Documente Academic
Documente Profesional
Documente Cultură
Separation of Radionuclides From High Level Waste Using Diglycolamide Extractants
Încărcat de
sernoorTitlu original
Drepturi de autor
Formate disponibile
Partajați acest document
Partajați sau inserați document
Vi se pare util acest document?
Este necorespunzător acest conținut?
Raportați acest documentDrepturi de autor:
Formate disponibile
Separation of Radionuclides From High Level Waste Using Diglycolamide Extractants
Încărcat de
sernoorDrepturi de autor:
Formate disponibile
Separation Studies on Long Lived
Radionuclides Using Novel Extractants
A
Thesis submitted to the
UNIVERSITY OF MUMBAI
for theDegreeof
DOCTOR OF PHI LOSOPHY
In
CHEMISTRY
By
SERAJ AHMAD ANSARI
Under theguidanceof
Prof . V. K. MANCHANDA
Radiochemistry Division
Bhabha Atomic Research Centre
Mumbai 400 085
December 2007
i
STATEMENT BY THE CANDIDATE UNDER ORDINANCE 770
As requiredbytheUniversityOrdinance770, I wish to statethat thework
embodiedin this thesis entitledSeparation Studies on LongLived
Radionuclides UsingNovel Extractants forms myown contribution to
theresearch work carriedout under theguidanceof Prof. V.K. Manchanda,
at theBhabha Atomic Research Centre, Mumbai 4000085. Thiswork has
not been submittedpreviously for any other degreeof either Mumbai
Universityor anyother University. Whenever references havebeen madeto
previousworksof others, it has been clearlyindicatedas such andincluded
in theBibliography.
(Ansari Seraj Ahmad)
Candidate
I hereby, certify that the above statement is correct.
(Prof. V. K. Manchanda)
Research Guide
Contents
ii
CONTENTS
Acknowledgements vii
Synopsis of the thesis viii
1. GENERAL INTRODUCTION
1-31
1.1. Nuclear Energy 1
1.2. Nuclear Fuel Cycle 2
1.2.1. Waste from Front End of Fuel Cycle 3
1.2.2. Waste from Back End of Fuel Cycle 4
1.3. Classification of Radioactive Waste 5
1.3.1. Low Level Waste 6
1.3.2. Intermediate Level Waste 6
1.3.3. High Level Waste 6
1.4. Impact of Radionuclides on Environment 7
1.5. Chemistry of Actinides 8
1.5.1. History 8
1.5.2. Electronic Configuration 9
1.5.3. Solution Chemistry of Actinides 9
1.5.3.1. Oxidation States 10
1.5.3.2. Disproportionation Reactions 12
1.5.3.3. Hydrolysis and Polymerization 13
1.5.3.4. Complexation of Actinides 14
1.5.3.5. Absorption Spectra 15
1.6. Separation of Metal Ions 16
1.7. Criteria for Selection of Extractants 18
1.8. Reprocessing of Spent Fuel 19
1.8.1. PUREX Process 19
1.9. Actinide Partitioning 20
1.9.1. TRUEX Process 21
1.9.2. TRPO Process 23
Contents
iii
1.9.3. DIDPA Process 23
1.9.4. DIAMEX Process 24
1.10. DIGLYCOLAMIDES: A Class of Promising Extractants
for Actinide Partitioning
25
1.10.1. Main Features of TODGA 26
1.11. Scope of the Thesis 27
1.12. References 28
2. EXPERIMENTAL
32-55
2.1. Synthesis of N,N,N,N-Tetraoctyl Diglycolamide 32
2.2. Characterization of Tetraoctyl Diglycolamide 34
2.3. Synthesis of Malonamide Functionalized Polymer 35
2.4. Characterization of Malonamide Grafted Polymer 36
2.5. Radiotracers (Separation and Purification) 38
2.5.1. Uranium-233 38
2.5.2. Thorium-234 38
2.5.3. Neptunium-239 39
2.5.4. Iron-59 39
2.5.5. Other Radiotracers 40
2.6. Preparation of Simulated High Level Waste 40
2.7. Methods and Equipments 41
2.7.1. Solvent Extraction Studies 41
2.7.2. Mixer-Settler Studies 42
2.7.3. Extraction Chromatography Studies 43
2.7.4. Membrane Studies 44
2.7.5. Hollow Fibre Membrane 45
2.7.6. Other Equipments 47
2.8. Analytical Instruments / Techniques 47
2.8.1. Liquid Scintillation Counter 48
2.8.2. NaI(Tl) Scintillation Counter 49
2.8.3. Surface Barrier Detector 49
2.8.4. High Purity Germanium Detector 51
Contents
iv
2.8.5. Estimation of Uranium 51
2.8.5.1. Spectrophotometry 51
2.8.5.2. Davis Gray Titration 52
2.8.6. Estimation of Thorium 52
2.8.6.1. Spectrophotometry 52
2.8.6.2. Complexometric Titration 53
2.8.7. Estimation of Neodymium 53
2.8.7.1. Spectrophotometry 53
2.8.7.2. Complexometric Titration 53
2.9. References 54
3. N,N,N,N-TETRAOCTYL DIGLYCOLAMIDE:
A PROMISING EXTRACTANT FOR THE
PARTITIONING OF ACTINIDES FROM
HIGH LEVEL WASTE
56-91
3.1. Introduction 56
3.2. Evaluation of Extractants for Actinide Partitioning 57
3.3. Basicity of TODGA 59
3.4. Extraction of Americium by TODGA 60
3.4.1. Effect of Anion 61
3.4.2. Effect of Ligand Concentration 62
3.4.3. Effect of Organic Diluent 65
3.4.4. Kinetics of Extraction 66
3.5. Thermdynamics of Extraction 66
3.5.1. Calculation of Thermodynamic Parameters 67
3.5.2. Effect of Temperature on Distribution of Actinides 70
3.5.3. Thermodynamic Parameters (G, H And S) 71
3.6. Neodymium Loading Studies 74
3.6.1. Evaluation of Phase Modifiers 76
3.7. Extraction of Actinides and Other Metal Ions 78
3.8. Stability of TODGA 82
3.9. Counter-Current Extraction 84
3.9.1. Optimization of Parameters 84
Contents
v
3.9.2. Mixer-Settler Runs 86
3.10. References 88
4. EXTRACTION CHROMATOGRAPHIC STUDIES
ON ACTINIDES AND OTHER METAL IONS USING
N,N,N,N-TETRAOCTYL DIGLYCOLAMIDE
AS THE STATIONARY PHASE
92-113
4.1. Introduction 92
4.2. Preparation of Chromatographic Resin 93
4.3. Batch Studies 95
4.3.1. Evaluation of Resin Materials 95
4.3.2. Kinetics of Extraction of Americium 96
4.3.3. Sorption of Metal Ions on TODGA/Chromosorb-W 97
4.3.4. Sorption of Am(III) Under Loading Conditions 100
4.3.5. Sorption of Am(III) from Nitrate and Sulphate Media 102
4.3.6. Sorption of Metal ions from Synthetic Waste Solution 103
4.4. Column Studies 104
4.4.1. Performance of Chromatography Column 104
4.4.2. Column Breakthrough for Am(III) 107
4.4.3. Column Elution Studies 108
4.4.4. Reusability of Column 110
4.5. References 111
5. SORPTION BEHAVIOUR OF ACTINIDES ON
N,N-DIMETHYL-N,N-DIBUTYL MALONAMIDE
GRAFTED POLYMER
114-135
5.1. Introduction 114
5.2. Sorption Kinetics for Actinides 115
5.3. Uranium Sorption Studies 118
5.3.1. Sorption Isotherms 118
5.3.2. Sorption Mechanism 123
5.4. Effect of Feed Acidity on Metal Ion Sorption 125
5.5. Desorption Studies 127
Contents
vi
5.6. Analytical Applications 128
5.6.1. Metal Loading Capacity 129
5.6.2. Tolerance of Metal ions on Sorption of Uranium 130
5.6.3. Column Separation of Am, Pu and U 130
5.6.4. Pre-concentration of Uranium and Thorium 133
5.7. References 133
6. TRANSPORT BEHAVIOUR OF LONG LIVED
RADIONUCLIDES ACROSS LIQUID MEMBRANES
USING N,N,N,N- TETRAOCTYL
DIGLYCOLAMIDE AS THE CARRIER
136-168
6.1. Introduction 136
6.2. Theory of Facilitated Transport 137
6.2.1. Distribution Equilibria at Aqueous Membrane Interface 138
6.2.2. Flux Equations for Permeation 139
6.3. Transport of Americium 142
6.3.1. Effect of Membrane Soaking Time 142
6.3.2. Effect of Feed Acidity 143
6.3.3. Effect of Carrier Concentration 145
6.3.4. Effect of Strippant 146
6.3.5. Effect of Nitrate ion Concentration 147
6.4. Transport of Metal ions from Nitric Acid 149
6.5. Transport of Metal ions from SHLW 153
6.6. Stability of Liquid Membrane 156
6.7. Hollow Fibre Liquid Membrane Studies 159
6.7.1. Permeation of Metal Ions across HFSLM 159
6.7.1.1. Transport of Neodymium from HNO
3
Solution 160
6.7.1.2. Transport of Americium from SHLW 164
6.7.2. Stability of Liquid Membrane in HFSLM 165
6.8. References 166
Summary and Conclusions 169
Statement Under Ordinance 771 173
vii
ACKNOWLEDGEMENTS
I am deeply indebted to Prof. V.K. Manchanda, Head, Radiochemistry
Division, Bhabha Atomic Research Centre, Mumbai for his invaluable guidance,
critical comments and constant encouragement during the entire course of this study.
I take this opportunity to state that his keen interest and valuable suggestions were of
immense help in improving the quality of work as well as enriching my knowledge.
It is my pleasure to express my sincere thanks to Dr. P.K. Mohapatra, Dr.
P.N. Pathak, Mr. A. Bhattacharyya and Mr. D.R. Prabhu for their active help and
continuous support at all stages of this work. I wish to express my sincere gratitude
to Dr. B.S. Tomar, Dr. M.S. Murali, Mrs. Neetika Rawat, Mr. Sumit Kumar, Ms.
Aishwarya Jain, Mr. R.B. Gujar, Mr. A.S. Kanekar and Mr. D.R. Raut for their
invaluable support and co-operation during the course of this work. I take this
opportunity to thank the technical and administrative staff of Radiochemistry
Division for their immense help during the entire course of this work.
I am thankful to Director, BARC and Director, RC & I Group, BARC for
allowing me to avail all the facilities required for the completion of this work.
Thanks are due to Department of Atomic Energy, Government of India for providing
me the fellowship during the course of this study.
Finally, my family being a constant source of inspiration to me, I take this
opportunity to express my profound gratitude to my beloved family.
viii
SYNOPSIS
of the Thesis submitted to the
UNI VERSI TY OF MUMBAI
for the Degree of
DOCTOR OF PHI LOSOPHY I N CHEMI STRY
-------------------------------------------------------------------------------------
Title of the Thesis : Separation Studies on Long Lived Radionuclides
Using Novel Extractants
Name of the Candidate : Seraj Ahmad F. A. Ansari
Name and Designation : Prof. V.K. Manchanda
of the Research Guide Head, Radiochemistry Division,
Bhabha Atomic Research Centre,
Mumbai 400 085
Place of research work : Radiochemistry Division,
Bhabha Atomic Research Centre,
Mumbai 400 085
Registration Number : BARC - 67
Date of Registration : 26 / 03 / 2004
Signature of the student Signature of the guide
(S. A. Ansari) (Prof. V. K. Manchanda)
Synopsis
ix
Synopsis
Separation Studies on Long Lived Radionuclides Using Novel Extractants
Nuclear energy has been projected as one of the potential sources of energy by
several nations including India. The basic nuclear reaction of neutron induced fission
results in the release of enormous amount of energy. However, due to limited natural
resources of the fissile material (
235
U), the future nuclear energy program largely
depends upon the availability of the man made fissile materials such as
239
Pu and
233
U. To sustain nuclear power programme beyond the availability of naturally
occurring
235
U, it is imperative to follow the closed fuel cycle option. The closed fuel
cycle emphasizes on recycling of the spent fuel and has been opted by several
countries including India. During reprocessing of the spent fuel, the valuable
plutonium and uranium are recovered by a hydrometallurgical process leaving behind
highly radioactive liquid waste solution referred to as High Level Waste (HLW).
This HLW solution comprises long-lived alpha emitting actinides such as
241
Am,
243
Am,
245
Cm and
237
Np (referred as minor actinides) apart from the small amounts of
un-recovered plutonium and uranium as well as beta / gamma emitting fission
products and significant concentrations of structural materials along with process
chemicals. Since the half lives of minor actinides and some of the fission products
range from few hundred to millions of years, HLW poses long term radiological risk
to the environment [1]. The sustainability of the future nuclear energy programme,
therefore, depends upon the effective radioactive waste management which must safe
guard the human health as well as the ecology.
The challenge for the final disposal of HLW is largely due to the radiotoxicity
associated with the minor actinides. At present, the most accepted concept for the
management of HLW is to vitrify it in the glass matrix followed by disposal in deep
geological repositories. Since the half lives of minor actinides concerned range
between a few hundred to millions of years, the surveillance of HLW for such a long
period is economically as well as environmentally daunting task. An alternative /
complimentary concept is the partitioning and transmutation (P&T), which envisages
the complete removal of minor actinides from HLW and their consequent burning in
reactors as mixed oxide fuels [2]. This process would lead to generation of extra
Synopsis
x
energy and at the same time would alleviate the need for long term surveillance of
geological repositories. After partitioning of the actinides along with the long lived
fission products, the residual waste can be vitrified and buried in subsurface
repositories at a much reduced risk and cost. Efforts are being made by radiochemists
/ separation chemists to develop efficient and environmentally benign processes for
the separation of long-lived radionuclides from HLW solution.
For the partitioning of actinides from HLW solution, several processes have
been proposed, viz. TRUEX, DIAMEX, DIDPA and TRPO which employ
octyl(phenyl)-N,N-diisobutyl carbamoyl methyl phosphine oxide (CMPO), N,N-
dimethyl-N,N-dibutyl tetradecyl malonamide (DMDBTDMA), diisodecyl
phosphoric acid (DIDPA) and trialkyl phosphine oxide (TRPO) as the extractants [3].
However, each of the above mentioned processes is associated with certain
limitations. The main drawbacks of the TRUEX process are: (a) the poor back
extraction of Am(III) and Cm(III), and (b) interference due to solvent degradation
products. On the other hand, DIDPA process cannot be applied to the concentrated
HLW solution without denitration which leads to the precipitation of actinides.
Similarly, the TRPO process works only at relatively lower acidity (1M HNO
3
) and,
therefore, cannot be applied directly to HLW conditions (3-4M HNO
3
). Though the
completely incinerable DMDBTDMA has been reported to be a promising candidate,
it is a moderate extractant for Am(III) / Cm(III) from HLW solution at acidity 3M
HNO
3
[4]. In order to improve the efficiency of diamides towards the forward
extraction of trivalent actinides, several structural modifications of the ligand have
been attempted. Recently, a series of diamide compounds have been synthesized by
introducing different substituents on amide nitrogen or introducing an ether oxygen
into the bridging chain of malonamide [5]. It has been observed that the introduction
of etherial oxygen between the two amide groups (diglycolamide) causes significant
enhancement in the extraction of trivalent actinides / lanthanides. Amongst the
several derivatives of diglycolamide studied, N,N,N,N-tetraoctyl diglycolamide
(TODGA) has been identified as one of the most promising extractants for the
partitioning of trivalent actinides and lanthanides from HLW solutions [6]. Some of
the salient features of TODGA include; (i) large extraction capacity for trivalent
actinides from moderate acidic aqueous solutions, (ii) low concentration of TODGA
Synopsis
xi
(0.1M) to be used, (iii) possibility of complete incineration as the constituent
elements are C, H, N and O, (iv) good radiolytic and hydrolytic stability, and (v) the
ease of synthesis. As TODGA exhibits excellent properties required by an extractant,
it was evaluated for the partitioning of actinides from HLW solution.
The main objective of the present work is to explore the separation of various
radionuclides (actinides / long lived fission products) from structural elements (Fe,
Co, Ni), process chemicals and daughter products of fission products present in
HLW. The present research work includes synthesis and characterization of
extractant / extraction chromatographic material, distribution behaviour of actinides
and other metal ions present in HLW and optimization of experimental parameters
for hollow fibre liquid membrane as well as for mixer-settler runs. Effort has been
made to understand the basic chemistry of TODGA interactions with actinides. An
insight into the sorption behaviour of actinide ions on a novel malonamide grafted
polymer has also been described. The thesis is structured into six chapters for
presentation of the present research work.
CHAPTER-1: GENERAL INTRODUCTION
This is the introductory chapter of the thesis that elaborates the importance of the
separation of minor actinides and long-lived fission products from radioactive waste
solutions. The source of these radionuclides and their impact on the environment has
been discussed. The radionuclides which are of major concern are the long lived
alpha emitting radioisotopes which belong to the actinide elements of the periodic
table. The chemistry of actinides is important for their separation and, therefore, the
chemistry of actinides in brief has been presented in this chapter. A brief overview of
the literature reports on the importance and separation of radionuclides by different
extractants has been presented. A brief background of the development of
diglycolamide extractants has been included in this chapter. This chapter also deals
with the aims and objectives of the present work.
CHAPTER- 2: EXPERIMENTAL
A general outline about different experimental techniques and instrumentation used
in the present work is given in this chapter. The synthesis, purification and
Synopsis
xii
characterization of TODGA have been described. Synthesis and characterization of a
novel malonamide grafted polymer has also been described. A brief mention about
the various analytical techniques followed is also made in this chapter. For
characterization of materials, techniques like UV-visible absorption spectroscopy,
infrared (IR) spectroscopy and nuclear magnetic resonance (NMR) spectroscopy
were employed. The gamma spectrometry was carried out using NaI(Tl) detector and
HPGe detector, whereas surface barrier detector and liquid scintillation counter were
employed for alpha spectrometry and gross assaying of alpha activity. The basic
principles of these detectors are also described. The preparation and purification of
various radiotracers is included in this chapter. The UV-visible absorption
spectrophotometry was followed for the analysis of Nd, Th and U when their
concentrations were in the range of microgram / mL quantities. The complexometric
titrations carried out for the analysis of various elements such as lanthanides, thorium
and uranium are also described in this chapter.
CHAPTER-3: N,N,N,N-TETRAOCTYL DIGLYCOLAMIDE: A
PROMISING EXTRACTANT FOR THE PARTITIONING OF ACTINIDES
FROM HIGH LEVEL WASTE
N,N,N,N-tetraoctyl diglycolamide (TODGA) has been evaluated as an extractant for
the partitioning of minor actinides from radioactive waste solutions [6]. This chapter
deals with the basic solvent extraction chemistry of actinides and fission products
with TODGA. The performance of TODGA for the extraction of actinides has been
compared with those of other extractants proposed for actinide partitioning, viz.
CMPO, TRPO and DMDBTDMA. Acid uptake studies suggested that TODGA is
more basic (K
H
: 4.1) as compared to CMPO (K
H
: 2.0) and DMDBTDMA (K
H
: 0.32).
In order to understand the effect of diluent on the complexation of TODGA with
trivalent actinides the distribution behaviour of Am(III) was studied employing
diluents with different dielectric constants. The effects of complexing anions such as
NO
3
-
, ClO
4
-
and Cl
-
were investigated to understand the mechanism of extraction for
the metal ions. The thermodynamics of extraction of actinide ions such as Am(III),
Pu(IV) and U(VI) from nitric acid medium by TODGA has also been discussed in
this chapter. The two-phase equilibrium constants and thermodynamic parameters,
Synopsis
xiii
viz. G, H and S for the extraction of actinides have been calculated and
compared with those of CMPO and DMDBTDMA.
One of the important criteria for a good extractant to be used in the solvent
extraction process is the high metal loading capacity in the organic phase. Though
TODGA exhibits high extraction behaviour for trivalent actinides, it forms third
phase at very low metal ion concentration and the limiting organic concentration
(LOC) for neodymium was found to be very low (~0.008M Nd by 0.1M TODGA /
dodecane at 3M HNO
3
). Third phase formation refers to the phenomenon in which
the organic phase splits into two phases, one is lighter in weight and rich in diluent,
and other is heavier in weight and rich in ligand-metal / ligand-acid complex. Third
phase formation is a natural phenomenon arising out of the incompatibility of the
polar metal solvate species (or acid ligand complex) with the highly non-polar
diluent like dodecane. The third phase is often eliminated by the addition of a
suitable diluent modifier which increases the polarity of diluent thereby increasing
the solubility of metal-ligand complex. In the present work, N,N-dihexyl octanamide
(DHOA) was found to be a promising phase modifier amongst a series of compounds
studied, viz., dibutyl decanamide, di(2-ethylhexyl) acetamide, di(2-ethylhexyl)
propionamide, di(2-ethylhexyl) isobutyramide, dihexyl decanamide, tri-n-butyl
phosphate and 1-decanol. The distribution behaviour of actinides / fission products
has been studied from pure nitric acid solution as well as from synthetic HLW
solution employing 0.1M TODGA +0.5M DHOA in n-dodecane. This chapter also
reports the applicability of TODGA for the extraction of lanthanides / actinides on
large scale in counter-current extraction using a mixer-settler system.
CHAPTER-4: EXTRACTION CHROMATOGRAPHIC STUDIES ON
ACTINIDES AND OTHER METAL IONS USING N,N,N,N-TETRAOCTYL
DIGLYCOLAMIDE AS THE STATIONARY PHASE
In the view of their continuous nature, solvent extraction processes are extensively
employed for plant scale operations for the recovery of metal ions in large scale.
However, the major problem associated with this technique is the generation of large
volume of secondary waste and handling of large volume of inflammable diluents,
particularly when the metal quantities involved are in the grams / milligrams
Synopsis
xiv
quantities. It is, therefore, imperative to look for an alternative technique where the
metal ions can be concentrated in a small volume with minimum generation of
secondary waste. In this context, several techniques like liquid membrane,
magnetically assisted chemical separation (MACS) and extraction chromatography
(EC) are promising alternatives [7-9]. Amongst these techniques EC is rather well
known.
This chapter deals with the preparation of a novel extraction chromatographic
resin impregnated with TODGA and its use to study the sorption behaviour of
actinides / fission products from nitric acid solutions as well as from SHLW solution.
The performance of the present resin has been compared with the resin prepared by
impregnation of other proposed extractants for actinide partitioning such as CMPO,
TRPO and DMDBTDMA. The possibility of the resin material to sorb trace
concentrations of Am(III) from nitric acid solutions containing relatively large
amounts of Nd(III), U(VI), Fe(III) as well as from SHLW solution has also been
reported. In the column chromatographic studies breakthrough capacity of the
column in the presence of macro concentrations of europium and uranium was
investigated. The breakthrough capacity of the column was found to be 20mg of Eu/g
of resin. Elution studies of Am(III) suggested that 0.01M EDTA was effective
amongst different eluents studied.
CHAPTER-5: SORPTION BEHAVIOUR OF ACTINIDE IONS ON
N,N-DIMETHYL-N,N-DIBUTYL MALONAMIDE GRAFTED POLYMER
Solid phase extraction has been increasingly used for the separation of trace as well
as ultra trace amounts of metal ions from complex matrices [10,11]. Chelating
polymers have been frequently used for solid phase extraction of metal ions as they
provide good stability and high sorption capacity. There are two approaches which
are frequently adopted for designing such chelating polymers. The first involves the
physical sorption of chelating ligands on the inert polymeric solid support as
discussed in chapter 4. The other is based on co-valent coupling of the ligands with
the polymer backbone through certain functional groups such as N=N- or CH
2
-
groups. The latter strategy renders the chromatographic system free from ligand
leaching problem which is often encountered in the former.
Synopsis
xv
Studies on substituted diamide suggested good metal extraction behaviour,
high radiolytic stability and complete incinerability [12]. However, despite these
features, amides do possess inherent limitations such as finite aqueous phase
solubility and third phase formation. In order to overcome these problems, the
synthesis of a novel malonamide grafted polymer was carried out using N,N-
dimethyl-N,N-dibutyl malonamide (DMDBMA) as chelating ligand and Merrifield
polymer as the support backbone. The synthesized polymeric material exhibited
superior binding for hexavalent and tetravalent metal ions such as U(VI) and Pu(IV)
over trivalent metal ions, viz. Am(III) and Pu(III). Various physico-chemical
properties of the polymer like phase adsorption kinetics, metal sorption mechanism
and metal sorption capacity have been studied in the static method. The kinetics for
the adsorption of Am(III), Th(IV) and U(VI) was found to follow the first order
Lagergren rate kinetics. Adsorption of U(VI) on the malonamide grafted polymer
followed the Langmuir adsorption isotherm. The metal sorption capacity for uranium
and thorium by the malonamide functionalized polymer is also reported in this
chapter. Batch extraction studies suggested the possible separation of uranium,
americium and plutonium from each other. The pre-concentration of thorium and
uranium from a large volume of dilute solution employing the grafted resin column is
also reported in this chapter.
CHAPTER-6: TRANSPORT BEHAVIOUR OF LONG LIVED
RADIONUCLIDES ACROSS LIQUID MEMBRANES USING N,N,N,N-
TETRAOCTYL DIGLYCOLAMIDE AS THE CARRIER
During the last two decades, the development of selective receptor molecules for
cationic as well as anionic, organic, or inorganic substrates led to their use as carrier
agents for facilitating selective transport through artificial or biological membranes.
Thus, the studies on transport processes were prompted by the design of synthetic
carrier molecules [13]. Liquid membrane transport processes, where the carrier
facilitates selective transportation, have many advantages over solvent extraction.
Liquid membrane processes are being widely employed for the separation of metal
ions involving bulk liquid, supported liquid, or emulsion liquid membranes [14,15].
Facilitated transport of metal ions through liquid membrane has potential
Synopsis
xvi
applications in the nuclear industry such as recovery of metals from
hydrometallurgical leach solutions, treatment and concentration of low level aqueous
waste from reprocessing plants and from waste streams of radiochemical laboratories
engaged in analytical and research activities. This is a fascinating separation
technique because of relatively small inventory of the extractant and low energy
consumption.
This chapter deals with the carrier mediated transport of actinides / fission
products from nitric acid medium across a membrane impregnated with TODGA in
n-dodecane. Microporous PTFE membranes have been used as the polymeric
support. The permeability of transported species through the liquid membrane is
explained in this chapter with the help of various diffusional parameters. Influence of
various parameters, viz. feed acidity, carrier concentration, nature of strippant and
effect of radiation dose on the transport of actinides has been reported. The effect of
macro concentration of neodymium, uranium and iron on the transport of Am(III) has
been illustrated in this chapter. The transport of actinides, fission products and
structural elements fromSimulated High Level Waste (SHLW) solution has also
been investigated. The effect of various strippants, namely distilled water, oxalic acid
and buffer solution on the transport of Am(III) has been explored. The membrane
stability was remarkably good when tested over 20 days of continuous operation. The
applicability of membrane separation process on a larger scale has been successfully
demonstrated in a liquid cell contactor (Hollow Fibre Module) for the separation of
lanthanide using TODGA as the extractant.
SUMMARY AND CONCLUSIONS
The present thesis describes the separation chemistry of actinides employing
N,N,N,N-tetraoctyl diglycolamide (TODGA) as the extractant. The synthesis,
characterization and interaction of TODGA with metal ions have been illustrated. A
novel dimethyl dibutyl malonamide grafted polymer has been synthesized and
sorption behaviour of actinide ions on this grafted polymer has been described. The
basic as well as applied aspects of extraction of actinide ions with TODGA have
been explored. Various techniques employed for the separation of actinides / fission
products were solvent extraction, extraction chromatography and liquid membranes.
Synopsis
xvii
In conclusion, TODGA exhibited high basicity and high extraction capacity
for trivalent lanthanides / actinides as compared to commonly proposed extractants
such as CMPO and DMDBTDMA. TODGA forms third phase at very low
concentration of Nd, however, DHOA has been evaluated as a suitable phase
modifier. The possible application of TODGA for the separation of actinides /
lanthanides from radioactive waste solutions has been successfully demonstrated on
large scale in counter-current extraction mode using a mixer-settler system. The
extraction chromatographic studies involving TODGA as the stationary phase
demonstrated the possible use of the material for the concentration of radionuclides
from a large volume of dilute waste solutions. The sorption behaviour of uranium
and thorium on malonamide grafted polymer was found to follow the first order
Lagergren rate kinetics. The sorption of uranium on malonamide grafted polymer
exhibited the Langmuir adsorption isotherm. The Langmuir monolayer adsorption
phenomenon was also confirmed by the theoretical approach based on adsorption
kinetics. The transport behaviour of radionuclides by TODGA liquid membrane has
been described with the help of various diffusional parameters. Distilled water has
been evaluated as a suitable strippant for actinides / fission products. Stability of the
TODGA liquid membrane was found to be excellent when monitored over a period
of twenty days of continuous operation. The possible application of TODGA-based
liquid membrane for the separation of metal ions on large scale has been
demonstrated using hollow fibre membrane modules.
REFERENCES
1. Status and Trends of Spent Fuel reprocessing, IAEA TECDOC-1103, 1999.
2. L.H. Baestle. Burning of Actinides: A complementary waste management
option? IAEA Bulletin, 34(3) (1992), 32.
3. J.N. Mathur, M.S. Murali and K.L. Nash, Solv. Extr. Ion Exch., 19 (2001) 357.
4. V.K. Manchanda and P.N. Pathak, Sep. Purif. Technol., 35 (2004) 85.
5. L. Spjuth, J.O. Liljenzin, M.J. Hudson, M.G.B. Drew, B.P. Iveson and C. Madic,
Solv. Extr. Ion Exch., 18 (2000) 1.
6. Y. Sasaki, Y. Sugo, S. Suzuki and S. Tachimori, Solv. Extr. Ion Exch., 19 (2001)
91.
Synopsis
xviii
7. P.R. Danesi, E.P. Horwitz and P.G. Rickert, J. phys. Chem., 87 (1983) 4708.
8. L. Nunez, B.A. Buchholz and G.F. Vandergrift, Sep. Sci. Technol., 30 (1995)
1455.
9. J.L. Cortina and A. Warshawsky, developments in solid-liquid extraction by
solvent impregnated resins, In Ion exchange and solvent extraction, J.A.
Marinsky and Y. Marcus (Eds.), Marcel Dekker, NY (1975), Vol. 13, P. 195-293.
10. V. Camel, Spectrochim. Acta Part B, 58 (2003) 1177.
11. N. Masque, R.M. Marce and F.B. Trends, Anal. Chem., 17 (1998), 384.
12. C. Musikas, Inorg. Chim. Acta, 140 (1987) 197.
13. G. Spach, Ed., "Physical Chemistry of Transmembrane Ion Motions", Elsevier:
Amsterdam, 1983.
14. R.M. Izatt, J.D. Lamb and R.L. Bruening, Sep. Sci. Technol., 23 (1988) 1645.
15. N.M. Kocherginsky, Q. Yang and L. Seelam, Sep. Purif. Technol., 53 (2007) 171.
------------------------
Chapter I
General Introduction
Chapter I
1
2%
7%
16% 17%
19%
39%
Coal
Gas
Nuclear
Hydro
Oil
Others
GENERAL INTRODUCTION
Our planet is witness to a constant increase in the population with a corresponding
increase in the needs of each individual. The demands for agricultural and industrial
output and essential services can only be met if the production of power (energy)
increases rapidly. While it is forecasted that the electrical power production in
industrialized countries will have to be doubled within the next 20years, the growth
rate of power generation will have to be much higher for developing countries like
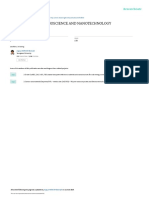
India. At present, vast bulk of the global energy is supplied by coal, natural gas,
hydroelectric and, to a small extent, by oil and nuclear energy (Fig. 1.1). Due to
limited resources of the fossil fuels the overwhelming demand of global energy can
only be achieved by utilization of other possible resources. Nuclear energy has been
projected as an alternate source to meet the considerable energy requirement of the
world.
1.1. NUCLEAR ENERGY
The nuclear power is characterized by the release of very large amount of energy
from a given amount of fuel generating relatively small amount of waste per unit
Fig. 1.1. World production of electricity in 2002 by various fuels.
Source: OECD/IEA world energy outlook 2004
Chapter I
2
production of electrical energy. The basic nuclear reaction, viz. neutron induced
fission of fissile materials like
235
U, results in the release of enormous amount of
energy. This fundamental nuclear reaction is utilized to obtain the controlled release
of energy in the nuclear power reactors. However, due to limited natural resources of
the fissile material (
235
U), the future nuclear energy program largely depends upon
the availability of the man made fissile materials like
233
U and
239
Pu. To sustain
nuclear power programme beyond the availability of naturally occurring
233
U, it is
imperative to follow the closed fuel cycle option. The closed fuel cycle emphasizes
on the recycling of the spent fuel and has been already opted by several nations
including India. During reprocessing of the spent fuel in the closed fuel cycle, the
valuable plutonium and uranium are recovered by the hydrometallurgical process
leaving behind highly radioactive liquid waste solution, referred to as High Level
Waste (HLW). The HLW solution contains long-lived alpha emitting actinides such
as
241
Am,
243
Am,
245
Cm and
237
Np (referred to as minor actinides) apart from the
small amount of un-recovered plutonium and uranium as well as beta / gamma
emitting fission products and significant concentrations of structural materials and
process chemicals [1,2]. Since the half lives of minor actinides and some of the
fission products range from few hundred to millions of years, the HLW possesses
long term radiological risk to the environment [3]. The sustainability of the future
nuclear energy program, therefore, depends upon the safe management of radioactive
waste which shall never jeopardize the human health as well as the ecology. For
efficient radioactive waste management it is desirable to understand the source and
composition of radioactive waste generated at various stages of the nuclear fuel
cycle.
1.2. NUCLEAR FUEL CYCLE
Nuclear fuel cycle comprises of front end and back end and comprises of various
stages like mineral exploration, mineral processing, purification of uranium /
thorium, fuel fabrication, reactor operation, spent fuel reprocessing, radioactive waste
management etc. (Fig. 1.2). The Front End includes stages from mining of the ore
to the reactor operation, and the Back End includes the removal of spent fuel from
the reactor and its subsequent reprocessing to recover valuables, and treatment and
disposal of high level waste.
Chapter I
3
Fig. 1.2. Nuclear Fuel Cycle
1.2.1. Waste from Front End of Fuel Cycle
The waste generated at the uranium mine site comprises decay products of
238
U /
233
U
and exists in the form of radioactive dust. At the mill, dust is collected and fed back
into the process, while radon gas is diluted and dispersed into the atmosphere. The
wastes from the milling operation include the radioactive radium which is reverted
back to the mine and covered with rock and clay. The uranium oxide produced from
the mining and milling of the ore is accompanied by only a fraction of total
concentration of decay products as most of them are diverted to the tailings.
Similarly, the step of turning uranium oxide concentrate into a useable fuel does not
produce significant radioactive waste. It is when uranium is burnt in the reactor that
significant quantities of highly radioactive fission / activation products are produced
(Table 1.1). More than 99.9% of the radioactivity produced in the reactor is retained
in the fuel rods, while less than 0.1% is distributed in other systems of the reactor.
Chapter I
4
Table 1.1: Major contributors to the radioactivity in the spent fuel after a
cooling period of 50 days
Nuclides Half life Nuclides Half life
3
H 12.3 yrs
131
I 8.05 days
85
Kr 10.8 yrs
137
Cs 30.0 yrs
89
Sr 50.6 days
140
Ba 12.8 days
90
Sr 28.8 yrs
140
La 40.2 days
90
Y 64.4 hrs
141
Ce 32.4 days
91
Y 58.8 days
143
Pr 13.6 days
95
Zr 65 days
144
Ce 285 days
95
Nb 35 days
144
Pr 17.3 min
103
Ru 39.6 days
147
Nb 11.1 days
106
Ru 367 days
147
Pm 2.62 yrs
129m
Te 34 days
1.2.2. Waste from Back End of Fuel Cycle
In the nuclear fuel cycle most of the radioactive waste is generated during
reprocessing of the spent fuel, i.e. at the back end of the fuel cycle. The fuel after
sufficient use in the reactor is referred as Spent Fuel. This irradiated spent fuel
contains long-lived alpha emitting transuranic elements (principally Np, Pu, Am and
Cm), which are formed in uranium fuelled reactors by neutron capture of
238
U
followed by a sequence of beta emission and neutron capture reaction of the daughter
products. Apart from this, the spent fuel also contains large amount of fission
products which are generally beta/gamma emitters and constitute major dose in the
waste [2]. Although nearly 200 radionuclides are produced during irradiation of the
fuel, the great majorities of them are relatively short lived and decay to low level
within few decades. The major contributors to the fission product activity after a
cooling period of 50 days are listed in Table 1.1. The spent fuel is often allowed to
cool for few years to allow short lived radionuclides to decay. After cooling the spent
fuel for about one year, only
106
Ru,
106
Rh,
90
Sr,
90
Y,
144
Ce,
144
Pr,
134
Cs,
137
Cs and
147
Pm contribute significantly to the activity [2]. During reprocessing of spent fuel
Chapter I
5
Fig. 1.3. Reprocessing of spent fuel
the irradiated fuel is dissolved in nitric acid solution, referred as dissolver solution,
and subsequently treated with tributyl phosphate (PUREX Process) to remove
valuable plutonium and uranium. A flow sheet for reprocessing of the spent fuel is
shown in Fig. 1.3. The aqueous raffinate remaining after the co-extraction of uranium
and plutonium from dissolver solution by PUREX process is concentrated into high
acidic liquid solution which is referred as High Level Waste (HLW). The HLW
solution thus contains minor actinides, fission products and left over uranium and
plutonium along with structural materials and process chemicals. One of the
challenges at the back end of the nuclear fuel cycle lies in the safe management of
HLW. Some of the radionuclides in HLW are very important and precious and hence
can be separated as wealth from the waste.
1.3. CLASSIFICATION OF RADIOACTIVE WASTE
Radioactive wastes are classified as low level waste, intermediate level waste and
high level waste depending upon the level of radioactivity which varies from curies
per litre to microcuries per litre.
Chapter I
6
1.3.1. Low Level Waste
When the total radioactivity of the waste is less than millicurie / litre, it is referred as
low level waste (LLW). It is generated as liquid from the decontamination of
equipments, radioactive laboratories, hospitals using radiopharmaceuticals as well as
from the nuclear fuel cycle. The level of radioactivity and half-lives of radioactive
isotopes present in LLW are relatively small. Storing the waste for a period of few
months allows most of the radioactive isotopes to decay, the point at which the
wastes can be disposed off safely. The LLW comprises about 90% of the total
volume of the radioactive wastes generated, but only <1% radioactivity of all the
wastes. To reduce the volume of solid LLW, it is often incinerated and compressed
before disposal. Usually it is buried in shallow landfill sites.
1.3.2. Intermediate Level Waste
When the radioactivity of the waste ranges from millicurie to curie / litre, the waste is
referred as intermediate level waste (ILW). The ILW contains higher amount of
radioactivity as compared to the LLW and, therefore, may require special shielding.
It typically comprises resins, chemical sludges, reactor components as well as
reprocessing equipments. The ILW comprises about 7% of the total volume of the
radioactive wastes, while it contains <4% radioactivity of all the radioactive wastes.
1.3.3. High Level Waste
When the radioactivity of the waste is greater than curie / liter, the radioactive waste
is referred as high level waste (HLW). The HLW is the waste emanating from the
reprocessing of spent fuel. While HLW comprises only about 3% of the total volume
of all the radioactive wastes, it contains more than 95% of the total radioactivity
generated in the nuclear fuel cycle. This waste includes uranium, plutonium and
other highly radioactive elements made up of fission products and alpha emitting
minor actinides. The challenge for the final disposal of HLW is largely due to the
radiotoxicity associated with the minor actinides which have half lives ranging from
few hundred to millions of years [4]. Efforts are being made by radiochemists /
separation chemists to meet the challenges of radioactive waste management by
developing efficient and environmentally benign processes for the separation of
Chapter I
7
10
1
10
2
10
3
10
4
10
5
10
6
10
-2
10
-1
10
0
10
1
10
2
10
3
10
4
10
5
P
a
r
t
i
t
i
o
n
i
n
g
Uranium Ore
No Partitioning
R
a
d
i
o
t
o
x
i
c
i
t
y
(
R
e
l
a
t
i
v
e
)
Time (Years)
various radionuclides from HLW solution. This would minimize the volumes of
radioactive wastes and costs of their final disposal.
1.4. IMPACT OF RADIONUCLIDES ON ENVIRONMENT
The long-lived radionuclides present in the raffinate of PUREX process after
reprocessing of the spent fuels are of great environmental concern. The radioactive
waste, whether natural or artificial, is a potential source of radiation exposure to the
human being through different pathways. The raffinate after PUREX process
generally contains un-extracted U, Pu and bulk of minor actinides such as Am, Np,
Cm and host of fission products like Tc, Pd, Zr, Cs, Sr and lanthanides as well as
activation products. At present the most accepted conceptual approach for the
management of HLW is to vitrify it in the glass matrix followed by disposal in deep
geological repositories [5,6]. Since the half lives of minor actinides concerned range
between a few hundred to millions of years, the surveillance of high active waste for
such a long period is debatable from economical as well as environmental safety
considerations. On the other hand, the vitrified mass of HLW will have to withstand
the heat and radiation damages caused by the decay of beta/gamma emitting fission
products such as
137
Cs and
90
Sr for about 100yrs. Therefore, it may create the
possible risk for the migration of long lived alpha emitting minor actinides from
Fig. 1.4. Partitioning of minor actinides- Impact on waste management
Chapter I
8
repository to the environment. The recommended activity level of 4000Bq per gram
in terms of alpha activity is considered benign enough to be treated as LLW. As
represented in Fig. 1.4, if actinides are not removed from the spent fuel, it will
require millions of years to reduce its radiotoxicity to this level. However, if one can
remove U, Pu and minor actinides from the waste its radiotoxicity could reach an
acceptable level after few hundreds of years. Therefore, strategy of P&T
(Partitioning of long-lived radionuclides followed by Transmutation) is being
considered by several countries around the world [7,8]. The P&T process envisages
the complete removal of minor actinides from radioactive waste and their subsequent
burning in the reactors / accelerators as mixed oxide fuel. This process will lead to
generation of extra energy and at the same time would alleviate the need for long
term surveillance of geological repositories. After partitioning of the actinides along
with the long lived fission products, the residual waste can be vitrified and buried in
subsurface repositories at a much reduced risk and cost.
