Documente Academic
Documente Profesional
Documente Cultură
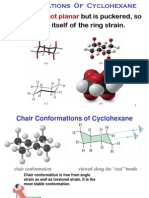
Loudon5ech07sec04 Cyclohexane Conformations
Încărcat de
F1234TDrepturi de autor
Formate disponibile
Partajați acest document
Partajați sau inserați document
Vi se pare util acest document?
Este necorespunzător acest conținut?
Raportați acest documentDrepturi de autor:
Formate disponibile
Loudon5ech07sec04 Cyclohexane Conformations
Încărcat de
F1234TDrepturi de autor:
Formate disponibile
7.
4 DISUBSTITUTED CYCLOHEXANES
281
0L F
fluorocyclohexane
(b) Estimate the energy difference between the gauche and anti conformations of 1-uoropropane. H3C L CH2 L CH2 L F
1-fluoropropane
7.5 Suggest a reason why the energy difference between conformations of ethylcyclohexane is about the same as that for methylcyclohexane, even though the ethyl group is larger than a methyl group.
7.4
DISUBSTITUTED CYCLOHEXANES
A. CisTrans Isomerism in Disubstituted Cyclohexanes
Consider a typical disubstituted cyclohexane, 1-chloro-2-methylcyclohexane. MCl ` % CH3
1-chloro-2-methylcyclohexane
In one stereoisomer of this compound, both the chloro and methyl groups are in equatorial positions. This compound is in rapid equilibrium with a conformational diastereomer in which both the chloro and methyl groups assume axial positions. H L L H3C H
LH
Cl CH 3 L
trans-1-chloro-2-methylcyclohexane
Either conformation (or the mixture of them) is called trans-1-chloro-2-methylcyclohexane. The designation trans is used with cyclic compounds when two substituents have an updown relationship.
down
Cl L CH3
up
H L
H3C H
Cl
LH
(7.8)
Cl
up (7.9) down
Notice that the updown relationship is unaffected by the chair interconversion.
282
CHAPTER 7 CYCLIC COMPOUNDS. STEREOCHEMISTRY OF REACTIONS
In a different stereoisomer of 1-chloro-2-methylcyclohexane, the chloro and methyl groups occupy adjacent equatorial and axial positions. L H
L
H3C
LH
Cl
L
H3C
Cl
LH
(7.10)
This compound, called cis-1-chloro-2-methylcyclohexane, is also a rapidly equilibrating mixture of conformational diastereomers. In a cis-disubstituted cycloalkane, the substituents have an upup or a downdown relationship.
down
cis-1-chloro-2-methylcyclohexane
H3C
LH
down down
H3C
Cl
Cl
LH
(7.11) down
Again, the cis relationship is unaltered by the chair interconversion. The same denition of cis and trans substitution can be applied to substituent groups in other positions of a cyclohexane ring, as illustrated by Study Problem 7.2.
Study Problem 7.2
Draw structures of the two chair conformations of trans-1,3-dimethylcyclohexane. Solution In a trans-disubstituted cyclohexane, the two substituent groups have an updown relationship. It doesnt matter which chair conformation is drawn rst, because the chair interconversion does not affect the trans relationship of the two methyl groups:
up
CH3
down
CH3
CH3
trans-1,3-dimethylcyclohexane
CH3
(7.12)
Notice that when the substituents in a disubstituted cyclohexane are on asymmetric carbons, the designations cis and trans specify the relative stereochemical congurations of the two asymmetric carbons, but they say nothing about the absolute congurations of these carbons. Thus, there are two enantiomers of cis-1-chloro-2-methylcyclohexane.
L
Cl
Cl L
CH3
(1R,2S)-1-chloro-2-methylcyclohexane (1S,2R)-1-chloro-2-methylcyclohexane enantiomers of cis-1-chloro-2-methylcyclohexane
L CH3
7.4 DISUBSTITUTED CYCLOHEXANES
283
Other Ways of Designating Relative Conguration
Further Exploration 7.1
When a ring contains more than two substituents, cistrans nomenclature is usually cumbersome. For such cases, other systems have been developed to designate relative conguration. (See Further Exploration 7.1.)
