Documente Academic
Documente Profesional
Documente Cultură
13 14 Immuno Signal Transduction Lymphocyte Activation 1-22-14
Încărcat de
lexi_2706Drepturi de autor
Formate disponibile
Partajați acest document
Partajați sau inserați document
Vi se pare util acest document?
Este necorespunzător acest conținut?
Raportați acest documentDrepturi de autor:
Formate disponibile
13 14 Immuno Signal Transduction Lymphocyte Activation 1-22-14
Încărcat de
lexi_2706Drepturi de autor:
Formate disponibile
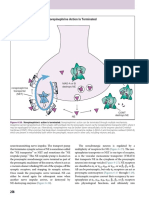
Signal Transduction & Lymphocyte Activation Leitenberg January 22, 2014 3:00pm-4:00pm NT #6 I apologize for going fast.
It would help to read the outline and watch the videos and if you need to you can read the textbook. I also try and underline and bold the molecules or concepts that I think you should know. Q: Can you summarize the sequence of phosphorylations? 1. Ag binds 2. SRC family tyrosine kinases (which are different for B & T cells) phosphorylate ITAM motif. 3. Other tyrosine kinase enzymes are recruited to this site. SRC family tyrosine kinases phosphorylate and activate these enzymes. 4. These enzymes will go on to phosphorylate other enzymes to produce signaling cascade. For example, they will phosphorylate PLC, which cleaves PIP2 into IP3 and DAG. Q: When do the co-receptors come in? Co-receptors are a way of explaining how the SRC family tyrosine kinases access the Ag receptor when it is being ligated. Cells without these co-receptors do not work as well. There is a defect in how quickly they initiate these different cascades. Its a graded process (its not all or none) but this works A LOT better with co-receptors. Q: What are some examples of co-receptors? For T-cells the co-receptors would be CD4 & CD8. For B-cells the co-receptors are CD21 & CD19. Slide 40: What happens after activation of PLC? Phospholipase C cleaves this lipid (PIP2) into IP3 and DAG. IP3 engages with an IP3 receptor on the ER and on the plasma membrane, opens up Ca2+ channels at those sites and causes an increase of Ca2+ in the cytosol. Slide 41: Cyclosporin A is a drug that inhibits this pathway by interfering specifically with T-cell activation. Another related drug is FK5O6. Here you can see PLC is activated, cleaves PIP2 into IP3 and DAG and then you can see there is an influx of Ca2+. Then we have this molecule called calcineurin, which is a phosphatase that dephosphorylates NFAT, which is usually highly phosphorylated in the cytosol. When it interacts with calcineurin it becomes dephosphorylated and triggers NFAT to move from
the cytosol to the nucleus where it can affect gene transcription. If you give a patient this drug it will inhibit calcineurin activity blocks NFAT from getting to nucleus negative affect on gene transcription and T-cell activation. This drug has revolutionized transplantation. You can do kidney transplants now without being too careful about matching HLA type as long as you treat with cyclosporine, which will decrease T-cell activity. Slide 42: G protein signaling is another signaling pathway. These G proteins are so called because they are active when bound to GTP and inactive when bound to GDP. So this is sort of a molecular switch. There are other factors in the cytosol that determine the amount of GDP/GTP available for G protein. For example guanine nucleotide exchange factor will actually displace GDP from inactive GDP-G-protein complex and replace it with GTP to activate the G-protein. One of these G proteins is called Ras. This happens in lots of cells, but in lymphocytes it happens as a consequence of Ag binding. What I want you to know about this is that small G proteins play a role in signal transduction and that they catalyze the reaction of GTP to GDP. Slide 43: A downstream consequence of activating a G protein, like Ras, is the activation of MAP Kinase cascade. ERK is a result of MAPK enzymatic cascade and it will go on to phosphorylate a transcription factor in the nucleus, which will regulate gene transcription and protein expression. Slide 44: What is the end result of all of these signaling cascades? Through these signal cascades we can activate a variety of transcription factors. When these factors bind on the DNA in the nucleus they direct gene transcription. One important gene that is transcribed as a result of all of this signaling is IL-2 cytokine gene. This is really important for Tcells. When T-cells make IL-2 it can bind to itself or cells in the neighborhood and initiate proliferation. So we think that IL-2 is really important in the initial clonal expansion, survival, and differentiation into effector cells. Slide 45: The T-cell receptor is not shown in this picture but when the ligand binds to the T-cell receptor we will make IL-2 and also make more and better IL-2 receptor. Since IL2 can act in an autocrine (self) manner, the IL-2 will bind on those