1.5. CHEMISTRY OF ACTINIDES
The work carried out in this thesis pertains to the separation chemistry of actinides
and fission products from radioactive waste solutions. The actinides include uranium,
neptunium, plutonium, americium and curium. It is quite essential to understand the
chemistry of actinides before their partitioning. A brief survey of the chemistry of
actinide elements is, therefore, considered relevant.
1.5.1. History
The existence of rare earth like series in the seventh row of periodic table, which was
suggested as early as 1926, gained wider acceptance with the discovery and study of
transuranium elements [9]. In 1945, Seaborg proposed that actinium and
transactinium elements form such a series in which the 5f electron shell is being
filled in a manner analogous to the filling of 4f shell in lanthanides [10]. Except for
uranium and thorium, which are well known actinide elements discovered in 1789
and 1828, respectively, all the other elements were discovered in twentieth century.
Among actinide elements uranium and thorium have isotopes with half-lives
exceeding the estimated life of this planet and hence occur in nature. Actinium and
protactinium owe their existence to the decay of long lived isotopes of uranium,
Chapter I
9
thorium and their daughter products. The rest of the elements in this series are
essentially man made with some evidence for the trace occurrence of neptunium
and plutonium in the nature formed by nuclear reactions involving uranium [11,12].
Among man made elements plutonium and, to a lesser extent, neptunium, americium
and curium are produced in the nuclear power reactors and are recovered from the
spent nuclear fuels. The elements beyond curium are generally produced through
heavy ion reactions of transplutonium elements in accelerators. With increasing
atomic number of actinides, the nuclei becomes rapidly less stable and only
einsteinium has an isotope with a half-life long enough to offer any possibility for
conventional chemical studies.
1.5.2. Electronic Configuration
The fourteen 5f electrons enter the actinide elements beginning formally with Th
(Z=90) and ending with Lr (Z=103). These fourteen elements following Ac are
placed in the 7
th
row of the periodic table separately analogous to lanthanides.
Intensive chemical studies have revealed many similarities between the lanthanides
and actinides. The ground state electronic configuration of lanthanides and actinides
is shown in Table 1.2. Though there is over all similarity between the two groups of
elements, some important differences also exist mainly because the 5f and 6d shells
are of similar energy in actinides and 5f electrons are not so well shielded as 4f
electrons in lanthanides [13]. The lighter actinides (Ac to Np) show greater tendency
to retain 6d electrons due to smaller energy differences between 6d and 5f orbitals
relative to that between 5d and 4f orbitals of lanthanides. In case of transition series
the relative energy of orbitals undergoing the filling process become lower as the
successive electrons are added. For actinides too the 5f orbitals of plutonium and
subsequent elements are of lower energy than 6d orbitals and, therefore, the
subsequent electrons are filled in 5f orbitals with no electrons in 6d orbitals.
1.5.3. Solution Chemistry of Actinides
As the processes of separation and purification of actinides on large scale are
essentially based on hydrometallurgical techniques, the study of solution chemistry
of actinides has received considerable attention. The actinide elements exist in
multiple oxidation states and most of their separation processes are based on the
Chapter I
10
Table 1.2: Electronic configuration of lanthanide and actinide elements
Lanthanides Actinides
Elements Atomic
numbers
Electronic
configurations
Elements Atomic
numbers
Electronic
configurations
La 57 5d
1
6s
2
Ac 89 6d
1
7s
2
Ce 58 4f
1
5d
1
6s
2
Th 90 6d
2
7s
2
Pr 59 4f
3
6s
2
Pa 91 5
f
2
6d
1
7s
2
Nd 60 4f
4
6s
2
U 92 5f
3
6d
1
7s
2
Pm 61 4f
5
6s
2
Np 93 5f
4
6d
1
7s
2
Sm 62 4f
6
6s
2
Pu 94 5f
6
7s
2
Eu 63 4f
7
6s
2
Am 95 5f
7
7s
2
Gd 64 4f
7
5d
1
6s
2
Cm 96 5f
7
6d
1
7s
2
Tb 65 4f
9
6s
2
Bk 97 5f
9
7s
2
Dy 66 4f
10
6s
2
Cf 98 5f
10
7s
2
Ho 67 4f
11
6s
2
Es 99 5f
11
7s
2
Er 68 4f
12
6s
2
Fm 100 5f
12
7s
2
Tm 69 4f
13
6s
2
Md 101 5f
13
7s
2
Yb 70 4f
14
6s
2
No 102 5f
14
7s
2
Lu 71 4f
14
5d
1
6s
2
Lr 103 5f
14
6d
1
7s
2
effective exploitation of these properties. It is, therefore, desirable to understand the
various oxidation states of actinides in solution.
1.5.3.1. Oxidation States
The trivalent oxidation state is the most stable for all lanthanides. However, this is
not so at least in the case of earlier members of actinide series. The 5f electrons of
actinides are subjected to a lesser attraction from the nuclear charge than the
corresponding 4f electrons of lanthanides. The greater stability of tetra positive ions
of early actinides is attributed to the smaller values of fourth ionization potential for
5f electrons compared to 4f electrons of lanthanides, an effect which has been
observed experimentally in the case of Th and Ce [14]. Thus, thorium exists in
aqueous phase only as Th(IV) while the oxidation state 3+becomes dominant only
Chapter I
11
Table 1.3: Oxidation states
*
of actinide elements
89 90 91 92 93 94 95 96 97 98 99 100 101 102 103
Ac Th Pa U Np Pu Am Cm Bk Cf Es Fm Md No Lr
(2) (2) 2 2
3 (3) (3) 3 3 3 3 3 3 3 3 3 3 3 3
4 4 4 4 4 4 4 4
5 5 5 5 5
6 6 6 6
7 7
* Those underlined are the most stable oxidation states in aqueous solution; those in
parentheses refer to oxidation states which are not known in solutions.
for transplutonium elements. The actinides existing in different oxidation states are
shown in Table 1.3, where the most stable oxidation states are under lined [13]. All
the oxidation states are well known except 7+states for Np and Pu which exist in
alkaline medium[15]. Penta and hexavalent actinide ions exist in acid solution as
oxygenated cations, viz. MO
2
+
and MO
2
2+
.
Fig. 1.5. Redox potential of actinide ions in 1M HClO
4
(Volts)
Chapter I
12
The redox potential diagrams of early actinides such as Th, U, Np and Pu at
25C in 1M HClO
4
are shown in Fig. 1.5 [16,17]. It has been found that the M
3+
/M
4+
and MO
2
+
/MO
2
2+
couples are reversible and fast as they involve the transfer of only
single electron. On the other hand, the other couples are irreversible and achieve
equilibrium slowly as they involve the formation or rupture of metal oxygen bonds.
1.5.3.2. Disproportionation Reactions
Disproportionation reaction is referred to as self oxidation reduction reaction. For
disproportionation reaction to occur an element must have at least three oxidation
states and these ions must be able to co-exist in solutions, which depend on the
closeness of the electrode potentials of redox couples involved. In case of Pu these
values are so close that the four oxidation states, viz. III, IV, V and VI are in
equilibrium with each other. The disproportionation reactions of U, Pu, Np and Am
have been well studied [13] and their equilibrium constant (logK) values are given in
Table 1.4. In general, disproportionation reactions of MO
2
+
(M=U, Pu or Np) ions
can be represented as follows,
2MO
2
+
+ 4H
+
M
4+
+MO
2
2+
+H
2
O (1.1)
Table 1.4: Disproportionation reactions of actinides in aqueous solutions
Element Oxidation Numbers Reaction logK
(25C)
U V =IV +VI 2UO
2
+
+4H
+
U
4+
+UO
2
2+
+2H
2
O 9.30
Np V =IV +VI 2NpO
2
+
+4H
+
Np
4+
+NpO
2
2+
+2H
2
O -6.72
Pu V =IV +VI 2PuO
2
+
+4H
+
Pu
4+
+PuO
2
2+
+2H
2
O 4.29
V =III +VI 3PuO
2
+
+4H
+
Pu
3+
+2PuO
2
2+
+2H
2
O 5.40
IV +V =III +VI Pu
4+
+PuO
2
+
Pu
3+
+PuO
2
2+
1.11
IV =III +VI 3Pu
4+
+2H
2
O 2Pu
3+
+PuO
2
2+
+4H
+
-2.08
Am IV +V =III +VI Am
4+
+AmO
2
+
Am
3+
+AmO
2
2+
12.5
IV =III +VI 3Am
4+
+2H
2
O 2Am
3+
+AmO
2
2+
+4H
+
32.5
IV =III +V 2Am
4+
+2H
2
O Am
3+
+AmO
2
+
+4H
+
19.5
Chapter I
13
It is clearly demonstrated from the equilibrium reaction (1.1) that the presence of
hydrogen ion and complexing ions like F
-
and SO
4
2-
, which complex strongly with
M
4+
and MO
2
2+
ions, have pronounced effect on disproportionation reactions.
1.5.3.3. Hydrolysis and Polymerization
In view of their large ionic potential, the actinide ions in various oxidation states
exist strongly as hydrated ions in the absence of complexing ions. The actinide ions
in divalent to tetravalent oxidation states are present as M
2+
, M
3+
and M
4+
,
respectively. The penta and hexavalent oxidation states are prone to more hydrolysis
as compared to lower oxidation states. These oxidation states exist as partially
hydrolyzed actinyl ions, viz. MO
2
+
and MO
2
2+
and can get further hydrolyzed under
high pH condition. The oxygen atoms of these ions are not basic in nature and thus
do not co-ordinate with protons. The degree of hydrolysis for actinide ions decreases
in the order: M
4+
>MO
2
2+
>M
3+
>MO
2
+
which is similar to their complex formation
properties [18]. In general the hydrolysis of the actinide ions can be represented as
follows,
M
n+
+ xH
2
O M(H
2
O)
x
n+
M(OH)
x
(n-x)+
+ xH
+
(1.2)
The hydrolysis behaviour of Th(IV) is quite different from those of other tetravalent
actinide ions [19]. For U(IV) and Pu(IV) the metal ion hydrolyses first in a simple
monomeric reaction (Eq. 1.2) followed by a slow irreversible polymerization of
hydrolyzed products. For Th(IV), however, various polymeric species exist even in
very dilute solutions. Whereas the polymer formation of Pu(IV) is irreversible, that
of Th(IV) is reversible. The hydrolysis of some of the trivalent actinides such as
Am(III), Cm(III) and Cf(III) is well studied which revealed the higher hydrolysis
constant values for trivalent actinides as compared to their lanthanides analogues
[13].
Though the polynuclear species of all actinide ions are of great interest, the
polymers of Pu(IV) have attracted particular attention because of practical
considerations. Pu(IV) polymers with varying molecular weights ranging from a few
thousand to as high as 10
10
have been observed [20]. In dilute HNO
3
or HCl
solutions, Pu(IV) polymer exists as a bright green colour with a characteristic
spectrum different from that of monomeric Pu(IV) in these solutions. The rate of
Chapter I
14
polymerization depends on acidity, temperature, Pu(IV) concentration as well as the
nature of ions present in the solution [21,22]. Polymerization rate for Pu(IV) is higher
when the ratio of acid to Pu(IV) concentration is low. Thus, Pu(IV) polymerization
can occur even at higher acidities if Pu(IV) concentration is raised. Depolymerization
of Pu(IV) is best accomplished by heating the Pu solution in 610M HNO
3
. Strong
complexing agents such as fluoride and sulphate ions as well as oxidizing agents
such as permanganate and dichromate promote depolymerization of plutonium.
1.5.3.4. Complexation of Actinides
The actinide ions in the aqueous solutions exhibit strong tendency to form
complexes. This property of actinides is widely exploited in devising methods for
their separation and purification. One of the most important factors that determines
the strength of the complex formed is the ionic potential (or charge density) of the
metal ions, which is the ratio of ionic charge to ionic radius. Higher the ionic
potential greater the electrostatic attraction between cations and anions and hence
stronger is the complex formed. The complexing strength of actinide ions in different
oxidation states follows the order: M
4+
>MO
2
2+
>M
3+
>MO
2
+
. Similarly, for the
given metal ions of same oxidation state, the complexing ability increases with the
atomic number due to increase in the ionic potential as a result of actinide contraction
[13]. However, the above generalized statement may be valid when complexation is
primarily ionic in nature. There are large number of instances where hybridization
involving 5f orbitals, steric effects and hydration of metal ions affect the tendency of
complexation. For anions the tendency to form complex with the given actinide ion
generally vary in the same manner as their abilities to bind with hydrogen ion [23].
For monovalent ligands the complexing tendency decreases in the order: F
-
>
CH
3
COO
-
>SCN
-
>NO
3
-
>Cl
-
>Br
-
>I
-
>ClO
4
-
. The divalent anions usually from
stronger complexes than the monovalent anions and their complexing ability
decreases in the order: CO
3
2-
>SO
3
2-
>C
2
O
4
2-
>SO
4
2-
. The complexing ability of
some of the organic ligands with Th(IV) varies as: EDTA >Citrate >Oxalate >
HIBA >Lactate >Acetate.
While discussing the stability of complexes between metal ions and ligands,
Pearson [24] proposed a scheme based on the concept of hard and soft acids and
bases. Those metal ions are called hard which have a small radius and high charge
Chapter I
15
and do not possess valence shell electrons that are easily distorted. The soft metal
ions have the opposite characteristics. When similar classification is applied to the
ligands it is observed that the hard metal ions form stronger complexes with hard
ligands and soft metal ions with soft ligands. Actinide ions behave as hard acids
and interact strongly with hard bases such as O or F rather than soft ligands like
N, S or P donors. However, as compared to lanthanides they show marked
preference for the soft donors which is commonly referred as covalent character due
to the f-orbital participation. The complex formation reactions involving hard acids
and bases are endothermic whereas the reverse is true for soft ions. This is because
the complex formation between hard metal ions and hard ligands require the breaking
of strong bonds between these metal ions and water molecules in the primary
hydration sphere which require large energy. The process of removal of water
molecules, however, results in large increase in entropy which contributes to the
driving force of these reactions [13]. When the primary hydration shell is broken
during complex formation, the complex formed is referred as inner sphere
complex. In contrast outer sphere complexes do not require breaking of the
primary hydration shell. The actinide ions interact with soft bases in organic solvents
of low solvating power, but not in aqueous solutions where the soft bases would have
to replace inner sphere water molecule which is a hard base. Thus, depending upon
the nature of ligand and medium actinide cations form inner or outer sphere
complexes.
1.5.3.5. Absorption Spectra
Similar to transition metal ions, the actinide ions display a rich variety of colours in
their aqueous solutions. The absorption spectra of actinides arise due to the electronic
transitions and absorption bands appear mainly from three types of transitions, viz. i)
f-f- transition, ii) f-d transition, and iii) charge transfer bands [13]. In f-f transitions,
the electronic transition occurs between the two 5f-5f orbitals of different angular
momentum. As the transitions occur between the orbitals of the same sub-shell they
are generally Laporate forbidden. The probabilities of transitions are, therefore, low
and the absorption bands are consequently low in intensity. However, the bands are
sharp because the transitions take place in the inner shell and are, therefore, not
affected much by the surrounding environment. The energy differences between the
Chapter I
16
various energy levels are of such an order of magnitude that the bands due to 5f-5f
transitions appear in UV, visible and near IR regions. The molar absorption
coefficient is in the range of 1050 M
-1
cm
-1
. On the other hand, in case of f-d
transitions the absorption bands are broad as these transitions are influenced by the
surrounding environment. As transitions take place between the orbitals of different
azimuthal quantum number they are Laporate allowed and, therefore, these bands are
relatively more intense. The molar absorption coefficient is of the order of ~10000
M
-1
cm
-1
. These bands appear invariably in the UV region due to large energy
differences between the d and f orbitals. In case of charge transfer transitions, the
absorption bands occur due to the transition between 5f orbitals of actinide ions and
ligand orbitals. Therefore, the nature of ligand plays an important role. These
transitions are significantly affected by the surrounding environment. As a
consequence, the charge transfer bands are broad. The absorption bands appear in the
UV region and are generally less intense than those resulting from f-d transitions.
The absorption spectra of actinide ions have been widely used in the
analytical chemistry. The absorption spectra of actinide ions in different oxidation
states differ widely, which have been successfully exploited for the quantitative
analysis of their mixtures present in different oxidation states. The absorption bands
of actinide ions have also been used for studying the redox reactions. Though the
transitions in actinide ions take place in an inner shell resulting in sharp bands,
complexing of metal ions can strongly affect the position as well as the intensities of
the individual absorption bands. Therefore, change in absorption spectra due to the
presence of ligands have often been used to establish complex formation, and in
some cases, even for the calculation of their stability constants. The complexes of
some of the actinides formed with many organic and inorganic ligands have very
high absorption in visible region. This property has been fruitfully exploited to
develop sensitive analytical methods for the detection and estimation of actinide ions.
1.6. SEPARATION OF METAL IONS
The scientific principles that govern the separation of metal ions from solutions are
chemical reaction equilibrium kinetics, fluid mechanics and mass transfer from one
phase to another. The theory of separation utilizes these principles in different
processes including solvent extraction, extraction chromatography as well as in
Chapter I
17
membrane processes. Amongst these techniques, solvent extraction is the most
versatile technique and is extensively used for separation, preparation, purification,
enrichment and analysis on micro scale to large industrial processes.
Solvent or liquid-liquid extraction is based on the principle that a solute can
distribute itself in a certain ratio between the two immiscible solvents, one of which
is usually water and the other is an organic solvent. In certain cases the solute can be
more or less completely transferred into the organic phase. The liquidliquid
distribution systems can be thermodynamically explained with the help of phase rule
[25]. Phase rule is usually stated as,
P +V =C +2 (1.3)
where P, V and C denote the number of phases, variances and components,
respectively. In general, a binary liquid-liquid distribution system has two phases (P
=2) and contains three or more components (two solvents and one or more solutes).
When a system contains only one solute (C =3), according to the phase rule the
variance is three, which means by keeping any two variables constant the system can
be defined by the third variable. In other words, at fixed temperature and pressure,
the concentration of solute in the organic phase is dependent on the concentration of
solute in the aqueous phase. Thus, when molecular species of the solute is same in
the two phases, its concentration in one phase is related to that in the other phase (the
distribution law). Consider following equilibrium reaction,
M
(aq.)
M
(org.)
(1.4)
where the subscripts (aq.) and (org.) represent aqueous and organic phases,
respectively. According to the distribution law, the distribution coefficient (k
d
) is
represented as,
[Mn]
(org.)
k
d
= ------------------ (1.5)
[Mn]
(aq.)
However, it has been observed that, in most cases, the molecular species of metal
ions are not the same in both the phases. Therefore, the term distribution ratio (D
M
)
is used in the solvent extraction which is defined as the ratio of the total
concentration of metal ions (in all forms) in the organic phase to that of in the
aqueous phase.
Chapter I
18
The solubility of charged metal ions in the organic solvents are very less as
they tend to remain in the aqueous phase due to ion-dipole interaction. For the
extraction of metal ions in the organic phase, the charge on the metal ions must be
neutralized so as to enhance the solubility in non-polar organic solvents. Therefore, a
suitable extractant (ligand) molecule is generally added in the organic solvent which
upon complexation with metal ions forms neutral hydrophobic species which is then
extracted in the organic phase. In such cases, the extraction of metal ions may follow
one of the following extraction mechanisms. (i) Solvation: The extraction of metal
ions by neutral ligands are followed by solvation mechanism. The extraction process
proceeds via replacement of water molecules from the co-ordination sphere of metal
ions by basic donor atoms such as O or N of the ligand molecules. The well
known example is the extraction of U(VI) by tri-n-butyl phosphate (TBP) from nitric
acid medium [26]. (ii) Chelation: The extraction of metal ions proceeds via the
formation of metal chelates with chelating ligands. The example of this type is the
extraction of Pu(IV) by thenoyltrifluoroacetone (HTTA) in benzene [27]. (iii) Ion
pair extraction: This type of extraction proceeds with the formation of neutral ion-
pair species between the metal ions and ionic organic ligands. Acidic ligands such as
sulphonic acids, carboxylic acids and organophosphoric acids provide anions by
liberating protons which then complexed with the metal cation to form ion-pair. On
the other hand, basic ligands provide cations which complex with aqueous anion
metal complex to form ion-pair. The best examples of basic extractants are
quaternary ammonium salts. (iv) Synergistic extraction: Synergism refers to the
phenomenon where the extraction of metal ions in the presence of two or more
extractants is more than that expected from the sum of extraction employing
individual extractants. Well known example of synergistic extraction is the extraction
of Pu(IV) from nitric acid medium by a mixture of HTTA and tri-n-octyl phosphine
oxide (TOPO) in benzene [28].
1.7. CRITERIA FOR SELECTION OF EXTRACTANTS
A number of factors are taken into consideration while selecting or designing a
particular extractant for the separation of metal ions for industrial applications [29].
Some of the important considerations are listed as follows,
i) High solubility in paraffinic solvents (non-polar solvents),
Chapter I
19
ii) Low solubility in the aqueous phase,
iii) Non-volatility, non-toxicity and non-inflammability,
iv) High complexation ability with the metal ions of interest,
v) High solubility of the metal-ligand complex in the organic phase, i.e. high
metal loading capacity in the organic phase,
vi) Ease of stripping of metal ions from the organic phase,
vii) Reasonably high selectivity for the metal ion of interest over the other metal
ions present in the aqueous solution,
viii) Optimum viscosity for ease of flow and optimum inter facial tension (IFT) to
enable a faster rate of phase disengagement,
ix) Ease of regeneration of the extractant for recycling,
x) High resistance to radiolytic and chemical degradation during operation, and
xi) Ease of synthesis / availability at a reasonable cost.
1.8. REPROCESSING OF SPENT FUEL
The fuel after use in the reactor is referred to as spent fuel. The spent fuel contains
man made fissile materials such as
239
Pu along with minor actinides and fission
products. Reprocessing of the spent fuel is important for the recovery of valuable
fissile materials to sustain the future nuclear energy programme. During reprocessing
of the spent fuel the valuable uranium and plutonium are recovered in the
hydrometallurgical process leaving behind highly radioactive liquid waste solution
(HLW). A brief mention about the reprocessing of the spent fuel by PUREX process
is presented here.
1.8.1. PUREX Process
The Plutonium Uranium Reduction Extraction (PUREX) process is employed for
reprocessing of the spent nuclear fuel throughout the world [30]. It involves
contacting a nitric acid solution of dissolved irradiated fuel with an organic solution
of tri-n-butyl phosphate (TBP) in a hydrocarbon diluent such as odourless kerosene
or n-dodecane. Typically, the TBP concentration is about 30% though the
concentration may be varied to effect a specific separation. The PUREX process is
based on the fact that TBP selectively extracts hexavalent uranium and tetravalent
plutonium over other actinides and fission products from moderately concentrated
Chapter I
20
(~3M) nitric acid solutions. By adjusting the valency of plutonium from tetravalent to
trivalent it may be partitioned from the organic phase to the aqueous solution, thus
providing an effective mean of separating plutonium from uranium. If the TBP
solution of extracted actinides is subsequently contacted with dilute (~0.1M) nitric
acid, the U(VI) may be easily back extracted (stripped) into the aqueous phase.
Though the PUREX process applied for reprocessing of the spent fuel
removes all uranium and plutonium, it rejects trivalent (Am and Cm) and pentavalent
(Np) actinides along with the fission products towards the aqueous raffinate. The
challenges for the disposal of aqueous raffinate generated during the PUREX process
(HLW) is largely due to the radiotoxicity associated with the transuranic actinides.
Thus, there is a need for the subsequent treatment of the aqueous raffinate to remove
all transuranic actinides before its disposal. Though PUREX process does not remove
all the actinides to the level necessary for their disposal (the process preferentially
recovers major actinides (U/Pu) present at ton/Kg scale leaving behind minor
actinides (Am/Cm/Np) present at mg/gm scale), it could provide a suitable clean feed
stream for the subsequent more efficient process of actinide partitioning [6].
1.9. ACTINIDE PARTITIONING
The selective extraction of trivalent actinides, namely Am(III) and Cm(III) present in
the HLW resulting from the reprocessing of the spent fuel is influenced by the
presence of trivalent lanthanides. The trivalent lanthanides have almost similar
chemical properties to those of trivalent actinides and have several times higher
concentration than the later, which represent about 1/3 of the total mass of the fission
products. So owing to the complexity of the selective removal of trivalent actinides,
the separation process can be split into two steps. The first step consists of co-
extraction of An(III) and Ln(III) aiming to eliminate all the alpha activities and 1/3 of
the fission products. The second step consists of the group separation of Ln(III) and
An(III) by several processes including Selective Actinide Extraction (SANEX)
process. During the last two decades, concerted research conducted around the world
has identified a number of promising extractants for actinide partitioning. The
performance and status of some of these extraction processes are briefed here.
Chapter I
21
Fig. 1.6. Structural formulae of some of the proposed extractants for actinide partitioning
1.9.1. TRUEX Process
The Trans Uranium Extraction (TRUEX) is a solvent extraction process designed to
separate transuranic elements from various types of high level waste solutions. The
key ingredient in this process is a phosphine oxide based extractant, viz.
octyl(phenyl)-N,N-diisobutyl carbamoyl methyl phosphine oxide (CMPO, Fig.
1.6(a)). Among several derivatives of phosphine oxide extractants, CMPO was found
(a) CMPO
(d) DMDBTDMA
(b) TRPO
(R: n-octyl and n-hexyl)
(e) Tetra alkyl diglycolamide
(c) DIDPA
N
C
C
H
2
O
CH
2
CH
2
P
C
8
H
17
O
CH
CH
H
3
C
H
3
C
H
3
C
H
3
C
P
O
R
1
R
3
R
2
P
O
O
OH
O
H
2
C
C
H
2
H
2
C
C
H
2
H
2
C
C
H
2
CH
CH
3
H
3
C
CH
2
H
2
C
CH
2
C
H
2
H
2
C
C
H
2
H
2
C
CH
CH
3
H
3
C
C
H
2
CH
C
C
N
N
O
O
C
4
H
9
CH
3
H
3
C
C
4
H
9
C
14
H
29
C
C
H
2
O
N
O
R
3
R
4
C
H
2
C
N
R
1
R
2
O
Chapter I
22
to possess the best combination of properties for actinide partitioning in a PUREX
compatible diluent system [31]. The TRUEX extractant is usually 0.2M CMPO +
1.2M TBP (used as a phase modifier) in paraffinic hydrocarbon like n-dodecane [32].
In TRUEX solvent, TBP suppresses third phase formation, contributes to better acid
dependencies for D
Am
, improves phase compatibility, and reduces hydrolytic and
radiolytic degradation of CMPO [33]. High distribution ratio of tri-, tetra- and
hexavalent actinides from solutions of moderate acid concentration and good
selectivity over fission products is the key feature of this extractant. Lanthanides such
as Eu, Ce and Pr behave similar to the trivalent actinides, viz. Am(III). Other fission
products, except Zr, show relatively small distribution values. Zirconium is also
extractable with TRUEX solvent; however, its extraction may be suppressed by the
addition of oxalic acid. From the process perspective, the insensitivity of distribution
values of actinides between 1M and 6M HNO
3
is important as it allows efficient
extraction of these ions from waste with little or no adjustment of feed acidity.
Due to high extraction of tetra- and hexavalent actinides such as Pu(IV) and
U(VI) by CMPO in a wide range of acidity the stripping of these metal ions with
dilute nitric acid is difficult. Therefore, more aggressive stripping, for example with
powerful diphosphonate actinides extractants, is required. Generally, 1-
hydroxyethylene-1,1-diphosphonic acid (HEDPA) is used for stripping of Am, Pu
and U from loaded organic phase. The oxidation state specific stripping of actinide
ions from loaded TRUEX solvent can be achieved in three steps: 0.04M HNO
3
to
remove trivalent actinides, dilute oxalic acid for selective stripping of tetravalent
actinides, and finally 0.25M Na
2
CO
3
for uranium recovery. A mixture of formic acid,
hydrazine hydrate and citric acid has shown promise for efficient stripping of Am
and Pu from TRUEX solvent loaded with HLW in both batch as well as counter
current modes [34,35].
Though CMPO shows high extraction efficiency and is a promising reagent
for the separation of actinides, TRUEX process exhibits certain limitations. Stripping
of trivalent actinides is cumbersome and requires several stages of contact with
0.04M HNO
3
. Degradation products of CMPO can also inhibit the stripping of Pu
and U. The presence of acidic extractants as degradation products increases the D
Am
values under stripping conditions. Such impurities must be removed from the used
Chapter I
23
TRUEX solvent prior to their recycling. More stringent stripping condition of metal
ions from the loaded organic phase is the major draw back of the TRUEX process.
1.9.2. TRPO Process
Trialkyl Phosphine Oxide (TRPO) process utilizes a mixture of four alkyl phosphine
oxides (Fig. 1.6(b)) as the extractant. The TRPO solvent has been tested for the
extraction of actinides, lanthanides and other fission products from HNO
3
and HLW
solutions [36,37]. It was observed that >99% of U(VI), Np(IV), Np(VI) and Pu(IV)
were extracted from 0.21M HNO
3
through a single extraction with 30% (v/v) TRPO
in kerosene [38]. Also >95% of Pu(III), Am(III) and Ln(III) could be extracted, while
fission products such as Cs, Sr, Ru were not extracted. Trivalent lanthanides and
actinides are generally stripped with 5M HNO
3
. On the other hand, tetravalent (Np
and Pu) and hexavalent (U) actinides are stripped with 0.5M oxalic acid and 5%
Na
2
CO
3
, respectively.
Though TRPO, with its relatively low cost and its high extraction efficiency,
is a promising extractant for actinide partitioning the process, however, it has certain
limitations. The TRPO process works only at relatively low acidity (0.1-1M HNO
3
)
and, therefore, the HLW solution (HLW is generally at ~3M HNO
3
) has to be diluted
several times to adjust the feed acidity. Poor stripping of actinide ions is also a
disadvantage of the TRPO process.
1.9.3. DIDPA Process
The extraction behaviour of actinides and other fission products with di-isodecyl
phosphoric acid (DIDPA, Fig. 1.6(c)) has been studied by Morita et al., at Japan
Atomic Energy Research Institute (JAERI). It has been shown that DIDPA can
simultaneously extract Am(III), Cm(III), U(VI), Pu(IV) and even Np(V) from a
solution of low acidity such as 0.5M HNO
3
[39,40]. The trivalent cations can be
separated from their tetravalent counterparts by appropriate back-extraction
procedures. The back extraction of trivalent actinides and lanthanides can be
achieved by 4M HNO
3
. On the other hand, tetravalent Np and Pu and hexavalent
uranium can be stripped by 0.8M oxalic acid and 1.5M Na
2
CO
3
solution,
respectively. For the partitioning of transuranic elements a mixture of 0.5M DIDPA
+0.1M TBP in dodecane has been proposed.
Chapter I
24
The major drawback of DIDPA process is the re-adjustment of the acidity of
HLW to about 0.5M HNO
3
prior to the processing. In this process, the reduction of
acidity and denitration is accomplished using formic acid. At such a low acidity,
molybdenum and zirconium form precipitates which carries about 90% of plutonium.
1.9.4. DIAMEX Process
Diamide extraction (DIAMEX) process was developed in France for the extraction of
transuranic elements from the HLW solutions. One of the major drawbacks of using
organophosphorus extractants is the solid residue that results upon their incineration
at the end of their useful life. French researchers utilized the CHON (carbon,
hydrogen, oxygen and nitrogen) principle for designing of the extractants, which can
be completely incinerated into gaseous products, thereby minimizing the generation
of solid secondary wastes at the end of the process.
Among the numerous diamides synthesized and tested for the extraction of
actinides, N,N-dimethyl-N,N-dibutyl tetradecyl malonamide (DMDBTDMA, Fig.
1.6(d)) has shown the greatest promise [41-46]. In France, this reagent is extensively
evaluated for actinide partitioning from HLW solution. DMDBTDMA dissolved in
dodecane does not give any third phase when contacted with 3-4M HNO
3
and hence
discourage the use of any phase modifier. Generally, 1M DMDBTDMA has been
proposed for actinide partitioning which gives D
Am
value of ~10 at 3M HNO
3
[45].
Zirconium(IV) is strongly extracted by DMDBTDMA, however, its extraction can be
suppressed to an acceptable level by complexing it with oxalic acid. Extraction of
molybdenum can be suppressed by complexation with hydrogen peroxide. Iron,
which is almost always present in HLW from corrosion of the process equipments,
also has high affinity for DMDBTDMA. However, the extraction kinetics for Fe(III)
is slow and it may be separated from actinides and lanthanides by the judicial choice
of contact time for their extraction [45].
Recently, a new diamide, viz. N,N-dimethyl-N,N-dioctyl-2-(2-hexylethoxy)
malonamide (DMDOHEMA) has been reported as a substitute of DMDBTDMA for
DIAMEX solvent [47]. Amongst several extractants described for actinide
partitioning, diamides have been found to be particularly promising in view of their
improved back extraction properties for Am(III)/Cm(III), their complete
incinerability, and the innocuous nature of their radiolytic and hydrolytic products
Chapter I
25
(mainly carboxylic acids and amines) that can be easily washed out. However, the
major draw back of DMDBTDMA is that it shows only moderate extraction of
trivalent actinides (Am and Cm) from HLW at acidity 3M HNO
3
[46]. Therefore, it
necessitates the structural modification of diamides so as to enhance the extraction
efficiency of trivalent actinides in particular.
1.10. DIGYLYCOLAMIDES: A Class of Promising Extractants for
Actinide Partitioning
The performance of some of the extraction processes developed for actinide
partitioning is briefly discussed in the earlier section. However, each of the described
processes has certain limitations. The main drawbacks of the TRUEX process are; (a)
the poor back extraction of Am(III) and Cm(III) at reduced acidity, and (b)
interference due to solvent degradation products. On the other hand, the TRPO
process works only at relatively low acidity (0.1-1M HNO
3
) and, therefore, cannot
applied at 3-4M HNO
3
conditions which is generally encountered in HLW. Similarly,
DIDPA process cannot be applied to the concentrated HLW solution without
denitration which leads to the precipitation of actinides. Though the completely
incinerable DMDBTDMA has been reported to be a promising candidate, it shows
only moderate extraction for Am(III)/Cm(III) from HLW at acidity 3M HNO
3
. In
order to improve the efficiency of diamides towards the forward extraction of
trivalent actinides, several structural modifications have been attempted. Recently, a
series of diamide compounds have been synthesized by introducing different
substituents on amide nitrogen or introducing an ether oxygen into the bridging chain
of malonamide [48]. It has been observed that the introduction of etheric oxygen
between the two amide groups (diglycolamide, Fig. 1.6(e)) causes significant
enhancement in the extraction of trivalent actinides / lanthanides.
The work on diglycolamides was started after Stephan et al., reported the
extraction of various metal ions with multidentate amido podands [49,50]. Sasaki and
Choppin were probably the first to report the extraction of lanthanides and actinides
with diglycolamides [51-55]. They used dimethyl dihexyl diglycolamide and its
analogous compounds for the solvent extraction studies on lanthanides and actinides.
These preliminary studies, however, were focused on the extraction of metal ions
from aqueous solutions of pH ranging from 1 to 4. Narita et al., studied the extraction
Chapter I
26
of lanthanides from acidic solutions employing dimethyl diphenyl diglycolamide
[56]. They proved by XRD and EXAFS studies that diglycolamide form tridentate
complex with lanthanides in solid as well as in solution [57].
Sasaki et al. [58] synthesized a series of diglycolamides having same central
frame with different alkyl chains (ranging from n-propyl to n-dodecyl) attached to
amidic nitrogen atoms (Fig. 1.6(e)). Various properties of the synthesized
diglycolamide derivatives are represented in Table 1.5. They found that the
diglycolamide derivatives with lower alkyl chain (propyl and butyl) were not soluble
in paraffinic solvents like n-dodecane due to presence of three polar oxygen atoms.
Though the higher homologues of diglycolamide were freely soluble in dodecane, the
distribution values for Am(III) were found to decrease due to the steric hindrance
during complexation of the bulky molecules. Amongst different derivatives
synthesized, tetraoctyl diglycolamide (TODGA) was demonstrated to be the best
candidate with reference to its free solubility in n-dodecane and significantly high
distribution values for trivalent actinides.
Table 1.5: Various properties of tetra- n-propyl, n-butyl, n-amyl, n-hexyl, n-
octyl, n-decyl and n-dodecyl diglycolamide
Diglycolamide
(DGA)
Solubility in water
[mM]
Solubility in
n-dodecane
D
Am
by 0.1M DGA
in n-dodecane
(1M HNO
3
)
TPDGA 57.0 Very poor --
TBDGA 2.3 poor --
TADGA 0.27 Soluble 100
THDGA 0.11 Soluble 40
TODGA 0.042 Freely soluble 30
TDDGA 0.042 Freely soluble 18
TDDDGA 0.040 Freely soluble 11
Chapter I
27
1.10.1. Main Features of TODGA
TODGA exhibits the excellent properties required for an extractant which can be
exploited for the partitioning of radionuclides from HLW. Salient features of
TODGA are listed as follows,
i) High extraction performance for minor actinides from moderate acidic
aqueous solutions,
ii) Ease of stripping of minor actinides,
iii) High solubility in paraffinic solvents (freely soluble in n-dodecane),
iv) Low concentration of TODGA to be used (~0.1M),
v) Good radiolytic and hydrolytic stability,
vi) Possibilities of complete incineration as the constituent elements are C, H, N
and O, and
vii) Ease of synthesis.
1.11. SCOPE OF THE THESIS
Though pentaalkyl malonamides have been proposed as promising extractants over
phosphorus based ligands for the partitioning of actinides, they have certain
limitations largely due to the poor extraction of trivalent actinides from moderate
acidity (3-4M HNO
3
). It was, therefore, desirable to develop alternative extractants
with better performances. In this context TODGA has been identified as one of the
most powerful ligands being considered for the partitioning of trivalent lanthanides /
actinides from HLW. In the present thesis, effort has been made to explore the basic
chemistry of TODGA for the separation of various metal ions (actinides and fission
products). A detailed insight into the thermodynamics of extraction of actinide ions
has been discussed. The feasibility of use of TODGA in counter-current extraction
operations has been investigated. Similarly, selective transport studies on metal ions
through liquid membrane using TODGA as carrier has been described. Separation of
metal ions on litres scale has been carried out using a membrane cell contactor
employing hollow fibre module.
Solid phase extraction has been increasingly used for the separation of trace
as well as ultra trace amounts of metal ions from complex matrices employing
chelating polymers. The extraction chromatographic separation studies on metal ions
were performed by impregnation of TODGA on an inert solid support. The
Chapter I
28
possibility of TODGA impregnated resin to sorb trace concentrations of Am(III)
from nitric acid solutions containing relatively large amounts of Nd(III), U(VI),
Fe(III) has been investigated. As an alternate to the impregnated resin, grafted
polymer was synthesized by chemical anchoring of malonamide ligand on a
polymeric solid support. Chemical anchoring is free from ligand leaching problem
which is generally encountered in the impregnated resin. Various physico-chemical
properties of the malonamide grafted polymer, like phase adsorption kinetics, metal
sorption mechanism, metal sorption capacity as well as the sorption behaviour of
actinides have been described in this thesis.
In conclusion, the work reported in this thesis has relevance to the separation
chemistry involving the partitioning of actinides and fission products from various
radioactive waste solutions. Basic as well as applied aspects of the extraction /
sorption behaviour of various metal ions have been explored in the present thesis.
1.12. REFERENCES
1. J. F. Ahearne, Special issue: Radioactive wastes, Physics Today, 50 (1997) 22.
2. J.V. Holder, Radiochim. Acta, 25 (1978) 171.
3. W.E. Kastenberg and L.J. Gratton, Hazardous of managing and disposing of
nuclear wastes, Physics Today, 50 (1997) 41.
4. K.D. Crowley, Nuclear waste disposal: The technical challenges, Physics Today,
50 (1997) 32.
5. Charles McCombie, Nuclear waste management world wide, Physics Today, 50
(1997) 56.
6. N.N. Egorov, M.A. Zakharov, C.N. Lazarev, R.I. Lyubtsev, A.S. Nikiforov, M.V.
Strakhov and E.A. Filippov, New approaches to solving the management
problem of long lived radionuclides, In proceedings of the third international
conference on nuclear fuel reprocessing and waste management, RECOD-91,
Vol. 1, P. 357.
7. Actinide and Fission Product Partitioning and TransmutationStatus and
Assessment report, OECD/NEA, 1999.
8. L.H. Baestle, Burning of Actinides: A complementary waste management
option? IAEA Bulletin, 34 (1992) 32.
Chapter I
29
9. J.C. McLean, A.B. McLay and H.G. Smith, Proc. Roy. Soc. (London), 112
(1926) 76.
10. G.T. Seaborg, Chem. Eng. News, 23 (1945) 2190.
11. M.S. Milyukova, N.I. Gusev, I.G. Sentyurin and I.S. Skyarenko, Analytical
Chemistry of Plutonium, Israel Prog. for Sci. Translation, J erusalem (1967), P.
5.
12. M. Taube, Plutonium, Pergamon Press, Oxford (1964), P. 5.
13. S. Ahrland, J.O. Liljenzin and J. Rydberg, Solution Chemistry, In
Comprehensive Inorganic Chemistry, J.C. Bailar Jr., H.J. Emeleus, R. Nyhlom
and A.F.T. Dickenson (Eds.), Pergamon Press, Oxford (1973), Vol. 5, P. 465-
535.
14. B.B. Cunningham, Proc. of XVII international congress of pure and applied
chemistry, Butterworths, London. Vol. 1, P. 64.
15. V.I. Spitsyn, A.D. Gelman, N.N. Krot, M.P. Mefodiyeva, F.A. Zacharova, Y.A.
Komokov, V.P. Shilov and I.V. Smirnovam, J. Inorg. Nucl. Chem., 31 (1969)
2733.
16. W.M. Latinmer, Oxidation Potentials, 2
nd
Ed., Prentice Hall (1952), P. 299.
17. J.J. Katz and G.T. Seaborg, The chemistry of actinide elements, Willy
Publications (1957), P. 315.
18. K.A. Kraus, Proc. First U.N. Conference on Peaceful Uses of Atomic Energy, 7
(1956) 245.
19. M. Haissinsky, Nuclear chemistry and its applications (English ed.), Addison-
Wesley (1964), P. 233.
20. O.J. Wick, Plutonium Hand Book: A Guide to the Technology, Gordon and
Breach, New York (1967), Vol. 1, P.438.
21. R.H. Rainey, USAEC Report CF-59-12-95 (1959).
22. J.M. Cleveland, The chemistry of plutonium, Gordon and Breach, New York
(1970), P. 84.