PROBLEMS
7.6 For each of the following compounds, draw the two chair conformations that are in equilibrium. (a) cis-1,3-dimethylcyclohexane (b) trans-1-ethyl-4-isopropylcyclohexane 7.7 For each of the compounds in Problem 7.6, draw a boat conformation.
B. Conformational Analysis
Disubstituted cyclohexanes, like monosubstituted cyclohexanes, can be subjected to conformational analysis. The relative stability of the two chair conformations is determined by comparing the 1,3-diaxial interactions (or gauche-butane interactions) in each conformation. Such an analysis is illustrated in Study Problem 7.3.
Study Problem 7.3
Determine the relative energies of the two chair conformations of trans-1,2-dimethylcyclohexane. Which conformation is more stable? Solution The rst step in solving any problem is to draw the structures of the species involved. The two chair conformations of trans-1,2-dimethylcyclohexane are as follows:
L
CH3
CH3
CH3 L CH3
B
Conformation A has the greater number of axial groups and should therefore be the less stable conformationbut by how much? Conformation A has four 1,3-diaxial methylhydrogen interactions (show these!), which contribute 4 X 3.7 = 14.8 kJ mol_1 (4 X 0.9 = 3.6 kcal mol_1) to its energy. What about B? You might be tempted to say that B has no unfavorable interactions because it has no axial groups, but in fact B does have one gauche-butane interactionthe one between the two methyl groups themselves, which have a dihedral angle between them of 60, just as in gauche-butane. This interaction can be seen in a Newman projection of the bond between the carbons bearing the methyl groups: H " L H " H CH3
CH3 L CH3 " H
%CH 3
gauche methyl groups
Newman projection
This gauche-butane interaction contributes 2.8 kJ mol_1 (0.7 kcal mol_1) to the energy of conformation B (Fig. 2.5, p. 54). The relative energy of the two conformations is the difference between their methylhydrogen interactions: 14.8 - 2.8 = 12.0 kJ mol_1 (or 3.6 - 0.7 = 2.9 kcal mol_1). The diaxial conformation A is less stable by this amount of energy.
284
CHAPTER 7 CYCLIC COMPOUNDS. STEREOCHEMISTRY OF REACTIONS
When two groups on a substituted cyclohexane conict in their preference for the equatorial position, the preferred conformation can usually be predicted from the relative conformational preferences of the two groups. Consider, for example, the chair interconversion of cis1-tert-butyl-4-methylcyclohexane. L HL L CH3
(7.13)
H3C H3C
CL CH3
(larger)
CH3
(smaller)
H3C H3C
The tert-butyl group is so large that its van der Waals repulsions control the conformational equilibrium (see Eq. 7.7, p. 280). Hence, the chair conformation in which the tert-butyl group assumes the equatorial position is overwhelmingly favored. The methyl group is thus forced to take up the axial position.
There is so little of the conformation with an axial tert-butyl group that chemists say sometimes that the conformational equilibrium is locked. This statement is somewhat misleading because it implies that the two conformations are not at equilibrium. The equilibrium indeed occurs rapidly, but simply contains very little of the conformation in which the tert-butyl group is axial. PROBLEMS
7.8 Calculate the energy difference between the two chair conformations of trans-1,4-dimethylcyclohexane. 7.9 Calculate the energy difference between cis-1,4-dimethylcyclohexane and the more stable conformation of trans-1,4-dimethylcyclohexane.
C. Use of Planar Structures for Cyclic Compounds
Although many cyclic compounds (such as cyclohexane and its derivatives) have nonplanar conformations, planar structures of these compounds can be used for situations in which conformational issues are not important. In this notation, the structures of cyclic compounds are represented by planar polygons with the stereochemistry of substituents indicated by dashed or solid wedges. In this type of structure, imagine viewing the ring from above. If a substituent is up, the bond to it is represented by a solid wedge; if it is down, the bond to it is represented as a dashed wedge. In this convention, one enantiomer of trans-1,2-dimethylcyclohexane can be drawn as follows:
L
down
CH3 CH L 3
`
up
L C % CH3 CH3 CH3
(7.14)
A planar structure for cis-1,2-dimethylcyclohexane can be drawn in either of two ways: L CH3 CH3 CH3 or (7.15) ` ` L CH3 CH3 CH3
cis-1,2-dimethylcyclohexane
7.4 DISUBSTITUTED CYCLOHEXANES
285
The two planar structures are derived, respectively, by viewing the ring from above or below. That the two structures are equivalent can be further demonstrated by turning either one over: ` CH3 CH3
turn 180
CH3 CH3
(7.16)
A planar structure does not convey any conformational information. That is, so long as all conformations are viewed from the same face of the ring, the chair interconversion does not interchange wedges and dashed lines because it does not change the up or down relationship of the ring substituents.
