Documente Academic
Documente Profesional
Documente Cultură
6268 Expt5 - PH Meter Titration
Încărcat de
megacobTitlu original
Drepturi de autor
Formate disponibile
Partajați acest document
Partajați sau inserați document
Vi se pare util acest document?
Este necorespunzător acest conținut?
Raportați acest documentDrepturi de autor:
Formate disponibile
6268 Expt5 - PH Meter Titration
Încărcat de
megacobDrepturi de autor:
Formate disponibile
62
Experiment #5. Titration of an Acid; Using a pH Meter
TITRATION OF AN ACID; USING A pH METER Introduction The pH meter is an instrument that measures the pH of a solution and affords a direct method of obtaining a titration curve. A titration curve is a graph of
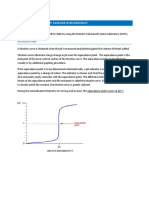
measured pH values versus the volume (milliliters) of titrant added. The figure below is an example of a titration curve, illustrating the numerous data points and the best smooth curve drawn through the points.
Figure 1. Titration Curve of acid HA The equivalence point is the point at which an equal amount of acid has been added to the amount of base present or vice versa. The equivalence point occurs on the titration curve in the region where there is a relatively large change in pH with a relatively small change in volume. The steeper the curve in the region of the equivalence point the more precisely it may be established. Once a titration curve is constructed and the equivalence point established the experimenter could then choose an indicator that would give a suitable endpoint (point at which the indicator changes colour).
63
Experiment #5. Titration of an Acid; Using a pH Meter
Selection of the equivalence point In this experiment you will graph the measured pH against the volume of standard NaOH solution added. The best smooth curve should be drawn through these points. The equivalence point can be established using the steepest tangent to the smooth curve where the pH changes rapidly. The equivalence point is the mid point between the two lines intersecting the volume axis. The method is summarized below:
Equivalence Point Determination 11 10 9 8
pH
7 6 5 4 3 23.5 24 24.5 25 25.5 26
Volume NaOH (mL)
Figure 2: Equivalence Point Determination for acid HA The equivalence point selected using this method is a more accurate method than using an indicator in the titration. A second method maybe used to determine the equivalence point. To use this method a graph is constructed of method.
! pH vsVaverage . The graph below illustrates this !V
64
Experiment #5. Titration of an Acid; Using a pH Meter
Equivalence Point Determination 50 40
dpH/dV
30 20 10 0 24 24.5 25
V average
25.5
26
26.5
27
Figure 3: Equivalence Point Determination for acid HA using the First Derivative Method The volume at the point where the graph reaches the maxima is the equivalence point of the titration. A disadvantage of the titration curve method is the time and effort required to make the measurements and to construct the graph. This disadvantage can be overcome by using a recording pH meter, which provides a chart record of the pH of a solution as a function of time. The pH of a solution is related to the H+ ion concentration by the equation: (1) pH = log[H+]
The ionization constant, Ka, for a generic acid HA is: HA (2) Ka = ! H+ + A [H+][A] [HA]
and the pKaof an acid is simply: (3) pKa = log Ka
If we take equation (2)
65
Experiment #5. Titration of an Acid; Using a pH Meter
(2)
Ka =
[H+][A] [HA]
and now take log of both sides: [A] log Ka = log[H+] log [HA] from equation (3): [A] pKa= -log[H+] log [HA] [A] pKa = pH log [HA] [A] pH = pKa + log [HA]
and therefore from (1):
But since pKais constant and pH varies: Therefore when [A] = [HA] pH = pKa
This is the midway point to the equivalence point. Therefore pKa values can also be directly read from your titration curves.
Procedure In preparation for this procedure, read Appendix B for directions on the proper use of a balance and how to weigh by difference, and Appendix C for directions on the proper use of a buret in a titration. Before lab have a draft of the procedures for the preparation and the standardization of ~300mL of ~1M NaOH solution ready for your instructor to look over. Obtain from your instructor an unknown acid sample. instructions on the pH meter before you start. You will also be given
Hint: A similar procedure was used in the Chemistry 1000 laboratory. SAFETY NOTE: Sodium hydroxide is a strong base and can cause burns if it is left on the skin for too long. If your hands feel slippery or soapy at any point during the lab, wash them well to remove the sodium hydroxide.
