Documente Academic
Documente Profesional
Documente Cultură
Organic Chemistry
Încărcat de
Pabloxd43Descriere originală:
Drepturi de autor
Formate disponibile
Partajați acest document
Partajați sau inserați document
Vi se pare util acest document?
Este necorespunzător acest conținut?
Raportați acest documentDrepturi de autor:
Formate disponibile
Organic Chemistry
Încărcat de
Pabloxd43Drepturi de autor:
Formate disponibile
Organic Chemistry from-the-text-book notes
Chapter 17 notes
Fossil fuels: a fuel formed from the remains of plants and animals that lived millions of years ago Fossil fuel formation: Dead sea plants and animals fall to the sea floor. They are buried under thick sediment (sand and mud) in anaerobic conditions. They slowly turn into oil and gas. Crude oil is a smelly mixture of hundreds of different compounds. They are organic compounds, which means they started off in living things. Most are also hydrocarbons they only contain carbon and hydrogen. Fossil fuels are non-renewable resources, since they are being used up far faster than they can be replenished. Fuels to know: -coal -natural gas (main constituent is methane) -petroleum (a mixture of hydrocarbons which can be separated into fractions in fractional distillation, this is called refining, the fractions are): 1. refinery gas bottled gas for heating and cooking 2. gasoline fraction fuel (petrol) in cars 3. naphtha fraction making chemicals 4. kerosene/paraffin fraction jet fuel, lamps 5. diesel oil/gas oil fraction fuel in diesel engines 6. fuel oil fraction fuel in ships and home heating systems 7. lubricating fraction lubricants, waxes and polishes 8. bitumen making roads Oil refinery in the lab: Heat the oil, it will start to evaporate. The lighter molecules evaporate first. The hot vapour rises, the thermometer reading rises too. The vapours condense in a cool test tube. Replace test tubes at 100C, 150C, 200C and 300C. Comparing the fractions: The larger the molecules are: -the higher the boiling point will be -the less volatile it will be -the more viscous it will be -the less flammable it will be After fractional distillation either 1) sulphur is removed because it is an impurity 2) fractions are separated into single compounds or 3) cracking Cracking: is a thermal decomposition reaction, in which an alkene (and sometimes hydrogen) is produced from an alkane. Cracking always produces a short chain compound with a C=C bond. Cracking of ethane will give ethene and hydrogen. In the lab it looks like this:
Using a catalyst it is called catalytic cracking. Cracking helps you to make the best use of oil, it can get you more reactive compounds or more desirable chain lengths, for example cracking the naphtha fraction can give you pentane which is suitable for petrol. Name ending compound-type name ane alkane ene alkene ol alcohol oic acid carboxylic acid yl, oate ester (as in ethyl ethanoate) Homologous series Homologous series: family of similar compounds with similar properties due to the presence of the same functional group. Characteristics of a homologous series: -all the compounds fit the same general formula -the chain length increases by 1 each time -as the chain gets longer, the compounds show a gradual change in properties. Structural isomers: have the same chemical formula, but different structures, they can be straight or branched. Branched isomers have lower boiling points, because branches prevent molecules getting close = less attraction Branched isomers are less flammable Alkanes The alkanes are the simplest family of compounds. Alkanes are hydrocarbonds, they only contain hydrogen and carbon. They have the general formula CnH2n+2 1. Found in oil and natural gas (natural gas is methane mostly, but also ethane, propane, butane) 2. First 4 are gases at RTP, next 12 are liquids, rest are solids 3. Each Carbon atom has four single covalent bonds 4. Reaction 1: They burn well in a good supply of oxygen, -combustion this can either be: -complete: meaning there is enough oxygen supply so water and carbon dioxide form. e.g. CH4 + 2O2 CO2 + 2H2O OR -incomplete: meaning there is not enough oxygen to burn them cleanly so either carbon monoxide and water or carbon and water form. 5. Reaction 2: They react with chlorine in sunlight 6. chlorine substitution: sunlight is necessary (or any light?). A chlorine atom replaces a hydrogen atom. This can happen to all of the hydrogen atoms if there is enough chlorine.