Cl
CH3
both: ` CH3
Cl
(7.17)
L
Cl
L
CH3
PROBLEMS
7.10 Draw a planar structure for each of the following compounds using dashed or solid wedges to show the stereochemistry of substituent groups. (a) cis-1,3-dimethylcyclohexane (b) trans-1,3-dimethylcyclohexane (c) (1R,2S,3R)-2-chloro-1-ethyl-3-methylcyclohexane (d) (1S,2R)-1-chloro-2-methylcyclopentane 7.11 Draw the more stable chair conformation for each of the following compounds: (a) CH3 (b) Cl Cl (c) CH3 ` ` Cl O 23
C(CH3)3
CH(CH3)2
D. Stereochemical Consequences of the Chair Interconversion
The chair interconversion has some interesting stereochemical consequences that can be illustrated with interconversion dimethylcyclohexanes. First, consider cis-1,2-dimethylcyclohexane. The chirality of either conformation can be demonstrated by showing that its mirror image is noncongruent.
L
mirror
CH3
H3C L L CH3
noncongruent mirror images of cis-1,2-dimethylcyclohexane
L CH3
286
CHAPTER 7 CYCLIC COMPOUNDS. STEREOCHEMISTRY OF REACTIONS
However, the chair interconversion converts one enantiomer into the other:
L
CH3
chair interconversion
(7.18)
H3C
A
H3C
enantiomers
From the way they are drawn, structures A and B may not look like enantiomers, but they are! You can see this by turning structure B 120 about a vertical axis:
120
STUDY GUIDE LINK 7.1
Relating Cyclohexane Conformations
is the same as H3C
B
H3C L L CH3
enantiomer of structure A
L CH3
B
(7.19)
In other words, cis-1,2-dimethylcyclohexane is a mixture of conformational enantiomers (Sec. 6.10A). Because these enantiomers are interconverted very rapidly, they cannot be separated at ordinary temperatures. Consequently, cis-1,2-dimethylcyclohexane is not optically active at ordinary temperatures. Cis-1,3-dimethylcyclohexane, another molecule with two asymmetric carbons, is a mixture of conformational diastereomers:
L
L CH3 CH3
chair interconversion
CH3
diastereomers
Yet, even though each conformation has two asymmetric carbons, neither conformation is chiral because each has an internal plane of symmetry.
L
CH3
CH3
planes of symmetry
In other words, both conformations are meso. Cis-1,3-dimethylcyclohexane is thus a rapidly interconverting mixture of two different meso compounds, each a diastereomer of the other. Like any meso compound, cis-1,3-dimethylcyclohexane is achiral and optically inactive. The planar structures of both cis-1,2- and cis-1,3-dimethylcyclohexane have internal mirror planes (planes of symmetry).
CH3
CH3 CH3
(7.20)
CH3
(7.21)
7.4 DISUBSTITUTED CYCLOHEXANES
287
CH3
plane of symmetry
CH3
cis-1,2-dimethylcyclohexane
` CH3
CH3
plane of symmetry (7.22)
cis-1,3-dimethylcyclohexane
As weve just seen, this plane corresponds to an actual plane of symmetry in either conformation of cis-1,3-dimethylcyclohexane (Eq. 7.21). However, this is not a plane of symmetry in the case of cis-1,2-dimethylcyclohexane, because each conformation is chiral and has no symmetry plane. The symmetry of the planar structures of cis-1,2- and cis-1,3-dimethylcyclohexane thus conceals a subtle difference between the two isomers. Cis-1,2-dimethylcyclohexane is a mixture of chiral (and enantiomeric) conformations in rapid equilibrium. Although it does not exhibit optical activity at ordinary temperatures, at a temperature so low that the rate of the chair interconversion would be negligible, it could in principle be separated into enantiomers, each of which would be optically active. Cis-1,3-dimethylcyclohexane, on the other hand, is a mixture of meso conformations. Although these, too, might be separated at a very low temperature, neither conformation would be optically active because neither is chiral. Generally speaking, then, if a cyclic compound has asymmetric carbons, and if its planar structure has an internal mirror plane, one of two things most be true: either the compound is a rapidly equilibrating mixture of conformational enantiomers, or the compound is meso. In either case, though, the compound cannot be isolated in optically active form at room temperature. The enantiomers of trans-1,2-dimethylcyclohexane represent a different situation. We can see from its planar structures that trans-1,2-dimethylcyclohexane is chiral.
