Documente Academic
Documente Profesional
Documente Cultură
Intro Bioseparation
Încărcat de
syazaismailTitlu original
Drepturi de autor
Formate disponibile
Partajați acest document
Partajați sau inserați document
Vi se pare util acest document?
Este necorespunzător acest conținut?
Raportați acest documentDrepturi de autor:
Formate disponibile
Intro Bioseparation
Încărcat de
syazaismailDrepturi de autor:
Formate disponibile
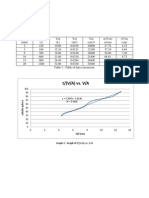
OBJECTIVE The objective of this experiment is to determine the effect of ammonium sulfate concentration towards protein precipitation
INT O!"CTION #rotein precipitation can be occurred b$ h$drophobic a%%re%ation& This can be happened b$ disruptin% the folded structure of the protein and exposin% more of the h$drophobic interior to the solution and also can be happened b$ deh$dratin% the shells of water molecules that form over h$drophobic patches on the surface of properl$ folded proteins& Once the proteins start a%%re%atin% into lar%er structures' the amount of water per protein will be drops' enhancin% the densit$ differences between the proteins and the solute& Once these a%%re%ates %row lar%e enou%h' it will disrupt the path of li%ht throu%h the solution' %ivin% it a cloud$ appearance& In addition' the densit$ differences become %reat enou%h for the a%%re%ates to be readil$ pellet in the centrifu%e& Because these methods depend on the protein molecules (findin%) one another in solution and formin% these a%%re%ates' the efficienc$ of this method depends on the concentration of the protein bein% precipitated& *hen the concentration is low' more harder for it to form a%%re%ates& #ractical rea%ents that has been used in this experiment to precipitate proteins is ammonium sulfate& +altin% proteins out of solution was discovered over ,-. $ears a%o b$ /ran0 1ofmeister when he noticed that the addition of different salts caused precipitate to form in solutions of e%% whites& 1e ordered the anions and cations b$ their abilit$ to precipitate proteins in what is 2nown as the 1ofmeister series& The current' mostl$3accepted
mechanism sa$s that saltin% proteins out of solution occurs when the water molecules are titrated awa$ from the solvent shells around the protein to the solvent shells around the ions that ma2e up the salt& +alts hi%h in the 1ofmeister series are the most efficient at protein precipitation because of the lar%e' stable solvent shells the$ maintain& This increases the surface tension of the solution' which effectivel$ increases the h$drophobic effect' which also stabili0es the protein structure while at the same time encoura%in% the h$drophobic re%ions on the surfaces of different molecules to interact' effectin% a%%re%ation& Because of this' proteins with a lar%er amount of h$drophobic surface character precipitate at lower salt concentrations than one with little h$drophobic surface character' and these protein to protein differences are exploited durin% protein purification procedures&
S-ar putea să vă placă și
- Water Potential LabreportDocument3 paginiWater Potential LabreportmichyyyyyÎncă nu există evaluări
- RheOlogy of Bio Process FluidsDocument3 paginiRheOlogy of Bio Process FluidsSagarika GhoshÎncă nu există evaluări
- Precipitation ChapterDocument12 paginiPrecipitation ChapterMaricica Gorceag50% (2)
- Contoh Report Jar TestDocument12 paginiContoh Report Jar TestIzzat75% (4)
- Hofmeister Series: Ions Franz Hofmeister ProteinsDocument11 paginiHofmeister Series: Ions Franz Hofmeister ProteinsRajeshwari SridharanÎncă nu există evaluări
- Biochemistry Experiment On OsmosisDocument5 paginiBiochemistry Experiment On OsmosisEna FarillasÎncă nu există evaluări
- Chemistry PracticalDocument4 paginiChemistry Practicalsuleiman kassimÎncă nu există evaluări
- Membrane Separation Processes: Reverse OsmosisDocument20 paginiMembrane Separation Processes: Reverse OsmosisSP ManjunathÎncă nu există evaluări