enhanced receptors and signal them to induce clonal expansion and proliferation. This is the first step in adaptive immunity and these IL-2 are also critical for differentiation of cells with different effector function. Slide 46: Why is A wrong? The question is specific for calcinuerin receptor. B is wrong because NFAT would be localized to the cytosol not the nucleus. D is wrong because this step is upstream from the calcinuerin effect. E is wrong because this is also upstream. We wouldnt expect anything upstream to be affected by what were doing in this question. The answer is C. I just told you this is what calcinuerin does. Slide 47: What weve talked about so far is really based on the Ag point of view. But there are other things that are also necessary for T-cell activation. The most important molecule for nave T-cell stimulation is called CD28. These are on the T-cell and they bind with a ligand called B7 (also called CD80) on the antigen-presenting cell. There is a
lot of cell-cell mediated interaction going on here. You have interactions outside of MHC:peptide and T-cell interaction. Slide 48: Now well talk about signaling through co-stimulatory molecules like CD28. We talked about pathogen recognition receptors when we talked about innate immunity. And well talk about signaling through cytokine receptors. Slide 49: This is a cartoon of the immunological synapse. We have talked a lot about MHC interaction with T-cells but we havent talked about this other stuff yet. There are a variety of other molecules that get engaged at the immunological synapse that positively and negatively regulate activation of T-cells. CD28 is one of these and it is constitutively expressed by nave CD8 T-cells. When CD28 recognizes MHC presented by a professional APC (with co stimulatory molecules) it will trigger the cell to express additional molecules on the cell surface that can further engage in interaction with MHC on the APC. CD40 ligand on the T-cell is another one of these molecules at the immunological synapse that interacts with CD40 molecule on the APC. There is bidirectional signaling going on here. Slide 50: Well talk about CD28 first. This is the two-signal model of T-cell activation. Signal 1 is a signal derived from the Ag receptor interacting with MHC-peptide complex. Another signal is required for nave T-cell stimulation and this is done via the CD28 molecule. This is essential to activate virgin T-cells in secondary lymphoid organs. Only professional APCs can do this, like we talked about before, because they express costimulatory molecules like the B7 molecule, which are required for interaction with CD28. This is less important for previously activated effector cells. Slide 51: What does CD28 do? Binding of B7 with CD28 initiates a variety of important molecular events. Binding promotes cell survival, protects the cell from programmed cell death, promotes transcription of IL-2 so the cell can make more and better IL-2 and there is also rearrangement of the cytoskeletal framework to optimize the immunological synapse. Dont worry about the specifics here. CD28 is important for naive T-cell activation. Slide 52: Adhesion molecules also play a key role. LFA-1, presented on the surface of Tcells, interacts with ICAM-1 on APC molecules and maintains a long-lived interaction between these two cells. We talked about these interactions in another context earlier where they were mediating leukocyte migration. Slide 53: When the T-cell is in a lymphoid organ, scanning the microenvironment, the Tcell can get activated by adhesion molecules. Adhesion molecules, like LFA-1, can loosely interact with the ICAM on APC, and if, at the same time the T-cell recognizes an MHC-peptide complex you will get a strengthening of this reaction. This is called inside-out signaling. There is something going on within the cell that changes the interaction of MHC-peptide and T-cell on the outside of the cell. The change in affinity of LFA-1 for ICAM-1 is changed by something intrinsic to the LFA-1 molecule. Slide 54: The 2 signal model also works for B cell activation. The first signal would be the B-cell receptor interacting with Ag and the second signal would be CD40 molecule