e.g. CH4 + Cl2 (light) HCl + CH3Cl / CH2Cl2 / CHCl3 / CCl4 these compounds are called chloromethane / dichloromethane / trichloromethane / tetrachloromethane Alkenes They have the general formula: CnH2n Functional group is the C=C bond Cracking: is a thermal decomposition reaction, in which an alkene (and sometimes hydrogen) are produced from an alkane. Cracking always produces a short chain compound with a C=C bond. Cracking of ethane will give ethene and hydrogen. In the lab it looks like this:
Saturated hydrocarbons: -have NO double bonds -do not react with aqueous bromine, so the mixture stays orange. Unsaturated hydrocarbons: -have double bonds -react with aqueous bromine, turning the mixture from orange to colourless. Poly(ethene) / Polythene: is a polymer produced from ethene by addition polymerisation. A polymer is a compound with very long carbon chains made up of monomer units.
Alkenes Addition Reactions: -with bromine: (the test for saturation) e.g. ethene (g) + bromine (aq) 1,2-dibromomethane (l) -with steam: forms alcohols with heat, pressure and a catalyst e.g. ethene (g) + steam (g) ethanol (l) -with hydrogen: double bond breaks down to for an alkane with heat, pressure and a catalyst e.g. ethene (g) + hydrogen (g) ethane (g)
Alkenes are much more reactive than alkanes, because the double bond can break to form single bonds Alkenes are highly flammable Alcohols Alcohols have the general formula CnH2n+1OH
Functional group is the OH group
Ethanol can be formed in to ways: 1) By fermentation: enzymes in yeast break down glucose (a simple sugar) to ethanol and carbon dioxide, giving out heat (exothermic). This can be done with any substance that contains cellulose, starch or glucose. It is done by grinding the source (e.g. corn or grapes) and treating it with enzymes to break down cellulose and starch into glucose. Leave it to ferment. Fractional distillation is used to get the ethanol from the mixture of substances. 2) Ethene is obtained by cracking long-chain alkenes from oil. The ethene reacts with steam (reversibly) in the following conditions: 570C, 60-70atm and a catalyst (phosphoric acid). Low temperature gives a better yield, but high temperature is used to give a better rate of reaction. *They can be compared like this (in the syllabus it says describe so I dont know how much they want you to know): Fermentation From ethene Advantages: Advantages: -renewable source -fast -good use of waste organic material (e.g. the apples -continuous process which dont look nice enough to be sold in shops.) -pure ethanol -smaller containers Disadvantages: Disadvantages: -Lots of material needed to produce just 1 litre of ethanol -oil is a non-renewable resource so lots of big fermentation tanks needed. -lots of energy to make steam and get the right conditions -Fractional distillation is expensive -a lot of ethene is un-reacted, (and then recycled) -Slow process -Batch process Ethanol burns well in oxygen, giving out plenty of heat, as well as carbon dioxide and water. Ethanol is used as a: -solvent: to dissolve the things than water cannot. It evaporates easily, so it is used a solvent in glues, printing inks, perfumes and aftershave. -fuel: added to or instead of petrol, because it burns cleanly -also to make esters, alcoholic drinks, Ethanol can be dehydrated to ethane using Aluminium (II) oxide and heat Ethanol is miscible mixes completely with water When oxidised alcohol form carboxylic acids Alcoholic drinks affect coordination and judgement, make you aggressive, depression and cirrhosis, high blood pressure, cancers, brain damage etc. Carboxylic acids and esters Carboxlic acids have the carboxylic functional group COOH General formula CnH2n+1COOH Formation of ethanoic acid: -oxidation of ethanol -with acidified potassium mangenate (VII) Ethanoic acid is a typical weak acid: it has a high (as in closer to 7 than 1) pH for an acid, and only dissociates a little bit. Carboxylic acids react with bases to form salts e.g. CH3COONa Carboxylic acids react with alcohols to give esters, in a condensation reaction, for example: Ethanoic acid + ethanol ethyl ethanoate + water (the alcohols name becomes -yl part and the carboxylic acids name becomes the -oate part.