- Jar Test Lab ReportDocument20 paginiJar Test Lab Reportkhairulhakam33% (3)
- Salting In, Salting Out, and Dialysis of ProteinsDocument5 paginiSalting In, Salting Out, and Dialysis of ProteinsspeknatsÎncă nu există evaluări
- Details of Method: The Outline of This Method Has Already BeenDocument10 paginiDetails of Method: The Outline of This Method Has Already BeenAlexÎncă nu există evaluări
- Practical Biochemistry (STBP2012) : Experiment 3: Purification & Characterization Of α -Lactalbumin, A Milk ProteinDocument20 paginiPractical Biochemistry (STBP2012) : Experiment 3: Purification & Characterization Of α -Lactalbumin, A Milk ProteinfaizzudDENT100% (2)
- Folio Biology A+Document67 paginiFolio Biology A+Nina IsmailÎncă nu există evaluări
- Raoult's Law For Ideal Solutions: Experiment #5Document6 paginiRaoult's Law For Ideal Solutions: Experiment #5Ibraheem KhressÎncă nu există evaluări
- Emulsion PolymerisationDocument5 paginiEmulsion PolymerisationLuizaÎncă nu există evaluări
- AW and Glass Transistion Review - LabuzaDocument29 paginiAW and Glass Transistion Review - LabuzalosparritÎncă nu există evaluări
- Colloids 02 00002Document8 paginiColloids 02 00002Sritam SwapnadarshiÎncă nu există evaluări
- Solubility of CrystalDocument26 paginiSolubility of Crystalbliprawira2470Încă nu există evaluări
- Precipitation, BioseparationDocument30 paginiPrecipitation, Bioseparationdhriti9Încă nu există evaluări
- Forward OsmosisDocument3 paginiForward Osmosismuratout3447Încă nu există evaluări
- ProcedureDocument3 paginiProcedureroshanjimaxoutÎncă nu există evaluări
- Osmosis in Plant CellDocument8 paginiOsmosis in Plant CellsandalailaÎncă nu există evaluări
- IT Bio F4 Topical Test 3 (BL)Document8 paginiIT Bio F4 Topical Test 3 (BL)Banu PriyaÎncă nu există evaluări
- Characterization of Proteins Using Ion Exchange Chromatography and Gel Filtration ChromatographyDocument4 paginiCharacterization of Proteins Using Ion Exchange Chromatography and Gel Filtration ChromatographyEricka GalangÎncă nu există evaluări
- Rent.: Emulsion. DemulsifirsDocument3 paginiRent.: Emulsion. Demulsifirslulalala8888Încă nu există evaluări
- SCI207 W2 LabReportingFormDocument10 paginiSCI207 W2 LabReportingFormacountryman27Încă nu există evaluări
- Bioseparation Dr. Kamal E. M. Elkahlout: PrecipitationDocument45 paginiBioseparation Dr. Kamal E. M. Elkahlout: PrecipitationKemal ELkahloutÎncă nu există evaluări
- Performance of RO Units at High TemperaturesDocument10 paginiPerformance of RO Units at High TemperaturesRoy JudeÎncă nu există evaluări
- Bio411 Lab Report 3Document12 paginiBio411 Lab Report 3Nur Aqillah100% (1)
- Principles and Practices of Reverse OsmosisDocument9 paginiPrinciples and Practices of Reverse OsmosisMohamadÎncă nu există evaluări
- Spe 27368 MSDocument14 paginiSpe 27368 MSDaniel Camilo BustosÎncă nu există evaluări
- Biology - Diffusion Through Membranes Lab Analysis QuestionsDocument2 paginiBiology - Diffusion Through Membranes Lab Analysis Questionslanichung0% (3)
- Osmosis Manual EnglishDocument46 paginiOsmosis Manual EnglishIacob Daniel100% (1)
- 1st Hand - Cell Membrane StructureDocument3 pagini1st Hand - Cell Membrane StructureYue CaoÎncă nu există evaluări
- Nephrotic Syndrome: Laporan Kasus 8Document31 paginiNephrotic Syndrome: Laporan Kasus 8OktaniaPutriKusnawanÎncă nu există evaluări
- Super-Absorbant Polymers PDFDocument13 paginiSuper-Absorbant Polymers PDFGayathri SelvarajÎncă nu există evaluări
- Protein Denaturation and HydrolysisDocument28 paginiProtein Denaturation and HydrolysisNEHA VAGHELAÎncă nu există evaluări
- Chapter 02 Fall 05Document8 paginiChapter 02 Fall 05shibhiÎncă nu există evaluări
- Microemulsion and Optimal Formulation Occurrence in pH-Dependent Systems As Found in Alkaline-Enhanced Oil RecoveryDocument25 paginiMicroemulsion and Optimal Formulation Occurrence in pH-Dependent Systems As Found in Alkaline-Enhanced Oil RecoveryJesus Miguel Cuervo100% (1)