binding to CD40 ligand on the B-cell. The B cell is activated by Ag binding which results in signaling events. This facilitates Ag presentation of the Ag molecule by a helper Tcell. The Helper T-cell and the B-cell will physically engage and the T-cell will become activated to express CD40 molecule on its surface. This CD40 molecule will interact with the CD40 ligand and will help the B-cell to become further activated via promotion of Ig switching, development of memory function, and germinal center formation. Slide 55: The same principle applies when T-cells interact with macrophages and dendritic cells. These cells also express CD40 ligand. Consequence of activation of this system for those cells might be production of pro-inflammatory cytokines. This is how the adaptive immune response feeds back on the innate immune response. Slide 56: This is the structure of CD40 engaging with CD40 ligand family members. CD40 ligand belongs to the Tumor necrosis factor receptor superfamily. This family is responsible for activating transcription factor, NFK-B. Depending on the cell that NFK-B is activated in will determine the effect. Activation of NFK-B in T cells and B cells promotes cell survival. In macrophages and dendritic cells, activation of NFK-B promotes cytokine production. This is a common way that cells get signaled. We can develop drugs to interfere with this pathway. Slide 57: CD40 signaling is important for B-cell activation and for T-cell help in B-cell activation but there are some Ags that dont go this route. We refer to Ags that activate the cell via PRR and B-cell receptor interaction alone, as T-independent antigens, which can activate these other guys independent of T-cell help. There is no CD40 signaling here. These interactions dont generally promote isotype switching, germinal center formation, or development of memory function. Slide 58: This is an example of how Toll like receptors signal. They use an intracellular molecule, an adaptor protein, that acts as a bridge between the receptor and additional cascade signaling events that ultimately activates NFK-B transcription factor. MyD88 is an example of one of these adaptor proteins, which will result in NFK-B activation. Just know that PRRs can result in NFK-B activation and MyD88 is commonly involved in this. Slide 59: Class I and II cytokine receptors regulate a lot of important immune responses. One example is the receptor for IL-2. Class I and Class II receptors all use the JAKSTAT signaling pathway. Slide 60: The cytokine engages with cytokine receptor, stabilizes the receptor and activates tyrosine kinases called JAK kinases, Janus associated kinases, so named after the mythological creature with two heads. These two JAK proteins oligomerize together to activate each other and the cytoplasmic tail of the receptor via phosphorylation. This recruits a STAT protein to the site of cytokine receptor signaling. The STAT molecules then get phosphorylated and dimerize. Then they get transported to the nucleus where they can act as transcription factors. This is a simple pathway. There are different STAT proteins that regulate different patterns of transcription. Different cytokines will signal different JAKS and STATS and therefore cause transcription of different genes.
Slide 61: Chemokine receptors are important and regulate where cells go in the body. This is done via mediation by large G proteins, as opposed to the small G proteins that we talked about earlier. The large G proteins are assemblies of different subunits of alpha and beta chains but they do the same thing as the small G proteins. Their activity in initiating signal transduction pathways is dependent on that switching of GDP-GTP. Slide 62: There is also negative signal transduction. Ex: the Fas molecule mediates cell death via down regulation of immune responses. This occurs when the Fas molecule trimerizes and initiates a signaling cascade with the result of apoptosis. You end up killing off activated cells. Slide 63: These signal transduction events determine proliferation, survival, and activation. These events are not necessarily distinct from each other. They may be happening at the same time, or over several days and you can have repetitive and simultaneous engagement of these receptors. Slide 64-66: How does this work? Within the cytoplasmic tails of inhibitory receptors there are ITIM motifs instead of ITAM motifs. When these ITIM motifs are phosphorylated as a result of Ag binding at receptor the signaling cascade is inhibited rather than propagated. When ITIM motifs are phosphorylated they recruit phosphatases, which cleave the phosphate from tyrosine kinases to turn them off. Slide 67: Another way to down regulate signaling is that you can degrade the proteins involved in these pathways. One way to do this is to tag these proteins with a protein called ubiquitin, which marks that cell for denaturation, by a proteosome. Ubiquitin ligases (ex: CBL) add ubiquitin to these components. If you add ubiquitin to ZAP70, a signaling molecule, you are marking it for destruction. If cells have less ZAP70 they wont be signaling as much. Down regulation of signaling can be important in autoimmune disorders. Down regulation of CBL, and therefore lower levels of ZAP70 will cause these cells to be less active, and this is example of anergy. You can do this in mice and potentiate autoimmune disease. Slide 68: The answer is A. Slide 69: This is the outline of everything that we talked about today. And some of this stuff we will revisit as we go along in the course.