mirror
CH3 CH3
H3C H3C
noncongruent mirror images enantiomers of trans-1,2-dimethylcyclohexane
The chair interconversion converts each enantiomer into a conformational diastereomer:
L
enantiomers
CH3 CH3
H3C L H3C L
chair interconversion
conformational diastereomers
chair interconversion
CH3 L
CH3
enantiomers
conformational diastereomers
H3C
H3C
(7.23)
288
CHAPTER 7 CYCLIC COMPOUNDS. STEREOCHEMISTRY OF REACTIONS
Thus, each of the two enantiomers of trans-1,2-dimethylcyclohexane is a rapidly interconverting mixture of conformational diastereomers. Because trans-1,2-dimethylcyclohexane is chiral, each enantiomer is capable of independent existence and can be isolated in optically active form.
PROBLEMS
7.12 Determine whether each of the following compounds can in principle be isolated in optically active form under ordinary conditions. (a) trans-1,3-dimethylcyclohexane (b) 1,1-dimethylcyclohexane (c) cis-1,4-dimethylcyclohexane (d) cis-1-ethyl-3-methylcyclohexane 7.13 (a) Does trans-1,4-dimethylcyclohexane contain asymmetric carbons? If so, identify them. (b) Does trans-1,4-dimethylcyclohexane have any stereocenters? If so, identify them. (c) What is the stereochemical relationship of the two chair conformations of trans1,4-dimethylcyclohexane? (d) Is trans-1,4-dimethylcyclohexane chiral? 7.14 What is the relationship of the two structures in each set? That is, are they identical molecules, enantiomers, or diastereomers? (a) CH3 CH3 CH3 (b) CH3 ` ` ` ` Cl CH3 Cl CH3 (c) ` Cl Cl ` Cl Cl
7.5
CYCLOPENTANE, CYCLOBUTANE, AND CYCLOPROPANE
A. Cyclopentane
Cyclopentane, like cyclohexane, exists in a puckered conformation, called the envelope conformation (Fig. 7.10). This conformation undergoes very rapid conformational changes in which each carbon alternates as the point of the envelope. The heats of formation in Table 7.1 (p. 269) show that cyclopentane has somewhat higher energy than cyclohexane. The higher energy of cyclopentane is due mostly to eclipsing between hydrogen atoms, which is also shown in Fig. 7.10. Substituted cyclopentanes also exist in envelope conformations, but the substituents adopt positions that minimize van der Waals repulsions with neighboring groups. For example, in methylcyclopentane, the methyl group assumes an equatorial position at the point of the envelope. H " L
equatorial axial
CH3
" CH3
(7.24)
"
S-ar putea să vă placă și
- GeneralChem LS 25 PDFDocument25 paginiGeneralChem LS 25 PDFSunil NahataÎncă nu există evaluări
- Chapter 4 With Video LinksDocument37 paginiChapter 4 With Video LinksDoom RefugeÎncă nu există evaluări
- Cyclohexane Conformational AnalysisDocument17 paginiCyclohexane Conformational AnalysiscclatrumÎncă nu există evaluări
- Practice Questions-Conformational AnalysisDocument4 paginiPractice Questions-Conformational AnalysisHarry Zgambo100% (1)
- Resonance and Inductive Effects in Organic ChemistryDocument36 paginiResonance and Inductive Effects in Organic Chemistryeagl33yeÎncă nu există evaluări
- Organic ChemistryDocument14 paginiOrganic ChemistryStuteeÎncă nu există evaluări
- Isolobal AnalogyDocument4 paginiIsolobal Analogyindu priyaÎncă nu există evaluări
- 1 Alkene Practice Problems MOC PDFDocument8 pagini1 Alkene Practice Problems MOC PDFsamkarthik47Încă nu există evaluări