Esters have the ester link (COO), they are found in: 1) tastes and smells of fruit and vegetables 2) oils and fats from plants and animals 3) Artificial esters are used in industry, for tastes in ice cream, foods, and soft drinks and for smell in soaps, shampoos and perfumes
Chapter 18 notes
A polymer is any substance containing very large molecules, formed lots of small molecules join together. Monomers are the small molecules which join together to form much larger molecules called macromolecules. A polymer is a substance made of macromolecules, there are 2 types of polymer: A synthetic polymer is a polymer made in a factory (e.g. Nylon, Lycra, Chewing gum, Polystyrene, Hair gel), a natural polymer is umm... natural A polymerisation reaction, thousands of small molecules join to give macromolecules. The product is a polymer. There are 2 types of polymerisation reaction: 1. Addition polymerisation: double bonds in molecules break and the molecules add on to each other. There are always double bonds in the monomers in an addition reaction.
2. Condensation reaction: two different monomers join; double bonds do not break. Instead, the monomers join by eliminating small molecules. Addition polymers: As they are inert, they are non-biodegradable. Polyalkenes are inert, although can burn. Catalysts are used for the polymerisation, and the exact mechanism depends on the catalyst and alkene (it is not electrophilic addition it is usually catalytic addition). Condensation polymers: Two different monomers join in a condensation reaction to eliminate small molecules Many natural polymers are condensation polymers, e.g. silk, starch and DNA. The two main types are polyesters and polyamides. Polyesters: Esters are formed on reaction between carboxylic acids and alcohols. Polyesters are formed when dicarboxylic acids react with diols.
Polyesters are hydrolysed (in the presence of strong acid or a specific enzyme) into their constituent acid and alcohol, so they are bio-degradable .
Polyamide: Amides are formed on reaction between carboxylic acids and amines. Polyamides are formed when dicarboxylic acids react with diamines. Polyamides are hydrolysed (in the presence of strong acid or a specific enzyme), so they are bio-degradable. Making nylon - the 2 monomers are:
They are represented using blocks as only the functional groups take part in the reaction.
No double bonds break single bonds break and new ones form. Hydrogen chloride is eliminated.
Making terylene a polyester; the 2 monomers are:
Only the functional groups take place in the reaction, (the acid and the alcohol groups)
The monomers join by eliminating a water molecule.
Plastics synthetic polymers. Plastic means can be moulded into shape without breaking. The properties of plastics are: 1. 2. 3. 4. 5. 6. do not usually conduct electricity or heat are unreactive, most are not affected by air, water, acids or other chemicals = good for storage usually light to carry dont break when you drop them are strong their long molecules are attracted to each other = hard to tear do not catch fire easily although when heated they soften and melt and some char
Changing properties: changing reactions are changed, or mixing other chemicals High density on the left below (50C, 3 or 4 atm, and a catalyst) and low density (200C, 2000atm, oxygen) polythene:
Uses of plastics: Polymer polythene polychloroethane (PVC) polypropene polystyrene Teflon
nylon Terylene Pollution problems from plastics: -choke birds, fish and other animals that try to eat them. Or they fill up the animals stomachs so that they cant eat proper food, and starve to death. -they clog up drains and sewers and cause flooding. -they collect in rivers, and get in the way of fish. Some river beds now contain a thick layer of plastic -they blow into trees and onto beaches. So the place looks a mess. Tourists become put off. Solutions: 1. Recycling: a. some are melted down and made into new plastic bags, and things like soles for shoes and fleeces
Example of uses plastic bags and gloves, clingfilm (low density), mugs, bowls, chairs, dustbins (high density) water pipes, wellingtons, hoses, covering for electricity cables crates, ropes used as expanded polystyrene in fast-food cartons, packaging, and insulation for roofs and walls coated on frying pans to make them non-stick, fabric protector, windscreen wipers, flooring ropes, fishing nets and lines, tents, curtains clothing (especially mixed with cotton), thread
b. some are melted and their long chains cracked, to make small molecules that can be polymerized into new plastics c. some are burned to make electricity 2. Degradable plastics a. biodegradable: contains additives such as starch that bacteria can feed on b. photodegradable: additives that break down in sunlight 3. Bio-polymers: are renewable and biodegradable. They are grown inside plants or by bacteria living in tanks Macromolecules in food:
Carbohydrates: cellulose, starch, glycogen and simple sugars. They contain only carbon, hydrogen and oxygen. Plants turn glucose into starch and cellulose by polymerisation 1. A glucose molecule is represented like this:
2. Two glucose molecules join to form a disaccharide (maltose)
3. When many glucose molecules join to give starch, a polysaccharide, a complex carbohydrate
Starch: is found in rice, wheat, millet, maize, pasta and bread. Cellulose is a polysaccharide, but the glucose units are joined differently. Cell walls are made of cellulose. It is indigestible. It is called fibre. It is found in cereals, vegetables and fruit. Glycogen: is another carbohydrate used for storage in humans. Proteins are made up of amino acids. Amino acids contain carbon, hydrogen, oxygen, nitrogen and sometimes sulphur. An amino acid is a carboxylic acid with an amino (NH2) group.