S-ar putea să vă placă și
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryDe la EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryEvaluare: 3.5 din 5 stele3.5/5 (231)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)De la EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Evaluare: 4.5 din 5 stele4.5/5 (119)
- Never Split the Difference: Negotiating As If Your Life Depended On ItDe la EverandNever Split the Difference: Negotiating As If Your Life Depended On ItEvaluare: 4.5 din 5 stele4.5/5 (838)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaDe la EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaEvaluare: 4.5 din 5 stele4.5/5 (265)
- The Little Book of Hygge: Danish Secrets to Happy LivingDe la EverandThe Little Book of Hygge: Danish Secrets to Happy LivingEvaluare: 3.5 din 5 stele3.5/5 (399)
- Grit: The Power of Passion and PerseveranceDe la EverandGrit: The Power of Passion and PerseveranceEvaluare: 4 din 5 stele4/5 (587)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyDe la EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyEvaluare: 3.5 din 5 stele3.5/5 (2219)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeDe la EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeEvaluare: 4 din 5 stele4/5 (5794)
- Team of Rivals: The Political Genius of Abraham LincolnDe la EverandTeam of Rivals: The Political Genius of Abraham LincolnEvaluare: 4.5 din 5 stele4.5/5 (234)
- Shoe Dog: A Memoir by the Creator of NikeDe la EverandShoe Dog: A Memoir by the Creator of NikeEvaluare: 4.5 din 5 stele4.5/5 (537)
- The Emperor of All Maladies: A Biography of CancerDe la EverandThe Emperor of All Maladies: A Biography of CancerEvaluare: 4.5 din 5 stele4.5/5 (271)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreDe la EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreEvaluare: 4 din 5 stele4/5 (1090)
- Her Body and Other Parties: StoriesDe la EverandHer Body and Other Parties: StoriesEvaluare: 4 din 5 stele4/5 (821)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersDe la EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersEvaluare: 4.5 din 5 stele4.5/5 (344)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceDe la EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceEvaluare: 4 din 5 stele4/5 (890)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureDe la EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureEvaluare: 4.5 din 5 stele4.5/5 (474)
- The Unwinding: An Inner History of the New AmericaDe la EverandThe Unwinding: An Inner History of the New AmericaEvaluare: 4 din 5 stele4/5 (45)
- The Yellow House: A Memoir (2019 National Book Award Winner)De la EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Evaluare: 4 din 5 stele4/5 (98)
- Psychopharmacology Drugs, The Brain, and BehaviorDocument13 paginiPsychopharmacology Drugs, The Brain, and Behavior陳琮方5% (21)
- On Fire: The (Burning) Case for a Green New DealDe la EverandOn Fire: The (Burning) Case for a Green New DealEvaluare: 4 din 5 stele4/5 (73)
- Walking Gait-MUSCLES Involved PDFDocument2 paginiWalking Gait-MUSCLES Involved PDFlexi_2706Încă nu există evaluări
- Biochemistry 1.3 NeurotransmittersDocument10 paginiBiochemistry 1.3 Neurotransmitterslovelots1234Încă nu există evaluări
- Unit 8. Cholinergic and AnticholinergicsDocument50 paginiUnit 8. Cholinergic and AnticholinergicsApril Mergelle LapuzÎncă nu există evaluări
- SMHS HIPAA Compliance Quiz ResultsDocument3 paginiSMHS HIPAA Compliance Quiz Resultslexi_2706Încă nu există evaluări
- Morphine Drug StudyDocument2 paginiMorphine Drug StudyNo Vem BerÎncă nu există evaluări