66
Experiment #5. Titration of an Acid; Using a pH Meter
Weigh accurately 2.00 - 2.25 g (+ 0.0001 g) of your sample into a 250 mL beaker. Dissolve the sample in 50 mL of water. Prepare your buret for titration (clean, rinse, etc.) and fill the buret with standardized ~1 M NaOH solution. Obtain a pH probe from your instructor and set up your apparatus as shown. Check with your instructor before starting to be sure everything is set up correctly. Proceed to add standardized ~1 M base at the rate of 1 mL per addition taking the buret reading and also the pH reading after each addition. Once the pH starts to change more rapidly the size of the additions should be reduced to 0.5 mL or less. As the pH changes become larger (this may occur at a pH of about 4 to 5) reduce the size of the NaOH additions until single drops are being added. Continue until you are satisfied the equivalence point has been passed or the pH is approximately 11 to 11.5. When you are finished, remove your apparatus, rinse the pH electrode and return the pH probe to your instructor. Note that your unknown acid will have more than one equivalence point if it is polyprotic. Report 1. 2. Construct a titration curve graph of pH values versus mL of 1M NaOH solution added. ! pH Construct a graph of vsVaverage . Using this graph determine the !V equivalence point for the titration. Illustrate on the titration curve the pKa(s) of your unknown acid. Calculate and report the Ka value(s) and the molar mass of your unknown sample. Give a balanced, generic reaction equation for your unknown acid.
3. 4.
67
Experiment #5. Titration of an Acid; Using a pH Meter
Titration of an Acid; Using a pH Meter DATA SHEET: Partner Credit: Y
(Circle if yes)
Name: _______________________ Partner's Name: ________________ Lab Section: _______________
Unknown # or letter:
_______________
Instructor's signature:
_______________________
68
Experiment #5. Titration of an Acid; Using a pH Meter
Experimental Procedure (draft only): Please prepare before the start of class.
Instructor's signature:
_______________________
S-ar putea să vă placă și
- Titration Lab ReportDocument38 paginiTitration Lab Reportadillaanis100% (4)
- Weak Acid Strong Base Titration LabDocument8 paginiWeak Acid Strong Base Titration Labapi-265089380100% (1)
- Experiment 1 Preparation of Buffer SolutionsDocument16 paginiExperiment 1 Preparation of Buffer Solutionsmohamad ashaziq89% (56)
- Acid/Base Titration LabDocument5 paginiAcid/Base Titration LabDavid GrahamÎncă nu există evaluări
- Lab Report Experiment 2aaa - EditDocument17 paginiLab Report Experiment 2aaa - EditAtikah Jembari100% (1)
- Discussion On Potentiometric TitrationsDocument16 paginiDiscussion On Potentiometric TitrationsKcirtap Zketh60% (5)
- Determination of Ka of Unknown AcidDocument23 paginiDetermination of Ka of Unknown AcidShasha0% (1)
- AP Chemistry Investigation 4 - Judy, Paul, AnthonyDocument13 paginiAP Chemistry Investigation 4 - Judy, Paul, AnthonyAnthony HowerÎncă nu există evaluări
- Experiment 11 Results and Discussion Report: Potentiometric Determination of The Purity and Dissociation Constant of Potassium Hydrogen PhthalateDocument4 paginiExperiment 11 Results and Discussion Report: Potentiometric Determination of The Purity and Dissociation Constant of Potassium Hydrogen PhthalateNathalie Dagmang80% (10)