Amino acids join together in a condensation polymerisation.
Proteins are needed for: 1. 2. 3. 4. 5. making enzymes making collagen for skin, bones and teeth making keratin for hair making haemoglobin making hormones
Proteins are found in: fish, cheese, yoghurt, milk, eggs, soya beans etc. Fats and oils: are esters, meaning they are formed from an alcohol (glycerol aka propan-1,2,3-triol) and an acid (fatty acids). Some examples of fatty acids are palmitic acid. How fats are formed:
Fats are found in meat, oily fish, butter, cheese, cream, nut and seeds etc. It is used for energy, combined into new fats to make the cell membranes of cell, some cells store fat droplets for insulation. Unsaturated fats are runny and have a carbon-carbon double bond are healthier than hard, saturated fats found in meat and cheese, that cause heart disease. In digestion, the macromolecules are broken down again by reacting with water. This is called hydrolysis. Hydrolysis: a reaction in which molecules are broken down by reaction with water: starch glucose proteins amino acids fats fatty acids and glycerol Enzymes are used to break down the macromolecules in digestion. Enzymes are proteins and biological catalysts. In the lab, unless you have enzymes, you have to boil the complex carbohydrate (or proteins or fats) in acid. But if hydrolysis is not complete, the macromolecules are not completely broken down. So you get a mixture of molecules of different sizes for example for starch you get, glucose, maltose (2 glucose units) and maltotriose (3 glucose units). Chromatography can be used to identify the products and the substances. However, amino acids and sugars are colourless when dissolved in water, so a locating agent is used. The substances can be identified using the Rf values or by matching them with spots which are horizontal.
S-ar putea să vă placă și
- Groups Rings and Modules CambridgeDocument95 paginiGroups Rings and Modules CambridgePabloxd43Încă nu există evaluări
- Lecture Notes - 19 PDFDocument9 paginiLecture Notes - 19 PDFPabloxd43Încă nu există evaluări
- 07 General Solution of The One-Dimensional Wave EquationDocument11 pagini07 General Solution of The One-Dimensional Wave EquationJuan SebastiánÎncă nu există evaluări
- Analysis CambridgeDocument62 paginiAnalysis CambridgePabloxd43Încă nu există evaluări
- Mildura Former Shire of Walpeup Heritage Study Stage 1 August 2009Document160 paginiMildura Former Shire of Walpeup Heritage Study Stage 1 August 2009Pabloxd43Încă nu există evaluări
- Tottle, Douglas - El Fraude, La Hambruna y El Fascismo. El Mito Del Genocidio Ucraniano de Hitler A Harvard (1987) - Stalin - HolodomorDocument176 paginiTottle, Douglas - El Fraude, La Hambruna y El Fascismo. El Mito Del Genocidio Ucraniano de Hitler A Harvard (1987) - Stalin - HolodomorJRMDRHÎncă nu există evaluări
- Lots of Time or Lots of MoneyDocument1 paginăLots of Time or Lots of MoneyPabloxd43Încă nu există evaluări
- Mildura Former Shire of Walpeup Heritage Study Stage 1 August 2009Document160 paginiMildura Former Shire of Walpeup Heritage Study Stage 1 August 2009Pabloxd43Încă nu există evaluări
- Ricci FlowDocument134 paginiRicci FlowPabloxd43100% (2)
- O Level Maths Notes, PDFDocument67 paginiO Level Maths Notes, PDFMahad Imran85% (181)
- O Level Maths Notes, PDFDocument67 paginiO Level Maths Notes, PDFMahad Imran85% (181)
- Solucionario ThomasDocument11 paginiSolucionario ThomasValeria MedinaÎncă nu există evaluări