- Stine Helene Falsig Pedersen (Editor) - Reviews of Physiology, Biochemistry and Pharmacology. 177-Springer (2021)Document155 paginiStine Helene Falsig Pedersen (Editor) - Reviews of Physiology, Biochemistry and Pharmacology. 177-Springer (2021)shuvvro dhaÎncă nu există evaluări
- GABA ReceptorDocument6 paginiGABA ReceptorAthena NocetoÎncă nu există evaluări
- Basics of How To Read ScansDocument12 paginiBasics of How To Read Scanslexi_2706Încă nu există evaluări
- 13 14 Biochemistry Elliot Enzymes Regulation and Signal Transduction 01-10-14Document6 pagini13 14 Biochemistry Elliot Enzymes Regulation and Signal Transduction 01-10-14lexi_2706Încă nu există evaluări
- 01 - 06 - 14 Protein Structure and Function Part 2Document8 pagini01 - 06 - 14 Protein Structure and Function Part 2lexi_2706Încă nu există evaluări
- 13 14 Biochemistry Elliot AminoAcidsProteinStructure-Function Continued 1-3-14Document5 pagini13 14 Biochemistry Elliot AminoAcidsProteinStructure-Function Continued 1-3-14lexi_2706Încă nu există evaluări
- 13 14 Immunology Leitenberg SignalTransductionPart1!01!22 14Document7 pagini13 14 Immunology Leitenberg SignalTransductionPart1!01!22 14lexi_2706Încă nu există evaluări
- 13 14 Immunology Leitenberg Lymphocytedevelopment2!01!17 14Document6 pagini13 14 Immunology Leitenberg Lymphocytedevelopment2!01!17 14lexi_2706Încă nu există evaluări
- 13 14 Biochemistry Elliot Enzymes I 01-08-14Document12 pagini13 14 Biochemistry Elliot Enzymes I 01-08-14lexi_2706Încă nu există evaluări
- Med Immunology Self-Assessment Questions Exam 1Document14 paginiMed Immunology Self-Assessment Questions Exam 1lexi_2706100% (1)
- 13 14 Immunology Lecture7TCellImmunityLeitenberg 01-27-14Document20 pagini13 14 Immunology Lecture7TCellImmunityLeitenberg 01-27-14lexi_2706Încă nu există evaluări
- Protein TLDocument1 paginăProtein TLlexi_2706Încă nu există evaluări
- 13 14 Immunology Leitenberg TCellImmunity 01-27-14Document8 pagini13 14 Immunology Leitenberg TCellImmunity 01-27-14lexi_2706Încă nu există evaluări
- 13 14 Immunology Leitenberg Antigen Recognition 01-10-14Document9 pagini13 14 Immunology Leitenberg Antigen Recognition 01-10-14lexi_2706Încă nu există evaluări
- My Immune GlossaryDocument24 paginiMy Immune Glossarylexi_2706Încă nu există evaluări
- 13 14 Immunology Leitenberg SignalTransductionPart1!01!22 14Document7 pagini13 14 Immunology Leitenberg SignalTransductionPart1!01!22 14lexi_2706Încă nu există evaluări
- 13 14 Immunology Leitenberg MHC&AgPresentation 01-15-13Document3 pagini13 14 Immunology Leitenberg MHC&AgPresentation 01-15-13lexi_2706Încă nu există evaluări
- 13 14 Immunology Leitenberg InnateImmune System 01-08-14Document8 pagini13 14 Immunology Leitenberg InnateImmune System 01-08-14lexi_2706Încă nu există evaluări
- Muscles NervesDocument1 paginăMuscles NervesKyle D. MillsÎncă nu există evaluări
- 13 14 Biochemistry Elliot Enzymes 1-8-14Document7 pagini13 14 Biochemistry Elliot Enzymes 1-8-14lexi_2706Încă nu există evaluări
- Hormonetherapy and StrokeDocument13 paginiHormonetherapy and Strokelexi_2706Încă nu există evaluări
- Sickle Cell Disease PathophysiologyDocument35 paginiSickle Cell Disease Pathophysiologylexi_2706Încă nu există evaluări
- HP-VT PCL OsteoPamphletDocument5 paginiHP-VT PCL OsteoPamphletlexi_2706Încă nu există evaluări
- Adrenal, Thyroid and Parathyroid GlandsDocument2 paginiAdrenal, Thyroid and Parathyroid Glandslexi_2706Încă nu există evaluări
- Nervous Tissue - PooneDocument25 paginiNervous Tissue - Poonelexi_2706Încă nu există evaluări
- Sickle Cell Disease PathophysiologyDocument35 paginiSickle Cell Disease Pathophysiologylexi_2706Încă nu există evaluări