23. A.D. Janes and G.R. Choppin, Actinides Rev., 1 (1969) 311.
24. R.G. Pearson, J. Am. Chem. Soc., 85 (1963) 3533.
25. T. Sekine and Y. Hasegawa (Eds.), Solvent Extraction Chemistry: Fundamentals
and Applications, Marcel Dekker, New York (1977), P. 60.
Chapter I
30
26. S.V. Bagawade, P.R. Vasudeva Rao, V.V. Ramakrishna and S.K. Patil, J. Inorg.
Nucl. Chem., 40 (1978) 1913.
27. A. Ramanujam, M.N. Nadkarni, V.V. Ramakrishna and S.K. Patil, J. Radioanal.
Chem., 42 (1978) 349.
28. S.K. Patil, V.V. Ramakrishna, P.K.S. Kartha and N.M. Gudi, Sep. Sci. Technol.,
15 (1980) 1459.
29. M. Benedict, T.H. Pigford and H.W. Levi, Nuclear Chemical Engineering, 2nd
Edition, McGraw Hill Book Company (1981), P. 172.
30. H.A.C. McKay, J.H. Miles and J.L. Swanson, Applications of Tributyl Phosphate
in Nuclear Fuel Reprocessing, In Science and Technology of Tributyl
Phosphate, W.W. Schulz, L.L. Burger, J.D. Navratil and K.P. Bender (Eds.),
CRC Press Inc., Boca Raton, Florida (1990), Vol. 3, P. 55.
31. B.M. Rapko, Synthesis, characterization and actinide extraction behaviour of
bridge modified carbamoyl methyl phosphonates and phosphine oxides, In
Separation of f-elements, K.L. Nash and G.R. Choppin (eds.), Plenum Press,
NY (1995), P. 99.
32. E.P. Horwitz, D.G. Kalina, G.F. Vandrift and W.W. Schulz, Solv. Extr. Ion
Exch., 3 (1985) 75.
33. W.W. Schultz and E.P. Horwitz, Sep. Sci. Technol., 23 (1988) 1191.
34. R.R. Chitnis, P.K. Wattal, A. Ramanujam, P.S. Dhami, V. Goparkrishnan, A.K.
Bauri and A. Banergy, J. Radioanal. Nucl. Chem., 240 (1999) 721.
35. R.R. Chitnis, P.K. Wattal, A. Ramanujam, P.S. Dhami, V. Goparkrishnan, A.K.
Bauri and A. Banergy, J. Radioanal. Nucl. Chem., 240 (1999) 727.
36. Y. Zhu, R. J io, S. Wang, S. Fan, B. Leu, H. Zheng, S. Zhou and S. Chen, An
extractant (TRPO) for the removal and recovery of actinides from high level
radioactive liquid waste, ISEC-1983, Denver, Colorado, USA.
37. Y. Zhu and R. J iao, Nucl. Technol., 108 (1994) 361.
38. R. J iao, S. Wang, S. Fan, B. Liu and Y. Zhu, J. Nucl. Radiochem., 7 (1985) 65.
39. Y. Morita and M. Kubota, J. Nucl. Sci. Technol., 24 (1987) 227.
40. Y. Morita, I. Yamaguchi, Y. Kondo, K. Shirahashi, I. Yamagishi, T. Fujirawa and
M. Kubota, Safety and environmental aspects of partitioning and transmutation of
actinides and fission products, IAEA-TECDOC-783, IAEA Vienna (1995), P. 93.
Chapter I
31
41. C. Cuillerdier, C. Musikas, P. Hoel, L. Nigond and X. Vitart, Sep. Sci. Technol.,
26 (1991) 1229.
42. C. Cuillerdier, C. Musikas and L. Nigond, Sep. Sci. Technol., 28 (1993) 155.
43. L. Nigond, C. Musikas and C. Cuillerdier, Solv. Extr. Ion Exch., 12 (1994) 261.
44. L. Nigond, C. Musikas and C. Cuillerdier, Solv. Extr. Ion Exch., 12 (1994) 297.
45. G.R. Mahajan, D.R. Prabhu, V.K. Manchanda and L.P. Badheka, Waste
Management, 18 (1998) 125.
46. L.B. Kumbhare, D.R. Prabhu, G.R. Mahajan, S. Sriram and V.K. Manchanda,
Nucl. Technol., 139 (2002) 253.
47. D.S. Purroy, P. Baron, B. Christiansen, J.P. Glatz, C. Madic, R. Malmbeck and
G. Modolo, Sep. Purif. Technol., 45 (2005) 157.
48. L. Spjuth, J.O. Liljenzin, M.J. Hudson, M.G.B. Drew, B.P. Iveson and C. Madic,
Solv. Extr. Ion Exch., 18 (2000) 1.
49. H. Stephan, K. Gloe, J. Bager and P. Muhl, Solv. Extr. Ion Exch., 9 (1991) 435.
50. H. Stephan, K. Gloe, J. Bager and P. Muhl, Solv. Extr. Ion Exch., 9 (1991) 459.
51. Y. Sasaki and G.R. Choppin, Anal. Sci., 12 (1996) 225.
52. Y. Sasaki and G.R. Choppin, J. Radioanal. Nucl. Chem., 207 (1997) 383.
53. Y. Sasaki, T. Adachi and G.R. Choppin, J. Alloy comp., 271-273 (1998) 799.
54. Y. Sasaki and G.R. Choppin, Radiochim. Acta, 80 (1998) 85.
55. Y. Sasaki and G.R. Choppin, J. Radioanal. Nucl. Chem., 246 (2000) 267.
56. H. Narita, T. Yaita, K. Tamura and S. Tachimori, Radiochim. Acta, 81 (1998)
223.
57. H. Narita, T. Yaita and S. Tachimori, Extraction Behavior for Trivalent
Lanthanides with Amides and EXAFS Study of their Complexes. In Solvent
Extraction for the 21
st
Century, Proceedings of ISEC99, Barcelona, Spain, July,
1999; M. Cox, M. Hidalgo, and M. Valiente (Eds.), Society of Chemical
Industry: London (2001), Vol. 1, P. 693.
58. Y. Sasaki, Y. Sugo, S. Suzuki and S. Tachimori, Solv. Extr. Ion Exch., 19 (2001)
91.
------------------------
Chapter II
Experimental
Chapter II
32
EXPERIMENTAL
In the present work, the extraction behaviour of various metal ions such as Am(III),
U(VI), Pu(IV), Np(IV), Tc(VII), Mo(VI), Zr(IV), Fe(III), Ru(III), Eu(III), Pd(II),
Sr(II) and Cs(I) has been investigated under different experimental conditions
employing N,N,N,N-tetraoctyl diglycolamide (TODGA) as the extractant. The
sorption behaviour of actinide ions on malonamide grafted polymer has also been
described. This chapter, therefore, deals with the synthesis and characterization of
TODGA as well as malonamide grafted polymer. The techniques used for the
separation studies of metal ions were solvent extraction, extraction chromatography
and liquid membrane. The details of various apparatus, materials, experimental
techniques as well as analytical techniques used in the present work are discussed in
this chapter.
2.1. SYNTHESIS OF N,N,N,N-TETRAOCTYL DIGLYCOLAMIDE
N,N,N,N-tetraoctyl diglycolamide (TODGA) was synthesized with slight
modifications to the method reported elsewhere [1]. In a reaction flask containing 1
mol of diglycolic anhydride (dissolved in 600mL of dichloromethane) connected
with moisture and oxygen free nitrogen gas stream, 1 mol of dioctyl amine dissolved
in ~300mL of dichloromethane was added dropwise with constant stirring
maintaining the temperature 0-5
o
C. After complete addition of dioctyl amine the
reaction mixture was stirred for 16hrs at room temperature. Subsequently to the
reaction mixture (at 0-5C), 1.3 mol of N,N-dicyclohexyl carbodiimide (DCC)
dissolved in 100mL dichloromethane was added dropwise with constant stirring. It
should be noted that ~30% extra DCC was added (DCC was used as a dehydrating
agent) as it is liable to decompose easily. After complete addition of DCC the
reaction mixture was stirred at the same temperature for 30min followed by addition
of the next batch of dioctyl amine (1 mol dissolved in dichloromethane) dropwise
with constant stirring and maintaining the temperature at 0-5C. After complete
addition of dioctyl amine, the reaction mixture was stirred for six days at room
temperature. The white precipitate of N,N-dicyclohexyl urea formed was discarded
after filtering and washing with excess of dichloromethane. The dichloromethane
Chapter II
33
H
2
C C
O
O
C
O
C
H
2
O
+
HN
C
8
H
17
C
8
H
17
Dig ly co lic Anhy dr ide
Dio ct yl ami ne
DCM
Sti rr a t RT
fo r 8 day s
N
C
8
H
1 7
C
8
H
17
C
O
C
H
2
O
C
H
2
C
O
OH
+
HN
C
8
H
17
C
8
H
17
0 - 5 C
16 hr s
N
C
8
H
1 7
C
8
H
1 7
C
O
C
H
2
O
C
H
2
C
O
N
C
8
H
1 7
C
8
H
1 7
T ODGA
Yie ld 70%
DCC
was then removed from the filtrate by rotavaporator to get the crude product. The
crude product thus obtained was purified by ethyl acetate when the insoluble
impurities were discarded. The other soluble volatile impurities were removed by
vacuum distillation (2.5mm) at 120-140C to get the product in the flask. The
product was further purified by silica gel column. The final pure product of TODGA
was obtained as a pale yellow viscous liquid with about 70% yield. The synthesis
scheme for TODGA is represented in Fig. 2.1.
Fig. 2.1. Synthesis scheme of TODGA
Chapter II
34
Table 2.1: PMR spectral data of TODGA
Functional groups No. of protons
#
Chemical shift (ppm)
-N- CH
2
CH
2
(CH
2
)
5
- CH
3
12 0.92 (t)
-N- CH
2
(CH
2
)
5
CH
2
- CH
3
40 1.26 (m)
-N- CH
2
(CH
2
)
5
CH
2
- CH
3
8 1.56 (m)
-N- CH
2
CH
2
(CH
2
)
5
- CH
3
8 3.18(t)
-CO-CH
2
- O- CH
2
CO- 4 4.39 (s)
#
s: singlet; t: triplet; m: multiplet
2.2. CHARACTERIZATION OF N,N,N,N-TETRAOCTYL DIGLYCOLAMIDE
The synthesized TODGA was characterized by PMR spectroscopy, FT-IR
spectroscopy as well as by elemental analysis. Fig. 2.2 represents the C, H, N & S
elemental analyzer used in the present work. In the PMR spectrum of TODGA the
signal peaks were multiplets at 1.26 and 1.56, triplets at 0.92 and 3.18 and singlet at
4.39 ppm. These peaks were assigned to the protons of different groups listed in
Table 2.1. Similarly, the analytical data of synthesized TODGA are represented in
Table 2.2. The appearance of characteristic vibrational frequency at 1640cm
-1
in FT-
IR spectrumdemonstrated the presence of carbonyl groups of diglycolamide. The
CHN elemental analysis of the synthesized product yielded values very close to that
of the theoretical expected values, suggesting the successful synthesis of TODGA.
Table 2.2: Analytical data of TODGA
Formula C
36
H
72
N
2
O
3
Product Yield ~ 70 %
C (%) 73.1 (74.5)
#
H (%) 12.8 (12.4)
#
N (%) 5.2 (4.8)
#
>C=O
1640cm
-1
#
Values in parentheses are the expected values
Chapter II
35
2.3. SYNTHESIS OF MALONAMIDE FUNCTIONALIZED POLYMER
For chemical anchoring of the malonamide ligand, chloromethylated polystyrene-
divinyl benzene polymer was reacted with N,N-dimethyl-N,N-dibutyl malonamide
(DMDBMA) in dried dimethyl formamide (DMF). In a reaction flask containing 30
mmol of NaH, connected with moisture and oxygen free nitrogen gas stream, 30
mmol of DMDBMA (dissolved in ~50mL DMF) was added dropwise at 0-5C. After
complete addition of DMDBMA, the reaction mixture was stirred at the same
temperature for 2hrs. Subsequently, 5g of vacuum dried chloromethylated polymer
was added and the solution was stirred for 1hr. Finally, the reaction mixture was
refluxed on an oil bath for 16hrs at 60C. The final product was purified from the
excess of reactants by washing with methanol and water. The resultant grafted
polymer was vacuum dried to constant weight. The synthesis scheme for anchoring
of the amide moiety on the polymeric matrix is represented in Fig. 2.3.
Fig. 2.2. Elemental analyzer
Chapter II
36
2.4. CHARACTERIZATION OF MALONAMIDE GRAFTED POLYMER
The solid state
13
C-NMR spectra of the functionalized and non- functionalized
polymer are shown in Fig. 2.4.
13
C solid-state NMR spectra of the chloromethylated
polystyrene-divinyl benzene polymer exhibited a resonance signal at 40.1ppm,
corresponding to the alkyl group (-CH
2
-Cl), was shifted to 48.4ppm in the
DMDBMA functionalized polymer due to the substitution of additional malonamide
moiety. The presence of lateral methyl (-CH
3
) group of the malonamide was
confirmed by the resonance signal at 14.2ppm. In addition to this, a broad resonance
signal of carbonyl carbon (>C=O) of malonamide in the grafted polymer was
observed at 169.5ppm, which was absent in the non-functionalized polymer,
suggesting the successful anchoring of the chelating ligand.
Fig. 2.3. Synthesis scheme for malonamide functionalized polymer
N
CH
3
C
4
H
9
C
CH
2
C
N
H
3
C C
4
H
9
+ N
C
4
H
9
H
3
C
C
C
H
C
N
CH
3
C
4
H
9
NaH
O
O
O
O
+
H
2
C
Cl
( ) n
DMDBMA
Chloromethylated polymer
H
2
C
CH
C
C
N
O
O
N
C
4
H
9
CH
3
H
3
C
C
4
H
9
Product
( ) n
DMF
Chapter II
37
200 150 100 50 0
145.6
135.3
128.7
40.1
ppm
Chloromethylat ed polymer
34.7
14.2
48.4 128.5
169.5
Malonamide graf ted polymer
Fig. 2.4. Solid state
13
C-NMR spectra of non- functionalized and malonamide functionalized
polymer
Fig. 2.5. FT-IR spectra of non- functionalized and malonamide functionalized polymer
3000 2500 2000 1500 1000 500
Mal onami de graft ed pol ymer
Chl orometyl at ed pol ymer
822
1136
573
1390
1645
1610
823
673
1443
1264
2930
2921
%
T
r
a
n
s
m
i
t
t
a
n
c
e
Wavenumber (cm
-1
)
Chapter II
38
The FT-IR spectra of the functionalized polymer exhibited enhanced
stretching vibrations between 30002800cm
-1
corresponding to =CH-, -CH
2
- and -
CH
3
groups. Appearance of characteristics band of carbonyl group (>C=O) at
1645cm
-1
, in addition to a band at 1390cm
-1
due to C-N stretching vibration,
suggested the presence of amide moiety on the grafted polymer. Similarly,
disappearance of the vibrational band at 673cm
-1
, which corresponds to CH
2
Cl
group in the non-functionalized polymer, confirmed the chemical modification of the
chloromethylated polymer. The FT-IR spectrum of the grafted and non-grafted
polymer is shown in Fig. 2.5. The elemental analysis of the functionalized polymer
yielded 6.58% of nitrogen against 7.23% of the expected value, suggesting >91%
anchoring of the malonamide moiety on the polymeric support. This nitrogen content
corresponded to 60%w/w loading of the malonamide ligand on the polymeric
support.
2.5. RADIOTRACERS (Preparation and Purification)
The separation studies on metal ions reported in the present thesis were carried out
with radiotracers. Procurement, preparation as well as purification of various
radiotracers is given below.
2.5.1. Uranium-233
233
U tracer (t
1/2
=1.59x10
5
years) was produced by irradiation of
232
Th followed by
its purification [2]. Purification of
233
U from the daughter / decay products of
232
U
was carried out from 6M HCl using anion exchange procedure [3]. The uranium in
6M HCl solution forms anionic complex which is held on the column, whereas
thorium and daughter products are not retained on the column. The column was
washed with excess of 6M HCl to remove any adsorbed impurity. Finally, the loaded
uranium was eluted with 0.01M HNO
3
and used as stock solution (at 0.5M HNO
3
).
The purity of
233
U tracer was ensured by alpha spectrometry.
2.5.2. Thorium-234
234
Th is a daughter product of
238
U and, therefore, it is separated from the natural
uranium [4]. Uranium solution at 8M HCl was loaded in 30% Aliquat 336 (tricapryl
methyl ammonium chloride) dissolved in chloroform. The organic phase was then
Chapter II
39
stored for about 6 months to allow the growth of
234
Th activity (t
1/2
=24.1days) when
a secular equilibrium with
238
U (t
1/2
=4.47x10
9
years) was attained.
234
Th thus
produced was stripped in the aqueous phase by contacting the uranium loaded
organic phase with 8M HCl. At this stage the stripped thorium is contaminated with
the parent uranium. Therefore, the tracer was further purified by preferential
extraction with 0.01M HPBI (3-phenyl-4-benzoyl-5-isooxazolone) in xylene at 0.5M
HNO
3
.
234
Th loaded organic phase was then scrubbed with 0.5M HNO
3
to remove
any trace of uranium. Finally, the pure
234
Th tracer was stripped with 8M HNO
3
and
used as stock. The purity of the purified product was confirmed by gamma
spectrometry.
2.5.3. Neptunium-239
239
Np (t
1/2
=2.35days) was prepared by irradiation of
238
U followed by purification by
anion exchange procedure [5]. After irradiation the target was dissolved in 8M HCl
when U and Np form anionic complex and retained on the anion exchange column.
The column was then washed with sufficient quantity of 8M HCl to remove any
impurities of fission products. Finally, Np was selectively eluted with a mixture of
4M HCl +0.05M HF. The eluted Np was then repeatedly dried in a Teflon crucible
with HClO
4
to remove the traces of HF. Finally, the tracer was dissolved in 3M
HNO
3
and used as stock of
239
Np. The radiochemical purity of the tracer was ensured
by gamma spectroscopy employing HPGe detector.
2.5.4. Iron-59
For various studies involving radiotracers, it is desirable to employ carrier free and
radiochemically pure tracers. However, in case of
59
Fe, the tracer is usually
contaminated with
60
Co. Since gamma energies of
59
Fe (1099 and 1292 keV) and
60
Co (1173 and 1333 keV) are very close to each other, it is often difficult to resolve
them using commonly used NaI(Tl) scintillation detector. In the present work,
59
Fe
was procured from Board of Radiation and Isotope Technology (BRIT), Mumbai and
was found to be contaminated with
60
Co. The
59
Fe tracer was, therefore, purified
prior to its use by the procedure developed in our laboratory [6]. The Fe(III) tracer
was quantitatively extracted by 30%TRPO in dodecane at 7M HNO
3
leaving behind
Co(II) in the aqueous phase. Fe(III) was then stripped with 0.2M oxalic acid after
Chapter II
40
scrubbing the organic phase with 7M HNO
3
to remove any traces of Co(II).
Subsequently, the oxalic acid from aqueous solution was destroyed by repeated
heating with a mixture of concentrated perchloric acid and concentrated nitric acid.
Finally, the tracer was obtained in nitric acid solution and its radiochemical purity
was ascertained by gamma spectrometry using HPGe detector.
2.5.5. Other Radiotracers
The radiochemical tracers such as
152-154
Eu,
99
Tc,
85,89
Sr and
137
Cs were procured
from BRIT, Mumbai and their radiochemical purity was ascertained by gamma
spectrometry.
147
Nd (t
1/2
=11days),
140
La (t
1/2
=40.3hrs),
177
Lu (t
1/2
=6.7days) were
prepared by irradiation of their spectrochemical pure oxide salts. The irradiated
samples were dissolved in 6M HNO
3
and used as stock.
95
Zr (t
1/2
=64days) was
obtained by irradiation of zirconyl chloride (ZrOCl
2
). The irradiated salt was
converted into the nitrate from by repeatedly evaporating with concentrated nitric
acid under IR lamp. Radiotracers such as
147
Pm,
241
Am were procured and purified
by standard procedures [7]. Pu (principally
239
Pu) was purified by the reported
procedure and its radiochemical purity was ascertained by gamma spectrometry for
the absence of
241
Am [8]. The valency of Pu was adjusted to Pu(IV) with sodium
nitrite and subsequently extracted by 0.5M HTTA (2-thenoyltrifluoro acetone) in
xylene at 1M HNO
3
followed by stripping with 8M HNO
3
. The resulting plutonium
solution was used as stock for Pu(IV). Further, plutonium valency in the aqueous
phase was adjusted and maintained in tetravalent state by the addition of 0.05M
NaNO
2
+0.005M NH
4
VO
3
(holding oxidants). On the other hand, Pu was reduced to
Pu(III) by addition of a mixture of 0.2M hydroxyl ammonium nitrate (HAN) and
0.2M hydrazinium nitrate (HN). A mixture of 0.2M HAN and 0.2M HN was
optimized as an efficient reductant for Pu as reported earlier from our laboratory [9].
2.6. PREPARATION OF SIMULATED HIGH LEVEL WASTE
The simulated high level waste (SHLW) solution was prepared by dissolving the
metal ions salts in nitric acid solution. Salts in the nitrate form were preferred and in
the cases where nitrate salts could not be arranged, metal powders were employed.
Care was taken to dissolve each of the salt separately in hot concentrated nitric acid
before their addition to the mixture. Finally, the acidity of SHLW was adjusted as
Chapter II
41
required with distilled water / HNO
3
. The acidity of the SHLW was ascertained by
acid-base titration in the presence of neutral saturated K
2
C
2
O
4
solution. The
concentration of various metal ions in SHLW was ascertained by ICP-AES / EDXRF
analysis.
2.7. METHODS AND EQUIPMENTS
Though several techniques for the separation of metal ions are known, the techniques
such as solvent extraction, extraction chromatography and liquid membrane were
employed in the present studies. The aqueous metal-ligand complexation, ligand-
nitric acid complexation, thermodynamics of metal ion extraction etc. were studied
by the solvent extraction technique. The feasibility of the separation process on large
scale was demonstrated in counter-current extraction employing a mixer-settler
system. The extraction chromatographic studies on metal ions were performed by
impregnation of the ligand as well as by chemical anchoring of the ligand on the inert
solid support. The basic understanding for the transport of metal ions through liquid
membrane was developed on batch studies using a transport cell. Liquid membrane
separation on large scale was demonstrated by using liquid cell contactor i.e. hollow
fibre membrane. Various experimental setups and methodologies used in the present
work are briefed below.
2.7.1. Solvent Extraction Studies
For metal ion distribution studies, suitable volume (0.5-2mL) of aqueous phase at
desired acidity containing required metal ion tracer was equilibrated in glass
stoppered equilibration tube with equal volume of organic phase with desired
concentration of extractant in the chosen diluent. For all the studies, except stated
otherwise, the organic phase was pre-equilibrated with the respective acid solutions.
The agitation of the two phases was carried out by rotary motion in a thermostated
water bath maintained at 250.1C. For thermodynamic studies, however, the
temperature of the water bath was maintained between 15-45C within 0.1C (Fig.
2.6). Care was taken to maintain the required temperature of the equilibration tubes
till the completion of aliquoting of the sample. After equilibration the two phases
were centrifuged and assayed by aliquoting suitable volumes (25-500L) from both
Chapter II
42
the phases. The distribution ratio of the metal ions was calculated as the
concentration of metal ions (in terms of counts of radionuclides per unit time per unit
volume) in the organic phase to that in the aqueous phase. During acid distribution
studies the hydrogen ion concentrations in the two phases were obtained by titration
with standard alkali solution using phenolphthalein as the indicator. The organic
phase was titrated in aqueous ethanol medium previously neutralized to
phenolphthalein end point. Each distribution value was obtained in duplicate or
triplicate and the agreement between these values was within 2%. A good material
balance (95%) was usually obtained in all the experiments.
2.7.2. Mixer-Settler Studies
A 16-stage mixer-settler unit made up of polypropylene with an air-pulsed ejector
technology fabricated at Reprocessing Research and Development Division, IGCAR,
Kalpakkam, was employed for counter current extraction studies. The unit had a total
hold up volume of ~640mL with a mixer volume of ~10mL and settler volume of
~30mL. The mixing of the two phases was achieved by air pulsing by applying
vacuum and venting alternatively with the help of a solenoid valve. The flow rates of
Fig. 2.6. Thermostat water bath used for maintaining constant temperature
Chapter II
43
feed solutions to the mixer settler units were maintained constant (4.50.2mL/min)
by means of pneumatic pumps equipped with precise flow control. In the integral
run, 9-16 stages were used for extraction, whereas 1-8 stages were employed for
stripping. A schematic diagram representing the mixer-settler stages for the integral
run is represented in Fig. 2.7. The system was operated for ~7hrs and the attainment
of steady state was confirmed by periodic estimation of Nd(III) and acidity in the
product and raffinate. After running the system for ~7hrs, the organic as well as the
aqueous samples were collected from each settler units and analyzed for Nd(III) as
well as HNO
3
content.
2.7.3. Extraction Chromatography Studies
The sorption of radionuclides on the resin materials was investigated in batch studies
by equilibrating a known volume (1-2mL) of aqueous solutions at desired acidity
containing metal ions with a known amount of resin (25-50mg). The agitation of the
two phases was carried out in a thermostated water bath maintained at 250.1C for
45 minutes. This time was found to be sufficient to attain the equilibrium condition.
Subsequently, the tubes were centrifuged; the aqueous layer was separated and
centrifuged second time. Suitable aliquots of the aqueous phases were taken before
and after equilibration for assaying the metal ions. The distribution coefficient (K
d
)
of the metal ions was calculated by employing the following formula,
16
S
15
M
14
S
13
M
12
S
11
M
10
S
9
M
8
S
7
M
6
S
5
M
4
S
3
M
2
S
1
M
16
M
15
S
14
M
13
S
12
M
11
S
10
M
9
S
8
M
7
S
6
M
5
S
4
M
3
S
2
M
1
S
Raffinate Out Strippant In Product Out Aq. Feed In
Extractant In Lean Organic Out
Fig. 2.7. Schematic diagram of the mixer- settler stages
Chapter II
44
K
d
=[ (C
o
C) / C ] x V / W (mL/g) (2.1)
where C
o
and C are the concentrations of metal ions (in counts per unit time per unit
volume) before and after equilibration, V is the volume of aqueous phase used (mL)
and W is the weight of the resin material employed (g). For determining K
d
-HNO
3
,
equilibration experiments were carried out in similar way in the absence of metal
ions. The concentration of HNO
3
in the aqueous phase was determined
volumetrically before and after equilibration. The agreement between the determined
value and the calculated value was within 5%.
The chromatographic columns were prepared by packing ~500mg of resin
material in borosilicate glass column of 4mm inner diameter. The bed volume and
the bed density were calculated from the column dimensions and the weight of the
packed chromatographic resin material. The volume of stationary phase (V
s
) and
volume of mobile phase (V
m
) were estimated by the reported method [10]. The
columns were preconditioned by passing excess of appropriate nitric acid solutions
prior to the introduction of the sample solutions. All the column operations were
carried out at room temperature (25.01.0C) at required flow rate. The breakthrough
curves and elution profiles were obtained by plotting radioactivity in the effluent /
eluent vs volume of solution passed.
2.7.4. Membrane Studies
The batch transport experiments with supported liquid membrane (SLM) were
carried out by using a Pyrex glass cell consisting of two compartments, viz. the feed
side and the strip side. As represented in Fig. 2.8, both the compartments of the glass
`
Fig. 2.8. A typical membrane transport cell
used in the present studies
Stirring bar
Stirring bar
SLM
Chapter II
45
cell were joined by glass flanges with SLM in between. The two phases were stirred
at 200 rpm using high speed magnetic stirrer equipped with precise speed control.
The stirring rate of 200 rpm was set to ensure minimal thickness of the aqueous
boundary layers without causing any damage to the membrane [11]. The volumes of
aqueous feed and strip solutions were kept constant (20mL) during all the
experiments.
For the preparation of SLM the microporous PTFE membranes were soaked
in the carrier solution (usually 0.1M TODGA in n-dodecane) for about 10 minutes
prior to use. Subsequently, the submerged membrane was removed from the solution
and wiped carefully with a tissue paper to clear it of the excess fluid at the outer
surface of the support. This impregnation technique leads to SLMs which were
reproducible within 5% with respect to their Am(III) transport behaviour. The
cumulative transport (%T) of the metal ions at the given time was determined by the
following equation,
% T =100 x (C
o
C
t
) / C
o
(2.2)
where C
o
and C
t
are the concentration of metal ions in the aqueous feed at the start of
experiment (t =0) and at time t, respectively.
2.7.5. Hollow Fibre Membrane
Applicability of TODGA liquid membrane for the separation of lanthanides /
actinides was demonstrated on larger scale using Hollow Fibre Modules. A 28.25cm
long module with 6.65cm diameter was employed in the present studies. The total
effective surface area was 1.4 square meters with ~10,000 fibers. The specifications
of the hollow fibre module used in the present work are summarized in Table 2.3.
The supported liquid membrane (SLM) was prepared by pumping the ligand solution
through the lumen side of module at a pressure of 3psi in recycling mode. To ensure
the complete soaking of the membrane pores, the ligand solution was circulated for
~30min when the solution started percolating from lumen side to shell side. After
complete soaking the excess of ligand solution was washed out with plenty of
distilled water, prior to the introduction of the feed and strip solutions. The feed
solution was passed through lumen side while strip solution was passed through the
shell side of the module in all the experiments. The flow rates of the feed and strip
Chapter II
46
Table 2.3: Specifications of hollow fiber membrane contactor
Fibre type Polypropylene
Number of fibers 9950
Fiber internal diameter (m) 240
Fiber outer diameter (m) 300
Fiber wall thickness (m) 30
Effective pore size (m) 0.03
Porosity (%) 40
Tortuosity 2.5
Effective fiber length (cm) 15
Effective surface area (m
2
) 1.4
solutions were maintained constant at 200mL/min with the help of gear pumps
equipped with precise flow controller. A schematic diagram of hollow fibre unit is
shown in Fig. 2.9. Similarly, the unit of hollow fibre system employed in the present
work is shown in Fig. 2.10.
Fig. 2.9. A schematic diagram of Hollow Fibre Membrane unit used in the present studies
Chapter II
47
Fig. 2.10. Hollow Fibre unit used in the present work
2.7.6. Other Equipments
Elemental (C, H, N) analysis was performed using an elemental analyzer EA 1110
from Carlo-Erba Instruments. The FT-IR spectra were recorded as thin film in IR
spectrophotometer in the range 4000 400 cm
-1
. The PMR spectra of the amide
products were recorded in CDCl
3
medium using Bruker 200MHz instrument
employing TMS (tetramethyl silane) as an internal standard. Jasco V-530 UV-
Spectrometer was employed for UV-Visible spectrophotometeric analysis. Viscosity
of the samples was determined using a digital viscometer from DJ Scientific, model
AV250VAC.
2.8. ANALYTICAL INSTRUMENTS / TECHNIQUES
The radioanalytical techniques employed for the analysis of alpha emitting
radionuclides were liquid scintillation counter and surface barrier detector, whereas
NaI(Tl) scintillation counter and HPGe detector were used for the estimation of
gamma emitting radionuclides. For the analysis U, Th and lanthanides,
spectrophotometric as well as volumetric methods were followed.
Chapter II
48
2.8.1. Liquid Scintillation Counter
Liquid scintillation counter is the most widely used detector for the quantitative
analysis of alpha emitters. Nearly 100% detection efficiency of this detector is of
great advantage and even as low as few Bq of alpha activity can be assayed with
good precision. A scintillator is a material that luminesces in a suitable wavelength
region when ionizing radiation interacts with it. Interaction of charged particles
(alpha particles) with the scintillator results in emission of photons and the intensity
of the emitted light is a quantitative measure of the incident radiation. The light
emitted from scintillator is then collected by the photomultiplier tube (PMT) which
produces signal representative of the primary radiation. In the case, when scintillator
emits photons in UV region, a wavelength shifter is added to the scintillator which
has intermediate energy levels. In such cases the de-excitation takes place via these
intermediate energy levels and hence the wavelength of the emitted photons is shifted
from UV to visible region which is subsequently recorded in the PMT (as
photocathodes of most PMTs are compatible with visible light). The liquid
scintillation counter is used to monitor gross alpha activity as it can not distinguish
between alpha energies and thus can not be used for alpha spectrometry.
Many organic compounds are versatile scintillators for radiation
measurements [12,13]. The liquid scintillation cocktail comprises of a solvent like
dioxane or toluene, a scintillator like PPO (2,5-diphenyl oxazole) and a wavelength
shifter such as POPOP [1,4-bis-2-(5-phenyl oxazolyl)-benzene]. The solvent is the
main stopping medium for radiation and must be chosen to give efficient energy
transfer to the scintillating solute. In case of toluene based scintillator a suitable
extractant such as di(2-ethylhexyl) phosphoric acid (HD2EHP) is also added which
facilitates the aqueous samples determinations by transferring the radionuclides from
aqueous phase to the organic phase. In the present work, generally toluene based
liquid scintillator was employed which consisted of 10%(v/v) HD2EHP, 0.7%(w/v)
PPO and 0.03%(w/v) POPOP. Suitable aliquots (25-100L) containing alpha activity
were taken in glass vials containing liquid scintillator solution. When aqueous phase
was added to the toluene scintillator, the two phases were mixed vigorously for
~2min using an ultrasonic agitator to transfer the radionuclides into the organic
phase. Each sample was counted for sufficient time so as to get more than 10,000
counts to restrict the statistical counting error within 1%.
Chapter II
49
2.8.2. NaI(Tl) Scintillation Counter
Sodium iodide activated with 0.10.2% of thallium, NaI(Tl) (Fig. 2.11), is by far the
most widely used inorganic scintillator for the assay of gamma emitting
radionuclides. Salient features of the detectors are the low cost, ease of operation and
ruggedness [14,15]. The band gap in NaI crystal is of the order of 5-6eV. When a
charged particle (or gamma ray) falls on the detector its energy is used up either for
excitation of electrons from valence band to conduction band or for the ionization of
atom. De-excitation of electrons from conduction band to valance band leads to the
emission of photons in UV region as the band gap is large. To shift the emitted
photons in visible region, which is requisite of PMT, NaI crystal is doped with
activator impurity like Tl which forms the intermediate level conduction band. The
resolution of NaI(Tl) detector is about 7% at 662 keV.
In the present work, a 3 x 3 well type NaI(Tl) detector coupled with a
multichannel analyzer has been used for gamma counting. Nearly 100% detection
efficiency for moderate energy photons in a well type Na(Tl) detector offers great
advantages for counting of low activity samples. A suitable aliquot (0.1 - 0.5mL) of
the desired analyte solution was taken in glass counting tubes which was then placed
in the cavity of detector coupled with PMT and associated electronics. Each sample
was counted for sufficient time so as to get more than 10,000 counts to restrict the
statistical counting error within 1%.
2.8.3. Surface Barrier Detector
A semiconductor can be referred to as an ionization chamber in which the detection
medium is a solid [16]. When radiation falls on the detector, it creates electrons and
holes which are collected at the appropriate electrodes. Under ideal conditions, the
signal is proportional to the number of electron-hole pair created in the medium
which in turn is proportional to the energy deposited in the medium by the radiation.
In order that the semiconductor works as a detector, the heavy background noise due
to the free exchange of electrons between the semiconductor and the metal electrodes
has to be eliminated. This is achieved by employing a p-n junction, which acts as a
blocking and prevents the entry of majority of electrons into the sensitive space of
the detector. When a reverse bias is applied to the p-n junction all the electrons and
Chapter II
50
holes are sweeped out thereby widening the charge depletion zone, thus presenting a
larger sensitive space to the radiation from the source.
For charged particle spectroscopy (alpha spectroscopy) an n-type
semiconductor like silicon is used in surface barrier detector. This is made by
evaporating a thin layer of gold to an n-type silicon of very high resistivity
(~80kcm). It is believed that a very thin oxide film between the gold and the silicon
is formed which acts as a p-layer, thus, a p-n junction is formed [17]. By applying
high voltage, depletion region upto 2-3mm has been achieved in silicon barrier
detector. The typical energy resolution of this detector is about 15-20 keV at
5.846MeV of
241
Am. The efficiency of this detector is low and is about
5-10%. A typical alpha spectrometer consist of a vacuum chamber fitted with a
microdot to BNC vacuum feed through connector to connect the detector with pre-
amplifier, a spectroscopy amplifier, detector bias (HV) unit and multichannel
analyzer. Sample for alpha spectrometry is prepared as a very thin layer so as to
minimize the alpha energy loss in the sample. The detector is operated in vacuum at a
pressure less than 10
-2
torr. In the present work the sample was prepared by
evaporating a thin layer of analyte solution (~5L) on a stainless steel planchette
followed by mounting the activity by heating the planchette to red hot.
Fig. 2.11. NaI(Tl) detector used for gamma spectrometry
Chapter II
51
2.8.4. High Purity Germanium Detector
In the present work, high purity germanium (HPGe) detector coupled with
multichannel analyzer was employed for gamma spectroscopy to check the
radiochemical purity of the radionuclides. The HPGe detector is made up of
exceptionally pure germanium in which the impurity level is around 10
10
atoms/g.
This is referred to as high purity germanium which approaches the theoretical pure
semiconductor. HPGe is the most widely used semiconductor detector for gamma
spectrometry and can be called as the workhorse of gamma ray spectroscopy. The
high energy resolution (typically 1.9keV at 1332 keV) is the key feature of this
detector due to low energy band gap (0.7eV). The great advantage of HPGe detector
over Ge(Li) detector is that it can be stored at room temperature [18]. However,
while operating it has to be cooled to liquid nitrogen temperature. The HPGe
detectors are of two types, p-type and n-type. In case of p-type the outer surface of
the germanium crystal is heavily doped with n-type impurity. As a result the
detection efficiency falls drastically below 100keV. On the other hand, n-type
detectors are sensitive to wider range of photon energy. The n-type detector has
added advantages in that it is more resistant to radiation damage in a neutron field as
compared to a p-type detector.
2.8.5. Estimation of Uranium
2.8.5.1. Spectrophotometry
Uranium in the aqueous phase as well as in the organic phase could be determined by
spectrophotometry using 2-(5-Bromo-2-pyridylazo)-5-(diethyl amino) phenol (Br-
PADAP) as a chromogenic reagent [19]. Uranyl ion forms stable intense violet
coloured complex with Br-PADAP at pH 7-8 in the alcoholic medium buffered with
triethanolamine (TEA) which shows absorption maxima at 575nm with molar
extinction coefficient of ~70,000. To a known volume of uranium solution in
standard flask (10mL), 1mL of complexing solution (1.25g CDTA +0.25g NaF +
3.25g sulphosalicylic acid dissolved in 100mL water adjusted to pH 7.8 with conc.
NaOH), 1mL buffer solution (14g TEA dissolved in 100mL water adjusted to pH 7.8
with perchloric acid) and 0.8mL Br-PADAP solution (50mg Br-PADAP dissolve in
100mL ethanol) were added, respectively. For organic samples the final volume
(10mL) was made up with ethanol. On the other hand, for aqueous samples the final
Chapter II
52
volume (10mL) was adjusted with distilled water after addition of 4mL of ethanol.
The final absorption measurements were performed after 30min of colour
development at 575nm. This method was found to be very sensitive and no
interference of Pu, Th, Al and Fe was observed. The calibration curve was plotted in
the concentration range of 1x10
-5
M to 1x10
-6
M with standard uranium solution. The
concentrations of unknown samples were determined from the calibration plot.
2.8.5.2. Davis Gray Titration
Uranium in the concentration range 50-200g/mL was estimated volumetrically by
Davis-Gray method employing potentiometric end point detection [20,21]. The
sample size was varied between 1-3mL. This method involves the reduction of U(VI)
to U(IV) by Fe(II) in the presence of concentrated phosphoric acid solution
containing sulphamic acid. Then the excess Fe(II) is selectively oxidized by nitric
acid in the presence of Mo(VI) which acts as a catalyst. The role of sulphamic acid is
to destroy any trace of nitrous acid present in the solution which may oxidize Fe(II)
and U(IV). The resulting U(IV) phosphate solution is then titrated with standard
potassium dichromate solution to potentiometric end point. A small amount of
vanadium(IV) sulphate is added in the solution which sharpens the end point. The
concentration of uranium in the analyte solution is calculated from the volume of
standard potassium dichromate solution consumed.
2.8.6. Estimation of Thorium
2.8.6.1. Spectrophotometry
Thorium in microgram quantities in aqueous samples could be determined by
spectrophotometric method [22]. Thorium forms a red coloured complex with
Arsenazo-III at 5-6M acidity which shows absorption maxima at 665nm with a molar
extinction coefficient of ~100,000. For sample preparation a suitable aliquot of Th
was taken in a standard flask (10mL) followed by addition of 1mL of 1M sulphamic
acid, 4mL of concentrated nitric acid and 1mL of 0.1% Arsenazo-III. The final
volume was made up with distilled water and absorbance was recorded at 665nm
after 15min of the colour development. The role of sulphamic acid is to remove any
trace of nitrous acid which interferes in the method. The calibration plot was
constructed by measuring the absorbance of standard solutions of thorium between
Chapter II
53
the concentration range of 1x10
-5
M to 1x10
-6
M. The concentration of unknown
analyte samples was determined from the calibration curve.
2.8.6.2. Complexometric Titration
When the concentration of thorium was in milligram quantities, the conventional
complexometric titration was followed. A suitable aliquot of Th solution was titrated
against standard EDTA (ethylenediamine tetraacetic acid) solution at pH 3 using
xylenol orange as an indicator [23]. The end point of the reaction was the change of
colour from deep purple to lemon yellow. The precision of these analyses was 2%
(~5mg Th). Similarly, the organic phase was also titrated with a precision of 5%. In
the case of organic samples, the aliquot size was restricted to 0.5mL.
2.8.7. Estimation of Neodymium
2.8.7.1. Spectrophotometry
Neodymium in microgram quantities present in the aqueous phase as well as in the
organic phase was determined by spectrophotometry using Arsenazo-I as the
chromogenic agent. Neodymium forms stable intense violet coloured complex with
Arsenazo-I at pH 7-8 in a solution buffered with triethyl amine (TEA) which shows
absorption maxima at 580nm with molar extinction coefficient of ~10
5
. For sample
preparation a suitable aliquot of Nd was taken in a standard flask (10mL) followed
by addition of 3mL of 10% TEA, 0.8mL of 0.1% Arsenazo-I. The final volume was
made up with distilled water and absorbance was recorded at 580nm after 15min of
the colour development. For organic samples, the TEA buffer as well as Arsenazo-I
solutions were prepared in ethanol medium and final volume (10mL) of the sample
was made up with ethanol. Separate calibration plots were constructed for organic as
well as aqueous samples by measuring the absorbance of standard solutions of Nd
between the concentration range of 1x10
-5
M to 1x10
-6
M. The concentration of
unknown analyte samples was determined from the calibration curve.