- Retrosynthetic Analysis PDFDocument6 paginiRetrosynthetic Analysis PDFNoleÎncă nu există evaluări
- Spec Prob Set 315 CurrentDocument20 paginiSpec Prob Set 315 CurrentUmang Agarwal57% (7)
- Reaction Guide by James Ashenhurst. 1-James AshenhurstDocument76 paginiReaction Guide by James Ashenhurst. 1-James AshenhurstSankar AdhikariÎncă nu există evaluări
- Organic Chemistry Practice ProblemsDocument1 paginăOrganic Chemistry Practice ProblemsSushant KumarÎncă nu există evaluări
- Chem 206: Introduction to Frontier Molecular Orbital TheoryDocument22 paginiChem 206: Introduction to Frontier Molecular Orbital TheoryeraborÎncă nu există evaluări
- Hückel's MO Treatment of BenzeneDocument12 paginiHückel's MO Treatment of BenzeneRichard Allen0% (1)
- HybridizationDocument18 paginiHybridizationSoub kuopÎncă nu există evaluări
- ChemistryDocument34 paginiChemistryraghuram_allaÎncă nu există evaluări
- ALKANES2Document41 paginiALKANES2Shiki Asagami BrunestedÎncă nu există evaluări
- Qoi0809t1 ConfDocument13 paginiQoi0809t1 ConfTahirat NasiruÎncă nu există evaluări
- Ether: Navigation Search AetherDocument7 paginiEther: Navigation Search AetherMuhammad Wahyu Nugraha0% (1)
- Organic Chemistry 32-235 Practice Questions For Exam #2: 2. Consider The SDocument9 paginiOrganic Chemistry 32-235 Practice Questions For Exam #2: 2. Consider The Ssweta KushwahaÎncă nu există evaluări
- Stereochemistry Basic Concepts Useful Notes For StudentsDocument26 paginiStereochemistry Basic Concepts Useful Notes For StudentsReddappaÎncă nu există evaluări
- Symmetry NotesDocument8 paginiSymmetry NoteslillyammalÎncă nu există evaluări
- Stereochemistry Qs: Fischer, R/S, ID pairs, Optical ActivityDocument2 paginiStereochemistry Qs: Fischer, R/S, ID pairs, Optical ActivityShilajit BaruaÎncă nu există evaluări
- NMR Organic ChemistryDocument17 paginiNMR Organic Chemistrysallymoon34Încă nu există evaluări
- Stereochemistry 22Document21 paginiStereochemistry 22Ahmed SideegÎncă nu există evaluări
- Organic Chemistry 2021Document76 paginiOrganic Chemistry 2021Arah Mae BonillaÎncă nu există evaluări
- Problems On Named ReactionsDocument103 paginiProblems On Named ReactionsBapu ThoratÎncă nu există evaluări
- Molecular OrbitalsDocument80 paginiMolecular Orbitals1balamanian100% (1)
- Stereochemistry CHM456Document82 paginiStereochemistry CHM456notmeÎncă nu există evaluări
- 429 SampleDocument40 pagini429 Samplejerrica thomasÎncă nu există evaluări
- Stereoisomerism - Geometric IsomerismDocument4 paginiStereoisomerism - Geometric IsomerismGopi KupuchittyÎncă nu există evaluări
- Introduction of Organic Chemistry by Eyes of Ajnish Kumar Gupta (AKG)Document24 paginiIntroduction of Organic Chemistry by Eyes of Ajnish Kumar Gupta (AKG)ajju_208180% (5)
- Hyperconjugation, The Anomeric Effect, and More: Chem 206 D. A. EvansDocument12 paginiHyperconjugation, The Anomeric Effect, and More: Chem 206 D. A. Evansomkar9996767Încă nu există evaluări
- Stereo Chemistry QuestionsDocument19 paginiStereo Chemistry QuestionsfritzÎncă nu există evaluări
- Lab ManualDocument19 paginiLab Manualanon_467104036Încă nu există evaluări
- PDF - Test Bank PDF - Test BankDocument85 paginiPDF - Test Bank PDF - Test BankKathy YellaÎncă nu există evaluări
- M.Sc. Part-II Organic Chemistry Revised Syllabus (Semester III & IVDocument17 paginiM.Sc. Part-II Organic Chemistry Revised Syllabus (Semester III & IVArnab ChakrabortyÎncă nu există evaluări