- 13 14 MicroscopicAnatomy Johnson MaleReproductiveSystem 11-20-13Document12 pagini13 14 MicroscopicAnatomy Johnson MaleReproductiveSystem 11-20-13lexi_2706Încă nu există evaluări
- Outline NeuroendocrineDocument3 paginiOutline Neuroendocrinelexi_2706Încă nu există evaluări
- Muscles NervesDocument1 paginăMuscles NervesKyle D. MillsÎncă nu există evaluări
- CNS NeurotransmitterDocument67 paginiCNS NeurotransmitterGreenÎncă nu există evaluări
- Christina H. Parks: Magna Cum Laude, Chemistry HonorsDocument2 paginiChristina H. Parks: Magna Cum Laude, Chemistry HonorsTim BrownÎncă nu există evaluări
- Chapter 10 Adrenoceptor Antagonist DrugsDocument7 paginiChapter 10 Adrenoceptor Antagonist DrugsChristine Annmarie TapawanÎncă nu există evaluări
- Review Test Submission: Quiz #2: Dallas College Included Program Community My ServerDocument4 paginiReview Test Submission: Quiz #2: Dallas College Included Program Community My ServerAkash PatelÎncă nu există evaluări
- Guided Reading - Chapter 11 and 45Document5 paginiGuided Reading - Chapter 11 and 45Katara HokaidaÎncă nu există evaluări
- 2 Effect of Drugs On Isolated Frog HeartDocument10 pagini2 Effect of Drugs On Isolated Frog HeartVidhiÎncă nu există evaluări
- Fungsi Protein - Andri Josua SianiparDocument30 paginiFungsi Protein - Andri Josua SianiparAndri Josua SianiparÎncă nu există evaluări
- Imunologi Asma PPOKDocument14 paginiImunologi Asma PPOKRamanda Cahya UmbarraÎncă nu există evaluări
- Stahl' Essential Psychopharmacology 276 PDFDocument1 paginăStahl' Essential Psychopharmacology 276 PDFMuhammad AzkaÎncă nu există evaluări
- Pharmacology Answer Key-PINK PACOPDocument54 paginiPharmacology Answer Key-PINK PACOPKaguraÎncă nu există evaluări
- Cholinergic DrugsDocument32 paginiCholinergic DrugsApt FianÎncă nu există evaluări
- Tutorial 2Document4 paginiTutorial 2Lina KhanÎncă nu există evaluări
- Significance of Mammalian N, N-Dimethyltryptamine (DMT) : A 60-Year-Old DebateDocument15 paginiSignificance of Mammalian N, N-Dimethyltryptamine (DMT) : A 60-Year-Old Debatesam_rastuÎncă nu există evaluări
- Cardiolab Practical-PharmacologyDocument2 paginiCardiolab Practical-PharmacologyGul HassanÎncă nu există evaluări
- Dexmedetomidina en Dolor Visceral: Dexmedetomidine in Visceral PainDocument6 paginiDexmedetomidina en Dolor Visceral: Dexmedetomidine in Visceral PainSteve Andy Jose Silva RomeroÎncă nu există evaluări
- Articulo de CancerDocument13 paginiArticulo de Cancer11249421Încă nu există evaluări
- Neuroscience Perspective on Abnormal PsychologyDocument22 paginiNeuroscience Perspective on Abnormal Psychologynad101Încă nu există evaluări
- Intraneuronal Signalling Pathways: Chair Person: Dr. S. Kiran Kumar Presenter: Dr. G - Sangha MitraDocument20 paginiIntraneuronal Signalling Pathways: Chair Person: Dr. S. Kiran Kumar Presenter: Dr. G - Sangha Mitrasangha_mitra_2Încă nu există evaluări
- Nav Structure-IntroductionDocument26 paginiNav Structure-IntroductionLorraine Joy dela CruzÎncă nu există evaluări
- 2014 Neurogenic NeuroinflammationDocument11 pagini2014 Neurogenic NeuroinflammationrendyjiwonoÎncă nu există evaluări
- Alpha7 Nicotinic Acetylcholine Receptor Is A Target in Pharmacology and ToxicologyDocument20 paginiAlpha7 Nicotinic Acetylcholine Receptor Is A Target in Pharmacology and ToxicologyJean Pierre Chastre LuzaÎncă nu există evaluări
- NPS Update 76Document14 paginiNPS Update 76Arif Al-AmarÎncă nu există evaluări
- Acetylcholinesterase InhibitorsDocument8 paginiAcetylcholinesterase InhibitorsCobra PrsjÎncă nu există evaluări
- Selalu Kurang Dari 6 Menit (Satu Dari 10 Tikus Memiliki 8Document9 paginiSelalu Kurang Dari 6 Menit (Satu Dari 10 Tikus Memiliki 8Annisa Dwi YantiÎncă nu există evaluări