2.8.7.2. Complexometric Titration
Estimation of neodymium (in mg range) in the aqueous phase as well in the organic
phase was carried out by complexometric titrations employing standard EDTA
solution with methyl thymol blue (MTB) as the indicator. A suitable aliquot of Nd
Chapter II
54
solution was taken in a beaker containing distilled water. The pH of the solution was
adjusted to 6 with the help of pH meter using a concentrated solution of
hexmethylene tetramine. Finally, the solution was titrated with standard EDTA
solution using MTB as the indicator when the colour changed from violet to lemon
yellow indicating the end point. Determination of Nd from the organic phase was
also carried out by titrating the biphasic sample with a precision of 5%. In case of
organic samples, the aliquot size was restricted to 0.5mL.
2.9. REFERENCES
1. Y. Sasaki, Y. Sugo, S. Suzuki and S. Tachimori, Solv. Extr. Ion Exch., 19 (2001)
91.
2. N. Srinivasan, M.N. Nadkarni, G.R. Balasubramanian, R.T. Chitnis and H.R.
Siddiqui, BARC Report, Report-643, Bhabha Atomic Research Centre, Mumbai,
India, 1972.
3. S.S. Rattan, A.V.R. Reddy, V.S. Mallapurkar, R.J. Singh and Satya Prakash, J.
Radioanal. Nucl. Chem., 67 (1981) 85.
4. P. Thakur, R. Veeraraghavan, P.K. Mohapatra, V.K. Manchanda and K.C. Dash,
Talanta, 43 (1996) 1305.
5. R. Artinger, C.M. Marquardt, J.I. Kim, A. Seibert, N. Trautmann and J.V. Kratz,
Radiochim. Acta, 88 (2000) 609.
6. S.A. Ansari, M.S. Murali, P.N. Pathak and V.K. Manchanda, J. Radioanl. Nucl.
Chem., 262 (2004) 469.
7. J.N. Mathur, M.S. Murali, P.R. Natarajan, L.P. Badheka and A. Banerji, Talanta,
39 (1992) 493.
8. D.E. Ryan and A.W. Wheelright, Ind. Eng. Chem., 51 (1959) 60.
9. P.N. Pathak, D.R. Prabhu, G.H. Rizvi, P.B. Ruikar, L.B. Kumbhare, P.K.
Mohapatra and V.K. Manchanda, Radiochim. Acta, 91 (2003) 91.
10. E.P. Horwitz, R. Chiarizia and M.L. Dietz, Solv. Extr. Ion Exch., 10 (1992) 313.
11. P.R. Danesi, E.P. Horwitz and P.G. Rickert, J. Phys. Chem., 87 (1983) 4708.
12. W.J. McDowell, Organic scintillators and liquid scintillation counting, D.L.
Horrocks, L.T. Peng (Eds.), Academic Press, Inc., New York (1971), P. 937.
Chapter II
55
13. H. Ihle, M. Karayannis and A. Murrenhoff, Organic scintillators and liquid
scintillation counting, D.L. Horrocks, L.T. Peng (Eds.), Academic Press, Inc.,
New York (1971), P. 879.
14. G.F. Knoll, J. Radioanal. Nucl. Chem., 243 (2000) 125.
15. D.D. Sood, A.V.R. Reddy and N. Ramamoorthy, Fundamentals of
Radiochemistry, 2
nd
ed., IANCAS publication, BARC, Mumbai (2004), P. 115.
16. H. J. Arnikar, Essentials of nuclear chemistry, fourth edition, New Age
International Pvt. Ltd. Publishers, New Delhi, 2001, P. 322.
17. G.F. Knoll, Radiation detection and measurement, 3
rd
ed., John Willy and sons,
New York (2000), P. 377.
18. G.F. Knoll, Radiation detection and measurement, 3
rd
ed., John Willy and sons,
New York (2000), P. 406.
19. W.J. Maeck, Anal. Chem., 31 (1955) 1130.
20. W. Davies and W. Gray, Talanta, 11 (1964) 1203.
21. F.P. Roberts, R. J. Brouns, K. R. Byers, J. E. Fager, B. W. Smith, K. L. Swinth
(Eds.), NUREG/CR-3584, Method 3.0, 1984.
22. G.H. Jeffery, J. Bassett, J. Mendham and R. C. Denney (Eds.), Vogels
Textbook of Quantitative Analysis, 5th ed., ELBS Longman Publishers, UK
(1996), P. 329.
23. G.R. Balasubramanian, R.T. Chitnis, A. Ramanujam, M. Venkatesan, BARC
Report, Report-940, Bhabha Atomic Research Centre, Mumbai, India, 1977.
------------------------
Chapter III
N,N,N,N-Tetraoctyl
Diglycolamide: A Promising
Extractant For The Partitioning Of
Actinides From High Level Waste
Chapter III
56
N,N,N,N-TETRAOCTYL DIGLYCOLAMIDE: A PROMISING
EXTRACTANT FOR THE PARTITIONING OF ACTINIDES
FROM HIGH LEVEL WASTE
3.1. INTRODUCTON
Steady growth of global fuel reprocessing activities implies a vital role of
radiochemists not only in developing efficient procedures for the separation and
purification of actinides, but also for devising safe procedure for the management of
high level waste (HLW) emanating in the PUREX process [1-3]. Possibility of the
isolation of some pure radionuclides as valuable by products makes actinide
partitioning a further attractive strategy for HLW treatment. Application of universal
fuel reprocessing extractant like tri-n-butyl phosphate (TBP) for actinide partitioning
is not possible without producing waste streams with high salt content. Thus, there is
a need to develop versatile reagents capable of partitioning of actinides under the
prevailing conditions of HLW.
In recent years, completely incinerable substituted diamides have been the
focus of numerous studies in the back end of nuclear fuel cycle [4,5]. These
extractants offer several advantages over organophosphorus compounds, especially
with respect to (i) the innocuous nature of their degradation products, viz. carboxylic
acids / amines; and (ii) the possibility of complete incineration of the spent solvent
leading to reduced volume of the secondary waste [6-8]. In addition, the physico-
chemical properties of this class of ligands can be tuned by the judicious choice of
alkyl groups. In the last decade, several investigations have been conducted on the
modification of diamide structure in order to further improve its extracting
properties. It has been observed that the introduction of etheric oxygen between the
two amide groups (diglycolamide) causes significant enhancement in the extraction
of trivalent actinides / lanthanides. Diglycolamide (DGA) was initially introduced by
Stephan et al. [9,10] for the extraction of divalent and trivalent cations. Studies
involving various DGA derivatives concerning fundamental as well as application
have been reported extensively [11-15]. The physico-chemical properties of DGA
can be changed by the attachment of different alkyl chains to N atoms. For example,
DGA ligands with short alkyl groups (-CH
3
, -C
2
H
5
) are aqueous soluble and have
Chapter III
57
Fig. 3.1. Tetraoctyl Diglycolamide (TODGA)
C
C
H
2
O
C
H
2
C
O
N
O
N
C
8
H
17
C
8
H
17
C
8
H
17
C
8
H
17
been utilized as masking agent [16]. On the other hand, DGA molecules with long
alkyl chains (-C
8
H
17
, -C
10
H
21
, -C
12
H
25
) can be dissolved in non-polar organic diluents
and hence can be utilized for in solvent extraction process for the separation of metal
ions. Amongst several derivatives of diglycolamide studied for separation processes,
recently developed N,N,N,N-tetraoctyl diglycolamide (TODGA, Fig. 3.1) has been
identified as one of the most promising extractants for the partitioning of trivalent
actinides and lanthanides from HLW solution [17-23].
Utilization of TODGA for the partitioning of actinides in the back end of
nuclear fuel cycle would heavily rely on the solvent extraction technique employing
contactors like mixer-settlers, centrifugal extractors or pulse columns. It is imperative
to carry out a systematic solvent extraction study to understand the extraction
behaviour of actinides and long-lived fission products employing TODGA as the
extractant. This chapter deals with the effects of (i) complexing anions such as NO
3
-
,
ClO
4
-
and Cl
-
, (ii) organic diluents, and (iii) temperature on the extraction of metal
ions to understand the extraction mechanism. There is temperature enhancement of
HLW due to the decay heat of / emitting radionuclides. The acid uptake constant
of TODGA and the effect of feed acidity on the extraction of metal ions were studied.
The hydrolytic as well as radiolytic stability of the ligand was also investigated. A
detailed study on the metal loading capacity in the organic phase and the effect of
various phase modifiers has been discussed in this chapter. This chapter also reports
the applicability of TODGA for the extraction of lanthanides/actinides on large scale
in counter-current extraction using a mixer-settler system.
3.2. EVALUATION OF EXTRACTANTS FOR ACTINIDE PARTITIONING
The most commonly proposed extractants for actinide partitioning are octyl(phenyl)-
N,N-diisobutyl carbamoyl methyl phosphine oxide (CMPO), trialkyl phosphine
oxide (TRPO) and N,N-dimethyl-N,N-dibutyl tetradecyl malonamide
Chapter III
58
0 1 2 3 4 5 6
10
-4
10
-3
10
-2
10
-1
10
0
10
1
10
2
10
3
0.1M TODGA
30% TRPO
1.0M DMDBTDMA
0.2M CMPO +1.2M TBP
D
A
m
[HNO
3
], M
(DMDBTDMA). These extractants are employed in TRUEX, TRPO and DIAMEX
processes, respectively and are briefed in Chapter-I. It is worth comparing the
performance of TODGA with these extractants. The extraction profile of Am(III)
with TODGA, TRUEX, DIAMEX and TRPO solvents from nitric acid solution is
presented in Fig. 3.2. In the case of TRUEX solvent, usually 1.2M TBP is employed
as phase modifier which improves phase compatibility and reduces hydrolytic and
radiolytic degradation of CMPO. The distribution ratio with TRUEX solvent
increased sharply upto 0.5M HNO
3
and then a plateau was observed upto 6M HNO
3
.
On the other hand, TRPO shows moderate D
Am
values only at lower acidity (0.5-1M
HNO
3
), suggesting that the extractant is effective only at acidity 1M HNO
3
. At
acidity 1M HNO
3
. At higher acidity, TRPO shows stronger complexation with
nitric acid resulting in lowering of D
Am
value. Unlike TRPO, CMPO shows required
acid dependence due to the presence of additional carbamoyl group, which provides
intermolecular buffering effect to the molecule [24]. Similarly, for DMDBTDMA
the D
Am
value increased gradually with nitric acid concentration and reached only to
Fig. 3.2. Variation of D
Am
with HNO
3
concentration; Diluent:
n-dodecane; Temperature: 25C
Chapter III
59
a moderate value of 11 at 3M HNO
3
. In case of TODGA, however, D
Am
increased
gradually upto 2M HNO
3
and exhibited very high distribution values between 2-6M
HNO
3
. Such distribution behaviour of Am(IIII) towards TODGA demonstrates its
desirable performance for the extraction of trivalent actinides.
3.3. BASICITY OF TODGA
The basicity of the ligand is expressed in terms of acid uptake constant (K
H
) which
plays an important role in determining the strength of complex formed with the metal
ions. This constant for TODGA was determined by solvent extraction procedure after
measuring the concentration of nitric acid in the organic phase. Assuming that
TODGA (A) interacts with nitric acid as follows,
K
H
H
+
(aq.)
+NO
3
-
(aq.)
+ nA
(org.)
\
=======
\
HNO
3
nA
(org.)
(3.1)
where K
H
, the acid uptake constant, is defined as,
[HNO
3
nA]
(org.)
K
H
= ---------------------------------------- (3.2)
[H
+
]
(aq.)
[NO
3
-
]
(aq.)
[A]
n
(org. free)
where the subscripts (aq.) and (org.) represent the aqueous and the organic phases,
respectively. Taking logarithm on both sides of the Eq. (3.2) and rearranging,
log[H
+
]
(org.)
- 2log[H
+
]
(aq.)
= nlog[A]
(org. free)
+ log K
H
(3.3)
where, [A]
(org. free)
=[A]
(initial)
- [H
+
]
(org.)
and [H
+
]
(org.)
=[HNO
3
nA]
(org.)
. A plot of
{log[H
+
]
(org.)
- 2log[H
+
]
(aq.)
} against log[A]
(org. free)
would allow to evaluate the
number of ligand molecules involved in the formation of TODGAHNO
3
adduct (n)
and the basicity of ligand (log K
H
) from the slope and intercept of the linear fit,
respectively. The activity of protons in the aqueous phase was calculated from the
dissociation constant of HNO
3
(K
a
=23.5). In view of the lack of data for the activities
of HNO
3
and free TODGA in the organic phase, the concentration terms have been
used and the two-phase equilibrium constant is referred to as conditional acid
uptake constant. As a simplified case, for different concentrations of TODGA
equilibrated with 1M HNO
3
, the plot of {log[H
+
]
(org.)
- 2log[H
+
]
(aq.)
} versus
log[TODGA]
(org. free)
is shown in Fig. 3.3. The slope of the curve (0.950.05)
suggests the formation of [TODGAHNO
3
] adduct in the organic phase. The value of
Chapter III
60
-3.0 -2.5 -2.0 -1.5
-2.1
-1.8
-1.5
-1.2
-0.9
-0.6
l
o
g
[
H
+
]
o
r
g
.
-
2
l
o
g
[
H
+
]
a
q
.
K
H
: 4.1 0.4
l og[ A]
free
, M
Slope : 0.95 0.05
Intercept : 0.61 0.09
R
2
: 0.95
K
H
was calculated as 4.10.4 from the intercept, suggesting that TODGA is more
basic as compared to CMPO (K
H
=2.0) [25], DMDBTDMA (K
H
=0.32) [26] and
TBP (K
H
=0.20) [27]. On the other hand, the K
H
value of TODGA was lower as
compared to phosphine oxide ligands, viz. TRPO (Cyanex-923, K
H
=8.30) [27] and
TOPO (K
H
=8.90) [28]. Higher basicity of TODGA as compared to diamides could
be attributed to the presence of third donor oxygen atom in the molecule.
3.4. EXTRACTION OF AMERICIUM BY TODGA
The extraction of trivalent americium by neutral ligand, TODGA, from aqueous acid
solution can be expressed by the following equilibrium reaction,
K
ex
Am
3+
(aq.)
+3X
-
(aq.)
+ nTODGA
(org.)
\
=======
\
AmX
3
nTODGA
(org.)
(3.4)
where the subscripts (aq.) and (org.) represent the aqueous and the organic phases,
respectively, X
-
is the counter anion present in the aqueous phase and n is the number
of ligand molecules involved in the metal-ligand complexation. In order to explain
the extraction mechanism, it is necessary to investigate several parameters affecting
Fig. 3.3. Uptake of HNO
3
by TODGA; Diluent: n-dodecane;
Temperature: 25C
Chapter III
61
the extraction of Am(III) such as effects of anions, ligand concentration as well as
those of organic diluents.
3.4.1. Effect of Anion
Preliminary studies on the distribution of Am(III) from HCl, HNO
3
and HClO
4
medium have shown that for a given concentration of TODGA, the distribution
values vary in the order: HCl <HNO
3
<HClO
4
[29]. The effect of anions on the
distribution of Am(III) is shown in Fig. 3.4. In case of HNO
3
, the D
Am
value
increased sharply with nitric acid concentration upto 3M, beyond which a plateau
was observed. Increase in the distribution value with acidity can be explained by the
law of mass action (Equilibrium reaction 3.4) due to increased nitrate concentration.
However, with increased acidity the law of mass action was countered by decreased
free ligand concentration due to the formation of TODGA-HNO
3
adduct, thereby
giving plateau at higher acidity. On the other hand, lower D
Am
value observed in HCl
medium did not show any significant change upto 2M HCl. However, there was a
steep increase in D
Am
values beyond 2M HCl. Lower distribution value in HCl
medium was attributed to the weaker aqueous complexation of Am(III) with Cl
-
ion
Fig. 3.4. Variation of D
Am
with aqueous phase acidity; Extractant:
0.1M TODGA in n-dodecane; Temperature: 25C
0 1 2 3 4 5 6
10
-4
10
-3
10
-2
10
-1
10
0
10
1
10
2
10
3
HCl
HNO
3
HClO
4
D
A
m
[HNO
3
], M
Chapter III
62
as compared to than with NO
3
-
ion during formation of the neutral extracted species.
Jensen, et al. [30] have reported that the extraction of metal ions by TODGA is
facilitated by the extraction of acid and water in the organic phase. The extraction of
mineral acids and water by common extractants is known to vary greatly as the
identity of acid is changed, as recently described for TBP extraction system [31]. In
the case of TODGA extraction system, both acid and water are poorly extracted from
HCl solutions than from HNO
3
solutions. Despite considerably higher aqueous
acidity of 4M HCl system, only 0.002M acid and 0.008M of H
2
O are extracted as
compared to HNO
3
system where 0.02M acid and 0.025M H
2
O are extracted even at
1M HNO
3
. Lower extraction of Am(III) from HCl solution is associated with this
behaviour. It is interesting to note that the distribution values in perchloric acid
medium were relatively higher and displayed minor variation (19520) with aqueous
phase acidity (0.01-6M). High D
Am
in HClO
4
was attributed to the perchlorate effect
where large perchlorate ions disrupt the ordered hydrated aqua-metal complex in the
aqueous phase, thereby promoting the phase transfer of metallic cations from
aqueous to organic phase.
3.4.2. Effect of Ligand Concentration
The stoichiometry of the extracted species of Am(III)-TODGA/n-dodecane from
nitric acid medium was investigated by determining the D
Am
values at different
ligand concentration. As a simplified case for equilibrium reaction (3.4), the
extraction constant (K
ex
) for the extraction of Am(III) by TODGA from nitric acid
medium can be represented as follows,
[Am(NO
3
)
3
nTODGA]
(org.)
K
ex
= ------------------------------------------------ (3.5)
[Am
3+
]
(aq.)
[NO
3
-
]
3
(aq.)
[TODGA]
n
(org.)
Since, the distribution ratio of the metal ions (D
M
) is the ratio of total concentration
of metal ions in the organic phase to that in the aqueous phase. Therefore, Eq. (3.5)
can be modified as follows,
D
Am
K
ex
= --------------------------- (3.6)
[NO
3
-
]
3
[TODGA]
n
Chapter III
63
Here the subscripts have been omitted to simplify the equation. Taking logarithm of
the Eq. (3.6) and rearranging,
logD
Am
= logK
ex
+ 3log[NO
3
-
]
+ nlog[TODGA] (3.7)
The slope analysis of Eq. (3.7) allows the interpretation of stoichiometry of the
extracted metal complex. The plot of logD
Am
versus log[TODGA] is shown in Fig.
3.5. The value of n was estimated from the slope of the linear fit as ~3 at 0.5M HNO
3
and ~4 at 1M HNO
3
, suggesting that Am(III) is predominantly extracted as a tetra-
solvated species at moderate acid concentration. Moreover, four tridentate molecules
of TODGA in the extraction of Am(III)-nitrate appears more than that can be
reasonably accommodated in the inner co-ordination sphere of trivalent actinide ions.
Small angle neutron scattering (SANS) studies on 0.1M TODGA/n-dodecane
solution equilibrated with aqueous nitric acid solution greater than 0.7M have shown
the presence of monomers, dimers, trimers and small reverse micelles of TODGA
tetramers [30]. However, no such species were observed in free TODGA/n-dodecane
solution as the interparticle attractions are negligible. After extraction of sufficient
acid and water molecules, the interparticle attraction facilitates the formation of
Fig. 3.5. Variation of D
Am
with TODGA concentration;
Diluent: n-dodecane
0.01 0.1
0.01
0.1
1
10
100
[TODGA], M
D
A
m
1.0M HNO
3
; Solpe: 3.78 0.23
0.5M HNO
3
; Slope: 3.22 0.13
Chapter III
64
different aggregates of TODGA. The micellar TODGA tetramers rapidly become
dominant species in the organic phase when the equilibrium aqueous acidity exceeds
1M HNO
3
. The extraction of Am(III)-4TODGA species in the organic phase
suggested that the trivalent metal ion was predominantly extracted by tetramer
aggregates of TODGA. The extraction of lower solvated species at 0.5M HNO
3
confirms the extraction of Am(III) by TODGA aggregates as SANS studies
confirmed the presence of trimer TODGA at 0.5M HNO
3
[30]. The formation of
reverse micelle and the extraction of metal ions by ligand aggregates have been
reported in alkyl substituted malonamides [32-34]. The existence of tetramers in
TODGA/alkane solution determines the nature of the extracted complex and provides
a specific mechanism to explain the unusual extraction behavior of trivalent f-
element cations. On the other hand, all other metal ions, including Pu(IV) and U(VI)
form complexes with three TODGA molecules [23]. Smaller tetravalent actinides
form stronger NO
3
-
complexes and have restricted space around them for TODGA
molecules. Tetravalent actinides may form intrinsically stronger metal-ligand bonds
with TODGA, as dictated by electrostatic considerations [35]. However, such cations
will have to expend more free energy to re-organize the TODGA aggregates to suit
lower metal-TODGA stoichiometries. By contrast, the binding of trivalent f-element
ions does not disrupt the (TODGA)
4
aggregates, and under solution conditions where
the concentration of the (TODGA)
4
tetramer is significant, Am(III) and Ln(III)
cations that form 1:4 complexes and are preferentially extracted over other metal ions
which form complexes with lower stoichiometries. Consequently, the pre-
organization of TODGA to form reverse-micelles of (TODGA)
4
in aliphatic organic
solvents appears to be the principal reason for the unusual selectivity of TODGA for
the trivalent f-cations. Nevertheless, in polar organic solvents, which inhibits
amphiphilic aggregation and micellization [36,37], only 1:2 M
3+
/TODGA ratio was
observed (as elaborated in the latter section, Table 3.1). Moreover, EXAFS study
proved that in Nd(III)TODGA / n-dodecane complex, only two of the four TODGA
molecules are bonded in tridentate fashion with some water molecules in the inner
coordination sphere and additional ligand molecules do not interact directly with the
metal ions [38]. It has been shown that the extraction of Am(III) by TODGA follows
the ion-pair mechanism [39], where two TODGA molecules and three water
Chapter III
65
molecules are present in the inner-sphere and two TODGA molecules and nitrates are
present in the outer-sphere of the complex
3.4.3. Effect of Organic Diluent
The effect of organic diluent on D
Am
by TODGA has been listed in Table 3.1. In
general, the D
Am
increased with the dielectric constant of the diluent. However, lower
D
Am
values were observed for chlorinated and aromatic solvents like 1,2-
dichloroethane and toluene which was attributed to their interaction with the donor
oxygen atoms of diglycolamide (as halogenated and aromatic solvents have high
acceptor numbers) leading to decrease in the ligand concentration [23]. Horwitz, et
al. have studied the distribution behaviour of Am(III) with several diluents
employing CMPO as the extractant and reported that the D
Am
was higher in n-
dodecane as compared to either toluene or CCl
4
[40]. In the present work, if we
divide the diluents into two groups, i.e. halogenated and non-halogenated diluents,
then an increasing trend of D
Am
with the dielectric constant of the diluent was
observed, i.e. from dodecane to nitrobenzene as well as from CCl
4
to dichloroethane.
Sasaki, et al. [39] have also reported increase in distribution of lanthanides and
actinides by TODGA with the polarity of the diluents. These observations further
support the ion-pair mechanism for the extraction of Am(III) with TODGA.
Ligand variation studies suggested that the stoichiometry of the Am(III)
extracted species changes with the diluent. The slope values for different diluents are
listed in Table 3.1. As described in the earlier section, the extraction of metal ions
inn-dodecane takes place by tetramer TODGA reverse micelle where only two
ligands are coordinated with the metal ions and the other ligand molecules are only
attached to the bonded TODGA molecules. In polar diluents, which inhibit
amphiphilic aggregation and micellization, only 1:2 Am(III) / TODGA ratio was
observed [30]. In other words, M(TODGA)
4
is not formed in solvents which do not
favour the formation of (TODGA)
4
micelles. It seems that the polar solvents like
nitrobenzene, octanol and dichloroethane do not allow the formation of TODGA
aggregates due to dipole-dipole interaction of diluent and ligand molecules. This
behaviour was subsequently confirmed by recording IR spectrum where ~30cm
-1
down shift in the >C=O stretching frequency was observed for TODGA / octanol
solution. Such shift was not observed when n-dodecane was used as the diluent.
Chapter III
66
Table 3.1: Distribution data and slope values for Am(III) obtained in different
diluents; Extractant: 0.1M TODGA; Aqueous phase: 1M HNO
3
Diluents Dielectric constant D
Am
Slope (n)
n-Hexane 1.91 33 --
n-Dodecane 2.01 30 3.78 0.23
1-Octanol 10.3 81 2.33 0.03
Nitrobenzene 34.8 220 1.76 0.06
Tetrachloromethane 2.24 0.09 --
Chloroform 4.90 0.12 3.28 0.18
1,2-dichloroethane 10.4 9.90 1.92 0.07
Benzene 2.28 0.39 3.70 0.11
Toluene 2.42 0.30 3.58 0.09
3.4.4. Kinetics of Extraction
The extraction kinetics of the metal ions was evaluated by determining the time
dependence D
Am
with 0.1M TODGA/n-dodecane. It was observed that the
equilibrium condition was attained within 5 minutes of the equilibration implying the
fast reaction between TODGA and actinides. The D
Am
value observed at 1M HNO
3
was 28 2 even after 3 hrs of equilibration. It is worth mentioning here that Fe(III)
was not extracted by TODGA. However, earlier studies involving ligands like
DMDBTDMA and CMPO reflected that Fe(III) could be extracted after longer time
of equilibration, i.e. with slow kinetics [24,26]. It was, therefore, of interest to
investigate the extraction behaviour of Fe(III) as a function of time using 0.1M
TODGA/n-dodecane. It was observed that unlike DMDBTDMA and CMPO systems,
Fe(III) was practically un-extracted even after 3 hrs of equilibration. The D
Fe
values
observed at 1M HNO
3
was ~10
-3
. Selective extraction of actinides over Fe(III) by
TODGA is an advantage in actinide partitioning from HLW which contains
significant amount of structural materials.
3.5. THERMDYNAMICS OF EXTRACTION
Preliminary studies on the distribution of actinide ions by 0.1M TODGA has shown
Chapter III
67
that the D
M
values followed the order: Am(NO
3
)
3
~Pu(NO
3
)
4
>UO
2
(NO
3
)
2
. The
unusual higher extraction of trivalent actinide ions over hexavalent one could not be
explained on the basis of their ionic potential. An attempt has been made to
understand insight into the extraction behaviour of these metal ions with the help of
thermodynamic quantities involved during the extraction process.
3.5.1. Calculation of Thermodynamic Parameters
Similar to equilibrium reaction (3.4), a general two phase equilibrium representing
the extraction of metal ions (M
n+
) from nitric acid medium with TODGA (A) can be
represented as,
K
ex
M
n+
(aq.)
+n NO
3
-
(aq.)
+x A
(org.)
\
=======
\
M(NO
3
)
n
xA
(org.)
(3.8)
where the subscripts (aq.) and (org.) represent the aqueous and the organic phases,
respectively and x is the number of ligand molecules present in the metal ligand
complex. Thus, the equilibrium constant for the reaction (3.8) can be written as,
[M(NO
3
)
n
xA]
(org.)
M(NO
3
)
n
xA
K
ex
= ------------------------------------------------------------------------ (3.9)
[M
n+
]
(aq.)
[NO
3
-
]
n
(aq.)
[A]
x
(org. free) .
M
n+
n
NO
3
-
x
A
where the squared brackets and represent the concentrations and activity
coefficients of the respective species. The concentration of free ligand [A]
(org. free)
was
calculated from the basicity of TODGA (K
H
=4.1) with the assumption that the K
H
value does not change in the temperature range selected for the present study. The
equilibrium constants (K
ex
) for the extraction of metal ions were calculated using the
following approximations: (a) the activity coefficients of metal ions in the aqueous
phase as well as that of the metal solvate in the organic phase were assumed to be
unity since the concentration of metal ions was at the tracer level, and (b) [A]
(org. free)
was assumed to be equal to the activity of free TODGA. Therefore, the equilibrium
constant is referred to as conditional extraction constant and can be written as,
[M(NO
3
)
n
xA]
(org.)
K
ex
= -------------------------------------------- (3.10)
[M
n+
]
(aq.)
{a
NO
3
-
}
n
(aq.)
[A]
x
(org. free)
Here the activity of nitrate ions in the aqueous phase was used from the literature
Chapter III
68
[41]. The experimentally determined distribution ratio (D
M
) is the ratio of the
concentration of metal ions in the organic phase to the total concentration of the
metal ions in the aqueous phase and is expressed as,
[M(NO
3
)
n
xA]
(org.)
D
M
= ------------------------- (3.11)
[M
t
n+
]
(aq.)
where the subscript t refers to the total metal ion concentration in the aqueous phase.
Because of the presence of substantial amount of NO
3
-
ions, the metal ion species
present in the aqueous phase will be M
n+
, M(NO
3
)
(n-1)+
, M(NO
3
)
2
(n-2)+
etc.
Thus, the total concentration of the metal ion in the aqueous phase can be written as,
[M
t
n+
]
(aq.)
=([M
n+
] + [M(NO
3
)
(n-1)+
]
+ [M(NO
3
)
2
(n-2)+
] +-----)
[M
t
n+
]
(aq.)
=[M
n+
] (1+
1
M
[NO
3
-
] +
2
M
[NO
3
-
]
2
+------)
[M
t
n+
]
(aq.)
=[M
n+
] (1+
n
M
[NO
3
-
]
n
) (3.12)
Here the term
n
M
represents the overall stability constant for the given metal
complex, which gives the extent of nitrate complexation. Therefore, Eq. (3.10) takes
the following form,
[M(NO
3
)
n
xA]
(org.)
(1+
n
M
[NO
3
-
]
n
)
K
ex
= ---------------------------------------------------- (3.13)
[M
t
n+
]
(aq.)
{a
NO
3
-
}
n
(aq.)
[A]
x
(org. free)
From Eqs. (3.11) and (3.13), following expression can be obtained,
D
M
(1+
n
M
[NO
3
-
]
n
)
K
ex
= ---------------------------------- (3.14)
{a
NO
3
-
}
n
(aq.)
[A]
x
(org. free)
The ligand variation studies for Am(III), Pu(IV) and U(VI) employing TODGA/n-
dodecane have shown the extraction of predominant species in the organic phase as:
Am(NO
3
)
3
4TODGA, Pu(NO
3
)
4
3TODGA and UO
2
(NO
3
)
2
3TODGA, respectively
[23]. With the knowledge of stoichiometry of the extracted species, the equilibrium
reaction (3.8) for Am(III), Pu(IV) and U(VI) by TODGA (A) can be written as
follows,
K
Am
Am
3+
(aq.)
+3 NO
3
-
(aq.)
+4 A
(org.)
\
=======
\
Am(NO
3
)
3
4A
(org.)
(3.15)
Chapter III
69
K
Pu
Pu
4+
(aq.)
+4 NO
3
-
(aq.)
+3 A
(org.)
\
=======
\
Pu(NO
3
)
4
3A
(org.)
(3.16)
K
U
UO
2
2+
(aq.)
+2 NO
3
-
(aq.)
+3 A
(org.) \
=======
\
UO
2
(NO
3
)
2
3A
(org.)
(3.17)
Assuming that the values of the term (1+
n
M
[NO
3
-
]
n
) and the stoichiometry of the
extracted species do not change significantly in the chosen temperature range [44-
50], the value of K
ex
for Am(III), Pu(IV) and U(VI) for the above equilibrium
reactions can be calculated on the basis of Eq. (3.14) as follows,
D
Am
(1+
n
Am
[NO
3
-
]
n
)
K
Am
= -------------------------------- (3.18)
{a
NO
3
-
}
3
(aq.)
[A]
4
(org. free)
D
Pu
(1+
n
Pu
[NO
3
-
]
n
)
K
Pu
= --------------------------------- (3.19)
{a
NO
3
-
}
4
(aq.)
[A]
3
(org. free)
D
U
(1+
n
U
[NO
3
-
]
n
)
K
U
= -------------------------------- (3.20)
{a
NO
3
-
}
2
(aq.)
[A]
3
(org. free)
Similarly, the Vant Hoff equation can be used to calculate the enthalpy change (H)
associated with the extraction process as follows,
lnK
ex
- H
------------ = ----------- (3.21)
T RT
2
On simplification and rearranging, Eq. (3.21) can be expressed as follows,
- H
logK
ex
= ---------------- (3.22)
2.303 R T
where R is the gas constant. Thus, H can be obtained from the slope of the linear
plot of logK
ex
versus temperature (1/T). Similarly, the Gibbs free energy change
(G) associated with the extraction process of the above metal ions can be calculated
from the value of K
ex
employing the following equation,
G = - RT x 2.303 x logK
ex
(3.23)
From the knowledge of the Gibbs free energy change (G) and the enthalpy change
Chapter III
70
(H), the entropy change (S) of the system is calculated from Gibbss Helmholtz
equation as follows,
G = H - TS (3.24)
3.5.2. Effect of Temperature on Distribution of Actinides
The distribution data of Am(III), Pu(IV) and U(VI) by 0.1M TODGA/n-dodecane at
different temperatures (15-45
o
C) are listed in Table 3.2. It is evidence that the D
M
values of actinide ions decreased with increase in the temperature, suggesting
exothermic nature of the biphasic reaction. Similar observations have been reported
earlier for the extraction of actinides with DMDBTDMA, CMPO and other neutral
organophosphorus extractants like tri-n-butyl phosphate (TBP) and tri-n-amyl
phosphate (TAP) [44-50]. The logK
ex
values for Am(III), Pu(IV) and U(VI) by
TODGA were calculated employing Eqs. (3.18), (3.19) and (3.20), respectively.
However, to enable such calculations it is essential to evaluate the value of
(1+
n
M
[NO
3
-
]
n
) for each of these metal ions. In the present work, the values of
nitrate complexation factors for Am(III), U(VI) and Pu(IV) at 1M HNO
3
were used
from the literature [45,48,49]. Table 3.3 compares the logK
ex
values for Am(III),
Pu(IV) and U(VI) of TODGA with those of DMDBTDMA and CMPO from nitrate
medium. In general, it was observed that logK
ex
values followed the order: TODGA
>CMPO >DMDBTDMA, which followed the order of the basicity (K
H
) of these
extractants. In case of DMDBTDMA, the logK
ex
values for different actinide ions
followed the order: Pu(IV) >U(VI) >Am(III), which was in accordance to the ionic
potential of these metal ions. On the other hand, logK
ex
values for TODGA and
Table 3.2: Distribution ratio of actinide ions at different temperature; Aqueous
phase: 1M HNO
3
; Organic phase: 0.1M TODGA / n-dodecane
Temperature
(K)
D
M
Am(III) Pu(IV) U(VI)
288 55.62 75.69 0.53
298 23.84 58.61 0.27
308 7.37 25.02 0.15
318 1.77 17.64 0.08
Chapter III
71
Table 3.3: Extraction constants for actinide ions by different extractants from
nitrate medium; Diluent: n-dodecane; Temperature: 25C
Extractants Extraction constant (logK
ex
) Reference
Am(III) Pu(IV) U(VI)
TODGA 9.4 0.04 8.6 0.03 4.9 0.01 Present work
CMPO 5.7 0.04 4.7 0.10 2.8 0.09 [37,48]
DMDBTDMA 1.3 0.06 5.4 0.02 2.2 0.04 [26,44]
CMPO were in the order: Am(III) >Pu(IV) >U(VI). The unusual observation with
respect to trivalent metal ion by TODGA and CMPO was attributed to the
stoichiometry of their extracted species. The stoichiometry of the extracted species
for actinide ions by these extractants are reported as: (i) TODGA:
Am(NO
3
)
3
4TODGA, Pu(NO
3
)
4
3TODGA and UO
2
(NO
3
)
2
3TODGA, (ii) CMPO:
Am(NO
3
)
3
3CMPO, Pu(NO
3
)
4
2CMPO and UO
2
(NO
3
)
2
2CMPO [51], and (iii)
DMDBTDMA: Am(NO
3
)
3
3DMDBTDMA, Pu(NO
3
)
4
3DMDBTDMA and
UO
2
(NO
3
)
2
2DMDBTDMA [26]. In case of TODGA and CMPO, higher solvation
number for Am(III) as compared to U(VI) and Pu(IV) could be responsible for
enhancement in the organophilicity of their complexes leading to higher logK
ex
values. As indicated by SANS studies [30], the existence of tetramers in
TODGA/alkane solution also provides a specific mechanism to explain the unusual
extraction behavior of Am(III), where tetravalent and hexavalent cations expend
more free energy to reorganize the TODGA aggregates to suit lower metal-TODGA
stoichiometries.
3.5.3. Thermodynamic Parameters (G, H and S)
The thermodynamic quantities are the important parameters for determining the
feasibility and efficiency of any process. The enthalpy change (H) for the extraction
process of actinides by TODGA was calculated from the slope of the linear
regression fits of the plot logK
ex
vs temperature (Fig. 3.6). Similarly, the G and S
values were calculated from Eqs. (3.23) and (3.24), respectively. Table 3.4 lists the
calculated thermodynamic parameters, viz. G, H and S for the extraction of
Am(III), Pu(IV) and U(VI) by TODGA. The data shows that the Gibbs free energy
Chapter III
72
3.1 3.2 3.3 3.4 3.5
4
6
8
10
Am slope : 4.578 0.46
Pu sl ope : 2.069 0.33
U slope : 2.486 0.05
l
o
g
K
e
x
1000/T (K)
Table 3.4: Thermodynamic parameters for the extraction of actinide ions at
25C; Aqueous phase: 1M HNO
3
; Organic phase: 0.1M TODGA/n-dodecane
Metal ion logK
ex
G (kJ/mol) H (kJ/mol) S (J/mol/K)
Am(III) 9.4 0.04 -53.6 0.2 -87.6 8.8 -114.1 5.5
Pu(IV) 8.6 0.03 -49.1 0.2 -39.6 6.3 31.9 3.5
U(VI) 4.9 0.01 -27.9 0.1 -47.6 2.5 -66.1 2.5
change (-G) for actinide ions followed the order: Am(III) > Pu(IV) > U(VI). More
negative G value for Am(III) shows that the formation of tetra-solvated Am(III)-
TODGA complex is thermodynamically more favoured as compared to tri-solvated
complexes of Pu(IV) and U(VI). The thermodynamics data supports the observations
made in SANS studies which suggested that the complexation of trivalent metal ions
did not disrupt the (TODGA)
4
aggregates thereby showing more negative G value
[30]. In contrast, tetra-valent and hexa-valent cations spent some free energy to
reorganize the TODGA aggregates to suit (TODGA)
3
metal stoichiometries thereby
showing lower free energy (-G) values. Similarly, higher exothermic enthalpy value
Fig. 3.6. Variation of logK
ex
with temperature; Organic phase:
0.1M TODGA/n-dodecane; Aqueous phase: 1M HNO
3
Chapter III
73
(-H) for Am(III) suggests the stronger binding of TODGA with Am(III) as
compared to Pu(IV) and U(VI) in the metal complex.
In general, the enthalpy and entropy changes in the complexation of metal
ions are largely associated with the change in the hydration of these cations [52,53].
Complexation of ligand with the metal ions results in decrease in their hydration
which increases the entropy as a result of increase in the randomness of the system.
On the other hand, dehydration process of the metal ions makes an endothermic
enthalpy contribution (+H) as a result of the breakage of metal-water and water-
water bonding in the hydrated species. Consequently, higher exothermic enthalpy
change in case of Am(III) as compared to Pu(IV) (Table 3.4) is due to less hydration
of the former metal ion which requires lesser energy for dehydration as compared to
Pu(IV). However, the over all enthalpy change depends on several contributing
factors such as, (i) dehydration of metal ions (H
1
), (ii) formation of neutral
extracted species (H
2
), and (iii) dissolution of metal complex in the organic phase
(H
3
). The values of H
3
should be more exothermic for Am(III) as the solubility of
the tetra-solvated species in the organic phase is more as compared to tri-solvated
Pu(IV) and U(VI) complex. Consequently, higher exothermic enthalpy value (-H)
was observed for Am(III) extraction. On the other hand, positive entropy contribution
during the extraction process of Pu(IV) reflects the net gain in the degrees of freedom
when the extractant molecule replaces relatively large number of water molecules
from the primary co-ordination sphere of the metal nitrate complex. On the other
hand, negative entropy changes (-S) in case of Am(III) and U(VI) extraction could
be due to the loss of translational and rotational entropy of TODGA upon
complexation in the bulky extracted species, viz. Am(NO
3
)
3
4TODGA and
UO
2
(NO
3
)
2
3TODGA. Negative entropy change also suggests the presence of few
water molecules in the extracted metal nitrate complexes.
Figure 3.7 compares the thermodynamic quantities for extraction of
Am(NO
3
)
3
by TODGA, DMDBTDMA [44] and CMPO [45]. The order of Gibbs free
energy change (-G) and enthalpy change (-H) for the extraction of Am(NO
3
)
3
was:
TODGA >CMPO >DMDBTDMA. The figure clearly demonstrates the stronger
complexation of trivalent actinide ions with TODGA as compared to CMPO and
DMDBTDMA which may be attributed to the tridentate nature of the ligand.
Consequently, it can be concluded that TODGA is a better extractant for trivalent
Chapter III
74
-100
-80
-60
-40
-20
0
TS H G
E
n
e
r
g
y
(
k
J
/
M
o
l
)
DBDBTDMA
CMPO
TODGA
actinides as compared to the other probable extractants for actinide partitioning, viz.
CMPO (TRUEX solvent) and DMDBTDMA (DIAMEX solvent). In case of
TODGA, less favorable entropy may arise due to the loss of rotational and
vibrational entropy of the large ligand molecule on strong complexation with
Am(NO
3
)
3
.
3.6. NEODYMIUM LOADING STUDIES
Though solvent extraction is a versatile technique used for the bulk separation of
metal ions on industrial scale, one of the major limitations of the technique is the
formation of third phase. Third phase formation is a phenomenon of splitting of the
organic phase into two parts, one rich in the metal/ligand or acid/ligand complex and
the other is lean in ligand and rich in diluent. When the concentration of polar metal-
ligand complex (or acid-ligand complex) in the organic phase exceeds certain limit, it
becomes insoluble in the non-polar aliphatic diluents, thus forming third phase. The
maximum concentration of the metal ions that can be loaded in the organic phase
without third phase formation is referred to as Limiting Organic Concentration
(LOC). Since the concentrations of metal ions present in HLW are large, many of the
proposed extractants for actinide partitioning (such as CMPO and DMDBTDMA)
Fig. 3.7. Thermodynamic parameters for extraction of Am(NO
3
)
3
by different extractants; diluent: n-dodecane; Temperature: 25C
Chapter III
75
Table 3.5: Extraction of Nd and HNO
3
by TODGA; Aqueous phase: 3M HNO
3
;
Diluent: n-dodecane; Temperature: 25C
[TODGA], M LOC of Nd, g/L D
H
+
0.1 1.06 0.034
0.2 1.73 0.077
0.3 4.46 0.110
0.5 8.64 0.173
0.8 14.4 0.281
form third phase under HLW loading conditions, and hence require suitable phase
modifiers [44,45]. Thus, the phenomenon of third phase formation is of great
importance in actinide partitioning which explains our motivation to study this
phenomenon in detail for TODGA system.