- Isomerism in Coordination Compounds PDFDocument7 paginiIsomerism in Coordination Compounds PDFmf720383270100% (1)
- SYMMETRY AND GROUP THEORYDocument27 paginiSYMMETRY AND GROUP THEORYAnonymous Tph9x741Încă nu există evaluări
- 太傻论坛精华帖Document30 pagini太傻论坛精华帖Alisa100% (1)
- Stereochemistry PDFDocument3 paginiStereochemistry PDFbencleese100% (1)
- Alkanes: Structure, Naming, Properties and ReactivityDocument73 paginiAlkanes: Structure, Naming, Properties and ReactivityChona TuyÎncă nu există evaluări
- Mass Spectrometry Problem Set II: Identify Structures from SpectraDocument22 paginiMass Spectrometry Problem Set II: Identify Structures from SpectraAnanda ReddyÎncă nu există evaluări
- Electron Delocalization and ResonanceDocument9 paginiElectron Delocalization and ResonanceMariana LizethÎncă nu există evaluări
- 01 StereochemistryDocument6 pagini01 StereochemistryGundum Bodyz100% (1)
- Chapter 6Document47 paginiChapter 6Lauren ZimmermanÎncă nu există evaluări
- QOI0809 AlkenesDocument30 paginiQOI0809 Alkenesmtucker17Încă nu există evaluări
- Molecular Spectroscopy: BackgroundDocument45 paginiMolecular Spectroscopy: Backgroundsavvy_as_98100% (1)
- Stereochemistry Sem 1 2013Document82 paginiStereochemistry Sem 1 2013Vaibhav RanaÎncă nu există evaluări
- CHEM F111: General Chemistry II-Semester Lecture 35 (12-04-2019Document20 paginiCHEM F111: General Chemistry II-Semester Lecture 35 (12-04-2019Rachit ShahÎncă nu există evaluări
- Chapter5 Symmetry After LectureDocument173 paginiChapter5 Symmetry After LecturekentanghkÎncă nu există evaluări
- E1 and E2 ReactionsDocument30 paginiE1 and E2 ReactionsVidhu Pandey100% (1)
- Experiment 2. Separation of Compounds by Paper ChromatographyDocument11 paginiExperiment 2. Separation of Compounds by Paper ChromatographybidinÎncă nu există evaluări
- Metal Complexes or Coordination Compounds: Kfecn 4K Fe CNDocument90 paginiMetal Complexes or Coordination Compounds: Kfecn 4K Fe CNPavan Boro100% (1)
- Chapter 05Document47 paginiChapter 05AC BañaresÎncă nu există evaluări
- Electron Transfer Reactions of Complex Ions in SolutionDe la EverandElectron Transfer Reactions of Complex Ions in SolutionÎncă nu există evaluări
- Transition Metal ToxicityDe la EverandTransition Metal ToxicityG. W. RichterÎncă nu există evaluări
- Strategies for Palladium-Catalyzed Non-directed and Directed C bond H Bond FunctionalizationDe la EverandStrategies for Palladium-Catalyzed Non-directed and Directed C bond H Bond FunctionalizationAnant R. KapdiÎncă nu există evaluări
- Organic Functional Group Analysis: Theory and DevelopmentDe la EverandOrganic Functional Group Analysis: Theory and DevelopmentÎncă nu există evaluări
- Stereochemistry of DrugsDocument68 paginiStereochemistry of DrugsDini Hara TriastutiÎncă nu există evaluări
- Chem 215-216 HH W11 Quiz 1Document1 paginăChem 215-216 HH W11 Quiz 1Sankar AdhikariÎncă nu există evaluări
- Cahn-Ingold-Prelog Priority RulesDocument5 paginiCahn-Ingold-Prelog Priority RulesBer GuzÎncă nu există evaluări
- Lista de Exercicios Sobre AlcanosDocument4 paginiLista de Exercicios Sobre AlcanosquelfisicaÎncă nu există evaluări
- A2 Optical IsomerismDocument35 paginiA2 Optical IsomerismteddaboyÎncă nu există evaluări
