder: over temperat!re controls is re<!ired to every f!rnace. =henever test piece re<!irements are given in drawing: they have to +e processed along with parts. &aily chec- ( Ann!al cali+ration of Hardness testing machines is a m!st. After Hardening & #empering: Hardness to +e chec-ed & record '?oc-well: Aic-ers or ,rinnel methods.* Bor s!rface hardening processes the following specimens to +e processed & chec-ed.
"as carburising: )py #est specimen @ Bor case depth: <!ality: hardness >ear c!t specimen ')im!lated #.".* @ Case depth: <!ality: hardness Car+on potential test specimen @ "ercentage of car+on on s!rface ?etained A!stenite test specimen @ #o chec- for retained a!stenite in the case )itriding: )py #est specimen @ Bor case depth: <!ality: hardness 8mpact test specimen @ to!ghness: #emper Em+ittlerment Cyaniding: )py #est specimen @ Case depth: <!ality & Hardness e!tral salt !sed for Hardening to +e chec-ed periodically Cyanide content to +e chec-ed +efore !se. L!enching oil to +e chec-ed for contamination & acidity periodically #richloro3 ethylene to +e chec-ed for contamination & acidity.
S-ar putea să vă placă și
- CNC Machine Operator Job Description Free PDF DownloadDocument4 paginiCNC Machine Operator Job Description Free PDF DownloadErkan Oztemel100% (1)
- Aluminum Alloys HTDocument6 paginiAluminum Alloys HTsaaÎncă nu există evaluări
- Aluminum Alloys HTDocument6 paginiAluminum Alloys HTsaaÎncă nu există evaluări
- How To Achieve World Class Efficiency PDFDocument3 paginiHow To Achieve World Class Efficiency PDFNagaraj ThakkannavarÎncă nu există evaluări
- Budget Highlights 2010: Key PointsDocument10 paginiBudget Highlights 2010: Key PointsNagaraj ThakkannavarÎncă nu există evaluări
- How To Achieve World Class Efficiency PDFDocument3 paginiHow To Achieve World Class Efficiency PDFNagaraj ThakkannavarÎncă nu există evaluări
- How To Achieve World Class Efficiency PDFDocument3 paginiHow To Achieve World Class Efficiency PDFNagaraj ThakkannavarÎncă nu există evaluări
- Aero Gas Turbine DesignDocument182 paginiAero Gas Turbine DesignNagaraj Thakkannavar50% (2)
- Chap 003Document25 paginiChap 003Nagaraj ThakkannavarÎncă nu există evaluări
- Material Science: Cast IronDocument40 paginiMaterial Science: Cast IronNagaraj ThakkannavarÎncă nu există evaluări
- Population and EnvironmenDocument23 paginiPopulation and EnvironmenNagaraj ThakkannavarÎncă nu există evaluări
- Comp 2Document31 paginiComp 2Sudheer AyazÎncă nu există evaluări
- Shoe Dog: A Memoir by the Creator of NikeDe la EverandShoe Dog: A Memoir by the Creator of NikeEvaluare: 4.5 din 5 stele4.5/5 (537)
- Never Split the Difference: Negotiating As If Your Life Depended On ItDe la EverandNever Split the Difference: Negotiating As If Your Life Depended On ItEvaluare: 4.5 din 5 stele4.5/5 (838)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureDe la EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureEvaluare: 4.5 din 5 stele4.5/5 (474)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeDe la EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeEvaluare: 4 din 5 stele4/5 (5782)
- Grit: The Power of Passion and PerseveranceDe la EverandGrit: The Power of Passion and PerseveranceEvaluare: 4 din 5 stele4/5 (587)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceDe la EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceEvaluare: 4 din 5 stele4/5 (890)
- The Yellow House: A Memoir (2019 National Book Award Winner)De la EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Evaluare: 4 din 5 stele4/5 (98)
- On Fire: The (Burning) Case for a Green New DealDe la EverandOn Fire: The (Burning) Case for a Green New DealEvaluare: 4 din 5 stele4/5 (72)
- The Little Book of Hygge: Danish Secrets to Happy LivingDe la EverandThe Little Book of Hygge: Danish Secrets to Happy LivingEvaluare: 3.5 din 5 stele3.5/5 (399)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryDe la EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryEvaluare: 3.5 din 5 stele3.5/5 (231)
- Team of Rivals: The Political Genius of Abraham LincolnDe la EverandTeam of Rivals: The Political Genius of Abraham LincolnEvaluare: 4.5 din 5 stele4.5/5 (234)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaDe la EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaEvaluare: 4.5 din 5 stele4.5/5 (265)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersDe la EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersEvaluare: 4.5 din 5 stele4.5/5 (344)
- The Emperor of All Maladies: A Biography of CancerDe la EverandThe Emperor of All Maladies: A Biography of CancerEvaluare: 4.5 din 5 stele4.5/5 (271)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyDe la EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyEvaluare: 3.5 din 5 stele3.5/5 (2219)
- The Unwinding: An Inner History of the New AmericaDe la EverandThe Unwinding: An Inner History of the New AmericaEvaluare: 4 din 5 stele4/5 (45)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreDe la EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreEvaluare: 4 din 5 stele4/5 (1090)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)De la EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Evaluare: 4.5 din 5 stele4.5/5 (119)