In the case of TODGA/n-dodecane, though D
Am
value is high, yet it shows
third phase formation at very low concentration of metal ions in the organic phase.
The LOC value for Nd(III) at different TODGA concentration is listed in Table 3.5,
which shows increased Nd(III) LOC values with TODGA concentration. At the same
time, the extraction of HNO
3
(D
H+
) also increased with TODGA concentration which
opposes the back extraction of Nd(III) from organic phase [54]. For example, Nd(III)
extracted by 0.1M TODGA could be back extracted completely in two contacts with
distilled water. On the other hand, when 0.5M TODGA was used, more than five
contacts were required for complete stripping of the metal ion. In addition, large
ligand concentration may result in co-extraction of unwanted metal ions (which do
not fall in the category of long-lived radionuclides) thereby affecting the efficiency of
the process. Therefore, low concentration of TODGA (0.1M) is recommended. The
LOC of Nd(III)(III) by 0.1M TODGA/n-dodecane at different HNO
3
concentration is
presented in Fig. 3.8. The LOC values of Nd(III)(III) decreased with increased
aqueous feed acidity which could be due to enhanced extraction of HNO
3
at higher
acidities. At 3M HNO
3
, the LOC of Nd(III)(III) was quite low (~1g/L). It is logical to
conclude that TODGA will form third phase under HLW conditions which contains
significant concentration of lanthanides and other extractable ions. The formation of
third phase during counter-current extraction may cause flooding affecting severely
Chapter III
76
1.0 1.5 2.0 2.5 3.0 3.5
0.0
0.4
0.8
1.2
1.6
2.0
L
O
C
o
f
N
d
,
g
/
L
[HNO
3
], M
the hydrodynamics of the contactors. It is, therefore, imperative to evaluate suitable
phase modifiers so as to eliminate the third phase formation and also to increase the
metal loading capacity in the organic phase if TODGA has to be used on the process
scale.
3.6.1. Evaluation of Phase Modifiers
Third phase formation is a phenomenon arising due to the incompatibility of the
polar metal solvate species with non-polar diluents (such as n-dodecane)
recommended for industrial processes [55]. The third phase is often eliminated by
addition of diluent modifiers such as long chain alcohols, monoamides, organic
phosphates, etc., which are capable of solvation of metal-ligand / acid-ligand
complexes through either dipole-dipole interaction or hydrogen bonding [55]. In this
context, N,N-dialkyl monoamides with varying alkyl groups and chain lengths, viz.
dibutyl decanamide (DBDA), di(2-ethylhexyl) acetamide (D2EHAA), di(2-
ethylhexyl) propionamide (D2EHPRA), di(2-ethylhexyl) isobutyramide (D2EHIBA),
dihexyl octanamide (DHOA) and dihexyl decanamide (DHDA) were evaluated as the
phase modifiers. Use of long chain alkyl amides as phase modifiers has been reported
Fig. 3.8. Limiting organic concentration of Nd at different feed acidity;
Extractant: 0.1M TODGA/n-dodecane; Temperature: 25C
Chapter III
77
Table 3.6: Limiting organic concentration of Nd(III) at 3M HNO
3
; Organic
phase: 0.1M TODGA and phase modifier; Diluent: n-dodecane; Temp.: 25C
0.1M TODGA +
Phase modifier
LOC of Nd(III), (g/L)
1-Decanol D2EHAA DHOA TBP
0.0 1.06 1.06 1.06 1.06
0.2 2.25 2.61 2.70 3.20
0.5 3.74 4.25 4.90 6.17
0.8 3.89 4.31 4.85 6.91
1.0 3.92 4.32 4.90 7.05
in the literature [56]. For 0.1M TODGA +0.5M amides / n-dodecane solution, the
D
Am
values at 1M HNO
3
varied in the order: 12.9 (D2EHAA) >10.7 (DHOA) >7.5
(DBDA) >7.0 (DHDA) >6.5 (D2EHPRA) >6.0 (D2EHIBA). In view of higher D
Am
values, Nd(III) loading studies were conducted using D2EHAA as phase modifier
and the results were compared with the values of DHOA. In addition to monoamides,
a long chain alcohol such as 1-decanol and an organic phosphate like TBP were also
studied as the phase modifiers. The loading of Nd(III) in 0.1M TODGA/n-dodecane
containing varying concentration of phase modifiers (0.11M) was investigated and
the results are summarized in Table 3.6. The LOC value increased with the phase
modifier concentration and reached the maximum value at 0.5M for phase modifiers
studied. It should be noted that no third phase was observed beyond 0.5M
concentration of phase modifiers even when Nd(III) concentration in the aqueous
phase was 20g/L Nd(III).
The loading of Nd(III) in 0.1M TODGA +0.5M phase modifiers as well as
D
Nd
as a function of initial Nd(III) concentration are given in Fig. 3.9. The Nd(III)
concentration in the organic phase increased gradually and attained the maximum
values for DHOA, D2EHAA and Decanol. However, in case of TBP, the maximum
loading value could not reach in the concentration range presented in the figure.
Though LOC value of Nd(III) was higher when TBP was employed as phase
modifier, better loading as well as D
Nd
was observed for DHOA at lower aqueous
metal concentration. It should be noted that the total concentration of lanthanides in
HLW is lower than 4g/L and depends on the burn up of the fuel. Therefore, data in
Fig. 3.9 referring to Nd(III) concentration in this range is relevant to the partitioning
Chapter III
78
2 4 6 8 10 12 14 16
0
1
2
3
4
5
6
7
0
1
2
3
4
5
6
7
D
N
d
[Nd]
int.
, g/L
[
N
d
]
o
r
g
.
,
g
/
L
DHOA
D2EHAA
TBP
Decanol
DHOA
D2EHAA
TBP
Decanol
of actinides from HLW. As it is evident from Fig.3.9, DHOA seems to be a better
choice as phase modifier as compared to the others for HLW. Use of DHOA would
also be preferred over TBP as the latter is a phosphorus based molecule which
requires special chemical treatment during disposal of the spent extractant after their
useful life. Further studies were, therefore, carried out employing 0.5M DHOA as the
phase modifier.
3.7. EXTRACTION OF ACTINIDES AND OTHER METAL IONS
The extraction behaviour of several metal ions, which are important from the actinide
partitioning point of view, was investigated from nitric acid medium. Fig. 3.10
depicts the distribution behaviour of different metal ions, viz. Am(III), U(VI),
Pu(IV), Fe(III), Eu(III), Sr(II) and Cs(I) from HNO
3
solution using 0.1M TODGA +
0.5M DHOA as the extractant. The D
M
values increased gradually for Eu(III),
Am(III), Pu(IV) and U(VI) with aqueous phase acidity upto 2M HNO
3
and were
nearly steady thereafter for all the metal ions. Increase in the D
M
value with acidity
can be explained by the law of mass action as depicted in the equilibrium reaction
(3.8). However, with increased acidity, law of mass action is countered by decreased
Fig. 3.9. Nd loading and D
Nd
as a function of Nd concentration; Extractant: 0.1M TODGA +
0.5M phase modifiers / n-dodecane; Aqueous phase acidity: 3M HNO
3
; Temperature: 25C
Chapter III
79
0 1 2 3 4 5 6 7
10
-4
10
-3
10
-2
10
-1
10
0
10
1
10
2
10
3
Am; U
Pu; Sr
Eu; Cs
Fe
D
M
[HNO
3
],M
free ligand concentration due to the formation of TODGA-HNO
3
adduct, thereby
giving plateau at higher acidity. Steady D
M
values of metal ions between 2-6M HNO
3
is important for the process applications as it implies that frequent adjustment of the
feed acidity is not required. The distribution values of actinide (An) ions followed the
order: An(III) ~An(IV) >An(VI). As depicted by the thermodynamic data (Section
3.4.3), more negative G values of Am(III) suggested that the tetra-solvated An(III)-
TODGA complex is thermodynamically more favoured as compared to tri-solvated
species of An(IV) and An(VI) ions. It is worth mentioning that TODGA also shows
some extraction of Sr(II) from nitric acid medium. Suzuki, et al. [57] reported that
D
Sr
value with 0.1M TODGA/n-dodecane increased with the nitric acid concentration
to maximum of 4 at 3M HNO
3
and decreased thereafter. On the other hand, the same
extractant exhibited constant D
Am
values between 2-6M HNO
3
(>300). The
separation factor between Am(III) and Sr(II) (SF =D
Am
/D
Sr
) was ~100 at 1-2M
HNO
3
. However in present extraction system, the addition of 0.5M DHOA as phase
modifier reduced the D
Sr
value significantly from 4 (in the absence of DHOA) to 0.9
at 3M HNO
3
without affecting the distribution ratio of Am(III) giving a separation
Fig. 3.10. Variation of D
M
with aqueous phase acidity; Extractant:
0.1M TODGA + 0.5 DHOA in n-dodecane; Temperature: 25
o
C
Chapter III
80
factor (SF) value of ~300. Though Sr(II) is partially co-extracted along with
actinides, it can be selectively stripped (scrubbed) with 6M HNO
3
separating it from
actinides. Negligible extraction of Fe(III) and Cs(I) was observed as D
M
values for
both the metal ions were <10
-2
in the entire range of acidity investigated. These
findings suggested the possible application of TODGA extraction system for
selective extraction of actinides over fission products and structural materials from
HLW solution.
Fig. 3.11 shows the extraction behaviour of actinides, fission products and
structural element from simulated high level waste (SHLW). The concentrations of
various metal ions in SHLW solution are given in Table 3.7. It should be noted that
~20g/L of U is also present in HLW which is eliminated quantitatively by repeated
Table 3.7: Composition of a typical Simulated High-Level Waste (SHLW)
solution of Pressurised Heavy Water Reactor; Acidity: 3M HNO
3
Constituent Concentration (mg/L) Constituent Concentration (mg/L)
Se
b
12.3 Rb
a
74.5
Sr
a
186.3 Y
d
99.0
Zr
a
771.3 Mo
b
731.3
Ru
d
463.8 Co
a,f
127.5
Pd
d
267.5 Ag
a
18.6
Cd
a
16.3 Sn
b
15.6
Sb
b
4.7 Te
b
102.8
Cs
a
543.8 Ba
a
308.8
La
d,e
263.8 Ce
a
532.5
Nd
c
862.5 Eu
c
22.6
Sm
c
163.8 Fe
b
500
Na
a
3000 Cr
a
100.0
Ni
a
100 Mn
a,g
181.3
U
a
20000 Tb
c
5.0
Pr
c
243.8 Dy
c
2.0
Gd
c
165.0
a: Nitrate salt; b: Metal powder; c: Oxide; d: Chloride salt; e: Taken in place of Pm;
f: Taken in place of Rh, and g: Taken in place of Tc.
Chapter III
81
1 2 3 4 5 6 7
10
-4
10
-3
10
-2
10
-1
10
0
10
1
10
2
10
3
Am; U; Pu
Sr; Eu; Cs; Fe
D
M
[HNO
3
], M
contacts with 30% TBP to avoid unwanted loading of the organic phase during
partitioning of long-lived actinides, viz.
241
Am,
243
Am,
244
Cm and
237
Np.
Accordingly, SHLW was prepared without uranium. As shown in Fig. 3.11, the D
M
values were lower for all the metal ions in the presence of SHLW as compared to
pure tracers, which is attributed to the co-extraction of other metal ions present in
SHLW. Though D
M
value was lower in the presence of SHLW, it was significantly
higher for the quantitative and efficient extraction of actinides. Typically for SHLW
at 3M HNO
3
, D
M
values for Eu(III), Am(III), Pu(IV), U(VI), and Sr(II) were 98, 62,
110, 6.2 and 0.08, respectively. Though there was a decrease in the distribution ratio
of all metal ions, D
Sr
value decreased significantly (~one tenth of the pure tracer
value) to an acceptable level. Typical SF values for the metal ions observed at 3M
HNO
3
was: 1375 (Pu/Sr), 1225 (Eu/Sr) and 775 (Am/Sr).
The selective extraction of trivalent actinides, viz. Am(III) and Cm(III)
present in HLW is very complex owing to the large competition effect due to
presence of trivalent lanthanides (Ln(III)). The Ln(III) have almost similar chemical
properties to those of An(III) and present at several times higher concentration than
Fig.3.11. Variation of D
M
with aqueous phase acidity of SHLW; Extractant:
0.1M TODGA + 0.5M DHOA in n-dodecane; Temperature: 25
o
C
Chapter III
82
the later. So owing to the complexity of the selective removal of An(III), the
separation process can be split into two steps. The first step consists of co-extraction
of An(III) and Ln(III) aiming to eliminate all the alpha activities along with the
fission products from HLW. The second step consists of the group separation of
Ln(III) and An(III). Several processes for lanthanide/actinide partitioning have been
discussed in the recent review [58]. Our findings clearly demonstrate the possible
application of TODGA extraction system for co-extraction of An(III) and Ln(III)
from HLW solution.
3.8. STABILITY OF TODGA
In order to use the given extraction system for the separation of metal ions from
HLW, it is essential to acquire the knowledge of radiation as well as hydrolytic
stability of the given reagent. Sugo, et al. [59] have demonstrated the superior
hydrolytic stability of TODGA. They found no degradation product when 0.1M
TODGA in dodecane was contacted with 3M HNO
3
for four weeks. In the present
work, reusability of the organic phase was investigated by contacting with SHLW
followed by stripping with 0.01M HNO
3
in five successive cycles. The stability of
the resultant lean organic phase was then evaluated by determining D
Am
value at 1M
HNO
3
(D
Am
at 1M HNO
3
was significant from analytical point of view). It was
observed that even after five successive cycles of loading and stripping, the D
Am
was
Table 3.8: Distribution data of Am(III) in five successive recycling stages;
Organic phase: 0.1M TODGA + 0.5M DHOA / n-dodecane
Number of cycles D
Am
at 1M HNO
3
1 10.01
2 10.93
3 9.89
4 10.38
5 10.86
Mean 10.41
S.D. 0.48
R.S.D. (%) 4.57
Chapter III
83
almost constant with the original value of 100.5 (Table 3.8), suggesting the good
stability of the TODGA-DHOA-n-dodecane system.
The radiation stability of TODGA was evaluated by determining D
Am
for the
irradiated organic phase. A solution of 0.1M TODGA in dodecane was irradiated at
room temperature with gamma rays from
60
Co source at an exposure dose of 1.8
kGyh
1
. Samples were removed after suitable time interval to get an irradiation dose
ranging from 0.0 to 1.8MGy. The distribution ratio of Am(III) as well as the physical
properties such as density and viscosity of the irradiated sample was determined and
listed in Table 3.9. It was observed that the D
Am
value at 3M HNO
3
did not show any
significant change upto 0.46MGy, however, it decreased almost linearly with the
irradiation dose from 0.46MGy to 1.8MGy. Though most of the radioactivity of the
HLW is due to beta / gamma activities of the fission products, alpha particles from
the minor actinides have higher linear energy transfer (LET) and hence, can cause
significant damage to the reagent. Alpha dose rate of HLW is ~0.4MGy/h in a
solution containing 1g/L
241
Am. Under similar conditions of dose exposure, no
significant decrease in the D
Am
was observed (Table 3.9). This observation is an
evidence suggesting that TODGA can be used for treating HLW without significant
radiolysis, though it undergoes radiolysis at higher radiation dose. As listed in Table
3.9, the physical parameters of the irradiated TODGA are influenced by the
concentrations of the various degradation products. It was observed that though the
density was almost insensitive to the dose, the dynamic viscosity increased steadily
Table 3.9: Distribution data for Am(III) and physical parameters of the
irradiated 0.1M TODGA / n-dodecane solution
Dose
(MGy)
Density
(gm/L)
Dynamic viscosity
(mPa)
D
Am
( 3M HNO
3
)
0.00 0.759 1.753 248.84
0.26 0.760 1.761 247.24
0.46 0.760 1.768 210.51
0.78 0.761 1.789 42.01
1.12 0.762 1.831 5.31
1.81 0.764 1.956 0.13
Chapter III
84
(by ~10%) on irradiating the sample. Sugo, et al. [59] studied the radiolytic stability
of TODGA and reported that the G-value (number of molecules decreased after
absorption of 100eV of energy) as 8.5 0.6 molecules / eV. They reported an
increased radilolytic stability of TODGA with the addition of benzene as well as
DHOA. The main degradation products of TODGA are dioctylamine, dioctyl
acetamide, dioctyl glycolamide and dioctyl formamide because of the breaking of
amide bonds as well as bonds in the vicinity of the ether oxygen.
3.9. COUNTER-CURRENT EXTRACTION
Solvent extraction is a versatile technique used in the industrial scale separation of
metal ions utilizing centrifugal extractors or pulsed columns. The performance of
TODGA for the extraction of metal ions on pilot scale was demonstrated in counter
current mode for the extraction of Nd(III) employing a 16 stages mixer settler
system. The details of the mixer-settler units have been described in Chapter-II.
3.9.1. Optimization of Parameters
Counter-current extraction of Nd(III) was carried out using 0.1M TODGA +0.5M
DHOA / NPH as the extractant. However, various process parameters were
optimized in the batch experiments before the actual mixer-settler runs. In order to
find out the number of contacts required for quantitative extraction of Nd(III), fresh
organic phase was contacted repeatedly with the same aqueous phase O/A=1. As
Table 3.10: Batch extraction and stripping of Nd(III); Organic phase: 0.1M
TODGA + 0.5M DHOA / NPH; Aqueous phase: 3.87g/L Nd(III) at 3.5M HNO
3
;
Strippant: 0.01M HNO
3
; O/A=1
No. of stages Loading stages; [Nd], g/L Stripping stages; [Nd], g/L
Org. phase Aq. phase Org. phase Aq. phase
I 3.41 0.46 0.66 3.19
II 0.46
#
ND 0.01 0.65
III
#
ND
#
ND
#
ND 0.01
IV
#
ND
#
ND
#
ND
#
ND
#
: Not detectable
Chapter III
85
0 1 2 3 4 5 6
0
1
2
3
4
5
[
N
d
]
o
r
g
.
,
g
/
L
[Nd]
aq.
, g/L
shown in Table 3.10, two contacts were sufficient for the complete extraction of
Nd(III) from a solution containing 3.87g/L of Nd(III) at 3.5M HNO
3
. Similarly,
stripping of the loaded organic phase was carried out repeatedly with 0.01M HNO
3
solution when quantitative stripping of Nd(III) was achieved in three contacts at O/A
ratio =1.
The number of theoretical stages required for quantitative extraction and
stripping of metal ions in dynamic conditions is determined by graphical method
using McCabe-Thiele diagram. In the construction of McCabe-Thiele diagram the
extraction isotherm, which represents the relation between the equilibrium
concentration of Nd(III) in the aqueous raffinate to that in the organic phase, was first
drawn as shown in Fig. 3.12. A vertical line is then drawn from the concentration of
Nd(III) in the feed solution on the x-axis. The operating line was then inserted, the
slope of which was equal to the phase ratio (A/O =1). The figure suggested that for
O/A=1, four extraction stages were needed for complete extraction of Nd(III) from a
solution containing 3.87g/L Nd(III). Similar plot was also constructed for stripping
stages employing 0.01M HNO
3
, which suggested that four stages were sufficient for
Fig. 3.12. McCabe-Thiele diagram for extraction of Nd; Extractant:
0.1M TODGA + 0.5M DHOA / NPH; Aqueous phase: 3.5M HNO
3
Chapter III
86
quantitative stripping of Nd(III) from the loaded organic phase at O/A =1. With the
knowledge of these parameters, counter-current extraction experiments were
conducted employing a 16 stages mixer settler system.
3.9.2. Mixer-Settler Runs
In the 16 stages integral mixer-settler run, 9-16 stages were used for extraction,
whereas 1-8 stages were employed for stripping. A schematic diagram representing
the mixer-settler stages for the integral run is shown in Chapter-II (Fig. 2.5). The
extractant employed was 0.1M TODGA +0.5M DHOA in NPH and the aqueous
feed solution was 3.8g/L Nd at 3.5M HNO
3
. Though 0.01M HNO
3
was found to be
effective for stripping of Nd(III) in batch experiments, preliminary experiments using
0.01M HNO
3
solution as strippant in the mixer-settler resulted in poor phase
separation (higher disengagement time). Subsequently, when 0.2M HNO
3
was
employed as strippant, satisfactory phase separation was achieved promptly. The
reason for the fast phase separation was attributed to increase in the interfacial
tension primarily due to increase in ionic strength. Quantitative stripping of Nd(III)
was also observed in three contacts with 0.2M HNO
3
in batch experiments. The flow
rates of feed solutions to the mixer settler units were maintained constant at
4.50.2mL/min to maintain the O/A ratio of one in extraction as well as in stripping
stages. The system was operated for ~7hrs; the organic as well as aqueous samples
from each settler units were analyzed for Nd(III) as well as acidity content
periodically. The results suggested that the steady state was attained within 2 hrs
when acidity as well as Nd(III) concentration in the aqueous product and in the
raffinate achieved a constant value.
Fig. 3.13 shows the profile of Nd(III) concentration in the organic phase as
well as in the aqueous phase at different stages of mixer-settler unit. It can be seen
from the figure that as the aqueous feed comes in contact with the organic phase at
9
th
stage, Nd(III) was rapidly extracted and quantitative extraction was achieved in
five stages. The extraction of Nd(III) in few stages underlines the fact that TODGA is
very efficient extractant for trivalent lanthanides / lanthanides. This feature was also
reflected in the aqueous profile of Nd(III) concentration at different stages when the
aqueous Nd(III) concentration decreased sharply (stages 9-13) and finally, the
aqueous raffinate comes out of the system without any Nd(III). Similarly, when the
Chapter III
87
Fig. 3.13. Nd concentration profile at different stages of mixer-settler units, Aqueous phase:
3.8g/L Nd at 3.5M HNO
3
; Extractant: 0.1M TODGA + 0.5M DHOA in NPH; Strippant:
0.2M HNO
3
; Extraction stages O/A = 1; Stripping stages O/A = 1; Temperature: 25C
loaded organic phase comes in contact with the strippant at 8
th
stage it could be
stripped quantitatively in three stages. The acid profile at different stages suggested
that ~0.37M HNO
3
was also extracted in the organic phase, which was subsequently
stripped in three stages. Therefore, the lean organic phase coming out from the
system was free from any acid and metal ion.
In another set of experiment, the organic to aqueous phase ratio was
maintained as one in the extraction stages, while it was maintained as two in the
stripping stages by reducing the flow rate of the strippant to half i.e. 2.2mL/min. It
was observed that the Nd(III) concentration profile in the extraction stages was
identical to that observed with the first run due to the identical conditions. However,
Nd(III) concentration in the stripping stages was significantly enhanced and
quantitative stripping could be achieved in four stages. The two times concentration
of Nd(III) in the product depicts the excellent extraction efficiency of the TODGA
system. Negligible loss of Nd(III) was observed for both the runs as the Nd(III)
concentration in the lean organic phase was <0.001g/L. Our findings suggested the
16 14 12 10 8 6 4 2
0
1
2
3
4
16 14 12 10 8 6 4 2
[Nd]
aq
[Nd]
org
[
N
d
]
,
m
g
/
m
L
Extractant In
Lean organic Out
Strippant In Product Out Aq. feed In
Aq. raffinate Out
Stripping stages
Stage Number
Extraction stages
Chapter III
88
possible application of the present solvent system for the partitioning of actinides
from HLW solution.
3.10. REFERENCES
1. R.J.M. Konings and J.L. Kloosterman, Progress in Nucl. Energy, 38 (2001) 331.
2. Proceedings of the Seventh Information Exchange Meeting on Actinide and
Fission Product Partitioning and Transmutation, J eju, South Korea, October 14
16, 2002.
3. C. Madic, M.J. Hudson, J.O. Liljenzin, J.P. Glatz, R. Nannicini, A. Facchini, Z.
Kolarik and R. Odoj, New Partitioning Techniques for Minor Actinides,
European Commission Report, EUR 19149, European Union, Brussels, 2000.
4. C. Cuillerdier, C. Musikas and L. Nigond, Sep. Sci. Technol., 28 (1993) 155.
5. V.K. Manchanda and P.N. Pathak, Sep. Purif. Technol., 35 (2004) 85.
6. T.H. Siddall, J. Phys. Chem., 64 (1960) 1863.
7. C. Musikas, Inorg. Chim. Acta, 140 (1987) 197.
8. C. Musikas, Sep. Sci. Technol., 23 (1988) 1211.
9. H. Stephan, K. Gloe, J. Bager and P. Muhl, Solv. Extr. Ion Exch., 9 (1991) 435.
10. H. Stephan, K. Gloe, J. Bager and P. Muhl, Solv. Extr. Ion Exch., 9 (1991) 459.
11. K. Matloka, A. Gelis, M. Regalbuto, G. Vandegrift and M.J. Scott, Dalton Trans.
(2005) 3719.
12. M. Hirata, P. Guilbaud, M. Dobler and S. Tachimori, Phys. Chem. Chem. Phys.,
5 (2005) 691.
13. Z. Zhu, Y. Sasaki, H. Suzuki and T. Kimura, Anal. Chim. Acta, 527 (2004) 163.
14. S.P. Kusumocahyo, T. Kanamori, T. Iwatsubo, K. Sumaru, T. Shinbo, H.
Matsuyama and M. Teramoto, J. Appl. Polym. Sci., 102 (2006) 4372.
15. E.A. Mowafy and H.F. Aly, Solv. Extr. Ion Exch., 25 (2007) 205.
16. Y. Sasaki, H. Suzuki, Y. Sugo, T. Kimura and G.R. Choppin, Chem. Lett., 35
(2006) 256.
17. Y. Sasaki and G.R. Choppin, Anal Sci., 12 (1996) 225.
18. Y. Sasaki and G.R. Choppin, J. Radioanal. Nucl. Chem., 207 (1997) 383.
19. Y. Sasaki, T. Adachi and G.R. Choppin, J. Alloy comp., 271-273 (1998) 799.
20. Y. Sasaki and G.R. Choppin, Radiochim. Acta, 80 (1998) 85.
21. Y. Sasaki and G.R. Choppin, J. Radioanal. Nucl. Chem., 246 (2000) 267.
Chapter III
89
22. H. Narita, T. Yaita, T. Kimura and S. Tachimori, Radiochim. Acta, 81 (1998)
223.
23. Y. Sasaki, Y. Sugo, S. Suzuki and S. Tachimori, Solv. Extr. Ion Exch., 19 (2001)
91.
24. E.P. Horwitz, K.A. Martin, H. Diamond and L. Kaplan, Solv. Extr. Ion Exch., 4
(1986) 449.
25. K.A. Martin, E.P. Horwitz and J.R. Ferraro, Solv. Extr. Ion Exch., 4 (1986) 1149.
26. G.R. Mahajan, D.R. Prabhu, V.K. Manchanda and L.P. Badheka, Waste
Management, 18 (1998) 125.
27. P.W. Naik, P.S. Dhami, S.K. Misra, U. Jambunathan and J.N. Mathur, J.
Radioanal. Nucl. Chem., 257 (2003) 327.
28. G. Suresh, M.S. Murali and J.N. Mathur, Radiochim. Acta, 91 (2003) 127.
29. S. Tachimori, Y. Sasaki and S. Suzuki, Solv. Extr. Ion Exch., 20 (2002) 687.
30. M.P. Jensen, T. Yaita and R. Chiarizia, Langmuir, 23 (2007) 4765.
31. R. Chiarizia and A. Briand, Solv. Ext. Ion Exch., 25 (2007) 351.
32. C. Erlinger, L. Belloni, T. Zemb and C. Madic, Langmuir, 15 (1999) 2290.
33. B. Abecassis, F. Testard, T. Zemb, L. Berthon and C. Madic, Langmuir, 19
(2003) 6638.
34. L. Lefrancois, J. Delpuech, H. Hebrant, J. Chrisment and C. Tondre, J. Phys.
Chem. B, 105 (2001) 2551.
35. G.R. Choppin and M.P. Jensen, Actinides in Solution: Complexation and
Kinetics, The Chemistry of the Actinide and Transactinide Elements, 3
rd
Ed.,
L.R. Morss, N.M. Edelstein and J. Fuger (Eds.), Springer, Netherlands (2006),
Vol. 4, P. 2524.
36. A.S. Ketres and H. Guttmann, Surfactants in Organic Solvents: The Physical
Chemistry of aggregation and Micellization, Surface and Colloid Science, E.
Matijevic (Ed.), Wiley Interscience, New York (1976), Vol. 8, P. 193-295.
37. O.A. El Seoud, E.J. Fendler, J.H. Fendler and R.T. Medary, J. Phys. Chem., 77
(1973) 1876.
38. H. Narita, T. Yaita and S. Tachimori, Extraction behavior of trivalent lanthanides
with amides and EXAFS study of their complexes. In Solvent Extraction for the
21
st
Century, Proceedings of ISEC99, Barcelona, Spain, July, 1999; M. Cox, M.
Chapter III
90
Hidalgo, M. Valiente (Eds.), Society of Chemical Industry: London (2001), Vol.
1, P. 693-696.
39. Y. Sasaki, P. Rapold, M. Arisaka, M. Hirata and T. Kimura, Solv. Ext. Ion Exch.,
25 (2007) 187.
40. E.P. Horwitz, K.A. Martin and H. Diamond, Solv. Ext. Ion Exch., 6 (1988) 859.
41. M. Gazith, Activity coefficients of various electrolytes, Israel Atomic Energy
Commission, Soreq Research Establishment, October 1964, IA-1004.
42. D.G. Kalina, G.W. Mason and E.P. Horwitz, J. Inorg. Nucl. Chem., 43 (1980)
159.
43. G.R. Choppin and W.F. Strazik, Complexation of trivalent lanthanide and
actinide ions. I. Outer-sphere ion pairs. Inorg. Chem., 4 (1965) 1250.
44. L.B. Kumbhare, D.R. Prabhu, G.R. Mahajan, S. Sriram and V.K. Manchanda,
Nucl. Technol., 139 (2002) 253.
45. J.N. Mathur and K.L. Nash, Solv. Extr. Ion Exch., 16 (1998) 1341.
46. S.V. Bagawade, P.R. Vasudeva Rao, V.V. Ramakrishna and S.K. Patil, J. Inorg.
Nucl. Chem., 40 (1978) 1913.
47. A. Ramanujam, V.V. Ramakrishna and S.K. Patil, J. Inorg. Nucl. Chem., 40
(1978) 1167.
48. T.G. Srinivasan, P.R. Vasudeva Rao and D.D. Sood, Solv. Extr. Ion Exch., 15
(1997) 15.
49. T.G. Srinivasan, P.R.V. Rao and D.D. Sood, Radiochim. Acta, 85 (1999) 97.
50. J.N. Mathur, P.B. Ruikar, M.V. Balramakrishna, M.S. Murali, M.S. Nagar and
R.H. Iyer, Radiochim. Acta, 73 (1996) 199.
51. J.N. Mathur, M.S. Murali and P.R. Natarajan, Talanta, 39 (1992) 439.
52. G.R. Choppin, Radiochim. Acta, 32 (1983) 43.
53. G.R. Choppin and A. Morgenstern, Solv. Extr. Ion Exch., 18 (2000) 1029.
54. Y. Sasaki, Y. Sugo, S. Suzuki and T. Kimura, Anal. Chim. Acta, 543 (2005) 31.
55. P.R. Vasudeva Roa and Z. Kolarik, Solv. Extr. Ion Exch., 14 (1996) 955.
56. B.F. Smith, K.V. Wilson, R.R. Gibson, M.M. Jones and G.D. Jarvinen, Sep. Sci.
Technol., 32 (1997) 149.
57. H. Suzuki, Y. Sasaki, Y. Sugo, A. Apichaibukol and T. Kimura, Radiochim.
Acta, 92 (2004) 463.
Chapter III
91
58. H.N. Dam, D.N. Reinhoudt and W. Verboom, Chem. Soc. Rev., 36 (2007) 367.
59. Y. Sugo, Y. Sasaki and S. Tachimori, Radiochim. Acta, 90 (2002) 161.
-------------------------
Chapter IV
Extraction Chromatographic
Studies On Actinides And Other
Metal Ions Using N,N,N,N-
Tetraoctyl Diglycolamide As The
Stationary Phase
Chapter IV
92
EXTRACTION CHROMATOGRAPHIC STUDIES ON ACTINIDES
AND OTHER METAL IONS USING N,N,N,N-TETRAOCTYL
DIGLYCOLAMIDE AS THE STATIONARY PHASE
4.1. INTRODUCTON
In view of their continuous nature, solvent extraction (SX) processes have been
extensively employed in industrial scale operations for the recovery of valuable metal
ions. However, the major problem associated with SX technique is the generation of
large volume of secondary waste and handling of large volume of inflammable
diluents. There are other techniques which are of relevance if the recovery /
separation of metal ions are demanded from limited volume solutions of low
concentrations where the secondary waste volume and volume of inflammable
diluents are reduced significantly. Such techniques include liquid membrane (LM)
[1-3], magnetically assisted chemical separation (MACS) [4-6] and extraction
chromatography (EXC) [7-10]. Amongst these techniques, EXC has played an
increasingly prominent role in the separation and pre-concentration of a number of
radionuclides, particularly actinides and selected fission products [11,12]. EXC is
often described as a technique that combines the selectivity of solvent extraction with
the ease of operation of chromatographic method. A general relationship between the
SX and EXC has been discussed by several authors [13-16]. Recently Horwitz, et al.
[16] illustrated the most significant similarities and differences between EXC and SX
systems. It was shown that the selectivities for all lanthanides were similar for the
two techniques for a given extractant. However, one difference between SX and EXC
is the change in the activities of the extractant and the extracted complex due to the
influence of solid support. The other difference is the non-attainment of
thermodynamic equilibrium when extraction chromatography is performed in a
column. Nevertheless, this does not influence the transfer of solutes from one phase
to another [14].
The liquid (or partition) chromatography (LC) is a separation technique
which involves predominantly a simple partitioning of solute between two
immiscible liquid phases, one is stationary and other the mobile phase. In classical
normal phase LC, the stationary phase is polar and mobile phase is non-polar. In
Chapter IV
93
contrast, the reverse phase LC utilizes a hydrophobic bonded stationary phase and a
polar mobile phase, usually an aqueous mobile phase. EXC resembles reverse phase
LC technique which is based on the preferential extraction of metal ions from an
aqueous solution by organic ligands physically impregnated or chemically anchored
on an inert solid support [17,18]. The extractant molecules are sorbed into the pores
of the solid support through interaction between the surface of the support containing
moderate polar groups (e.g. C=O, Si=O) and hydrophobic regions of the extractant
molecules by van der Waals interactions. The possibility of varying the capacity of
extraction chromatographic resins by loading varying amount of extractants on the
solid support is another interesting feature of these resins. It is also possible to
remove the entire amount of loaded extractants from the support material, which
would help in disposing off the resin materials after their useful life. Excellent
separations of elements from aqueous solutions containing high acid concentrations
have been achieved by this technique [19]. Several extractants such as tri-n-butyl
phosphate (TBP) [20], octyl(phenyl)-N,N-diisobutyl carbamoyl methyl phosphine
oxide (CMPO) [21], N,N-dimethyl-N,N-dibutyl tetradecyl malonamide
(DMDBTDMA) [22], trialkyl phosphine oxide (TRPO) [23], and bis-2-diethylhexyl
phosphoric acid (HDEHP) [24,25] have been used in EXC for the recovery of
actinides and other metal ions from aqueous solutions. Horwitz, et al. [17,18] have
used a mixture of CMPO and TBP (TRUEX solvent) in EXC technique to study the
recovery of various actinide elements.
N,N,N,N-tetraoctyl diglycolamide (TODGA) has shown good promise in
SX studies for the recovery of actinides / lanthanides from various acidic solutions.
The detailed SX studies of actinides / fission product elements by TODGA have been
discussed in Chapter-III. The present chapter deals with the extraction
chromatographic separation of several actinides and fission products employing
TODGA as the stationary phase. The performance of TODGA-EXC column has been
described in terms of column breakthrough capacity. The results obtained with this
resin have been compared with those of other proposed extractants for actinide
partitioning, viz. CMPO, DMDBTDMA and TRPO as the stationary phases.
4.2. PREPARATION OF CHROMATOGRAPHIC RESIN
The extraction chromatographic resin (EXC-resin) material was prepared by
Chapter IV
94
impregnating TODGA on chromosorb-W, an inert solid support. Chromosorb-W
(dimethyl dichlorosilane treated acid washed celite diatomaceous silica, 60-80
Mesh), obtained from Johns Manville, was washed with distilled water and acetone
to remove any adsorbed matter followed by air-drying before use. Chromosorb-W
has been reported to be an excellent solid support for actinide-partitioning employing
DMDBTDMA as the stationary phase [22]. In a flask containing a known amount of
TODGA diluted in acetone (1:1) was mixed with equal weight of the solid support.
The slurry was then equilibrated for 24 hours in a mechanical shaker followed by the
removal of solvent by flushing nitrogen gas with gentle stirring. The resultant resin
was vacuum dried to constant weight to get TODGA impregnated resin. This method
of resin preparation has been reported in literature using volatile solvents such as
methanol, acetone, dichloromethane, toluene etc. [16-26]. Mohapatra, et al. [22]
prepared the malonamide impregnated resin in acetone as well as in methanol and
shown that the material prepared by the two methods were identical in their
extraction behaviour. The weight percentage of the TODGA loaded on the
chromosorb-W support was calculated from the difference in the weight of the resin
before and after impregnation and was found to be ~50% w/w. The loading
percentage of the extractant on the solid support was confirmed by elemental (C, H,
N) analysis. Since chromosorb-W does not contain any nitrogen, determination of
nitrogen content of the impregnated resin could be used for the calculation of the
amount of TODGA loaded on the support. Elemental analysis results gave 2.27% of
nitrogen which corresponded to 47% w/w loading of the extractant on the
chromosorb-W. The density of the TODGA impregnated EXC-resin was determined
Table 4.1: Characteristics of the TODGA extraction chromatographic resin
used in the present work
Stationary phase TODGA
Support material Chromosorb-W
Mesh size 60-80
Extractant loading 47% w/w
Average density of resin 1.08 g/mL
Density of TODGA 0.891 g/mL
Chapter IV
95
by water displacement method. A weighed amount of the impregnated resin (~2g)
was suspended in water in a narrow neck standard calibrated volumetric flask
(25mL). Care was taken to allow the resin to wet completely to ensure that all the air
bubbles were excluded. Finally, the volume of displaced water was measured by
weighing the same. The average density of the prepared TODGA EXC-resin was
found to be 1.08g/mL. The characteristics of the TODGA EXC-resin used in the
present work are listed in Table 4.1.
4.3. BATCH STUDIES
Batch studies were performed to explain the extraction behaviour of various metal
ions by TODGA EXC-resin. Batch studies are also important for the optimization of
various parameters for column chromatography. The extraction of metal ions from
nitric acid medium by TODGA impregnated resin can be expressed by the following
equilibrium reaction,
M
n+
+n NO
3
-
+x TODGA
\
==
\
M(NO
3
)
n
. xTODGA (4.1)
where TODGA is the ligand moiety impregnated on the resin. The above equilibrium
reaction is an indication that the extraction of metal ions by TODGA impregnated
resin is influenced by aqueous nitric acid concentration as well as the extent of
loading of the extractant on the solid support.
4.3.1. Evaluation of Resin Materials
The extractants of TRUEX, TRPO and DIAMEX processes, viz. CMPO, TRPO and
DMDBTDMA, respectively have been evaluated by EXC for the partitioning of
actinides from various feed solutions [21-23]. A broad comparison of the
performances of these EXC-resin materials with the TODGA resin is, therefore,
considered relevant. Fig. 4.1 compares the sorption behaviour of Am(III) as a
function of nitric acid concentration for different extractants, viz. TODGA, CMPO,
TRPO and DMDBTDMA sorbed on chromosorb-W. It should be noted that the
loading of extractants on the solid support (chromosorb-W) was comparable (48
2% w/w) for all the resins. In analogy to the corresponding solvent extraction system,
the distribution coefficient (K
d
) of Am(III) by TODGA and CMPO resins increased
sharply upto 1M HNO
3
and remained constant upto 6M HNO
3
. However, for
Chapter IV
96
0 1 2 3 4 5 6
10
0
10
1
10
2
10
3
10
4
TODGA
CMPO
TRPO
DMDBTDMA
K
d
-
A
m
[HNO
3
], M
DMDBTDMA resin, sorption of Am(III) increased gradually with nitric acid
concentration and reached to moderate values above 3M HNO
3
. On the other hand,
TRPO resin exhibited reasonably good K
d
values only at lower acidity (K
d
=140 at
0.5M HNO
3
). As demonstrated earlier, TRPO extractant is applicable for the
recovery of actinides from solutions of low acidity (1M HNO
3
). The K
d
values of
Am(III) by different EXC-resins studied at 3M HNO
3
followed the order: 7200
(TODGA) > 2000 (CMPO) > 35.3 (DMDBTDMA) > 3.2 (TRPO), clearly
demonstrating the better performance of TODGA resin than others for the
partitioning of actinides.
4.3.2. Kinetics of Extraction of Americium
The extraction kinetics of Am(III) by TODGA EXC-resin was evaluated by
determining the distribution coefficient (K
d
) from 1M HNO
3
at different time
intervals. Being a dynamic process, column extraction chromatography is affected by
the kinetics of extraction and re-extraction of the metal ions under investigation. As
listed in Table 4.2, the extraction equilibrium was rapidly achieved and constant K
d
values were observed after 5min of equilibration. Similar fast extraction kinetics was
Fig. 4.1. Sorption of Am(III) as a function of HNO
3
concentration by
different extractants sorbed on chromosorb-W; Temperature: 25C
Chapter IV
97
Table 4.2: Rate of sorption of Am(III) by TODGA/chromosorb-W resin;
Aqueous phase: 1M HNO
3
; Temperature: 25C
Time of equilibrations
(min)
K
d
-Am(III)
(1M HNO
3
)
2 3001
5 5935
10 6129
20 6118
30 6384
60 6059
120 6371
also observed in solvent extraction studies as described in Chapter-III. These results
are consistent with the data reported for TODGA and other EXC-resins [15,27]. The
rapid attainment of the equilibrium for 5f elements may be explained by the rapid
interfacial mass transfer of the metal ions across the aqueous / organic interface of
the resin. As the metal ion extractants are interfacially active, the hydrophilic portion
of extractant is ideally positioned to complex the metal ions that diffuse into the
aqueous / organic interface [28]. In the present work, equilibration time of 45min was
employed for all the measurements.