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeDe la EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeEvaluare: 4 din 5 stele4/5 (5794)
- Shoe Dog: A Memoir by the Creator of NikeDe la EverandShoe Dog: A Memoir by the Creator of NikeEvaluare: 4.5 din 5 stele4.5/5 (537)
- The Yellow House: A Memoir (2019 National Book Award Winner)De la EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Evaluare: 4 din 5 stele4/5 (98)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceDe la EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceEvaluare: 4 din 5 stele4/5 (895)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersDe la EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersEvaluare: 4.5 din 5 stele4.5/5 (344)
- The Little Book of Hygge: Danish Secrets to Happy LivingDe la EverandThe Little Book of Hygge: Danish Secrets to Happy LivingEvaluare: 3.5 din 5 stele3.5/5 (399)
- Grit: The Power of Passion and PerseveranceDe la EverandGrit: The Power of Passion and PerseveranceEvaluare: 4 din 5 stele4/5 (588)
- The Emperor of All Maladies: A Biography of CancerDe la EverandThe Emperor of All Maladies: A Biography of CancerEvaluare: 4.5 din 5 stele4.5/5 (271)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaDe la EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaEvaluare: 4.5 din 5 stele4.5/5 (266)
- Never Split the Difference: Negotiating As If Your Life Depended On ItDe la EverandNever Split the Difference: Negotiating As If Your Life Depended On ItEvaluare: 4.5 din 5 stele4.5/5 (838)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryDe la EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryEvaluare: 3.5 din 5 stele3.5/5 (231)
- On Fire: The (Burning) Case for a Green New DealDe la EverandOn Fire: The (Burning) Case for a Green New DealEvaluare: 4 din 5 stele4/5 (73)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureDe la EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureEvaluare: 4.5 din 5 stele4.5/5 (474)
- Team of Rivals: The Political Genius of Abraham LincolnDe la EverandTeam of Rivals: The Political Genius of Abraham LincolnEvaluare: 4.5 din 5 stele4.5/5 (234)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyDe la EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyEvaluare: 3.5 din 5 stele3.5/5 (2259)
- The Unwinding: An Inner History of the New AmericaDe la EverandThe Unwinding: An Inner History of the New AmericaEvaluare: 4 din 5 stele4/5 (45)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreDe la EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreEvaluare: 4 din 5 stele4/5 (1090)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)De la EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Evaluare: 4.5 din 5 stele4.5/5 (120)
- Her Body and Other Parties: StoriesDe la EverandHer Body and Other Parties: StoriesEvaluare: 4 din 5 stele4/5 (821)
- CH 4 - ProblemsDocument5 paginiCH 4 - ProblemsKhris Griffis75% (8)
- Catalytic Reforming and Isomerization Tutorial SheetDocument2 paginiCatalytic Reforming and Isomerization Tutorial SheetRohit Sahu100% (1)
- Problems and Answers On Determination of Relative Configuration-02-8-2023Document6 paginiProblems and Answers On Determination of Relative Configuration-02-8-2023Solomon DereseÎncă nu există evaluări
- Chapter 23. Carbonyl Condensation ReactionsDocument19 paginiChapter 23. Carbonyl Condensation Reactions張湧浩Încă nu există evaluări
- Analisis Lemak & Minyak: By. Mulono ApriyantoDocument47 paginiAnalisis Lemak & Minyak: By. Mulono ApriyantoSalsabila NurÎncă nu există evaluări