- Her Body and Other Parties: StoriesDe la EverandHer Body and Other Parties: StoriesEvaluare: 4 din 5 stele4/5 (821)
- Ashish Bhateja, Aditya Varma, Ashish Kashyap and Bhupinder Singh _ TheStudy the Effect on the Hardness of Three Sample Grades of Tool Steel i.e. En-31, En-8, And D3 After Heat Treatment Processes Such As Annealing, Normalizing, and Hardening & TemperingDocument7 paginiAshish Bhateja, Aditya Varma, Ashish Kashyap and Bhupinder Singh _ TheStudy the Effect on the Hardness of Three Sample Grades of Tool Steel i.e. En-31, En-8, And D3 After Heat Treatment Processes Such As Annealing, Normalizing, and Hardening & TemperingProf.Bhateja AshishÎncă nu există evaluări
- Asme Section II A-2 Sa-660Document6 paginiAsme Section II A-2 Sa-660Anonymous GhPzn1xÎncă nu există evaluări
- Evolution of Microstructure and Mechanical Properties of D2 Tool Steel During Annealing Heat TreatmentDocument5 paginiEvolution of Microstructure and Mechanical Properties of D2 Tool Steel During Annealing Heat TreatmentLeydherIbarraRipollÎncă nu există evaluări
- Effects of Alloying Elements in SteelDocument12 paginiEffects of Alloying Elements in SteelJenna BaileyÎncă nu există evaluări
- IG 76 MESC 14d - 2021Document3 paginiIG 76 MESC 14d - 2021bmanojkumar16Încă nu există evaluări
- A STUDY ON THE TENSILE AND FATIGUE STRENGTH OF WELDED AND THREADED QUENCH TEMPERED/THERMO-MECHANICALLY TREATED REBARSDocument63 paginiA STUDY ON THE TENSILE AND FATIGUE STRENGTH OF WELDED AND THREADED QUENCH TEMPERED/THERMO-MECHANICALLY TREATED REBARSJohn Christopher AgutayaÎncă nu există evaluări
- Minimum Design Temperature (C) Chemical Composition and Heat Treatment Impact Test Temperature (C)Document3 paginiMinimum Design Temperature (C) Chemical Composition and Heat Treatment Impact Test Temperature (C)MohdNabhanFahmiZailaniÎncă nu există evaluări
- Complying With Nace Hardness RequirementsDocument5 paginiComplying With Nace Hardness Requirementsmanimaran100% (2)
- Asme Section Ii A-2 Sa-836 Sa-836mDocument4 paginiAsme Section Ii A-2 Sa-836 Sa-836mdavid perezÎncă nu există evaluări
- Essar Steel Rockstar 400Document2 paginiEssar Steel Rockstar 400HimanshuNarayanSinghÎncă nu există evaluări
- 15.welding Engineering PDFDocument15 pagini15.welding Engineering PDFEmad A.Ahmad100% (2)
- Manufacturing Process of Engine Valve PlantDocument30 paginiManufacturing Process of Engine Valve PlantVirat Dubey80% (5)
- Sa 387 2019Document6 paginiSa 387 2019CK CkkouÎncă nu există evaluări
- Engineering Failure Analysis: Colin R Gagg, Peter R LewisDocument19 paginiEngineering Failure Analysis: Colin R Gagg, Peter R LewisOssamaElKhalÎncă nu există evaluări
- Case Hardening Steels - Technical Delivery Conditions: British Standard Bs en 10084:2008Document40 paginiCase Hardening Steels - Technical Delivery Conditions: British Standard Bs en 10084:2008Senthil Kumar Ganesan100% (1)
- (p636-644) Metals Handbook. Volume 1, Properties and Selection Irons, Steels, and High-Performance Alloys PDFDocument9 pagini(p636-644) Metals Handbook. Volume 1, Properties and Selection Irons, Steels, and High-Performance Alloys PDFSethGraceÎncă nu există evaluări
- 420 Stainless Steel DS 201406 PDFDocument3 pagini420 Stainless Steel DS 201406 PDFAlexander Saavedra MambuscayÎncă nu există evaluări
- A867-03 (2013) Standard Specification For Iron-Silicon Relay SteelsDocument4 paginiA867-03 (2013) Standard Specification For Iron-Silicon Relay SteelsdcardonasterÎncă nu există evaluări
- Heintzberger Influence 2017Document61 paginiHeintzberger Influence 2017ashav patelÎncă nu există evaluări
- Ams4943l 2020Document8 paginiAms4943l 2020BauyrzhanÎncă nu există evaluări
- Evaluation of Mechanical Properties of Aluminum Alloy-Alumina-Boron Carbide Metal Matrix CompositesDocument8 paginiEvaluation of Mechanical Properties of Aluminum Alloy-Alumina-Boron Carbide Metal Matrix CompositesPremier PublishersÎncă nu există evaluări
- SA336Document10 paginiSA336ismaelarchilacastilloÎncă nu există evaluări
- Steel Plates For Pressure Vessels, Produced by Thermo-Mechanical Control Process (TMCP)Document10 paginiSteel Plates For Pressure Vessels, Produced by Thermo-Mechanical Control Process (TMCP)Sama UmateÎncă nu există evaluări
- Holland KingpinsDocument8 paginiHolland KingpinsPablo César Suárez NogalesÎncă nu există evaluări
- NBS Monograph 88 PDFDocument52 paginiNBS Monograph 88 PDFDavid C HouserÎncă nu există evaluări
- Is 1865Document13 paginiIs 1865Yogesh NadgoudaÎncă nu există evaluări
- CK45 (1.1191)Document3 paginiCK45 (1.1191)alextentwentyÎncă nu există evaluări
- A437A437Document3 paginiA437A437Fallo SusiloÎncă nu există evaluări
- PRC-2003G Heat Treating Nickel AlloysDocument9 paginiPRC-2003G Heat Treating Nickel AlloysHenryÎncă nu există evaluări
- Cast IronDocument15 paginiCast IronJohnÎncă nu există evaluări