4.3.3. Sorption of Metal Ions on TODGA/Chromosorb-W Resin
The sorption profiles of individual metal ions such as Am(III), Eu(III), Pu(IV),
U(VI), Fe(III), Sr(II) and Cs(I) were obtained from nitric acid medium employing
TODGA EXC-resin. Fig. 4.2 depicts the variation in the K
d
values of metal ions with
HNO
3
concentration. The sorption of Am(III), Eu(III) and Pu(IV) increased sharply
with the acidity upto 2M HNO
3
, beyond which saturation was observed. Increase in
the extraction of metal ions with nitric acid concentration can be explained by the
equilibrium reaction (4.1) which is favoured with nitrate ion concentration by law of
mass action. The order of K
d
observed for actinide ions was similar to their
distribution ratio (D
M
) in solvent extraction studies, i.e. An(III) >An(IV) >An(VI).
These results clearly demonstrate that TODGA molecules impregnated on
Chapter IV
98
0 1 2 3 4 5 6
10
-1
10
0
10
1
10
2
10
3
10
4
Am(III) U(VI)
Pu(IV) Eu(III)
Fe(III)
Sr(II))
Cs(I)
K
d
[HNO
3
], M
Table 4.3: Uptake of nitric acid by TODGA/chromosorb-W resin
[HNO
3
]
intial
(M)
Acid uptake
(mMole/g)
K
d
-HNO
3
(mL/g)
1.02 1.24 1.41
2.02 1.99 1.11
3.05 2.63 0.95
3.94 3.08 0.85
4.97 3.46 0.75
5.97 3.99 0.61
Chromosorb-W behave similar to that observed in liquid-liquid extraction systems.
Similar observation has been reported recently for the selectivity in the extraction of
lanthanides by HDEHP with the two techniques [15]. In case of Sr(II), the K
d
value
increased with the aqueous phase acidity and reached to maximum of ~100 mL/g at
2M HNO
3
and decreased thereafter. The solvent extraction studies suggested that
TODGA also extracts sufficient nitric acid due to its high basicity (K
H
=4.1). As
Fig. 4.2. Sorption of metal ions as a function of HNO
3
concentration
by TODGA / chromosorb-W resin; Temperature: 25C
Chapter IV
99
depicted in Table 4.3, the uptake of HNO
3
increased gradually with the aqueous acid
concentration. Relatively large extraction of acid at higher nitric acid concentration
suppresses the sorption of Sr(II) which was reflected by decrease in the K
d
values at
higher acidity (>4M HNO
3
). Though Sr(II) shows relatively good extraction between
1-3M HNO
3
, nevertheless, trivalent actinides can be selectively extracted at higher
acidity (~6M HNO
3
) where Sr(II) shows relatively low extraction (K
d
at 6M HNO
3
=
15mL/g). On the other hand, K
d
values for Cs(I) and Fe(III) were less than 0.5mL/g
in the entire range of acidity investigated, suggesting insignificant sorption of these
metal ions.
Table 4.4 compares the K
d
values of Am(III), Pu(IV) and U(VI) at 3M HNO
3
by TODGA resin with those of other commonly used extractants such as
DMDBTDMA, CMPO and TRPO sorbed on chromosorb-W. It can be seen that
TODGA represents better sorption (K
d
) for Am(III) as compared to other proposed
extractants for actinide partitioning. Higher extraction of Am(III) by diglycolamide
ligand as compared to diamide is attributed to the tridentate nature of the former
ligand where commonly used neutral diamides act as bidentate ligand [29]. However,
lower K
d
value for Am(III) by TRPO resin was expected as the ligand is well known
to extract trivalent actinides only at lower acidity (1M HNO
3
). On the other hand,
the differences between the CMPO and TODGA resins lay in the number of donor
oxygen atoms and the configuration of the extractants. As described by Horwitz, et
al. [30,31] the carbamoyl methyl phosphoryl group acts as a monodentate ligand with
the metal ions at the tracer-scale level. The diglycolamide (DGA) group, on the other
hand, has been shown to be tridentate in configuration, complexing the metal ions
Table 4.4: Sorption of metal ions by different extractants sorbed on
chromosorb-W at 3M HNO
3
; Temperature: 25C
Extractant loaded on
chromosorb-W
K
d
for actinides
Am(III) Pu(IV) U(VI)
TODGA 7.2 x 10
3
5.1 x 10
3
1.1 x 10
2
CMPO 2.0 x 10
3
3.0 x 10
5
1.0 x 10
5
DMDBTDMA 35 4.5 x 10
3
1.1 x 10
3
TRPO 3.2 1.5 x 10
3
1.1 x 10
3
Chapter IV
100
through two carbonyl oxygens and the ether oxygen forming two stable five-
membered chelate rings [32]. The higher extraction constants of the DGA class of
extractants compared to the corresponding bifunctional malonamide extractants is
also noteworthy and supports the importance of the tridentate co-ordination of the
DGA. Although the carbonyl oxygen is less electronegative than the phosphoryl
oxygen, the coordination of DGA to a tri-positive actinide probably involves less
ligand strain and / or rearrangement, which may compensate for the lower electron
density of the donor groups in DGA [15]. However, for Pu(IV) and U(VI) the K
d
values with CMPO were distinctly larger, suggesting significant role of steric factors
for TODGA. For DMDBTDMA, CMPO and TRPO EXC-resins, the order of K
d
values for actinides were: Pu(IV) >U(VI) >Am(III). This is the general order
observed for many neutral extractants, which followed the order of ionic potential of
actinide ions. In case of TODGA, however, the uptake of Am(III) was larger than
that of U(VI) probably due to the presence of two oxygen atoms in the uranyl ions
which might cause hindrance in complexation with the tridentate ligand. Though
TODGA and CMPO display comparable extraction properties for trivalent actinides,
there is distinct advantage of using TODGA over phosphorous-based extractants as
the former facilitates the secondary waste management of the spent extractants due
its complete incinerability.
4.3.4. Sorption of Am(III) Under Loading Conditions
The effect of some of the selected metal ions, viz. U(VI), Nd(III) and Fe(III) on the
extraction of Am(III) by TODGA EXC-resin was studied at 3M HNO
3
. Since, the
high level waste (HLW) generated during reprocessing of the spent nuclear fuel
contains significant amount of these metal ions (composition of a typical HLW is
given in Table 3.7), it is desirable to study their effect on the sorption of minor
actinides. Fig. 4.3 depicts the sorption of Am(III) by TODGA EXC-resin in the
presence of varying concentration of U(VI) and Nd(III). Uranium concentration upto
20g/L was studied as HLW may contain varying amount of uranium depending upon
the efficiency of the PUREX process. The results reflected that even the presence of
20g/L of uranium did not affect the sorption of Am(III) significantly. This was
attributed to the higher uptake of trivalent actinide as compared to hexavalent uranyl
ions. In case of Nd(III) (taken as a representative element of lanthanides), the K
d
Chapter IV
101
0 4 8 12 16 20
10
1
10
2
10
3
10
4
Varying Nd(III)
Varying U(VI)
2g/L Nd(III) + varying U(VI)
K
d
-
A
m
Concentration of Nd / U (g/L)
value of Am(III) decreased sharply with increase in the lanthanide concentration.
Similarly, the presence of 2g/L Nd(III) and varying concentration of uranium
suggested that the K
d
values of Am(III) was significantly affected by the presence of
lanthanides. These observations clearly indicate that the sorption of Am(III) on
TODGA EXC-resin is mainly affected by the presence of lanthanides.
Literature survey has revealed that the presence of Fe(III) significantly affects
the sorption of Am(III) as reported earlier for CMPO and DMDBTDMA EXC-resins
[21,22]. Since, HLW also contains significant concentration of Fe (Table 3.7), the
sorption of Am(III) was investigated in the presence of macro concentration of Fe (1-
6 g/L). Table 4.5 shows that there was marginal decrease in the sorption of Am(III)
even in the presence of 6g/L of Fe(III), suggesting the selective recovery of minor
actinides by TODGA EXC-resin without contamination of structural materials. It is
evident from the present studies that the behaviour of TODGA EXC-resin is quite
different from those of CMPO and DMDBTDMA EXC-resins, which is related to the
fact that whereas TODGA does not extract Fe(III); CMPO and DMDBTDMA have
significant distribution ratio for Fe(III) in the acidity range 3-6M HNO
3
.
Fig. 4.3. Sorption of Am under loading conditions of U and Nd(III)
by TODGA sorbed on chromosorb-W; Temperature: 25C
Chapter IV
102
Table 4.5: Sorption of Am(III) by TODGA sorbed on chromosorb-W in the
presence of macro amounts of iron; Temperature: 25C
[Fe], gm/L
K
d
, Am(III)
1M HNO
3
4M HNO
3
0.0 5895 7493
1.0 5902 7376
2.0 5704 7056
4.0 5893 6668
6.0 5401 6737
4.3.5. Sorption of Am(III) from Nitrate and Sulphate Media
The sorption of Am(III) in the presence of nitrate and sulphate is particularly relevant
for the recovery of minor actinides from the nitrate and sulphate bearing waste
solutions. Table 4.6 gives the batch distribution data for the sorption of Am(III) in
the presence of varying concentration of nitrate and sulphate. It can be seen that the
K
d
value of Am(III) increased many fold (~20 times) in the presence of 1M nitrate,
which was attributed to the law of mass action as well as salting out effect. On the
other hand, there was marginal change in the K
d
value of Am(III) even in the
presence of 10g/L (0.1M) of sulphate suggesting that minor actinides can be
effectively separated from the sulphate bearing waste by TODGA EXC-resin.
Table 4.6: Sorption of Am(III) by TODGA/chromosorb-W resin in the presence
of varying concentrations of nitrate and sulphate ions; Temperature: 25C
Nitrate ions at 0.1M HNO
3
Sulphate ions at 1M HNO
3
[NO
3
-
], M K
d
, Am(III) [SO
4
2-
], M K
d
, Am(III)
0.0 341 0.00 5895
1.0 6610 0.02 6674
2.0 6820 0.04 7447
4.0 6776 0.06 7645
6.0 6556 0.08 7596
- - 0.10 7513
Chapter IV
103
1 2 3 4 5 6
10
-1
10
0
10
1
10
2
10
3
Am U Pu
Eu Fe
Sr Cs
K
d
[HNO
3
], M
4.3.6. Sorption of Metal Ions from Synthetic Waste Solution
In order to evaluate the applicability of TODGA EXC-resin for the separation of
actinides from nuclear waste solution, the sorption behaviour of various metal ions
was studied from a simulated high level waste (SHLW) solution conforming to the
composition given in Table 3.7. Fig. 4.4 shows the variation of the K
d
values of
Am(III), Eu(III), Pu(IV), U(VI), Fe(III), Sr(II) and Cs(I) as a function of HNO
3
concentration from SHLW solution. Interestingly, the K
d
values were distinctly
higher for Pu(IV) as compared to those of Am(III) and Eu(III). Nevertheless, at any
acidity, the K
d
value of any given metal ion was less in the SHLW as compared to
that in pure HNO
3
(Fig. 4.2), apparently due to the co-extraction of other metal ions
present in SHLW. It is worth mentioning that the SHLW constituted about 2g/L
lanthanides and ~0.8g/L zirconium. Significant decrease in the sorption of Am(III)
from SHLW was attributed to the presence of these metal ions which show strong
sorption on TODGA EXC-resin. On the other hand, the structural materials and other
fission products such as Fe(III), Sr(II) and Cs(I) were not extracted from SHLW,
reflecting selective extraction of actinides and lanthanides from SHLW by TODGA
EXC-resin.
Fig. 4.4. Uptake of metal ions as a function of HNO
3
concentration
from SHLW by TODGA/chromosorb-W resin; Temperature: 25C
Chapter IV
104
4.4. COLUMN STUDIES
The chromatography column was prepared by packing ~500mg of TODGA EXC-
resin in a borosilicate glass column of 4.0mm diameter. Various column parameters
were estimated by the method reported in the literature [33]. The bed volume and the
bed density were calculated from the column dimensions, bed height and the weight
of the packed EXC-resin. The volume of mobile phase (V
m
), referred to as the free
column volume, was calculated from the difference in the bed volume and the
volume of resin in the bed, the latter being the weight of resin material in bed divided
by its density. The volume of liquid stationary phase (V
s
) was estimated from the
weight of the extractant impregnated on the support material packed in the column
divided by the density of the extractant. The former was obtained from the weight of
the resin packed in the column and the percentage of extractant loaded on the resin.
The calculated parameters and specifications of the packed EXC column are
summarized in Table 4.7. The column was preconditioned by passing excess of
appropriate nitric acid solution, prior to the introduction of the sample solution. The
column operations were carried out at ambient temperature (251C) at the flow rate
of about 0.4mL/minute. The breakthrough curves and elution profiles were obtained
by plotting radioactivity (in terms of counts per unit time per unit volume) in the
effluent versus volume of the solution passed.
Table 4.7: Characteristics of TODGA chromatographic resin packed column
Bed volume 1.257 mL
Bed density 0.398 g/mL
Density of stationary phase 0.891 g/mL
Volume of stationary phase 0.210 mL/mL of bed
Volume of mobile phase 0.632 mL/mL of bed
4.4.1. Performance of Chromatography Column
The concept of extraction chromatography is the combination of liquid-liquid
extraction with chromatography technique. According to the plate theory, the
chromatography column consists of a number of discrete plates, referred to as the
Chapter IV
105
number of theoretical plates (N). During the course of extraction of the
chromatographic process, many extractions and re-extractions occur between the
mobile phase and the stationary organic phase of each theoretical plate, which define
the efficiency of the column. Thus, more the number of theoretical plates more the
number of equilibrations, and hence better will be the performance of the column.
The number of theoretical plates is correlated with the bed height of the column (L)
by the following relation,
N = L / H (4.2)
where, H is the height equivalent to the theoretical plate (or plate height). Thus, for a
column of fixed bed height (L), the efficiency of the column will, therefore, depend
upon the value of H. In other words, the performance of the given chromatographic
column is governed by the height equivalent to the theoretical plate.
Over the last few decades, enormous amount of theoretical and experimental
efforts have been devoted to develop quantitative relationships describing the effect
of various parameters affecting the column performance [34]. In 1950s, Dutch
chemical engineers developed a mathematical approximation, known as Van
Deemter equation, to explain various parameters affecting the performance of the
column. The van Deemter equation can be written in the following form,
H = A + (B / U) + (C
s
+ C
m
) U (4.3)
where, H is the height equivalent to theoretical plate (cm), U is the linear velocity of
the mobile phase (flow rate: cm/sec), C
s
& C
m
are the mass transfer coefficients of
the stationary and the mobile phases, respectively. The coefficients A and B in Van
Deemter equation are the functions of the diameter of the packing material (Eddy
diffusion) and diffusion coefficient of species in the mobile phase (longitudinal
diffusion), respectively. For liquid phase chromatography, B << (C
s
+ C
m
), therefore,
H is a function of U suggesting that more is the flow rate more would be the height
of theoretical plates. For a fixed column length (L in cm), the number of theoretical
plates (N) would decrease with increase in the plate height (H), [N = L/H], thereby
reducing the efficiency of the column. In other words, due to the dynamic condition
of the column, its efficiency depends upon the phase contact time, which in turn
depends upon the flow rate of the mobile phase. This effect was demonstrated in an
independent experiment by evaluating breakthrough for Am(III) in the presence of
Chapter IV
106
0 5 10 15 20 25 30
0
20
40
60
80
100
0.3 mL/min
0.7 mL/min
1.0 mL/min
%
B
r
e
a
k
t
h
r
o
u
g
h
Volume of solution passed (mL)
1g/L Eu as a carrier using TRPO/chromosorb-W resin column. As depicted from Fig.
4.5, increase in the flow rate reduced the volume of aqueous feed solution that could
be passed through the column without breakthrough of Am(III). From Fig. 4.5, the
volume of aqueous feed solution for a fixed percentage breakthrough (VFPB) was
plotted for different flow rates and presented in Fig. 4.6. As depicted by the Van
Deemeters equation, the straight lines of the plots suggested a linear relationship
between the flow rate and the efficiency of the column when other parameters are
fixed. It has been shown experimentally by several authors that the basic Van
Deemter equation is quite satisfactory to understand the column efficiency [35]. Our
observation was in good agreement with the Van Deemter equation and observations
reported earlier [35]. These observations suggested that for a fixed column length,
the flow rate should not be very high for having good column performance. On the
other hand, flow rate should not be too low to result in blocking of the column.
Nevertheless, for an optimum flow rate, the performance of the column can be
improved by increasing the column length. Our further studies on TODGA EXC-
Fig. 4.5. Breakthrough curve for
241
Am at different flow rate on
TRPO/chromosorb-W column; Loading solution: 1.2 M HNO
3
containing 1g/L Eu spiked with
241
Am; Column length: 18.5cm
Chapter IV
107
0.2 0.4 0.6 0.8 1.0
10
15
20
25
30
1%
10%
100%
V
o
l
u
m
e
f
o
r
f
i
x
e
d
%
B
r
e
k
t
h
r
o
u
g
h
Flow rate (mL/min)
resin column were based on these observations and all the subsequent studies were
carried out at fixed flow rate of 0.4mL/min for a column length of about 10cm.
4.4.2. Column Breakthrough for Am(III)
The ability of the TODGA EXC-resin column to pre-concentrate the analytes from
large volumes of dilute samples was tested in terms of sample breakthrough.
Breakthrough curves for Am(III) were obtained from nitric acid solution under
different loading conditions and are presented in Fig. 4.7. It was observed that
~10mL solution containing 1g/L of Eu(III) could be passed through the column
without any breakthrough of
241
Am, both at 1M and 4M HNO
3
, suggesting that about
20mg of Eu(III) could be loaded per g of the TODGA EXC-resin (column contains
500mg of resin). This observation was attributed to the high K
d
values for trivalent
metal ions between 1-6M HNO
3
as reflected in batch distribution studies (Fig. 4.2). If
one calculates the amount of extractant present in the column, it corresponds to
~0.405mMol of TODGA for 500mg of resin present in the column. If we assume the
stoichiometry of the extracted species of `europium and TODGA as 1:3 (as suggested
Fig. 4.6. Volume for fixed percentage breakthrough (VFPB) at different
flow rate on TRPO/chromosorb-W column; Loading solution: 1.2 M
HNO
3
containing 1g/L Eu spiked with
241
Am; Column length: 18.5cm
Chapter IV
108
0 5 10 15 20
0
20
40
60
80
100
120
1g/L Eu at 1M HNO
3
1g/L Eu at 4M HNO
3
20g/L U at 1M HNO
3
%
B
r
e
a
k
t
h
r
o
u
g
h
o
f
2
4
1
A
m
Volume of solution passed [mL]
by the metal loading studies in solvent extraction) then the column should hold
~0.135mMol (~20mg) of Eu(III). The loading of 10mg of Eu(III) (~0.066mMol) on
the column suggested only50% loading of lanthanide before the column
breakthrough. Furthermore, the physical and chemical properties such as density of
the organic phase, aggregation and metal / extractant stoichiometry in EXC systems
may deviate from those of the corresponding liquidliquid extraction systems. On the
other hand, when a solution containing 20g/L U spiked with
241
Am tracer was passed
through the column, no breakthrough for
241
Am was observed even after passing
100mL of the feed solution. This observation suggested that U(VI) has no significant
effect on the loading of trivalent actinide which was also reflected in the batch
studies where the presence of 20g/L U did not show any significant decrease in the
K
d
value of Am(III).
4.4.3. Column Elution Studies
In order to study the elution behaviour of Am(III) from the loaded TODGA EXC-
resin column, different eluents were evaluated, viz. 0.01M HNO
3
, 0.01M EDTA,
Fig. 4.7. Breakthrough curve for
241
Am under different loading
condition on TODGA/chromosorb-W column; Flow rate: 0.4mL/min
Chapter IV
109
0 10 20 30 40 50
0
1x10
4
2x10
4
3x10
4
4x10
4
5x10
4
0.1M Imino Di acet ic Acid
2-Hydroxy Isobutyric Acid
0.01M HNO
3
0.01M EDTA
C
o
u
n
t
s
p
e
r
m
i
n
u
t
e
Vol ume of eluent passed [ mL]
0.1M-hydroxy isobutyric acid (-HIBA) and 0.1M imino diacetic acid (IDA). The
column was loaded with a solution containing 1g/L of Eu spiked with
241
Am tracer at
4M HNO
3
and subsequently washed with 10mL of 4M HNO
3
before the elution. It
should be noted that no activity release was observed in the washing solution. A
constant flow rate of 0.4mL/min was maintained throughout the elution studies. Fig.
4.8 shows the elution curves for Am(III) for different eluents. It was observed that
dilute nitric acid (0.01M HNO
3
) was not a good eluent for Ln(III) / An(III). It
appears that HNO
3
held in the column during the loading would eventually increase
the acidity of the eluent way beyond 0.01M HNO
3
. In case of complexing agents like
EDTA and IDA, ~98% Am(III) could be eluted within 20mL of the eluent. Similarly,
quantitative recovery of Am(III) was achieved within 25mL of 0.1M -HIBA
solution. The column studies suggested the possible application of TODGA EXC-
resin for the pre-concentration / separation of trivalent actinides / lanthanides from
other fission products present in relatively large volume of waste / analyte solutions.
Fig. 4.8. Elution curve for Am(III) by different eluents; Loading solution:
1g/L Eu containing
241
Am tracer at 4M HNO
3
; Flow rate: 0.4mL/min
Chapter IV
110
0 2 4 6 8 10 12 14 16 18
0
20
40
60
80
100
First Run
Second Run
Thi rd Run
Fourth Run
Fift h Run
(af ter 100 hours)
C
o
u
n
t
s
/
m
i
n
u
t
e
(
2
4
1
A
m
)
Volume of effl uent passed (mL)
4.4.4. Reusability of Column
The stability of the column is an important parameter which influences the
applicability of the system on the process scale. In this context, the stability of the
column was studied by determining loading and elution of the same column in
several cycles over a period of time. The column was loaded with a solution
containing 1g/L of Eu(III) spiked with
241
Am tracer at 4M HNO
3
and subsequently
eluted with 0.01M EDTA solution. Fig. 4.9 shows the breakthrough curves for the
successive loadings of Am(III) using the same column. In the first loading, the
column was loaded till the 100% breakthrough for Am(III) was observed and then all
the Am(III) activity was eluted with 0.01M EDTA. The column was subsequently
washed with 15mL water and conditioned with 4M HNO
3
before the second loading
was carried out. Subsequent loadings and elutions were carried out for five cycles in
a similar manner. It is interesting to note that there was no change in the
breakthrough of Am(III) after five cycles of loading even when EXC-resin was in
contact with loading solution / eluent for 100hours. The results clearly demonstrated
Fig. 4.9. Breakthrough curve for Am for successive loading and elution;
Loading solution 1g/L Eu spiked with
241
Am at 4M HNO
3
; Flow rate: 0.4mL/min
Chapter IV
111
the stability of the TODGA EXC-resin column. The leaching of the loaded
extractants from the solid support is reported by several authors. However, such
possibility is minimized when the operation is performed in column due to closed
packing of the resin.
4.5. REFERENCES
1. L. Boyadzhiev and Z. Lazarova, In Membrane Separations Technology:
Principles and Applications, R.D. Noble and S.A. Stern (Eds.), Elsevier Science
B.V. (1995), P. 283 - 300.
2. H.C. Visser, D.N. Reinhoudt, and F. de Jong, Chem. Soc. Rev., (1994) 75.
3. P.R. Danesi, E.P. Horwitz and P.G. Rickert, J. phys. Chem., 87 (1983) 4708.
4. L. Nunez, B.A. Buchholz and G.F. Vandergrift, Sep. Sci. Technol., 30 (1995)
1455.
5. M.D. Kamaniski and L. Nunez, Sep. Sci. Technol., 35 (2000) 2003.
6. M. D. Kaminski and L. Nunez, J. Magnetism and Magnetic Mater., 194 (1999)
31.
7. E.P. Horwitz, Extraction chromatography of actinides and fission products:
Principles and achievement of selectivity, In proceedings of the international
workshop on the application of extraction chromatography in radionuclide
measurements, IRMM, Geel 9-10, EUR 18974EN (1998), P. 27-37.
8. F. Sebesta, J. Radioanal. Chem., 6 (1970) 41.
9. E.P. Horwitz, D.R. McAlister, A.H. Bond, R.E. Jr. Barrans and J.M. Williamson,
Appl. Radiat. Isotopes, 63 (2005) 23.
10. A. Suresh, C.V.S. Brahmmananda Rao, R. Deivanayaki, T.G. Srinivasan and P.R.
Vasudeva Rao, Solv. Extr. Ion Exch., 21 (2003) 449.
11. P. Markl and E.R. Schmidt, Techniques in column chromatography, In
Extraction chromatography, T. Braun and G. Ghersini (Eds.), Elsevier NY
(1975), P. 45-66.
12. B. Zielinska, C. apostolidis, F. Bruchertseifer and A. Morgenterm, Solv. Extr. Ion
Exch., 25 (2007) 339.
13. J.L. Cortina and A. Warshawsky, developments in solid-liquid extraction by
solvent impregnated resins, In Ion exchange and solvent extraction, J.A.
Marinsky and Y. Marcus (Eds.), Marcel Dekker, NY (1975), Vol. 13, P. 195-293.
Chapter IV
112
14. I. Akaza, Correlation between extraction chromatography and liquid-liquid
extraction, In Extraction Chromatography, T. Braun and G. Ghersini (Eds.),
Elsevier NY (1975), P. 17-44.
15. E.P. Horwitz, D.R. McAlister, A.H. Bond and R.E. Barrans, Solv. Extr. Ion
Exch., 23 (2005) 319.
16. E.P. Horwitz, D.R. McAlister and M.L. Dietz, Sep. Sci. Technol., 41 (2006)
2163.
17. E.P. Horwitz, M.L. Dietz, D.M. Nelson, J.J. LaRosa and W.D. Fairman, Anal.
Chim. Acta, 23 (1990) 263.
18. E.P. Horwitz, R. Chiarizia, M.L. Dietz, H. Diamond and D.M. Nelson, Anal.
Chim. Acta, 28 (1993) 361.
19. J. Serranog and T. Kimura, J. Radioanal. Nucl. Chem., Articles, 172 (1993) 97.
20. M. Yamaura and H.T. Matsuda, J. Radioanal. Nucl. Chem., 224 (1997) 83.
21. J.N. Mathur, M.S. Murali, R.H. Iyer, A. Ramanujam, P.S. Dhami, V.
Gopalkrishnan, M.K. Rao, L.P. Badheka and A. Banergi, Nucl. Technol., 109
(1995) 216.
22. P.K. Mohapatra, S. Sriram, V.K. Manchanda and L.P. Badheka, Sep. Sci.
Technol., 35 (2000) 39.
23. S.A. Ansari, M.S. Murali, P.N. Pathak and V.K. Manchanda, Solv. Extr. Ion
Exch., 22 (2004) 1013.
24. J.L. Cortina, N. Miralles, M. Aguilar and A.M. Sastre, Solv. Extr. Ion Exch., 12
(1994) 371.
25. N. Sivaraman, R. Kumar, S. Subramaniam and P.R. Vasudeva Rao, J. Radioanal.
Nucl. Chem., 252 (2002) 491.
26. Y. Wei, M. Kumagai and Y. Takashima, Nucl. Technol., 132 (2000) 413.
27. K. Van Hecke and G. Modolo, J. Radioanal. Nucl. Chem., 261 (2004) 269.
28. E.P. Horwitz, R. Chiarizia, M.L. Dietz and H. Diamond, Anal. Chim. Acta, 28
(1993) 361.
29. E.P. Horwitz, W.H. Delphin, C.A. Bloomquist and G.F. Vandergrift, J.
Chromatogr., 125 (1976) 203.
30. E.P. Horwitz, A.C. Muscatello, D.G. Kalina and L. Kaplan, Sep. Sci. Technol.,
16 (1981) 417.
Chapter IV
113
31. E.P. Horwitz, D.G. Kalina, L. Kaplan, G.W. Mason and H. Diamond, Sep. Sci.
Technol., 17 (1982) 1261.
32. H. Narita, T. Yaita and S. Tachimori, Extraction behavior of trivalent lanthanides
with amides and EXAFS study of their complexes. In Solvent Extraction for the
21
st
Century, Proceedings of ISEC99, Barcelona, Spain, July, 1999, m. Cox, M.
Hidalgo, M. Valiente (Eds.), Society of Chemical Industry: London (2001), Vol.
1, P. 693-696.
33. E.P. Horwitz, R. Chiarizia and M.L. Dietz, Solv. Extr. Ion Exch., 10 (1992) 313.
34. D.A. Skoog, F.J. Holler and T.A. Nieman (Eds.), Principles of instrumental
analysis, 5
th
Ed., Saunders college publishing, Fort Worth (1998), P. 684.
35. E. Katz, K.L. Ogan and R.P.W. Scott, J. Chromatogr., 270 (1983) 51.
---------------------------
Chapter V
Sorption Behaviour Of Actinides
On N,N-Dimethyl-N,N-Dibutyl
Malonamide Grafted Polymer
Chapter V
114
SORPTION BEHAVIOUR OF ACTINIDES ON
N,N-DIMETHYL-N,N-DIBUTYL MALONAMIDE
GRAFTED POLYMER
5.1. INTRODUCTON
The determination of heavy metal ions at trace levels is mandatory in waste stream
emanating from industrial processes for environmental pollution. However, trace
analysis often necessitates pre-concentration and / or separation of trace elements
from the matrix to improve their detection limits. There are several methods
including solvent extraction and ion exchange which have been proposed for pre-
concentration / pre-treatment of trace metal ions before their analysis [1-3]. Solid
phase extraction has also been proposed as an alternate efficient technique for the
pre-concentration of trace and ultra trace amounts of elements from complex
matrices [4-7]. The use of chelating ligands in solid phase extraction has several
advantages, i.e. immiscibility with aqueous phase, low rate of physical degradation,
minimum release of toxic organic solvents as waste and recycling options [8-9]. Two
approaches are adopted for incorporating chelating ligands on inert polymeric
support for solid phase extraction. The first involves the physical sorption of
chelating ligands on the inert solid support. The other is based on co-valent coupling
of the ligands with the polymer backbone through certain functional groups such as
N=N- or CH
2
- groups. The extraction chromatography technique with physically
sorbed resin poses serious limitation to the reusability due to slow leaching of the
extractants from the pores of the support materials. However, the strategy of
chemical grafting of the ligands on the solid support renders rugged systems free
from ligand leaching problems. A number of chelating resins have been prepared by
incorporating different functional groups such as ethylene diamine tetraacetic acid
(EDTA) [10], imino diacetic acid (IDA) [11,12], succinic acid [13], 8-hydroxy
quinoline [14], etc. for the separation / pre-concentration of heavy metal ions.
Trace analysis of metal ions pose unique problem to analysis as it involves
rigorous requirements of versatility, specificity, sensitivity and accuracy during the
chemical analysis [15-20]. These factors are also crucial in the nuclear spent fuel
reprocessing units, where elemental quantification at trace quantities is immense
Chapter V
115
value. The selective extraction and / or pre-concentration of long lived actinides such
as
241
Am,
243
Am,
244
Cm,
239
Pu,
237
Np etc. from various waste streams are extremely
important not only from the analytical point of view, but also to reduce their quantum
as radioactive wastes [21-23]. Moreover, the recovery of plutonium from radioactive
waste solution is also relevant for effective resource management.
Green reagents such as substituted malonamides have shown good metal
extraction behaviour [24-26]. In the series of substituted malonamides, N,N-
dimethyl-N,N-dibutyl tetradecyl malonamide (DMDBTDMA) has shown promise in
solvent extraction as well as in extraction chromatography for the separation of
actinides from nuclear waste solutions [27-30]. However, in view of ligand leaching
possibilities in the case of physically sorbed extraction chromatographic resin, an
attempt was made to prepare a chemically anchored resin with substituted
malonamide ligand. In this context, N,N-dimethyl-N,N-dibutyl-malonamide
(DMDBMA) was functionalized with polystyrene-divinyl benzene polymer and
evaluated for solid phase extraction. The important features of this functionalized
resin towards the sorption of actinide ions were studied and are discussed in this
chapter. Analytical applications of this grafted resin for the separation and pre-
concentration of actinide ions were also evaluated.
5.2. SORPTION KINETICS FOR ACTINIDES
The rate of transfer of actinide ions, viz. Am(III), Th(IV) and U(VI) fromaqueous
phase to the solid phase was studied at 3M HNO
3
by equilibrating the malonamide
functionalized resin with aqueous solution containing metal ions tracer for different
periods. The solid phase extraction kinetics was monitored in terms of fractional
attainment of the equilibrium expressed as follows [31],
F =[M
R
]
t
/ [M
R
]
eq
(5.1)
where [M
R
]
t
and [M
R
]
eq
are the metal ions concentration on the solid phase at time t
and at equilibrium, respectively. The kinetics data were plotted in terms of (1-F)
values as a function of equilibration time and are shown in Fig. 5.1. It is evident that
the equilibrium condition was reached within 20 minutes for all the metal ions.
Negligible desorption of metal ions even after 2hrs of the equilibration suggested no
signature of degradation of the grafted resin. Fast extraction kinetics may be
Chapter V
116
0 10 20 30 40 50 60
0.0
0.2
0.4
0.6
0.8
1.0
Am(III)
U(VI)
Th(IV)
1
-
F
Ti me (mi n)
attributed to the macroporous nature of the resin matrix, which favoured the greater
accessibility of the neutral metal complex towards the anchored hydrophobic
malonamide moiety. Subramanian, et al. [21] also reported fast extraction kinetics
(equilibrium attained in 15min) of uranium and thorium for bis(2-ethylhexyl)
malonamide grafted resin. Present studies were carried out by maintaining the
equilibration time of 45minutes.
The sorption kinetics data of actinide ions on DMDBMA grafted resin was
tested for Lagergren first order rate kinetics which is expressed in the following
mathematical form [32],
log(q
e
q) =log q
e
(k
ads
/ 2.303) t (5.2)
where q and q
e
are the amount of metal ions sorbed per g of chelating polymer at
time t and at equilibrium condition, respectively and k
ads
is the sorption rate
constant for the given metal ions. Fit of the experimental data in Eq. (5.2) would
suggest the first order rate kinetics for the system. As depicted in Fig. 5.2, the linear
plots of log(q
e
q) versus t suggested that the sorption of tri-, tetra- and hexavalent
Fig. 5.1. Kinetics for the sorption of actinides on malonamide
functionalized polymer at 3 M HNO
3
; Temperature: 25C
Chapter V
117
0 4 8 12 16 20 24
2
3
4
5
6
Am(III) ; slope : -0.088 R
2
: 0.999
U(VI) ; slope : -0.151 R
2
: 0.999
Th(IV) ; slope : -0.183 R
2
: 0.998
l
o
g
(
q
e
-
q
)
Time (min)
Table 5.1: Sorption rate constant (k
ads
) and distribution coefficient (K
d
) of
actinide ions by DMDBMA grafted resin; Aqueous phase: 3M HNO
3
Metal ions k
ads
(min
-1
) K
d
(mL/g)
Th(IV) 0.42 0.02 880
U(VI) 0.35 0.01 410
Am(III) 0.20 0.01 48
actinide ions followed the first order rate kinetics. The k
ads
values obtained from the
slope of the plot are listed in Table 5.1. It was observed that the sorption rate constant
for actinide ions followed the order: Th(IV) >U(VI) >Am(III), which was in
accordance to their ionic potential. Due to higher ionic potential, tetravalent actinides
form stronger complex with malonamide ligands as compared to trivalent and
hexavalent actinides as reflected by the higher K
d
value of Th(IV) as compared to
U(VI) and Am(III) (Table 5.1). It should be noted that though the sorption kinetics of
Pu(IV) was not studied, its behaviour could be similar to Th(IV). The effect of metal
ions concentration on the extraction kinetics has been reported by Metilda, et al. [23]
Fig. 5.2. Lagergren plots for the sorption of actinide ions on
malonamide grafted polymer at 3 M HNO
3
; Temperature: 25C
Chapter V
118
for the sorption of uranium on succinic acid functionalized polymer. They reported
the k
ads
values for uranium as 0.18 0.01 min
-1
for aqueous phase containing 20mg,
40mg and 60mg/L of U(VI), reflecting no significant effect of metal ion
concentration on the sorption rate. The present work was carried out with trace metal
concentrations.
5.3. URANIUM SORPTION STUDIES
The sorption of uranium on malonamide grafted polymer was studied from aqueous
solution containing varying concentration of U at 1M and 3M HNO
3
. The
concentration of uranium in the aqueous phase was monitored using
233
U radiotracer.
The sorption phenomena are classified as physisorption or chemisorption, depending
upon the bond energies or the enthalpy of sorption. In physisorption, the bonding
takes place through weak van der Waals forces. On the other hand, chemisorption
involves a chemical bond (ionic, metallic or co-valent bonds) between sorbate
molecules and the sorbent surfaces [33].
5.3.1. Sorption Isotherms
The sorption data of uranium on the malonamide grafted polymer were fitted in
several types of isotherms such as Langmuir, Dubinin-Rodushkevich (D-R) and
Freundlich sorption isotherms. Langmuir isotherm is the most simple for the sorption
of sorbate on to the sorbent, which is based on the following assumptions [34]; i)
Only one monolayer of an sorbing molecule can be sorbed on the surface of the
sorbent, ii) All sites of sorbent are equivalent, iii) The ability of a molecule to sorb at
a given site is independent of the nature of the neighbouring sites, and iv) The
enthalpy of sorption is constant. The Langmuir sorption isotherm in the liquid phase
can be represented by the following equation [23],
C
e
/ q
e
=1 / q
o
b +C
e
/ q
o
(5.3)
where, C
e
is the equilibrium concentration of metal ions in the aqueous phase, q
e
is
the amount of metal ions sorbed on the solid phase at equilibrium; q
o
and b are the
Langmuir constant related to the sorption capacity of the metal ions and the sorption
energy, respectively. Applicability of the Langmuir model can be ensured by the
linear fit of the Eq. (5.3). As shown in Fig. 5.3, the linear plot of C
e
/q
e
versus C
e
Chapter V
119
0 50 100 150 200 250 300 350
0
10
20
30
40
50
60
C
e
/
q
e
C
e
1M HNO
3
; R
2
: 0.999
3M HNO
3
; R
2
: 0.999
suggested that the sorption of uranium on malonamide grafted polymer followed the
Langmuir sorption model. The correlation coefficients for the linear fits of the plot
were found to be 0.999 for both, at 1 M as well as at 3 M HNO
3
. The upper line in
the figure suggested that the equilibrium sorption of uranium (q
e
) at 1M HNO
3
is
lower than at 3M HNO
3
. This behaviour can be explained in terms of nitrate effect
where the formation of neutral UO
2
(NO
3
)
2
is facilitated at higher acidity. This
observation is further supported by the lower slope value at 3M HNO
3
due to higher
sorption capacity. The Langmuir constant q
o
and b were determined from the slope
and intercept of the curve and are listed in Table 5.2. The maximum sorption
capacities (q
o
) of uranium at 1M and 3M HNO
3
were found to be 32.610.33 mol
and 43.910.65 mol per g of resin, respectively, which represent the monolayer
coverage of metal ions on the malonamide grafted polymer. The value of constant b
in the Langmuir model indicates the strength of bonding between the sorbate and
sorbent. Large values of b indicate the stronger bond between uranium and
malonamide ligand. This observation is in good agreement with the reported value in
the literature for the sorption of uranium on succinic acid grafted polymer [23].
Fig. 5.3. Langmuir isotherm for the sorption of uranium on
malonamide functionalized polymer; Temperature: 25C
Chapter V
120
Table 5.2: Langmuir constant for the sorption of uranium on DMDBMA
grafted resin; Temperature: 25C
[HNO
3
] R
2
q
o
(mol/g) b (L/mol)
1.0 M 0.999 32.6 0.3 1878 122
3.0 M 0.999 43.9 0.7 4558 138
In order to obtain insight into the sorption energy of uranyl ions on
DMDBMA grafted resin, Dubinin-Rodushkevich (D-R) isotherm [35,36] was applied
in the following form,
lnq
e
=lnX
m
-
2
(5.4)
where q
e
is the amount of metal ions sorbed on the solid matrix at equilibrium, X
m
is
the maximum sorption capacity, is the activity coefficient which is related to the
mean sorption energy, and is the Polanyi potential [36] expressed as follows,
=RT ln(1 +1 /C
e
) (5.5)
where C
e
is the equilibrium concentration of metal ions in the aqueous phase, R is the
gas constant (kJ/mol) and T is the absolute temperature. Using the value of C
e
at a
given temperature, the value of Polanyi potential () was calculated and fitted in the
linear form of the Eq. (5.4). The plot of lnq
e
vs
2
yielded a straight line as shown in
Fig. 5.4 with the correlation coefficients of 0.992 and 0.991 at 1M and 3M HNO
3
,
respectively. The values of X
m
and were calculated from the slope and intercept of
the linear fit of the plot and are summarized in Table 5.3. The value of X
m
was
calculated to be 30.21.1 and 49.91.7 mol/g at 1M and 3M HNO
3
, respectively.
The maximum sorption capacity (X
m
) obtained by D-R model was very close to that
obtained by Langmuir model, which depends on the total specific active sites on the
polymer surface. The energy (E) for the sorption of metal ions on the solid surface is
related to the activity coefficient () as follows [37],
E =1 / (-2)
1/2
(5.6)
The mean sorption energy (E) was calculated by Eq. (5.6) using the value of from
the slope of the plot of Eq. (5.4). The value of E for the present system was estimated
Chapter V
121
-500 0 500 1000 1500 2000 2500
-8
-7
-6
-5
-4
-3
2
l
n
q
e
1M HNO
3
3M HNO
3
3000
Table 5.3: D-R constant for the sorption of uranium on DMDBMA grafted
resin; Temperature: 25C
[HNO
3
] R
2
X
m
(mol/g) E (kJ/mol)
1.0 M 0.992 30.2 1.1 15.1 0.7
3.0 M 0.991 49.9 1.7 17.2 0.9
to be 15.080.65 and 17.150.93 kJ/mol at 1M and 3M HNO
3
, respectively (Table
5.3). These values are of the same order of magnitude as reported for the sorption of
Zn(II) ions on polyurethane foam [37].