- Effect of Water and Grist On Mash PHDocument20 paginiEffect of Water and Grist On Mash PHenvisageamitÎncă nu există evaluări
- SPE-169060-MS The Effectiveness of MEOR Permeability Modification Beyond The Well BoreDocument12 paginiSPE-169060-MS The Effectiveness of MEOR Permeability Modification Beyond The Well BoreanderlethÎncă nu există evaluări
- Determination Formation RW Using Shale PropertiesDocument11 paginiDetermination Formation RW Using Shale PropertiesRosa K Chang HÎncă nu există evaluări
- A REVIEW Selection of Dissolution MediaDocument21 paginiA REVIEW Selection of Dissolution MediavunnamnareshÎncă nu există evaluări
- Protein Precipitation by SaltsDocument5 paginiProtein Precipitation by SaltsRüveyda Akçin100% (3)
- Efecto Del PH en EmulsionesDocument32 paginiEfecto Del PH en EmulsionesDaniel Acevedo MejíaÎncă nu există evaluări
- Mech of Action of Ppi PDFDocument6 paginiMech of Action of Ppi PDFIndri HadiansyahÎncă nu există evaluări
- (English (Auto-Generated) ) Precipitation Reactions of Proteins - Biochemistry (DownSub - Com)Document8 pagini(English (Auto-Generated) ) Precipitation Reactions of Proteins - Biochemistry (DownSub - Com)Isyam fawaidÎncă nu există evaluări
- Thermal Deathpoint Investigation of BeetrootDocument3 paginiThermal Deathpoint Investigation of BeetrootJohn OsborneÎncă nu există evaluări
- Coagulation and Flocculation ReportDocument13 paginiCoagulation and Flocculation Reportdrami94100% (2)
- Polymer Flooding ReviewDocument5 paginiPolymer Flooding ReviewChristian PradaÎncă nu există evaluări
- Precipitation PDFDocument28 paginiPrecipitation PDFMridul GuptaÎncă nu există evaluări
- SDS PAGE - Experiment and Report PDFDocument16 paginiSDS PAGE - Experiment and Report PDFHazar HiaryÎncă nu există evaluări
- 1 - Chapter02-Organic Medicinal and Pharmaceutical Chemistry - 12 TH EditionDocument1 pagină1 - Chapter02-Organic Medicinal and Pharmaceutical Chemistry - 12 TH EditionThuongNguyen1981Încă nu există evaluări
- SPE 132237 Scale Prediction For Oil and Gas ProductionDocument29 paginiSPE 132237 Scale Prediction For Oil and Gas Productionsuhaimi manÎncă nu există evaluări
- Water Treatment Final RevisionDocument26 paginiWater Treatment Final RevisionadhamÎncă nu există evaluări
- Pulsed Column Liquid - Liquid Extraction Unit.: Experiment 4Document16 paginiPulsed Column Liquid - Liquid Extraction Unit.: Experiment 4Dark_KiroÎncă nu există evaluări
- Surfactant FloodingDocument10 paginiSurfactant FloodinghkaqlqÎncă nu există evaluări
- Introduction Fermentech 1Document3 paginiIntroduction Fermentech 1syazaismailÎncă nu există evaluări
- CapsulesDocument6 paginiCapsulessyazaismailÎncă nu există evaluări
- ConclusionDocument1 paginăConclusionsyazaismailÎncă nu există evaluări
- Procedures Bioproduct Lab 1Document2 paginiProcedures Bioproduct Lab 1syazaismailÎncă nu există evaluări
- Lab Manual 3Document7 paginiLab Manual 3syazaismailÎncă nu există evaluări
- RESULT Discussion Spray DryerDocument7 paginiRESULT Discussion Spray Dryersyazaismail100% (1)
- LAB Manual 2Document4 paginiLAB Manual 2syazaismailÎncă nu există evaluări
- LAB Manual 1Document2 paginiLAB Manual 1syazaismailÎncă nu există evaluări
- Assignment 1Document1 paginăAssignment 1syazaismailÎncă nu există evaluări
- Acid Base TitrationDocument16 paginiAcid Base TitrationsyazaismailÎncă nu există evaluări
- Introduction To Capsule FillingDocument3 paginiIntroduction To Capsule FillingsyazaismailÎncă nu există evaluări
- Online Sales of New Cars: StudyDocument29 paginiOnline Sales of New Cars: StudyIson StudiosÎncă nu există evaluări
- New Public Service (Denhardt & Denhardt)Document12 paginiNew Public Service (Denhardt & Denhardt)Ika Hartantiningsih100% (1)