- Organic Chemistry 100 Must-Know Mechanisms (Roman A. Valiulin) (Z-Library)Document288 paginiOrganic Chemistry 100 Must-Know Mechanisms (Roman A. Valiulin) (Z-Library)Andrew GrantÎncă nu există evaluări
- Stereocontrolled Total Synthesis of ( ) - Englerin ADocument7 paginiStereocontrolled Total Synthesis of ( ) - Englerin ARiccardo SannaÎncă nu există evaluări
- Hydrocarbon and Alkyl Halide-1Document10 paginiHydrocarbon and Alkyl Halide-1Aarya Vardhan ShandilyaÎncă nu există evaluări
- Extraction of Total Lipids From Chicken Egg Yolk Column Chromatography and Qualitative Tests For LipidsDocument9 paginiExtraction of Total Lipids From Chicken Egg Yolk Column Chromatography and Qualitative Tests For LipidsLeslie Manuel Mendoza0% (1)
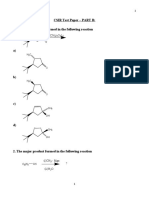
- CSIR Test Paper - PART B: 1. The Major Product Formed in The Following ReactionDocument33 paginiCSIR Test Paper - PART B: 1. The Major Product Formed in The Following ReactionVineeth V TÎncă nu există evaluări
- Amination by ReductionDocument43 paginiAmination by ReductionShreyashÎncă nu există evaluări
- Aliphatic N Aromatic SubDocument48 paginiAliphatic N Aromatic SubSahla Thasnim ckÎncă nu există evaluări
- EdExcel A Level Chemistry Unit 9 Mark Scheme Jan 2000Document3 paginiEdExcel A Level Chemistry Unit 9 Mark Scheme Jan 2000Nabeeha07Încă nu există evaluări
- QUIZ - 2 (ISOMERISM) (Laxman) 1Document5 paginiQUIZ - 2 (ISOMERISM) (Laxman) 1Sanjay Mani TripathiÎncă nu există evaluări
- Nomenclature of BenzeneDocument11 paginiNomenclature of BenzeneNajwa ZhafiraÎncă nu există evaluări
- H H H2O: H H C1 H H2O: H H H H H: 7-29 (C) Continued Without RearrangementDocument131 paginiH H H2O: H H C1 H H2O: H H H H H: 7-29 (C) Continued Without RearrangementKaren Elsy Gonzales CamachoÎncă nu există evaluări
- Iso 4327 1979Document9 paginiIso 4327 1979Isaí Nava CamachoÎncă nu există evaluări
- IntroductionDocument41 paginiIntroductionابوالنور عمارÎncă nu există evaluări
- Chemistry Revision Test - No. 5 (Haloalkanes & Haloalkanes & Alcohols, Phenols & Ethers)Document9 paginiChemistry Revision Test - No. 5 (Haloalkanes & Haloalkanes & Alcohols, Phenols & Ethers)Charles Murgasan Charles MurgasanÎncă nu există evaluări
- Fragmentation in Mass Spectrometry: - Introduction - Ionization - SeparationDocument38 paginiFragmentation in Mass Spectrometry: - Introduction - Ionization - SeparationRøccø RäjÎncă nu există evaluări
- Chapter 12 Aldehyde & KetonesDocument10 paginiChapter 12 Aldehyde & KetonesForzen flamesÎncă nu există evaluări
- Chapter 11 Carbon CompoundDocument50 paginiChapter 11 Carbon CompoundvaogerÎncă nu există evaluări
- Conjugation & ResonannceDocument10 paginiConjugation & Resonanncearya1234Încă nu există evaluări
- Department of Electrical and Information Engineering (Eie) : Staff ListDocument32 paginiDepartment of Electrical and Information Engineering (Eie) : Staff ListtemitopefunmiÎncă nu există evaluări
- Chemistry of CarbohydratesDocument66 paginiChemistry of CarbohydratesPraveen VundrajavarapuÎncă nu există evaluări
- 2CDocument28 pagini2CAbhinav BhatnagarÎncă nu există evaluări
- Semester Syllabus For M. Sc. in Chemistry: School of Chemistry (AutonomousDocument22 paginiSemester Syllabus For M. Sc. in Chemistry: School of Chemistry (AutonomousDachou GeetuÎncă nu există evaluări
- Boyles Resume 2015Document19 paginiBoyles Resume 2015David BoylesÎncă nu există evaluări
- Chromic Acid Oxidation of AlcoholsDocument3 paginiChromic Acid Oxidation of AlcoholsDiana Catalina CruzÎncă nu există evaluări
- CH 01Document201 paginiCH 01Khaled OsmanÎncă nu există evaluări