The uranium sorption data fitted to Langmuir and D-R isotherm model was
an evidence for the presence of monolayer chemisorption of uranium on a set of well-
defined localized sorption sites having same sorption energies on the malonamide
grafted polymer. Nevertheless, the possibility of multilayer sorption was also tested
using Freundlich isotherm model which assumes the multilayer sorption of metal
Fig. 5.4. D-R isotherm for the sorption of uranium on
malonamide functionalized polymer; Temperature: 25C
Chapter V
122
-0.5 0.0 0.5 1.0 1.5 2.0 2.5 3.0
-1.0
-0.5
0.0
0.5
1.0
l
o
g
(
x
/
m
)
l og C
e
1M HNO
3
3M HNO
3
ions on the solid surface. Where as Langmuir model assumes that the enthalpy of
adsorption is constant, Freundlich isotherm assumes that the enthalpy of adsorption
varies logarithmically with the gas pressure (or initial concentration of metal ions in
liquid phase). The Freundlich sorption isotherm in the liquid phase is represented by
the following equation [33,38],
log(x/m) =logK
f
+ (1/n) logC
e
(5.7)
where, C
e
is the concentration of metal ions in the aqueous phase at equilibrium, x/m
is the concentration of metal ions adsorbed per unit mass of the polymer. The
Freundlich constants K
f
and n are related to the sorption capacity and sorption
intensity, respectively. The Freundlich plot of log(x/m) versus logC
e
for uranium
sorption data is presented in Fig. 5.5. As can be seen from the figure, the observed
points do not fit on the straight line behaviour (dotted line) and coefficients of the
linear regression fits were 0.981 and 0.979 at 1M and 3M HNO
3
, respectively. This
observation reflected the non-applicability of the Freundlich isotherm for the present
system, confirming the absence of multilayer physisorption of uranium on the
malonamide grafted polymer. On the other hand, better linear fits for Langmuir
Fig. 5.5. Freundlich isotherm for the sorption of uranium on
malonamide functionalized polymer; Temperature: 25C
Chapter V
123
model (Fig. 5.3) represents monolayer chemisorption on a set of well-defined
localized sorption sites. However, to confirm the presence of monolayer sorption of
U(VI) on the malonamide grafted resin, a theoretical model based on sorption
kinetics was applied to the present system.
5.3.2. Sorption Mechanism
The Langmuir sorption isotherm model suggested that the sorption of uranium on the
malonamide functionalized polymer was a monolayer sorption process. However, to
understand the mechanism further, the sorption model described by Zhang, et al. [39]
was followed. If one assumes that the sorption of U(VI) on the DMDBMA
functionalized polymer was a monolayer process, then the sorption rate would
increase with the available concentration of U(VI), (C
o
-C
t
), and the effect of contact
time is not significant on the rate of sorption [39]. Then the sorption equation can be
expressed as follows,
dC
t
/ dt =K (C
o
C
t
) (5.8)
On integration, following expression can be obtained,
Ct
Co
dC
t
/ (C
o
C
t
) =
0
t
K dt (5.9)
ln[(C
o
C
t
) / C
o
] =K t (5.10)
ln[1- (C
t
/ C
o
)] =K t (5.11)
where C
o
and C
t
represent the sorbed amount of U(VI) at equilibrium and at time t,
respectively, and K is the sorption constant for monolayer sorption. According to the
above model, the plot of -ln[1- (C
t
/ C
o
)] versus time t should be a straight line with
slope K if the assumption for a monolayer sorption was correct. The sorption of
U(VI) on malonamide grafted polymer was investigated as a function of time at 3M
HNO
3
and Eq. (5.11) was plotted in Fig. 5.6. The linearity of the plot of -ln[1- (C
t
/
C
o
)] versus time t was an evidence that the sorption of U(VI) followed the
monolayer mechanism. The correlation coefficient of the linear regression fit was
observed to be 0.999 i.e. ~1. This observation confirmed that the Langmuir
monolayer sorption isotherm was correct.
Chapter V
124
0 5 10 15 20 25
0
2
4
6
8
-
l
n
[
1
-
(
C
t
/
C
0
)
]
t (min)
0 1 2 3
0
2
4
6
8
ln t
Similarly, if one assumes that the sorption of U(VI) on the grafted polymer
was multilayer molecule sorption, then the rate of U(VI) sorption on the polymer
usually increased with the available concentration of U, (C
o
-C
t
), and decreased with
the contact time [39]. Then the sorption equation can be expressed as follows,
dC
t
/ dt =K
m
(C
o
C
t
) / t (5.12)
Eq. (5.12) can be solved as follows,
Ct
Co
dC
t
/ (C
o
C
t
) =
0
t
K
m
dt / t (5.13)
ln[(C
o
C
t
) / C
o
] =K
m
ln t (5.14)
ln[1- (C
t
/ C
o
)] =K
m
ln t (5.15)
where K
m
is the sorption constant for multilayer sorption. From the above equation,
one should also get a straight line of the plot of -ln[1- (C
t
/ C
o
)] versus ln t with slope
K
m
, if the assumption for multilayer adsorption was correct. However, non-linearity
of the plot of -ln[1- (C
t
/ C
o
)] versus ln t (Fig. 5.6) suggested that the assumption for
multilayer sorption of U(VI) was incorrect. Similar observation was also exhibited by
Fig. 5.6. Relationship between ln[1- (C
t
/ C
o
)] and t as well as ln[1- (C
t
/ C
o
)]
and ln t for the sorption of uranium at 3 M HNO
3
; Temperature: 25C
Chapter V
125
Freundlich isotherm, where nonlinearity of the plot suggested the absence of
multilayer sorption mechanism. This observation confirmed that the sorption of
U(VI) on the malonamide functionalized polymer followed the monolayer
chemisorption mechanism instead of multilayer physisorption. Zhang, et al. [39] have
also reported the chemisorption of Sr(II) on a polymeric material containing
butylcyclohexano-18-crown-6 as the ligand. The chemisorption of uranyl ions on the
malonamide grafted resin takes place through co-ordination of the metal ion with the
carbonyl oxygen of the malonamide ligand.
5.4. EFFECT OF FEED ACIDITY ON METAL ION SORPTION
The role of nature of mineral acids such as HNO
3
, HCl and HClO
4
on the sorption
behaviour of uranium was investigated and the data are summarized in Fig. 5.7. The
distribution coefficient (K
d
) of U(VI) was lowest in HClO
4
medium which was
ascribed to the weak complexing ability of the ClO
4
-
ions. By contrast, the K
d
value
increased with increasing acidity in HCl as well as in HNO
3
media. This behaviour
was ascribed to the nature of amide ligands which extract neutral metal species after
Fig. 5.7. Distribution coefficient (K
d
) of U(VI) ions by malonamide
grafted resin from various mineral acid solutions; Temperature: 25C
0 1 2 3 4 5 6
10
0
10
1
10
2
10
3
HNO
3
HCl
HClO
4
Acid concent ration, M
K
d
Chapter V
126
0 1 2 3 4 5 6
10
-1
10
0
10
1
10
2
10
3
K
d
,
M
e
t
a
l
I
o
n
s
[HNO
3
], M
Am(III)
U(VI)
Pu(IV)
Pu(III)
Th(IV)
their charge neutralization using counter anions such as Cl
-
/ NO
3
-
ions. The K
d
values of U(VI) at 3M acid solution followed the order: 410 (HNO
3
) >180 (HCl) >
27 (HClO
4
). Higher K
d
value in nitric acid was attributed to the stronger
complexation of nitrate ions to form neutral metal-nitrate extractable species.
Fig. 5.8 shows the sorption profile of actinide ions, viz. Am(III), Pu(III),
Th(IV), Pu(IV) and U(VI) onto the DMDBMA grafted resin from HNO
3
solution.
The valency of Pu was adjusted to Pu(IV) as well as to Pu(III) by the procedure
described in Chapter-II. The sorption of metal ions from nitric acid medium on the
malonamide grafted resin can be expressed by the following equilibrium reaction,
M
n+
+n NO
3
-
+x DMDBMA---R
\
==
\
M(NO
3
)
n
. xDMDBMA---R (5.16)
where DMDBMA---R is the malonamide moiety attached to the resin. As depicted in
Fig. 5.8, the K
d
values of all the metal ions increased gradually with nitric acid
concentration. Higher K
d
values for tetravalent metal ions, viz. Th(IV) and Pu(IV)
were attributed to their stronger complexation with the bidentate malonamide
molecule due to higher charge density on the metal ions as compared to uranyl ions.
On the other hand, trivalent Am and Pu showed significant distribution coefficient
Fig. 5.8. Distribution coefficient (K
d
) of actinide ions by malonamide
grafted resin from varying concentration HNO
3
; Temperature: 25C
Chapter V
127
Table 5.4: Selectivity factor of actinide ions by malonamide grafted resin;
Aqueous phase: 1M HNO
3
; Temperature: 25C
Meta1 ions K
d
(mL/g) Selectivity factor
Am(III) 4.9 Pu
4+
/Am
3+
32.2
Pu(III) 7.4 UO
2
2+
/Am
3+
15.3
Pu(IV) 158 UO
2
2+
/Pu
3+
10.1
Th(IV) 66 Th
4+
/Am
3+
13.5
U(VI) 75 Th
4+
/Pu
3+
8.9
only above 3M HNO
3
. At 1M HNO
3
the K
d
values for Am(III) and Pu(III) were 5.2
and 7.4 mL/g, respectively, suggesting insignificant sorption of these metal ions at
low acidity. The batch distribution data of actinides suggested the possible analytical
application of the malonamide grafted resin for the separation of Am, Pu and U or
Am, Th and Pu from each other at low acidity (~1M HNO
3
). The analysis of K
d
values and the selectivity factors of these metal ions at 1M HNO
3
are listed in Table
5.4. Reasonably good separation factor for Pu
4+
/Am
3+
, Th
4+
/Am
3+
, UO
2
2+
/Am
3+
and
UO
2
2+
/Pu
3+
reflected that U(VI) along with Pu(IV) or Th(IV) along with Pu(IV) can
be selectively sorbed on the DMDBMA column leaving behind Am(III) in the
aqueous stream. Then Pu(IV) can be selectively eluted from the column after
reducing to Pu(III) using a reducing mixture of 0.2M HAN and 0.2M HN in 1M
HNO
3
. After eluting plutonium as Pu(III), the remaining fraction of uranium (or
thorium) can be eluted separately. Nevertheless, it is obvious from the data presented
in Fig. 5.8 that the developed resin matrix is highly suitable for the recovery of
actinide ions from their dilute solutions at moderate acidity (3-4M HNO
3
).
5.5. DESORPTION STUDIES
For desorption studies, 100mg of the resin was loaded with the actinide ions at 3M
HNO
3
by equilibrating with metal ions solution for 60min. Subsequently, the sorbed
actinide ions were desorbed from the resin phase using various eluting agents and the
results are summarized in Table 5.5. In the case of uranium, maximum desorption
was observed in first contact with Na
2
CO
3
due to strong complexation of carbonate
ions with uranyl ions which forms non-extractable [UO
2
(CO
3
)
3
]
4-
anionic
Chapter V
128
Table 5.5: Desorption of actinide ions from DMDBTDMA grafted resin; Each
desorption stages represent 2mL of eluting solution
Elements Eluting solution
% Desorption
I Stage II Stage III Stage
Am(III) 0.01M HNO
3
84.47 96.93 99.04
0.01M EDTA 85.51 99.63 99.87
Pu(IV
0.01M HNO
3
29.49 62.27 83.07
0.25M Oxalic acid 86.02 99.62 101.21
0.2M HN +0.2M
HAN (at 1M HNO
3
)
88.93 99.17 99.89
U(VI)
0.01M HNO
3
33.27 78.12 89.92
0.25M Na
2
CO
3
89.62 96.02 99.28
0.1M -HIBA 83.21 97.11 99.15
species. Nevertheless, quantitative desorption of uranium could be achieved by
Na
2
CO
3
as well as -hydroxy isobutyric acid (-HIBA) in three contacts. Similarly,
plutonium was effectively desorbed with 0.25M oxalic acid in two contacts. The
possibility of reductive desorption of plutonium was explored using a mixture of
0.2M HAN and 0.2M HN at 1M HNO
3
. The results showed that the Pu(IV) could be
reduced to Pu(III) and quantitative desorption was possible in two contacts. On the
other hand, Am(III) could be desorbed quantitatively with 0.01M EDTA solution as
well as dilute nitric acid solution. The desorption data was an evidence for the
possible reductive desorption of plutonium. These data were explored for the
analytical application of the resin column.
5.6. ANALYTICAL APPLICATIONS
The most important application of solid phase extraction is pre-concentration /
separation of trace elements, which increases the sensitivity of the analytical
technique and also the selectivity after separation of the metal ions of interest from
complex matrix. Some of the applications of the DMDBMA grafted resin were
explored and presented in this section.
Chapter V
129
5.6.1. Metal Loading Capacity
The loading capacities of uranium and thorium by DMDBMA functionalized
polymer were evaluated and the results were compared with different amide grafted
resins reported in the literature. The metal loading capacity was determined by
equilibrating 100mg of polymeric material with 5mL of individual solution of U and
Th (1mg/mL for each metal ions) for 24hrs. Suitable aliquots of the aqueous phases
were removed before and after equilibration for the estimation of uranium and
thorium. The analyte solutions were diluted suitably for spectrophotometeric
estimation using Arsenazo-III as the chromogenic agent. The calibration curve for
Th(IV) was obtained in the concentration range of 1x10
-6
to 1x10
-5
M giving molar
extinction coefficient () value of 1.0x10
5
M
-1
cm
-1
. Similarly, calibration curve for
U(VI) was constructed between the concentration range of 1x10
-5
to 5x10
-5
M, which
yielded an value of 2.0x10
4
M
-1
cm
-1
. The metal sorption capacities were found to
be 18.781.53 and 15.741.59mg/g resin for U and Th, respectively. Higher loading
capacity for U(VI) was attributed to the greater metal ligand complex stability and
also to the lesser steric hindrance involved for U(VI) complexation over Th(IV) as
later requires four nitrate ions for metal charge neutralization. Similar observation
was also reported for 2-ethylhexyl malonamide grafted resin [21]. Table 5.7 lists the
sorption capacities of uranium and thorium by various amide grafted resins obtained
from the literature. Though DMDBMA grafted polymer reflected lower metal
Table 5.7: Comparison of resin sorption capacity for U and Th by several
chemically grafted resins
Grafted resin
Aqueous
phase
Sorption capacity (mg/g)
U(VI) Th(IV)
Merrifield polymer-DMDBMA 4M HNO
3
18.78 15.74
Merrifield polymer-EDHBA [42] 6M HNO
3
84.97 157.78
Merrifield polymer-DB2EHMA [21] 5M HNO
3
62.50 38.20
Merrifield polymer-DAPPA [18] 4M HNO
3
211.82 143.64
Amberlite XAD-16-ADHA [43] 4M HNO
3
164.70 -
Chapter V
130
loading capacity as compared to other amide grafted resins, the resin is highly
suitable for the pre-concentration of actinide ions from their dilute solutions of
moderate acidity (3-4M HNO
3
).
5.6.2. Tolerance of Metal Ions on Sorption of Uranium
It was of interest to investigate the role of some of the common interfering elements
present in the radioactive waste as well as in the environmental samples on the
sorption of uranium. Therefore, the sorption behaviour of U(VI) on grafted resin was
investigated in the presence of large concentrations of various interfering metal ions,
viz. Fe, Al, V, Sr and Cs. As depicted in Table 5.8, the distribution coefficient values
of uranium were found to be practically unchanged in the presence of various metal
ions. The result clearly depicts the ion selectivity behaviour of the developed resin
matrix towards actinide ions.
Table 5.8: Distribution coefficient of uranium under different feed conditions
Meta Ions
K
d
-U at 3M HNO
3
100ppm 500ppm 1000ppm
Fe 419 407 417
Al 431 404 392
Sr 417 398 414
Cs 436 436 430
V 415 433 415
5.6.3. Column Separation of Am, Pu and U
An attempt was made for the separation of Am, Pu and U from a synthetic solution
(at 1M HNO
3
) employing malonamide grafted resin column. A glass column
(dimension: 5.5cm x 0.4cm) was packed with 0.3g of grafted resin beads. The bed
volume and the bed density were calculated from the column dimensions and the
weight of the packed chromatographic resin material. The optimized experimental
column parameters are listed in Table 5.9. The column was preconditioned by
passing excess of 1M HNO
3
solution, prior to the introduction of the sample solution.
Chapter V
131
Table 5.9: Optimized experimental parameters for separation of Am, Pu and U
Stationary phase DMDBMA
Support material Merrifield Resin
Mesh size 16-50
Bed volume 0.69cm
3
Bed density 0.43 g/cm
3
Flow rate 3drops/min
Eluent for Am 1M HNO
3
Eluent for Pu 0.2M HAN+0.2M HN at 1M HNO
3
Eluent for U 0.1M -hydroxy isobutyric acid
The column operations were carried out at ambient temperature (251C) at a flow
rate of 3drops / min. A synthetic mixture of known quantity of
241
Am,
233
U and
239
Pu
at 1M HNO
3
was prepared and the valency of Pu was adjusted to Pu(IV). The
resulting solution was subsequently loaded on the column at a flow rate of
3drops/min. Similarly, the sequential elution of the loaded metal ions was carried out
at the flow rate of 3drops/min. The first fraction of Am was eluted with 1M HNO
3
where all the activity could be recovered with in 25mL of the eluent. As shown in the
Fig. 5.9. Elution profile of Am, Pu and U from the malonamide grafted resin
column; Temperature: 25C
0 10 20 30 40 50 60 70 80 90
0.0
5.0x10
4
1.0x10
5
1.5x10
5
2.0x10
5
2.5x10
5
U by 0.1M -HIBA
Pu by 0.2M HN+
0.2M HAN at
1M HNO
3
Am by 1M HNO
3
C
o
l
u
m
n
w
a
s
h
i
n
g
w
i
t
h
1
M
H
N
O
3
c
p
m
/
m
L
Volume of eluent passed (mL)
Chapter V
132
batch distribution data, low complexation of the trivalent metal ions with DMDBMA
resulted in the elution of Am(III) at 1M HNO
3
, where tetravalent Pu and hexavalent
U were quantitatively complexed with the malonamide ligand on the column. The
next fraction of Pu was quantitatively eluted with 30mL of a reducing mixture of
0.2M HAN and 0.2M HN at 1M HNO
3
. As soon as the reducing mixture comes in
the contact of the resin, Pu(IV) could be reduced to Pu(III) and eluted from the
column due to low affinity to the malonamide. The flow rate of 3drops/min
(~150L/min) could provide sufficient time for the reduction of Pu [37]. Finally, the
remaining fraction of uranium was quantitatively eluted with 0.1M -HIBA. The
chromatogram resulting from the elution of actinide ions is presented in Fig. 5.9. The
first 10mL of 1M HNO
3
solution was passed to ensure the complete pre-conditioning
of the column. Subsequently, the column was loaded with the mixture of three metal
ions followed by their sequential elution with different eluents. The purity of the
eluted fraction was determined by alpha spectrometry and shown in Fig. 5.10. It was
observed from the spectrum that Am fraction was fairly pure with <0.01%
contamination of U and Pu. However, Pu fraction was found to be contaminated
with6% of Am and <0.1% of U. Similarly, U fraction was contaminated with 4% of
Am as well as 2% of Pu. The column performance can be further improved by
increasing the column length.
4.6 4.8 5.0 5.2 5.4 5.6
0
100
200
Energy, keV
241
Am
0
100
200
C
o
u
n
t
s
p
e
r
m
i
n
239
Pu
0
100
200
233
U
Fig. 5.10. Alpha spectrum of separated Am, Pu and U
Chapter V
133
Table 5.10: Pre-concentration of actinides by DMDBMA grafted resin column;
Feed: 1L solution containing metal ions at 3M HNO
3
; Eluent: 0.1M -HIBA
Metal
ions
Amount loaded
(mg)
Amount
eluted
a
(mg)
Percentage
Recovery
Pre-concentration
factor
Th(IV) 4.10 4.23 103.17 40
U(VI)
b
0.124 0.118 95.16 40
a
: As estimated from the 25 mL of the eluted fraction;
b
:Refers to
233
U
5.6.4. Pre-concentration of Uranium and Thorium
The Pre-concentration of trace metal ions such as uranium and thorium was carried
out from 3M HNO
3
using the DMDBMA grafted resin column. The column
parameters were identical as described in Table 5.9. The column was preconditioned
by passing excess of 3M nitric acid solution, prior to the introduction of the sample
solution. In independent experiments, one litre solution containing trace amount of
either uranium or thorium at 3M HNO
3
was passed through the column at a flow rate
of 3drops/min. It should be noted that no breakthrough was observed for both the
metal ions throughout the loading stages. After passing the complete loading
solution, the elution was performed with 0.1M -HIBA and the results of pre-
concentration are shown in Table 5.10. It was observed that the quantitative recovery
of both the metal ions was possible in 25mL eluting solution. The results suggested
the successful pre-concentration of actinide ions with a pre-concentration factor of
40. It is evident from the Table that about 4.10mg of Th and 0.124mg of U could be
loaded on the column and recovered quantitatively with 5% of the original
concentration.
5.7. REFERENCES
1. Y. Marcus and A.S. Kertes, Ion Exchange and Solvent Extraction of Metal
Complexes, Wiley Interscience, New York, 1969.
2. D. Kara and M. Alkan, Talanta, 55 (2001) 415.
3. J. Kubova, V. Neveral and V. Stresko, J. Anal. At. Spectrom., 9 (1994) 241.
4. N. Masque, R.M. Marce and F. Borull, Trends. Anal. Chem., 17 (1998) 384.
Chapter V
134
5. T. Prasada Rao and J.M. Gladis, Rev. Anal. Chem., 20 (2001) 145.
6. T. Prasada Rao and C.R. Preetha, Sep. Purif. Rev., 32 (2003) 1.
7. V. Camel, Spectrochim. Acta Part B, 58 (2003) 1177.
8. J.S. Lee, S.G. Salazar and L.L. Tavlarides, React. Funct. Polym., 49 (2001) 159.
9. G. Fang, J in Tam and Xiu-Ping Yan, Anal. Chem., 77 (2005) 1734.
10. E.M. Moyers and J.S. Fritz, Anal. Chem., 49 (1977) 418.
11. M. Marhol and K.L. Cheng, Talanta, 21 (1974) 751.
12. P. Figura and B. McDuffie, Anal. Chem., 49 (1977) 1950.
13. P. Metilda, K. Sanghamitra, J.M. Gladis, G.R.K. Naidu and T.P. Rao, Talanta, 65
(2005) 192.
14. W.M. Landing, C. Haraldsson and N. Paxeus, Anal. Chem., 58 (1986) 3031.
15. C. Kantipuly, S. Katragadda, A. Chow and H.D. Gesser, Talanta, 37 (1990) 491.
16. R.K. Sharma, S. Mittal and M. Koel, CRC Crit. Rev. Anal. Chem., 33 (2003)
183.
17. H. Nalwa, Advanced Functional Molecules and Polymers, Vol. 4, Gordon &
Breach, Singapore (2001), P. 323.
18. J.S. Fritz, Introduction and Principles: Analytical Solid Phase Extraction, Willey-
VCH, New York (1999), P. 1-14.
19. M. Torre and M.L. Marina, CRC Crit. Rev. Anal. Chem., 24 (1994) 237.
20. P.N. Pathak, R. Veerraghavan, D,R, Prabhu, G.R. Mahajan and V.K. Manchanda,
Sep. Sci. Technol., 34 (1999) 2601.
21. C. Madic and M.J. Hudson, Euro. Comm. Report, EUR18038EN, (1998), P. 5.
22. G.R. Choppin and A. Morgenstern, J. Radioanal. Nucl. Chem., 243 (2000) 45.
23. G. Thiollet and C. Musikas, Solv. Extr. Ion Exch., 7 (1989) 813.
24. C. Cuillerdier, C. Musikas and L. Nigond, Sep. Sci. Technol., 28 (1993) 155.
25. C. Musikas, Inorg. Chim. Acta, 140 (1987) 197.
26. V.K. Manchanda and P.N. Pathak, Sep. Purif. Technol., 35 (2004) 85.
27. G.R. Mahajan, D.R. Prabhu, V.K. Manchanda and L.P. Badheka, Waste
Management, 18 (1998) 125.
28. L.B. Kumbhare, D.R. Prabhu, G.R. Mahajan, S. Sriram and V.K. Manchanda,
Nucl. Technol., 139 (2002) 253.
29. L. Berthon, J.M. Morel, N. Zorz, C. Nicol, H. Virelizier, and C. Madic, Sep. Sci.
Technol., 36 (2001) 709.
Chapter V
135
30. P.K. Mohapatra, S. Sriram, V.K. Manchanda and L.P. Badheka, Sep. Sci.
Technol., 35 (2000) 39.
31. R. Chiarizia, E.P. Horwitz and S.D. Alexandratos, Solv. Extr. Ion Exch., 12
(1994) 211.
32. D.B. Singh, G. Prasad, D.C. Rupainwar and V.N. Singh, Water Air Soil Pollut.,
42 (1988) 373.
33. P.W. Atkins, Physical Chemistry, 5
th
Ed. Oxford: Oxford University press
(1994).
34. I. J. Langmuir, J. Am. Chem. Soc., 40 (1918) 1361.
35. M.M. Dubininn and L.V. Raushkevich, Proc. Acad. Sci. USSR, Phys. Chem.
Sect., 55 (1947) 331.
36. M. Polanyi, Trans. Faraday Soc., 28 (1932) 316.
37. M.H. Hasany, M.M. Saeed and M. Ahmed, J. Radioanal. Nucl. Chem., 252
(2002) 477.
38. G. McKay, H.S. Blair and J.R. Garden, J. Appl. Polym. Sci., 27 (1982) 3043.
39. A. Zhang, Y. Wei and M. Kumagai, React. Funct. Polym., 61 (2004) 191.
40. P.K. Mohapatra and V.K. Manchanda, Radiochim. Acta, 91 (2003) 91.
41. M.A. Maheshwari and M.S. Subramanian, Talanta, 65 (2005) 735.
42. M.A. Maheshwari and M.S. Subramanian, Solv. Extr. Ion Exch., 23 (2005) 249.
--------------------------
Chapter VI
Transport Behaviour Of Long
Lived Radionuclides Across
Liquid Membranes Using
N,N,N,N-Tetraoctyl
Diglycolamide As The Carrier
Chapter VI
136
TRANSPORT BEHAVIOUR OF LONG LIVED
RADIONUCLIDES ACROSS LIQUID MEMBRANES USING
N,N,N,N-TETRAOCTYL DIGLYCOLAMIDE AS THE CARRIER
6.1. INTRODUCTON
Membranes are physical / chemical barriers which allow selective permeation of
certain species. Membrane technology has contributed significantly for the
production of potable water from sea water in the last few decades. In the recent
years, membrane processes have been found to be effective in the treatment of
industrial effluents and gas purifications [1-3]. Advantages of membrane based
separation techniques are low energy requirements, low capital and operating costs,
the possibility of achieving high separation factors and simple modular design [4,5].
The use of liquid membranes containing a carrier has been proposed as an alternative
to solvent extraction for selective separation and concentration of metal ions from
dilute aqueous solutions [6-8]. The important feature of liquid membrane is that
unlike solvent extraction the extraction and stripping of the metal ions as well as
regeneration of the carrier are combined in a single stage.
Liquid membrane usually consists of a water immiscible (organic) layer
separating a source aqueous phase (feed) containing mixture of metal ions and a
receiving aqueous phase (receiver), where the metal ions of interest gets concentrated
preferentially. The liquid membrane may be a stirred organic phase separated by
aqueous feed and receiver phases (Bulk Liquid Membrane, BLM) or a dispersion of
water (strip phase) containing oil droplets in aqueous feed phase (Emulsion Liquid
Membrane, ELM). In contrast, supported liquid membrane (SLM) consists of water
immiscible organic phase immobilized in the pores of microporous polymeric film
(which acts as inert support) separating the two phases, the feed and the receiver
phases. Commonly two types of membrane support are used in SLM, a flat sheet
(FSSLM) or a hollow fibre (HFSLM). The performance of different types of liquid
membranes, viz. BLM, ELM, FSSLM and HFSLM has been reviewed by Izatt, et al.
[9]. Facilitated transport of metal ions through SLM has been described in a number
of papers [1-15]. In such transport systems, the metal ions can be transported across
the membrane against their concentration gradient, i.e. uphill transport. The driving
Chapter VI
137
force in such processes is provided by the chemical potential difference of the
chemical species present on the two opposite sides of the membrane. The
permeability of the transported species is decided by the parameters such as
membrane thickness, pore structure, aqueous diffusion coefficient of metal ions,
aqueous diffusion layer thickness, distribution and diffusion coefficients of the metal
bearing species in the organic liquid membrane phase, etc. [14-16]. While diffusion
coefficient of the metal species in the carrier solvent depend upon the chosen
membrane and the aqueous diffusion coefficient of the metal ions depend on the
stirring rate, the transport rates can be controlled through various parameters, viz.
feed acidity, solute concentration, carrier concentration, nature of diluent, strip phase
composition etc. [17].
Extensive work has been carried out by several laboratories (including our
laboratory) to investigate N,N,N',N'-tetraoctyl diglycolamide (TODGA) as the
extractant for the partitioning of long-lived radionuclides using solvent extraction
technique. The present chapter describes the carrier mediated transport of actinides
and few selected fission products from nitric acid solution across the SLM containing
TODGA/n-dodecane as the carrier. A detailed study was undertaken to optimize the
metal transport process by investigating various parameters such as nature of
strippant, carrier concentration, feed acidity etc. An attempt has also been made to
elucidate the transport mechanism. The stability of the liquid membrane was also
investigated. The applicability of TODGA-SLM technique on large scale separation
of lanthanides / actinides has been successfully demonstrated in a membrane cell
contactor employing Hollow Fibre Module.
6.2. THEORY OF FACILITATED TRANSPORT
In the facilitated transport process, the metal ion diffuses through the aqueous feed
boundary layer and reacts with the carrier molecule (present in the pores of the solid
support) at the aqueous feed-membrane interface resulting into the formation of
metal-carrier complex. The metal-ligand complex then diffuses through the liquid
membrane to the receiver phase because of its negative concentration gradient.
Finally, the metal-carrier complex releases the metal ion into the strip solution at the
membrane-aqueous strip interface. The quantitative description of the facilitated
transport, therefore, requires a detailed knowledge of the processes like (a) diffusion
Chapter VI
138
of the metal ions through the aqueous feed boundary layer, (b) reversible chemical
reaction at the aqueous feed-membrane interface, (c) diffusion of the metal-carrier
complex in the membrane phase, and (d) dissociation reaction of the metal-carrier
complex at the membrane-aqueous strip interface. The mathematical relationships for
the permeation of metal ions in the facilitated transport can be derived by simple
assumptions. The model described by Danesi, et al. [16] assumes that the chemical
reaction between the metal ions and the ligand occurs instantaneously (relative to
other rate controlling processes) at the aqueous-membrane interface. It implies that
the local equilibrium always occurs at the aqueous-membrane interface and the metal
ion transport rate will be determined by the diffusion rate through the aqueous
stagnant layers (Nernst films) and the diffusion rate through the liquid membrane.
6.2.1. Distribution Equilibria at Aqueous Membrane Interface
The chemical reaction involved during the extraction of metal ions (M
n+
) from
aqueous nitrate medium by neutral ligand, TODGA (L), can be described as,
M
n+
(aq)
+n NO
3
-
(aq)
+x L
(org)
M(NO
3
)
n
xL
(org)
(6.1)
where the subscripts (aq) and (org) represent the aqueous and the organic phases,
respectively. The term M(NO
3
)
n
xL represents the extracted species in the organic
phase. For simplicity we will represent this term as [ML] in this chapter. The
stoichiometry of the extracted species of TODGA and various actinide ions are
described in Chapter III. At the aqueous feed-membrane interface, the equilibrium
reaction (6.1) is shifted to right and the [ML] complex species then moves towards
the receiving phase within the membrane as a consequence of concentration gradient.
On the other hand, at the membrane-strip interface, the equilibrium reaction (6.1) is
completely shifted to the left because of unfavourable conditions in the strippant
phase. This results in dissociation of the [ML] complex leaving behind free ligand
molecules in the membrane phase. The free carrier molecules then move towards the
feed solution due to negative concentration gradient to complete the cycle. The
transport mechanism of metal ions through SLM is depicted in scheme-I. As per the
empirical WilkeChang equation [18,19], the bulk diffusion coefficient of the
transported species is defined as,
Chapter VI
139
D
o
=7.4x10
-8
(
0.5
M
0.5
T3600)/(V
m
0.6
) (6.2)
where M, and are the molecular weight, solvent association parameter and the
viscosity of the solvent, respectively, V
m
, the molar volume of the carrier and T is the
temperature. WilkeChang equation depicts that the diffusion coefficient of the
forward transported metal-ligand complex [ML] should be much lower than the
diffusion coefficient of the backward transported bare ligand molecules due to
smaller molar volume of the later. This fact implies that the concentration of ligand at
the feed membrane interface will always be in excess because of faster diffusion of
bare ligand as compared to bulky [ML] complex.
6.2.2. Flux Equations for Permeation
The equations describing flux across the stagnant aqueous diffusion layer and
membrane can be derived by applying Ficks diffusion law with simple assumptions
[16,17]: (a) the composition of the strip solution is such that the complex [ML] is
completely dissociated at the membrane-aqueous strip interface, (b) the membrane
polarity is low enough to neglect the presence of charged species in the membrane
phase, and (c) there is no interaction of metal ions with pure diluent. If one includes
the assumptions that the transport of metal ion occurs at the steady state, the
concentration gradients are linear, and the feed concentration of NO
3
-
is constant,
Scheme-I
Membrane phase
Strip phase Feed phase
M
n+
+ H
+
+ NO
3
-
M
n+
+ H
+
+ NO
3
-
ML
L
Chapter VI
140
Fig. 6.1. A schematic diagram representing the different phases and species in SLM
then the equations describing the aqueous diffusion film flux (J
a
) and membrane flux
(J
o
) are given as [16],
J
a
=D
a
/ d
a
{[M]
aq,f
[M]
aq,i
} (6.3)
J
o
=D
o
/ d
o
{[ML]
org,f
[ML]
org,s
} (6.4)
where D
a
is the aqueous diffusion coefficient of the metal ions, d
a
the thickness of the
aqueous stagnant film, [M]
aq,f
and [M]
aq,i
the metal ion concentrations in the aqueous
feed phase and at the aqueous feed-membrane interface, respectively, D
o
the
membrane diffusion coefficient of [ML] and d
o
the membrane thickness. The
concentration of carrier-metal complex at the aqueous feed-membrane and at the
membrane-aqueous strip interfaces are denoted by [ML]
org,f
and [ML]
org,s
,
respectively. The various parameters in Eqs. (6.3) and (6.4) are described in Fig. 6.1.
The distribution coefficient (K
d
) of the given metal ion at the aqueous feed-
membrane interface is reasonably high as equilibrium reaction (1) is shifted to the
right due to favourable conditions. However, due to unfavourable conditions on the
strip side, the equilibrium reaction (6.1) is shifted to the left as the K
d
value of the
given metal ion at the membrane-aqueous strip interface is much lower as compared
to the aqueous feed-membrane interface, i.e. [M.L]
org,s
<<[M.L]
org,f
. Therefore, Eq.
(6.4) can be reduced to,
Chapter VI
141
J
o
=D
o
/ d
o
[M.L]
org,f
(6.5)
If we assume that the carrier in the liquid membrane is not saturated with the metal
ions, then the concentration terms [ML]
org,f
and [M]
aq,i
are co-related to the K
d
by the
following equation,
K
d
=[ML]
org,f
/ [M]
aq,i
(6.6)
If the chemical reaction between ligand and the metal nitrate is assumed to be fast
compared to the diffusion rate, a local equilibrium at the interface is reached. Thus, at
steady state, J
a
=J
o
=J and Eqs. (6.3), (6.5) and (6.6) can be solved to get the
following expression,
J =K
d
[M]
aq,f
/ [K
d
(d
a
/ D
a
) +(d
o
/ D
o
)] (6.7)
Here the value of membrane thickness (d
o
) can be assumed to be equal to the nominal
thickness of the membrane support. Substituting the permeability coefficient, P =J /
[M]
aq,f
in the above equation, we get,
P =K
d
/ [K
d
(d
a
/ D
a
) +(d
o
/ D
o
)] (6.8)
Above equation can be used for predicting P from K
d
values. In general, however, P
is obtained experimentally using following equation [16],
ln([M]
t
/ [M]
o
) = P (Q/V) t (6.9)
where, [M]
t
and [M]
o
are the concentration of metal ion in the aqueous feed solution
at time t and initial concentration (t =0), respectively. Q is the effective membrane
area obtained from the total exposed membrane surface area A and the porosity c (Q
=Ac), V is the volume of the feed solution in cm
3
, and t is the permeation time
(seconds). A plot of ln([M]
t
/ [M]
o
) versus time allows to calculate the P value from
the slope of the linear fit. It should be noted that the above equation is valid only
when the carrier is not saturated and the flux decreases linearly with time. In the
present work, since all the experiments were carried out at tracer metal concentration,
Eq. (6.9) was applied for the calculation of P.
The membrane diffusion coefficient (D
o
) can be calculated from the
knowledge of permeability coefficient (P) and the distribution ratio of the metal ions
(D
M
) as per the following equation [20],
Chapter VI
142
D
M
P = ------------------------------ (6.10)
[D
M
(d
a
/D
a
) +(d
o
/D
o
)]
where is the tortuosity factor of the membrane. Assuming that the rate determining
step is diffusion of the bulky complex across the membrane, the first term in the
denominator of Eq. (6.10) (which refers to the transport of hydrated metal ions across
aqueous diffusion layer) can be ignored. Therefore, Eq. (6.10) can be rearranged as
follows,
D
o
D
M
P = ---------- (6.11)
d
o
In the present studies, PTFE membranes of 85m thickness (d
o
) were employed and
the tortuosity factor () for the membrane is reported as 2.4 [21]. Thus, from the
knowledge of P and D
M
, the value of D
o
of the metal-ligand complex in the
membrane can be obtained. Alternatively, D
o
can also be obtained experimentally by
determining the P value of metal ions for varying thickness of the membrane (d
o
). A
plot of P vs 1/d
o
would be a straight line and the value of D
o
can be obtained from the
slope of the linear fit.
6.3. TRANSPORT OF AMERICIUM
The conditions for the transport of lanthanides / actinides through TODGA-SLM
were optimized using Am(III) as the model element. Moreover, focus of the present
study was the separation of trivalent actinides and lanthanides from radioactive waste
solution. A detailed investigation on the effect of carrier concentration, feed acidity,
stripping solution, nitrate concentration etc. on the transport of Am(III) was carried
out.
6.3.1. Effect of Membrane Soaking Time
For the preparation of TODGA supported liquid membrane, the microporous PTFE
membranes (as solid support) were soaked in the carrier solution for 15min.
Subsequently, the submerged membrane was removed from the solution and wiped
carefully with a tissue paper to clear it of the excess fluid at the outer surface of the
support. This impregnation technique lead to SLMs which were reproducible within
Chapter VI
143
Table 6.1: Effect of carrier soaking time on the transport of Am(III); Carrier:
0.1M TODGA in n-dodecane; Membrane: 0.45m PTFE; Feed: 1M HNO
3
;
Strippant: Distilled water
Soaking time (min) P x 10
3
(cm/s)
15 2.100.03
30 2.08 0.07
60 2.06 0.04
120 2.09 0.06
1440 2.07 0.04
5% with respect to their Am(III) transport behaviour. However, there was a need to
optimize the soaking time to obtain a uniform and reproducible SLM. As shown in
Table 6.1, a soaking time of 15min gave the P value of 2.1010
3
cm/s which was
consistent with the data obtained after 24hrs of soaking. The results suggested that a
soaking time of 15min was sufficient for making a uniform SLM on PTFE support
using TODGA/n-dodecane as the carrier.
6.3.2. Effect of Feed Acidity
A systematic study was carried out to investigate the effect of feed acidity on the
transport of Am(III) using 0.1M TODGA/n-dodecane as the carrier. As depicted in
Fig. 6.2, quantitative transport of Am(III) was observed in 3hrs in the acidity range of
1-3M HNO
3
. On the other hand, more than 6hrs were required for a similar
Table 6.2: Correlation of permeability coefficient (P) with Feed acidity; Carrier:
0.1M TODGA in dodecane; Membrane: 0.45m PTFE; Strippant: Dist. water
[HNO
3
], M P x 10
3
(cm/s)
1.0 2.1 0.03
2.0 3.8 0.06
3.0 3.7 0.04
4.5 3.1 0.16
6.0 2.2 0.11
Chapter VI
144
0 50 100 150 200 250 300 350 400
0
20
40
60
80
100
%
A
m
T
r
a
n
s
p
o
r
t
Time (min)
1M HNO
3
2M HNO
3
3M HNO
3
4.5M HNO
3
6M HNO
3
transport of Am(III) at 6M HNO
3
. The P value of Am(III) at different feed acidity is
listed in Table 6.2. Similar to solvent extraction studies, where D
Am
increased with
the aqueous phase acidity at a fixed TODGA concentration, the transport rate of
Am(III) was also expected to increase with the aqueous feed acidity. However, the P
data of Am(III) revealed a gradual increase in the transport of Am(III) upto 2M
HNO
3
, and then there was a decline after 3M HNO
3
. Increase in the transport was
attributed to the higher D
Am
values at higher nitric acid concentration where the
formation of extractable neutral metal-ligand complex is favoured. On the other
hand, decrease in the P value at higher acidity (>3M HNO
3
) was ascribed to the
formation of TODGAHNO
3
adduct resulting in decrease of the free carrier
concentration. It was observed that nitric acid was also co-transported along with the
metal ion from feed to the receiver compartment (Table 6.3). There was a gradual
increase in the receiver phase acidity with HNO
3
concentration from 0.04M (feed:
1M HNO
3
) to 0.19M (feed: 6M HNO
3
). Enhanced acid transport at 6M HNO
3
could
also reduce the stripping efficiency of the receiver phase which suppresses the
effective transport of Am(III). Similar observation has also been reported for the
Fig. 6.2. Transport of Am(III) at different feed acidity; Membrane: 0.45m
PTFE; Carrier: 0.1M TODGA in n-dodecane; Strippant: Distilled water
Chapter VI
145
Table 6.3: Transport of acid from feed to the receiver side; Carrier: 0.1M
TODGA in n-dodecane; Membrane: 0.45m PTFE; Strippant: Distilled water
transport of uranium using bis-2-ethylhexyl isobutyramide (D2EHIBA) as the carrier
[22]. It is worth mentioning here that the transport of sufficient amount of acid
suppresses the hydrolysis of metal ions in the receiver phase which is distilled water.