- Chapter 3Document27 paginiChapter 3Siddarth PalletiÎncă nu există evaluări
- R-S Configuration Rules for Assigning ChiralityDocument7 paginiR-S Configuration Rules for Assigning ChiralityRyan Dave SuganoÎncă nu există evaluări
- Organic Chemistry Prochirality Lecture Outline: S-Configuration R-ConfigurationDocument3 paginiOrganic Chemistry Prochirality Lecture Outline: S-Configuration R-ConfigurationcfmonarquiaÎncă nu există evaluări
- Monosaccharides:, Dr. Shweta HardiaDocument28 paginiMonosaccharides:, Dr. Shweta HardiaArzoo RathoreÎncă nu există evaluări
- Stereochemistry shapes propertiesDocument11 paginiStereochemistry shapes propertiesWendell Kim LlanetaÎncă nu există evaluări
- SP StereochemistryDocument63 paginiSP StereochemistryMadhumitha KatreddyÎncă nu există evaluări
- Stereochemistry: Understanding 3D Structures and Isomers in Organic ChemistryDocument106 paginiStereochemistry: Understanding 3D Structures and Isomers in Organic ChemistryJoemer Absalon Adorna100% (1)
- Isomerism: Structural and StereoisomersDocument12 paginiIsomerism: Structural and StereoisomersDisha SharmaÎncă nu există evaluări
- Part 3Document200 paginiPart 3DgjnÎncă nu există evaluări
- DPP For IIT JEE CHEMISTRY By:PJOY From KOTADocument2 paginiDPP For IIT JEE CHEMISTRY By:PJOY From KOTAPrakash Joy86% (7)
- Ib HL Chem Unit 4 Practice 1 and 2 A PDFDocument22 paginiIb HL Chem Unit 4 Practice 1 and 2 A PDFojas surasÎncă nu există evaluări
- Estereoquimica AntimicrobianosDocument26 paginiEstereoquimica Antimicrobianosperezj891Încă nu există evaluări
- VSEPR theory predicts molecular shapesDocument7 paginiVSEPR theory predicts molecular shapesSaravananÎncă nu există evaluări
- Stereo ChemistryDocument17 paginiStereo ChemistryDeepak PradhanÎncă nu există evaluări
- Organic - Reagents FinalDocument27 paginiOrganic - Reagents FinalSankar AdhikariÎncă nu există evaluări
- Reaction Map For Alkene CombinedDocument3 paginiReaction Map For Alkene CombinedZÎncă nu există evaluări
- Organic Chemistry: Key ConceptsDocument15 paginiOrganic Chemistry: Key ConceptsDhruv Jyot SinghÎncă nu există evaluări
- Stereo PresentationDocument24 paginiStereo PresentationSuman BalyaniÎncă nu există evaluări
- Chiral CompoundsDocument20 paginiChiral CompoundsShubham RampalliwarÎncă nu există evaluări
- Epimers, Anomers, Enantiomers and MutarotationDocument7 paginiEpimers, Anomers, Enantiomers and MutarotationgayathriÎncă nu există evaluări
- StereochemistryDocument53 paginiStereochemistryalyssa_marie_ke100% (17)
- Chem113Lec-Activity 2-Carbohydrates ActivityDocument3 paginiChem113Lec-Activity 2-Carbohydrates ActivityAlliana Denice VicencioÎncă nu există evaluări
- Cycloalkane Ring Strain and ConformationsDocument18 paginiCycloalkane Ring Strain and ConformationsbgbscgvÎncă nu există evaluări
- Ebook Chemistry A Molecular Approach Canadian 1St Edition Tro Test Bank Full Chapter PDFDocument63 paginiEbook Chemistry A Molecular Approach Canadian 1St Edition Tro Test Bank Full Chapter PDFthuygladys5x0100% (9)
- S.SEETARAM SWAMY, M.Pharm.,: Asst. Professor, Dept. of Pharmaceutical Chemistry, Chilkur Balaji College of PharmacyDocument46 paginiS.SEETARAM SWAMY, M.Pharm.,: Asst. Professor, Dept. of Pharmaceutical Chemistry, Chilkur Balaji College of PharmacyAVVARI AMMUÎncă nu există evaluări