- Advances in Intelligent Systems and Computing PDFDocument807 paginiAdvances in Intelligent Systems and Computing PDFli250250250Încă nu există evaluări
- The Importance of MoneyDocument9 paginiThe Importance of MoneyLinda FeiÎncă nu există evaluări
- The Myth of HITLER's POPE - Hubert - LunsDocument13 paginiThe Myth of HITLER's POPE - Hubert - LunsHubert LunsÎncă nu există evaluări
- Hemzo M. Marketing Luxury Services. Concepts, Strategy and Practice 2023Document219 paginiHemzo M. Marketing Luxury Services. Concepts, Strategy and Practice 2023ichigosonix66Încă nu există evaluări
- Contract ManagementDocument26 paginiContract ManagementGK TiwariÎncă nu există evaluări
- Types of Drills PDFDocument8 paginiTypes of Drills PDFSummer nightsÎncă nu există evaluări
- Corpuz V Cuaderno, G.R. No. L-17860, March 30, 1962Document3 paginiCorpuz V Cuaderno, G.R. No. L-17860, March 30, 1962Lyle BucolÎncă nu există evaluări
- Isl Isl StyleguideDocument50 paginiIsl Isl StyleguideCole FranklinÎncă nu există evaluări
- Lesson 6 Intercultural ComDocument25 paginiLesson 6 Intercultural ComBrave MitraÎncă nu există evaluări
- Notes On The "Historical Turn" and The Uses of Theory in Film Studies - ARTDocument7 paginiNotes On The "Historical Turn" and The Uses of Theory in Film Studies - ARTaarmherÎncă nu există evaluări
- 1903 Er19031211Document22 pagini1903 Er19031211Tullio OpattiÎncă nu există evaluări
- 308 MSR Folktale Sir CedricDocument3 pagini308 MSR Folktale Sir Cedricapi-242415607Încă nu există evaluări
- Dwnload Full Social Psychology 4th Edition Gilovich Test Bank PDFDocument35 paginiDwnload Full Social Psychology 4th Edition Gilovich Test Bank PDFalilonghidotardlyq71i7f100% (8)
- Aarvee Denim & Export LTD.: CertificateDocument46 paginiAarvee Denim & Export LTD.: CertificateVinita AgrawalÎncă nu există evaluări
- Tales From The Wood RPGDocument51 paginiTales From The Wood RPGArthur Taylor100% (1)
- Tutorial-Midterm - CHEM 1020 General Chemistry - IB - AnsDocument71 paginiTutorial-Midterm - CHEM 1020 General Chemistry - IB - AnsWing Chi Rainbow TamÎncă nu există evaluări
- The AmazonsDocument18 paginiThe AmazonsJoan Grace Laguitan100% (1)
- A Minor Project Report Submitted To Rajiv Gandhi Prodhyogik Vishwavidyalaya, Bhopal Towards Partial Fulfillment For The Award of The Degree ofDocument53 paginiA Minor Project Report Submitted To Rajiv Gandhi Prodhyogik Vishwavidyalaya, Bhopal Towards Partial Fulfillment For The Award of The Degree ofwahidjaanÎncă nu există evaluări
- Degenesis Rebirth Edition - Interview With Marko DjurdjevicDocument5 paginiDegenesis Rebirth Edition - Interview With Marko DjurdjevicfoxtroutÎncă nu există evaluări
- Say. - .: Examining Contemporary: Tom Daley's Something I Want To Celebrity, Identity and SexualityDocument5 paginiSay. - .: Examining Contemporary: Tom Daley's Something I Want To Celebrity, Identity and SexualityKen Bruce-Barba TabuenaÎncă nu există evaluări
- DLP IN LitDocument9 paginiDLP IN LitLotis VallanteÎncă nu există evaluări
- Schedule Standard and Syllabus: Section A: Geomorphology and Remote SensingDocument6 paginiSchedule Standard and Syllabus: Section A: Geomorphology and Remote SensingPankaj SharmaÎncă nu există evaluări
- Developmental Stages WritingDocument2 paginiDevelopmental Stages WritingEva Wong AlindayuÎncă nu există evaluări
- 4 Modes Operations RC4Document37 pagini4 Modes Operations RC4Komal BansalÎncă nu există evaluări
- Daily Hora ChartDocument2 paginiDaily Hora ChartkalanemiÎncă nu există evaluări
- Internetworking Concepts Overview: © 1999, Cisco Systems, IncDocument50 paginiInternetworking Concepts Overview: © 1999, Cisco Systems, IncShashi Kant RaviÎncă nu există evaluări
- Elliot Kamilla - Literary Film Adaptation Form-Content DilemmaDocument14 paginiElliot Kamilla - Literary Film Adaptation Form-Content DilemmaDavid SalazarÎncă nu există evaluări
- Exercises 2Document7 paginiExercises 2Taseen Junnat SeenÎncă nu există evaluări