- Acid-Base Titrations Curve Formal LabDocument9 paginiAcid-Base Titrations Curve Formal LabAshley StraubÎncă nu există evaluări
- pH Titration Lab Experiment Ka DeterminationDocument4 paginipH Titration Lab Experiment Ka DeterminationxmusiqaÎncă nu există evaluări
- Mistakes in Quality Statistics: and How to Fix ThemDe la EverandMistakes in Quality Statistics: and How to Fix ThemÎncă nu există evaluări
- 06 and 07 Standardization of NaOH and Acid Base TitrationDocument16 pagini06 and 07 Standardization of NaOH and Acid Base TitrationTyler Hardy80% (5)
- Experiment 4 - Potentiometric TitrationDocument11 paginiExperiment 4 - Potentiometric TitrationJoemer Absalon Adorna100% (2)
- Experiment 6 Titration II - Acid Dissociation ConstantDocument8 paginiExperiment 6 Titration II - Acid Dissociation ConstantPanneer SelvamÎncă nu există evaluări
- Acetic Acid Dissociation Constant S11Document7 paginiAcetic Acid Dissociation Constant S11Ayesha ShahidÎncă nu există evaluări
- 24 Acid-Base TitrationDocument5 pagini24 Acid-Base Titrationgardarr11Încă nu există evaluări
- Determine Ka of Acetic Acid Using Half-TitrationDocument4 paginiDetermine Ka of Acetic Acid Using Half-TitrationSung Hoon ParkÎncă nu există evaluări
- Practical 4Document2 paginiPractical 4vimukthi gunasinghaÎncă nu există evaluări
- Mixture of Carbonate BicarbonateDocument9 paginiMixture of Carbonate BicarbonateIan Justine SanchezÎncă nu există evaluări
- Determination of pKa for Weak AcidDocument5 paginiDetermination of pKa for Weak AcidSonu DubeyÎncă nu există evaluări
- Lab Report Acid BaseDocument4 paginiLab Report Acid Basexuni34Încă nu există evaluări
- Potentiometric Titration Ex17Document10 paginiPotentiometric Titration Ex17Tien HaminhÎncă nu există evaluări
- Exp 6 - Acid Base Titration-2Document9 paginiExp 6 - Acid Base Titration-2liquidsnake007Încă nu există evaluări
- Potentiometric Titration of a Weak AcidDocument14 paginiPotentiometric Titration of a Weak AcidMay LeeÎncă nu există evaluări
- Lab Report Experiment 2 Determination of Ka Value of A Weak AcidDocument17 paginiLab Report Experiment 2 Determination of Ka Value of A Weak AcidarisyahariffÎncă nu există evaluări
- Determine Phosphoric Acid Content in Soft DrinksDocument4 paginiDetermine Phosphoric Acid Content in Soft DrinksNaveen KumarÎncă nu există evaluări
- Potentiometric Titration Curve Determines Unknown Acid pKaDocument3 paginiPotentiometric Titration Curve Determines Unknown Acid pKaDaniele Joseph HizonÎncă nu există evaluări
- Determination of The Ka Ofa Weak AcidDocument7 paginiDetermination of The Ka Ofa Weak AcidFikrie MuhdÎncă nu există evaluări
- Lab 2 Eng Chem LabDocument19 paginiLab 2 Eng Chem LabillyzlÎncă nu există evaluări
- Practical 04 - Estimation of PKa by Half Neutralization MethodDocument10 paginiPractical 04 - Estimation of PKa by Half Neutralization Methodsandi fernandoÎncă nu există evaluări
- Acid Base TitrationDocument12 paginiAcid Base TitrationMsfaeza HanafiÎncă nu există evaluări
- Chem 18.1 Titration AnalysisDocument5 paginiChem 18.1 Titration AnalysisNicole NatanauanÎncă nu există evaluări
- AP Chemistry - Titration Curves of Strong and Weak Acids and BasesDocument5 paginiAP Chemistry - Titration Curves of Strong and Weak Acids and BasesJonathan Chen100% (2)
- Quantitative Determination of Potassium Acid Phthalate KHPDocument17 paginiQuantitative Determination of Potassium Acid Phthalate KHPMichelle Cruz AbrilÎncă nu există evaluări
- 5: PH Measurement and Its Applications (Experiment) : ObjectivesDocument19 pagini5: PH Measurement and Its Applications (Experiment) : ObjectivesNajmi NasirÎncă nu există evaluări
- CH142Exp5Titration PDFDocument7 paginiCH142Exp5Titration PDFSako RasheedÎncă nu există evaluări
- Sinha TitrationcurvesDocument11 paginiSinha TitrationcurvesRadu StafiÎncă nu există evaluări
- PH Titration LabQuest PDFDocument3 paginiPH Titration LabQuest PDFAntónio MatiasÎncă nu există evaluări
- Titration Curves of Strong and Weak Acids and BasesDocument3 paginiTitration Curves of Strong and Weak Acids and BasesMatthew Runyon50% (2)
- GA7 Potentio Titr Rev7 99Document9 paginiGA7 Potentio Titr Rev7 99Jerome SadudaquilÎncă nu există evaluări
- Sample Lab Report For Experiment 2Document2 paginiSample Lab Report For Experiment 2Ashfaq AhmadÎncă nu există evaluări
- LSM1101 Practical 1Document6 paginiLSM1101 Practical 1givena2ndchance100% (1)
- NSCI/NENG 115 Chemical Principles LabDocument7 paginiNSCI/NENG 115 Chemical Principles LabIsaac SnitkoffÎncă nu există evaluări
- Determination Acetic AcidDocument21 paginiDetermination Acetic Acidameyakem100% (1)
- ABcurves SP 19Document8 paginiABcurves SP 19Sakshi BangarwaÎncă nu există evaluări
- Determine pH of samples using a pH meterDocument5 paginiDetermine pH of samples using a pH meterAjuba AbujaÎncă nu există evaluări
- Lab 3: Introduction To Acids Base Chemistry Part A Experimental Determination of Acid Dissociation Constant, KaDocument10 paginiLab 3: Introduction To Acids Base Chemistry Part A Experimental Determination of Acid Dissociation Constant, Kaenock yegonÎncă nu există evaluări
- Module Anachem Acid-Base 2Document9 paginiModule Anachem Acid-Base 2arejay castroÎncă nu există evaluări
- Chem 18.1 Experiment 6 Formal ReportDocument5 paginiChem 18.1 Experiment 6 Formal Reportlouize_1496Încă nu există evaluări
- Titration of A Diprotic Acid Identifying An Unknown: ObjectiveDocument9 paginiTitration of A Diprotic Acid Identifying An Unknown: ObjectivePuji WulandariÎncă nu există evaluări
- Determining Vinegar Acidity Through TitrationDocument15 paginiDetermining Vinegar Acidity Through TitrationDayledaniel SorvetoÎncă nu există evaluări
- Lab Report IonizationDocument6 paginiLab Report IonizationJasmeetSinghÎncă nu există evaluări
- Determining The Amount of Acetic Acid in VinegarDocument2 paginiDetermining The Amount of Acetic Acid in VinegarAadhi JÎncă nu există evaluări
- WINSEM2022-23 BBIT206P LO VL2022230503900 Reference Material I 23-12-2022 Experiment 2 Acid-Base Titration PH Meter FADocument4 paginiWINSEM2022-23 BBIT206P LO VL2022230503900 Reference Material I 23-12-2022 Experiment 2 Acid-Base Titration PH Meter FAGravity JaiÎncă nu există evaluări
- ProjectDocument2 paginiProjectCindy Mae A. PogoyÎncă nu există evaluări
- Determining the Ka of an Unknown Weak AcidDocument15 paginiDetermining the Ka of an Unknown Weak AcidNikMuhammadIzzatÎncă nu există evaluări
- Lab Format:: Lab 2: Determination of Carbonate/BicarbonateDocument5 paginiLab Format:: Lab 2: Determination of Carbonate/BicarbonateAnaya FatimaÎncă nu există evaluări
- Enzyme Kinetics: Rapid-Equilibrium Applications of MathematicaDe la EverandEnzyme Kinetics: Rapid-Equilibrium Applications of MathematicaÎncă nu există evaluări