The major source of trivalent actinides is the high level waste (HLW) generated as a
consequence of reprocessing of the spent nuclear fuel. In view of acidic condition of
the HLW (3-4M HNO
3
), all the subsequent studies were carried out at 3M HNO
3
.
6.3.3. Effect of Carrier Concentration
The organic phase ligand concentration has a significant effect on the transport of
metal ion across the SLM [23]. When the transport of metal ions occurs via carrier,
as in facilitated transport, the permeability coefficient is expected to increase with the
carrier concentration. The variation of P value for the transport of Am(III) with
TODGA concentration at fixed nitrate concentration (1M HNO
3
) is given in Fig. 6.3.
Since these data have been obtained at tracer metal concentration, the value of P is
time independent and the data can be quantitatively explained by Eq. (6.8). The P
value of Am(III) increased linearly from 3x10
-5
cm/s at 0.02M to 4x10
-4
cm/s at
0.15M TODGA concentration. The linear increase of P with TODGA concentration
is representative of membrane diffusion controlled permeation process. In this
region, the term K
d
(d
a
/D
a
) <<d
o
/D
o
in the denominator of Eq. (6.8) and hence, P =
Time (min) Nitric acid transport (M)
Feed: 1M Feed: 2M Feed: 3M Feed: 4.5M Feed: 6M
15 0.001 0.01 0.01 0.01 0.01
45 0.01 0.01 0.01 0.02 0.02
75 0.01 0.02 0.02 0.03 0.04
105 0.01 0.03 0.03 0.04 0.06
165 0.01 0.04 0.05 0.07 0.10
240 0.02 0.05 0.07 0.10 0.13
360 0.04 0.08 0.10 0.15 0.19
Chapter VI
146
0.00 0.05 0.10 0.15
0.000
0.001
0.002
0.003
0.004
0.005
P
(
c
m
/
s
)
[TODGA], M
K
d
(D
o
/d
o
). Similar effect has been reported by several authors using CMPO, diamide
(viz. N,N-dimethyl-N,N-dibutyl tetradecyl malonamide) as the carriers [16,21].
Even though the P value increased with TODGA concentration, a concentration of
0.1M TODGA was good enough for reasonably high transport of Am(III). Therefore,
0.1M TODGA/n-dodecane was selected as the carrier for the subsequent studies.
6.3.4. Effect of Nature of Strippant
The nature of strippant has a direct effect on the transport of metal ions. An efficient
strippant should be such that the equilibrium reaction (6.1) shifts to the left leading to
the dissociation of metal-carrier complex. In the present studies, strippants such as
distilled water, 0.1M alpha-hydroxy isobutyric acid (o-HIBA), 0.1M oxalic acid and
a buffer mixture (consisting of 0.4M formic acid +0.4M hydrazine hydrate and 0.1M
citric acid) were evaluated for the transport of Am(III). Fig. 6.4 compares the
transport data of Am(III) for different strippants. The transport of Am(III) for
different strippants upto 2hrs followed the order: buffer mixture >oxalic acid ~o-
HIBA >distilled water. However, at >4 hrs interval, all four strippants yielded near
Fig. 6.3. Permeability coefficient (P) of Am(III) with TODGA concentration;
Diluent: n-dodecane; Feed: 1M HNO
3
; Strippant: Distilled water
Chapter VI
147
0 50 100 150 200 250 300 350 400
0
20
40
60
80
100
%
A
m
T
r
a
n
s
p
o
r
t
Time (min)
Distilled water
0.1M -HIBA
0.1M Oxalic acid
Buffer mixture
#
quantitative transport. Use of buffer as the strippant is advisable when the transport
of acid is high which may suppress the stripping of the given metal ion. The buffer
mixture used in the present work was employed earlier for similar studies where the
stripping rates were lower in distilled water (as receiver phase) due to the transport of
acid [21]. Distilled water was, however, employed as the strippant in major part of
this work.
6.3.5. Effect of Nitrate Ion Concentration
The effect of nitrate ion on the transport of Am(III) was investigated in the presence
of varying concentration of nitric acid as well as sodium nitrate. Kusumocahyo, et al.
[24] have reported that neither proton nor sodium ion is transferred during the
transport of Ce(III) through a polymer inclusion membrane (PIM) containing
TODGA as the carrier and NPOE as the diluent. On the other hand, present studies
on SLM have quite clearly indicated for the transport of acid. The difference in the
acid transport behaviour could be attributed to the different diluents used in the two
studies. In fact, when NPOE was used as the diluent, a reversal in the relative
Fig. 6.4. Effect of strippants on the transport of Am(III); Feed: 1M HNO
3
;
Membrane: 0.45m PTFE; Carrier: 0.1M TODGA in dodecane;
#
Buffer mixture: 0.4M formic acid + 0.4M hydrazine + 0.1M citric acid
Chapter VI
148
extraction properties of TODGA was reported as compared to those observed with n-
dodecane as the diluent [24]. In the experiment carried out at varying acidity with
fixed nitrate ion concentration (3M with NaNO
3
), the P value of Am(III) increased
sharply from 0.01M to 2M HNO
3
. At higher nitric acid concentrations (>2M HNO
3
),
however, a plateau was observed indicating insignificant effect of acid on the
transport of Am(III) (Fig. 6.5(a)). This behaviour resembles the data obtained with
the solvent extraction studies under identical experimental conditions (Fig. 6.5(b))
confirming the importance of equilibrium at the aqueous feedmembrane interface.
The transport and distribution studies carried out at fixed acidity(0.01M H
+
) and
varying nitrate concentration (NaNO
3
) exhibited enhanced Am(III) pertraction with
Fig. 6.5. Comparison of extraction and transport data of Am(III) using 0.1M TODGA in
dodecane; (a) P with HNO
3
concentration at a fixed nitrate concentration of 3M (using NaNO
3
);
(b) D
Am
with HNO
3
concentration at a fixed nitrate concentration of 3M (using NaNO
3
); (c) P
with NaNO
3
concentration at a fixed H
+
concentration of 0.01M; (d) D
Am
with NaNO
3
concentration at a fixed H
+
concentration of 0.01M
0.0 0.5 1.0 1.5 2.0 2.5 3.0
0.0015
0.0020
0.0025
0.0030
0.0035
0.0040
(a)
(b)
D
A
m
P
(
c
m
/
s
)
[HNO
3
], M
D
A
m
P
(
c
m
/
s
)
[HNO
3
], M
0.0 0.5 1.0 1.5 2.0 2.5 3.0
0
50
100
150
200
0.5 1.0 1.5 2.0 2.5 3.0
0.0000
0.0004
0.0008
0.0012
0.0016
(c)
[NaNO
3
], M [NaNO
3
], M
0.5 1.0 1.5 2.0 2.5 3.0
0
5
10
15
20
(d)
Chapter VI
149
increased nitrate concentration. The profile of P vs [NaNO
3
] (Fig. 6.5(c)) resembles
that of D
Am
vs [NaNO
3
] (Fig. 6.5(d)) suggesting the extraction process is indeed a
key step for the transport process.
6.4. TRANSPORT OF METAL IONS FROM NITRIC ACID
The transport studies on various actinide ions and some of the selected fission
products were carried out from 3M HNO
3
employing 0.1M TODGA/n-dodecane as
the liquid membrane. The transport of actinides and lanthanides is shown in Fig. 6.6.
The quantitative transport of Am(III) and Eu(III) was observed within 3hrs.
However, for Pu(IV) and Np(IV), quantitative transport was observed only after 5hrs.
Molecular dynamics simulations studies for the complexation of lanthanide(III) and
UO
2
2+
ions by diglycolamide ligand suggested that the bond distance between UO
2
2+
and carbonyl oxygen (C=O) of diglycolamide is larger than that for corresponding
Ln
3+
bond [25]. Consequently, it can be concluded that the Ln
3+
/ An
3+
-TODGA
complex is stronger as compared to uranyl-TODGA complex thereby showing lower
permeation for the latter nuclide. As listed in Table 6.4, the P values of Ln / An by
TODGA followed the order: An(III)/Ln(III) >An(IV) >An(VI). Zhu, et al. [26] have
reported the relative extractabilities of trivalent lanthanides by TODGA as: Lu(III) >
Eu(III) > La(III), which follow their ionic potentials and also the complexing
Table 6.4: Permeability coefficient (P) of actinides / lanthanides by 0.1M
TODGA-SLM; Membrane: 0.45m PTFE; Feed: 3M HNO
3
; Strippant:
Distilled water
Metal ions P x10
3
(cm/s) % Transport (in 4 h)
La(III) 2.34 0.15 99.99
Eu(III) 3.49 0.15 99.99
Lu(III) 3.64 0.12 99.99
Am(III) 3.67 0.06 99.99
Pu(IV) 1.52 0.03 99.02
Np(IV) 1.50 0.04 97.21
U(VI) 0.94 0.01 91.16
Chapter VI
150
0 50 100 150 200 250 300
0
20
40
60
80
100
%
T
r
a
n
s
p
o
r
t
Time (min)
La(III)
Lu(III)
Am(III)
Pu(IV)
Np(IV)
U(VI)
abilities. However, the P values of all the three lanthanides in the present system
were comparable. The comparable transport behaviour of lanthanides at 3M HNO
3
was in line with the D
M
of these metal ions as described in Chapter III.
The transport of other fission products and structural elements at 3M HNO
3
is
summarized in Fig. 6.7. Similarly, the P values of these metal ions are listed in Table
6.5. As it is evident from the figure, ~8% Ru(III) transport was observed in 5hrs. The
speciation of Ru(III) is rather complex and it was not possible to predict the exact
nature of the species transported. Under identical condition ~85% of Sr(II) was also
transported in 5hrs. Nevertheless, solvent extraction studies of Sr(II) suggested that it
is possible to decontaminate Sr(II) at higher feed acidity (6M HNO
3
) [27]. At 3M
HNO
3
, both Pd(II) and Sr(II) display similar distribution pattern showing decreased
D
M
with increased acidity beyond 3M HNO
3
[26]. Accordingly, about 56% transport
(in 5hrs) of Pd(II) was also observed under identical conditions. Iodine can exist in
Fig. 6.6. Transport of actinides / lanthanides; Membrane: 0.45m PTFE; Carrier:
0.1M TODGA in n-dodecane; Feed acidity: 3M HNO
3
; Strippant: Distilled water
Chapter VI
151
0 50 100 150 200 250 300
0
20
40
60
80
100
120
%
T
r
a
n
s
p
o
r
t
Time (min)
Cs
Sr
Pd
Ru
Zr
Mo
Tc
I
Table 6.5: Permeability coefficient (P) of fission product ions by 0.1M TODGA-
SLM; Membrane: 0.45m PTFE; Feed: 3M HNO
3
; Strippant: Distilled water
Metal ions P x10
2
(cm/s) % Transport (in 5h)
Cs(I) --
a
0.17
Sr(II) 5.6 0.3 85.97
Pd(II) 3.2 0.1 56.15
Ru(III) 0.7 0.1 8.42
Zr(IV) 13.3 0.6 99.9
Mo(VI) --
a
0.75
Tc(VII) 1.2 0.1 31.2
Iodine 1.8 0.1 26.16
a : Insignificant transport of these metal ions
Fig. 6.7. Transport of fission products; Membrane: 0.45m PTFE; Carrier:
0.1M TODGA in n-dodecane; Feed acidity: 3M HNO
3
; Receiver: Distilled water
Chapter VI
152
0 50 100 150 200 250
0
20
40
60
80
100
Time (min)
%
T
r
a
n
s
p
o
r
t
Am(III)
Am(III) + oxalic acid
Zr(IV)
Zr(IV) + oxalic acid
various molecular / ionic species such as I
2
, I
-
, I
3
-
, IO
3
-
etc and predicting its transport
behaviour is cumbersome. Since all these species are in equilibrium, it was of interest
to see if all or some of these species were transported by TODGA-SLM. The
transport of
133
I indicated saturation in the transport after about 100min. One of the
possibilities for the transport of iodine is as counter anion in place of nitrate ion. To
prove this, additional experiment in the presence of Nd(III) was carried out. If iodine
would have transported as counter anion, the presence of excess Nd(III) would
increase the transport of iodine. However, no change in the transport ruled out the co-
transport of the anionic counter ions suggesting that molecular iodine as such may be
transporting.
The transport behaviour of Zr(IV) was similar to that was observed for
Pu(IV) which was in accordance with the distribution behaviour of tetravalent metal
ions [26]. However, significant transport of Zr(IV) implies that suitable modification
of the feed is necessary to suppress its transport to enhance the decontamination
factor (D.F.) with respect of trivalent actinides. Solvent extraction studies revealed
that the presence of 0.4M oxalic acid suppressed the extraction of tetravalent
zirconium without affecting the distribution of trivalent actinides. With this
Fig. 6.8. Effect of oxalic acid on transport of Am(III) and Zr(IV); Membrane: 0.45m PTFE;
Carrier: 0.1M TODGA in n-dodecane; Feed acidity: 3M HNO
3
; Strippant: Distilled water
Chapter VI
153
information, the transport of Am(III) and Zr(IV) was investigated in the presence of
oxalic acid. As depicted in Fig. 6.8, the presence of 0.4M oxalic acid suppressed the
transport of Zr(IV) significantly without affecting the transport of Am(III). It implies
that the trivalent actinides and lanthanides can be selectively separated from Zr(IV)
after selective complexation of the latter in the feed solution.
6.5. TRANSPORT OF METAL IONS FROM SHLW
The tracer studies employing TODGA-SLM for the transport of actinide and fission
product ions were very encouraging. However, the behaviour will be entirely
different when such a system is applied to the HLW which not only contains a host
of other metal ions at appreciable concentrations, but also encounters the radiation
and chemical stability issues. The HLW generated during the reprocessing of spent
fuel contains structural materials like Fe, Ni, Cr, Mo etc., and process chemicals like
Na and Al in addition to the actinides, fission and activation products. A typical
composition of PHWR-HLW is listed in Table 6.6. The distribution studies of metal
ions from SHLW solution exhibited lower D
M
values as compared to the tracer
values (Table 6.7). It was, therefore, of interest to investigate the transport behaviour
of Am(III) in the presence of SHLW conforming to Table 6.6. The transport
behaviour of Am(III) was also investigated in the presence of individual components
Table 6.6: Composition of Simulated High Level Waste (SHLW) used in the
present studies; Acidity: 3.12M HNO
3
Element Concentration, g/L Element Concentration, g/L
Fe 0.72 Ba 0.06
Cr 0.12 La 0.18
Ni 0.11 Ce 0.06
Na 5.50 Pr 0.09
K 0.22 Nd 0.12
Mn 0.43 Sm 0.086
Cs 0.32 Y 0.06
Sr 0.03 Zr 0.004
U 6.34 Mo 0.14
Chapter VI
154
0 50 100 150 200 250 300
0
20
40
60
80
100
Time (min)
%
T
r
a
n
s
p
o
r
t
Pure tracer
In presence of 6g/L Fe
In presence of 20g/L U
Table 6.7: Distribution ratio of metal ions from SHLW at 3M HNO
3
;
Extractant: 0.1M TODGA; Diluent: n-dodecane
Metal ions Distribution ratio
Tracer
#
SHLW
Am(III) 275 160
Eu(III) 263 192
U(VI) 9.50 5.97
Fe(III) 1x10
-4
1x10
-4
Sr(II) 0.91 0.27
Cs(I) 1x10
-4
1x10
-4
#
0.5M DHOA was added as phase modifier since TODGA forms third phase with SHLW
such as macro concentration of uranium and iron, which are generally present in
HLW in higher concentration as compared to those of trivalent actinides. Fig. 6.9
shows that the transport of Am(III) was not affected even in the presence of 20g/L
uranium as well as in the presence of 6g/L of Fe. This is attributed to the fact that
Fig. 6.9. Transport of Am(III) in the presence of U and Fe; Membrane: 0.45m PTFE;
Carrier: 0.1M TODGA in n-dodecane; Feed acidity: 3M HNO
3
; Strippant: Distilled water
Chapter VI
155
0 10 20 30 40 50
0
20
40
60
80
100
%
T
r
a
n
s
p
o
r
t
Time (Hrs)
Am(III) - Tracer
Am(III) - SHLW
Eu(III) - Tracer
Eu(III) - SHLW
Sr(II) - Tracer
Sr(II) - SHLW
while U(VI) is only moderately extracted by TODGA; Fe(III) has negligible
extraction (Chapter-III). Similar observation has been described in Chapter-IV for
extraction chromatography using TODGA as the stationary phase, where the sorption
of Am(III) was not affected by the presence of macro concentrations of U and Fe.
The transport of metal ions such as Am(III), Eu(III) and Sr(II) from SHLW was
studied and is shown in Fig. 6.10. The transport of these metal ions as pure tracer
from 3M HNO
3
is also presented in the figure for comparison purpose. It was
observed that the transport of these metal ions were lower in the presence of SHLW
as compared to that of pure tracer. Nevertheless, the quantitative transport of trivalent
lanthanides and actinides was possible within 15hrs of the experiment. It is worth
noting here that the SHLW used in the present studies contained ~0.6g/L of
lanthanides (Table 6.6) which suppressed the transport of Ln(III)/An(III). On the
other hand, transport of Sr(II) reduced significantly from ~85% in pure tracer to <5%
in SHLW in 5hrs. After 15h, quantitative transport of trivalent actinides and
lanthanides was possible with less than 20% contamination of Sr(II) indicating a D.F.
value of >5 with respect to Sr.
Fig. 6.10. Transport of metal ions from HNO
3
and SHLW; Membrane: 0.45m
PTFE; Carrier: 0.1M TODGA in n-dodecane; Strippant: Distilled water
Chapter VI
156
0 40 80 120 160
0
20
40
60
80
100
0 days
3 days
6 days
13 days
20 days
%
T
r
a
n
s
p
o
r
t
Time (min)
6.6. STABILITY OF LIQUID MEMBRANE
The stability of the SLM is one of the major limitations of the liquid membrane
separation technique. Therefore, it was of interest to investigate the stability of
TODGA-SLM employed in the present work. The stability of the SLM is explained
in terms of stability of solid membrane support, stability of the liquid membrane as
well as chemical and radiolytic stability of the carrier. In the present work, PTFE
membrane was employed as the inert solid support which is well known for its
chemical as well as physical stability. To investigate the physical stability of the
SLM (loss of carrier fromthe pores of the support), transport studies were performed
over a period of 20days (480h) by keeping the impregnated membrane in contact
with the feed and strip solutions. The results of the Am(III) transport over a period of
time are shown in Fig. 6.11. It is clear from the results that the transport rates were
reproducible (within experimental error limits). These observations thus led us to
conclude that the loss of carrier from the membrane phase was insignificant under the
experimental conditions. This is in conformity with the previous observations for the
transport of uranium using D2EHIBA/n-dodecane as the carrier [22]. On the other
hand, studies on uranium transport from HCl medium using Aliquat-336 in
Fig. 6.11. Successive transport experiments for Am(III) to evaluate the
stability SLM; Feed: 1M HNO
3
; Membrane: 0.45m PTFE; Carrier:
0.1M TODGA in dodecane; Strippant: Distilled water
Chapter VI
157
chloroform [28] as well as cesium transport using DTBuB18C6 in nitrobenzene +
toluene mixture [29] has revealed poor stability of the SLMs. As indicated by
Kempermann, et al. [30] the stability of the liquid membranes is greatly influenced
by the viscosity as well as the water miscibility of the carrier phase. In fact, several
other factors also influence the loss of liquid membrane phase such as solubility of
water in solvent, volatility of solvent, interfacial tension as well as pore size of the
support membrane [30-33]. The effect of diluent on the transport of metal ions
revealed n-dodecane as the best diluent in view of the stability of the SLM [21].
While chloroform is volatile, diluents such as nitrobenzene react with nitric acid
leading to its loss from the membrane pores [29].
In order to use the given system for the separation of metal ions from HLW it
is essential to understand the radiation as well as hydrolytic stability of the reagent. A
detailed study on the hydrolytic and radiolytic stability of TODGA has been
elaborated in Chapter-III. In the present chapter, the effect of radiation dose on the
transport of Am(III) has been described. A solution of 0.1M TODGA in dodecane
was irradiated at room temperature with gamma rays from
60
Co source at an exposure
dose of 1.8kGy/h. Parts of the sample were removed after suitable time interval to get
an irradiation dose ranging from 0.0 to1.8MGy. The permeability coefficient as well
as the D
Am
was determined with the irradiated TODGA and the results are shown in
Fig. 6.12. It was observed that the P value of Am(III) decreased almost linearly with
the irradiation dose beyond 0.46MGy. Similarly, the D
Am
value observed at 3M
HNO
3
also followed the similar trend to that of the P values. No significant decrease
in P as well as in D
Am
values was observed upto 0.46MGy. As described in Chapter-
III, a linear decrease in TODGA concentration was observed with the irradiation dose
which is in conformity with the D
Am
values at 1M HNO
3
where significant
differences in the distribution ratios were clearly reflected. It seems that though
TODGA concentration decreases with the irradiation dose, its concentration is still
sufficient upto 0.46MGy dose thereby showing nearly constant P and D
Am
values at
3M HNO
3
. The density and viscosity of the irradiated carrier solutions were also
measured and listed in Table 6.8. It can be seen that the density was almost
insensitive to the dose but viscosity increased steadily (upto ~10%) on irradiation.
The increased viscosity severely affects the diffusion coefficient of the transported
species as per the Stokes-Einstein equation as follows,
Chapter VI
158
0.00 0.25 0.50 0.75 1.00 1.25
10
-2
10
-1
10
0
10
1
10
2
10
3
10
-2
10
-1
10
0
10
1
10
2
10
3
Distribution ratio
D
A
m
Dose, MGy
P
x
1
0
0
0
Permeability coefficient
Table 6.8: Physical parameters of the irradiated liquid membrane; Extractant:
0.1M TODGA; Diluent: n-dodecane
Dose
(MGy)
Density
(gm/L)
Viscosity
(mPa)
0.00 0.759 1.753
0.26 0.760 1.761
0.46 0.760 1.768
0.78 0.761 1.789
1.12 0.762 1.831
1.81 0.764 1.956
D =kT/6tR (6.12)
where, D is the membrane diffusion coefficient, R the radius of the diffusing species
and the viscosity of the carrier. These observations suggested that though TODGA
is susceptible to radiolytic degradation, yet it can be used without any adverse effect
Fig. 6.12. Permeability coefficient and distribution ratio of Am(III) as a function of
radiation dose at 3M HNO
3
; Organic phase: 0.1M TODGA in n-dodecane; Feed
acidity: 3M HNO
3
; Strippant: Distilled water; Dotted line represents D
Am
at 1M HNO
3
Chapter VI
159
upto 0.5MGy dose. Considering the composition of HLW with respect to
radionuclides, such a high dose can be administered to the carrier solvent only when
used for several days.
6.7. HOLLOW FIBRE LIQUID MEMBRANE STUDIES
Although SLM techniques have been widely studied for the separation and
concentration of a variety of compounds, they present an important drawback, i.e.
their low stability and lower transport rate when used as flat sheet SLM. The
instability of SLM displays two characteristics. The first is the solute flux decline
with time, which is attributed to the loss of the carrier / or solvent from the support,
and the second is the channeling of the aqueous solution between the feed and the
strip phases through the liquid membrane [32,33]. Similarly, lower transport of metal
ions is associated with the small surface area encountered in FSSLM. In this sense,
the configuration of SLM by using tubular hollow fibres (HFSLM) have been found
to improve the lifetime of the liquid membrane and also the high metal transport flux
due to their hydrodynamic characteristics [34]. The HFSLM has major advantages
over flat sheet SLM due to very large membrane area and also due to the fact that
degraded membranes can be easily regenerated by passing the ligand solution
through the module [35]. The HFSLM configuration has been applied in various
fields, viz. clinical, pharmaceutical and environmental studies [36]. The HFSLM
module is operated in two modes, (a) the recycling mode, i.e. the feed solution is re-
circulated through the HFSLM module, and (b) the once through mode, i.e. the feed
solution passes only once through the module. Danesi, et al. [37] described a model
for the calculation of various parameters for the transport of metal ions in the above
two modes. A schematic representation of the HFSLM module operation has been
described in Chapter-II. The main objective of the present work was to develop a
HFSLM system for the separation of actinides and lanthanides using TODGA as the
carrier.
6.7.1. Permeation of Metal Ions across HFSLM
The performance of liquid membrane is described in terms of permeability
coefficient (P) of the transported species. However, the P value in flat sheet SLM can
not be correlated with that of HFSLM due to hydrodynamic condition of the latter.
Chapter VI
160
The permeation of the metal species through HFSLM in recycling mode is expressed
in terms of modified permeability coefficient (P
*
) described as follows [38-40],
ln(C
t
/C
o
) =- A P
*
/ V (|+1/|) t (6.13)
where, C
t
and C
o
are the respective concentrations of the metal ions in the feed
solution at an elapsed time t and at zero time, and V is the total volume of the feed
solution. Here, the parameter A represents the total internal area of the hollow fibre
which is calculated as follows [40],
A =2tRLNe (6.14)
where, R is the internal radius of the fibre, L is the length of the fibre, N is the
number of fibres and e is the membrane porosity. The parameter, | for a module
containing N number of fibres is expressed as follows,
| =Q
T
/ P
*
RLN (6.15)
where, Q
T
is the flow rate of the feed solution. The characteristics and various
parameters of the HF module employed in the present work are described in Chapter-
II (Table 2.3). By plotting ln(C
t
/C
o
) as a function of t a straight line is expected, and
according to Eqs. (13) and (15) the P
*
value for the given system can be obtained
from the fitted slope.
6.7.1.1. Transport of Neodymium from HNO
3
Solution
The transport of Nd(III) from nitric acid solution was studied in litre scale
(0.5-2L) with HFSLM system. In the recycling mode, the feed solution was passed
through the lumen side and the strip solution through the shell side at identical flow
rate of 200mL/min to maintain equal hydraulic pressure on both the sides of the
HFSLM. It has been shown that a better HFSLM stability can be obtained by
balancing equal hydraulic pressure inside and outside the lumen [15]. For all the
transport studies, a solution of 0.1M TODGA +0.5M DHOA in NPH was used as the
carrier and distilled water as the receiver phase.
The effect of feed acidity on the transport of Nd(III) was investigated from a
feed solution containing 1g/L Nd. The results revealed an enhanced permeation rate
of Nd(III) with the feed acidity upto 3.5M HNO
3
(Fig. 6.13). Further increase in the
feed acidity suppressed the metal permeation rate due to co-transport of significant
Chapter VI
161
0 10 20 30 40 50
0
1
2
3
4
-
l
n
(
C
t
/
C
o
)
0.5M HNO
3
1M HNO
3
2M HNO
3
3.5M HNO
3
6M HNO
3
Time (min)
Table 6.9: Permeation of acid from feed to the receiver side through HFSLM
system; Feed: 1g/L Nd; Receiver: Distilled water
Time
(min)
Nitric acid transport (M)
Feed: 0.5M Feed: 1M Feed: 2M Feed: 3.5M Feed: 6M
0 0.00 0.00 0.00 0.00 0.00
5 0.01 0.01 0.07 0.03 0.15
10 0.01 0.03 0.11 0.20 0.21
20 0.01 0.05 0.13 0.34 0.34
30 0.02 0.06 0.21 0.54 0.50
45 0.02 0.07 0.25 0.63 0.66
60 0.03 0.09 0.32 0.70 0.95
amount of acid in the receiver phase (Table 6.9). The P
*
value and the quantity of
Nd(III) transported in 45min is listed in Table 6.10. The P
*
value observed in
HFSLM was similar to that observed in FSSLM for the transport of Am(III) at
different feed acidity (Table 6.2). At feed acidity of 3.5M HNO
3
, quantitative
Fig. 6.13. Influence of feed acidity on the permeation of Nd(III) by
HFSLM module; Feed: 1g/L Nd; Receiver: Distilled Water
Chapter VI
162
transport of Nd(III) was observed in 45min of operation. It should be noted that for
the same feed in FSSLM, more than 25hrs were required for quantitative transport of
Nd(III). Higher transport in the case of HFSLM is very obvious due to large surface
area and favourable hydrodynamic condition of the system. At higher feed acidity
(6M HNO
3
), the P
*
value decreased significantly and only ~82% of Nd(III) transport
was noticed after 45min, which reached to ~93% after 60min. The receiver phase
acidity reached 0.95M HNO
3
after 60min, which suppressed the dissociation of the
metal-ligand complex at the membraneaqueous strip interface.
Table 6.10: Permeability coefficient (P
*
) of Nd(III) by HFSLM system; Feed:
1g/L Nd; Receiver: Distilled water
[HNO
3
], M P
*
(cm/min) % Transport in 45min
0.5 2.97x10
-4
3.52
1.0 9.97x10
-4
33.62
2.0 2.35x10
-3
87.85
3.5 4.32x10
-3
99.99
6.0 1.79x10
-3
82.62
In another set of experiments, the transport of Nd(III) was investigated at
various feed solute (Nd) concentration at 3.5M HNO
3
and the results are shown in
Fig. 6.14. When the feed concentration was 0.1g/L Nd, the permeation rate was very
high and it could be quantitatively transported in <20min. On the other hand, when
the feed solute concentration increased to 3g/L and 6g/L, the transport rate was
suppressed and only about 70% and 40% of Nd(III) was transported, respectively in
60min. The P
*
values calculated for 0.1, 1.0, 3.0 and 6.0g/L Nd were 8.80x10
-3
,
4.32x10
-3
, 9.78x10
-4
and 2.57x10
-4
cm/min, respectively. This behaviour was again
attributed to the acid transport which suppressed the dissociation of metal-ligand
complex at the membrane-aqueous strip interface. Lower P
*
value at higher feed
solute concentration also suggested the metal loading effect in the liquid membrane.
This behaviour was further confirmed by investigating the transport of Nd(III) at
different feed to strip volume ratio. The results represented in Fig. 6.15 were
obtained while working with 0.56.5L of the feed solution containing 1g/L Nd at
Chapter VI
163
0 50 100 150 200
0
1
2
3
4
5
0.1g/L
1g/L
3g/L
6g/L
-
l
n
(
C
t
/
C
o
)
Time (min)
0 25 50 75 100 125 150 175 200
0
20
40
60
80
100
%
T
r
a
n
s
p
o
r
t
Time (min)
1:1
2:1
4:1
13:1
Fig. 6.15. Transport of Nd(III) by HFSLM at different feed to strip ratio;
Feed: 1g/L Nd at 3.5M HNO
3
; Receiver: Distilled Water
Fig. 6.14. Effect of feed concentration on the permeation of Nd(III) by
HFSLM module; Feed: 3.5M HNO
3
; Receiver: Distilled Water
Chapter VI
164
3.5M HNO
3
and fixed volume of receiver phase (0.5L). It can be seen that the
saturation in the Nd(III) transports were obtained after 90min of operation with 98%
and 85% transport at feed to strip ratio of 2 and 4, respectively. At this stage, the
receiver phase acidity was 1.0M and 1.5M HNO
3
, respectively. When the receiver
phase acidity is <0.6M HNO
3
(in less than 60min), the transport of Nd(III) increased
sharply upto 60min. However, when strip phase acidity becomes greater than 0.6M
HNO
3
(after 60min), the transport is suppressed. Similarly, at volume ration of 13,
only 13% of Nd(III) transport was observed after 3hrs. At the same time, the strip
phase acidity reached >2M HNO
3
and hence the transport was suppressed. The pre-
concentration factor (PF) was obtained as, PF =C
st
/C
fo
, where C
st
is the concentration
of Nd(III) in the strip solution at an elapsed time t and C
fo
is the Nd(III)
concentration in the feed solution at zero time. After 60min, the PF values were: 1.0,
1.58, 2.56 and 2.20 for volume ratio of 1, 2, 4 and 13, respectively. Though the PF
value for volume ratio of 4 was higher, quantitative transport was not observed.
Nevertheless, quantitative transport may be achieved by replacing the strip solution
with fresh one after suitable time interval or neutralizing the receiver phase acidity.
6.7.1.2. Transport of Americium from SHLW
The main objective of the present work was the recovery of actinides from nuclear
waste solution (HLW). In this context, the transport of Am(III) was studied under
simulated condition of HLW. The composition of a typical HLW solution is given in
Table 6.6. Since, HLW contains lanthanides at several times higher concentration
than the minor actinides, it was of interest to investigate the effect of rare earth metal
concentration on the transport of Am(III). By taking Nd(III) as the representative
element of lanthanides, the transport of Am(III) was investigated in the presence of
1g/L Nd and the results are shown in Fig. 6.16. It was evident that the quantitative
transport of Am(III) was achievable in 45min of operation. Similarly, the quantitative
transport of Am(III) was achieved in 30min under SHLW conditions (Fig. 6.16). Fast
transport of Am(III) from SHLW was ascribed to lower concentration of lanthanides
(~0.6g/L) and salting out effect due to presence of large concentration of metal
nitrate salts. Present observation suggests that HFSLM offers a promising alternative
approach for actinide partitioning using TODGA as the carrier. The basic phenomena
which occur at SLMs separation processes are sufficiently well understood to permit
Chapter VI
165
0 10 20 30 40 50 60
0
20
40
60
80
100
F
r
a
c
t
i
o
n
o
f
A
m
(
I
I
I
)
i
n
f
e
e
d
F
r
a
c
t
i
o
n
o
f
A
m
(
I
I
I
)
i
n
s
t
r
i
p
Time (min)
1g/L Nd (Strip)
1g/L Nd (Feed)
SHLW (Strip)
SHLW (Feed)
the scale-up of laboratory units to commercial scale. However, more research effort
has to be undertaken to study the radiation stability of the HF modules before
applying the system to the actual HLW.
6.7.2. Stability of Liquid Membrane in HFSLM
The long term stability of the HFSLM is important from the standpoint of its
industrial application. For such stability test, the module was impregnated once with
the carrier solution and the system was run continuously in several successive cycles
for the transport of Nd(III) from a solution containing 1g/L Nd at 3.5M HNO
3
. The
permeability of Nd(III) was measured for 45min, and after this period of time, the
depleted feed and enriched strip solutions were replaced with fresh ones. From the
data reported in Fig. 6.17, it can be observed that the Nd(III) transport was consistent
within the error limit in all the ten runs. Person, et al. [41] described that probable
causes of membrane instability could be (a) loss of extractant by solubility in
adjacent aqueous solution, (b) progressive wetabilty of the support pores induced by
lowering of the organic-water interfacial tension which results from the surface
active nature of the carrier molecules, and (c) the differential pressures existing
Fig. 6.16. Transport of Am(III) from feed solution containing 1g/L Nd at 3.5M
HNO
3
and from SHLW spiked with
241
Am tracer; Receiver: Distilled Water
Chapter VI
166
0 10 20 30 40 50
0
20
40
60
80
100
Time (min)
%
T
r
a
n
s
p
o
r
t
between the inside and outside of the HFSLM caused by pumping of the solutions.
Danesi, et al. [15,42] reported that the loss of carrier from the pores are more
significant when carrier molecules are very strong surfactant such as alkylaryl
sulphonic acids and long chain quaternary ammonium salts. On the other hand, no
significant loss was observed when weaker surface active carriers such as CMPO,
TOPO and long chain amines were used. They reported that the HFSLM was quite
stable for 60days with polypropylene hollow fibre SLM containing tridodecylamine
in n-dodecane as the carrier, when outside and inside hydraulic pressures were
balanced. In the present work, TODGA being a weaker surface active carrier, we
expect a similar stability of HFSLM though our study was restricted to only several
successive cycles of continuous operation.
6.8. REFERENCES
1. H.K. Lonsdale, J. Membr. Sci., 10 (1982) 81.
2. E.L. Cussler (Ed.), Multicomponent Diffusion, Elsevier, Amsterdam (1976),
Chap-8.
Fig. 6.17. Successive transport experiments for Nd(III) to evaluate the stability
of HFSLM; Feed: 1g/L Nd at 3.5M HNO
3
; Receiver: Distilled Water
Chapter VI
167
3. N.M. Kocherginsky, Q. Yang and L. Seelam, Sep. Purif. Technol., 53 (2007) 171.
4. H.J, Fendler, J. Membr. Sci., 30 (1987) 323.
5. R.D. Noble, Sep. Sci. Technol., 22 (1987) 731.
6. E.L. Cussler and D.F. Evans, Sep. Purif. Meth., 3 (1974) 399.
7. R. Marr and A. Kopp, Int. Chem. Eng., 22 (1982) 44.
8. L.L. Tavlarides, J.H. Bae and C.K. Lee, Sep. Sci. Technol., 22 (1987) 581.
9. R.M. Izatt, J.D. Lamb and R.L. Bruening, Sep. Sci. Technol., 23 (1988) 1645.
10. J.D, Way, R.N. Noble, T.M. Flynn and E.D. Sloan, J. Membr. Sci., 12 (1982)
239.
11. H.C. Visser, D.N. Reinhoudt and F. de Jong, Chem. Soc. Rev., (1994) 75.
12. R.A. Bartsch, J.D. Way, L.A.J. Chrisstoffels, F. de Jong and D.N. Reinhoudt,
Chemical Separations with Liquid Membranes, R.A. Bartsch and J.D. Way
(Eds.), ACS Symposium Series Number 642, ACS, Washington DC (1996), P. 1-
10.
13. L. Boyadzhiev and Z. Lazarova, Membrane Separations Technology. Principles
and Applications, R.D. Noble and S.A. Stern (Eds.), Elsevier Science B.V.
1995, P. 283.
14. G. Spach, Ed., "Physical Chemistry of Transmembrane Ion Motions", Elsevier:
Amsterdam, 1983.
15. P.R. Danesi, Sep. Sci. Technol., 19 (1985) 857.
16. P.R. Danesi, E.P. Horwitz and P.G. Rickert, J. Phys. Chem., 87 (1983) 4708.
17. M. Rovira and A.M. Sastre, J. Membr. Sci., 149 (1998) 241.
18. M.R. Kamal and L.N. Canjar, Chem. Eng. Prog., 62 (1966) 83.
19. J.C. Charpentier, Advances in Chemical Engineering, T.B. Drew, G.R. Cokelet,
J.W. Hoopes and T. Vermeulin (Eds.), Academic Press, London (1981), Vol. 11,
Chapter 1.
20. S. Sriram, P.K. Mohapatra, A.K. Pandey, V.K. Manchanda and L.P. Badheka, J.
Membr. Sci., 177 (2000) 163.
21. S. Sriram and V. K. Manchanda, Solv. Extr. Ion Exch., 20 (2002) 97.
22. S. Shailesh, P.N. Pathak, P.K. Mohapatra and V.K. Manchanda, J. Membr. Sci.
272 (2006) 143.
23. J.P. Shukla, A. Kumar and R.K. Singh, Sep. Sci. Technol., 27 (1992) 447.
24. S.P. Kusumocahyo, K. Sumaru, T. Iwatsubo, T. Shinbo, T. Kanamori, H.
Chapter VI
168
Matsuyama and M. Teramoto, J. Membr. Sci., 280 (2006) 73.
25. M. Hirata, P. Guilbaud, M. Dobler and S. Tachimori, Phys. Chem. Chem. Phys. 5
(2003) 691.
26. Z. Zhu, Y. Sasaki, H. Suzuki and T. Kimura, Anal. Chim. Acta 527 (2004) 163.
27. H. Suzuki, Y. Sasaki, Y. Sugo, A. Apichaibukol and T. Kimura, Radiochim.
Acta, 92 (2004) 463.
28. P.K. Mohapatra, D.S. Lakshmi, D. Mohan and V.K. Manchanda, J. Membr. Sci.,
232 (2004) 133.
29. P.K. Mohapatra, D.S. Lakshmi, D. Mohan and V.K. Manchanda, Sep. Purif.
Technol., 51 (2006) 24.
30. A.J.B. Kemperman, D. Bargeman, V.D. Boomgard and H. Strathmann, Sep. Sci.
Technol., 31 (1996) 2733.
31. P. Debley, S. Delepine, M. Minter and H. Renon, Sep. Sci. Technol., 26 (1991)
97.
32. A.M. Neplenbroek, D. Bargeman and C.A. Smolders, J. Membr. Sci., 67 (1992)
121.
33. H. Takeuchi, K. Takahashi and W. Goto, J. Membr. Sci., 34 (1987) 19.
34. P.R. Danesi and P.G. Rickert, Solv. Extr. Ion Exch., 4 (1986) 149.
35. C. Fontas, C. Palet, V. Salvado and M. Hidalgo, J. Membr. Sci., 178 (2000) 131.
36. R.D. Noble and J.D. way (Eds.), Liquid Membrane: Theory and Applications,
ACS Symposium Series, Washington, DC (1987), Vol. 347.
37. P.R. Danesi, Solv. Extr. Ion Exch., 2 (1984) 115.
38. C. Fontas, V. Salvado and M. Hidalgo, J. Membr. Sci., 223 (2003) 39.
39. A. Uheida, Y. Zhang and M. Muhammed, J. Membr. Sci., 241 (2004) 289.
40. P.R. Danesi, J. Membr. Sci., 20 (1984) 231.
41. D. Pearson, Ion Exchange Membranes, D.S. Flett (Ed.), Society of Chemical
Industry, London (1983), P. 55-73.
42. P.R. Danesi and P.G. Rickert, Solv. Extr. Ion Exch., 4 (1986) 149.
------------------
Summary and Conclusions
Summary and Conclusions
169
SUMMARY AND CONCLUSIONS
The challenge for the final disposal of HLW is largely due to the radiotoxicity
associated with the minor actinides. At present, the option for the management of
HLW is to vitrify it in the glass matrix followed by disposal in deep geological
repositories. Since the half lives of minor actinides concerned range between a few
hundred to millions of years, the surveillance of HLW for such a long period is
economically as well as environmentally a daunting task. An alternative /
complimentary concept is the partitioning and transmutation (P&T), which envisages
the complete removal of minor actinides from HLW and their consequent burning in
high flux reactors / high energy accelerators. After partitioning of the actinides along
with the long lived fission products, the residual waste can be fixed in suitable
matrices and buried in subsurface repositories at a much reduced risk and cost. The
work reported in this thesis has relevance to the partitioning of minor actinides and
long lived fission products from nuclear waste solutions. Various techniques
employed for the separation of long lived radionuclides were solvent extraction,
extraction chromatography and supported liquid membrane.
The summary and conclusions of the work reported in this thesis are as
follows:
i. Extraction behaviour of laboratory synthesized N,N,N,N-tetraoctyl
diglycolamide (TODGA) towards actinides, lanthanides, long-lived fission
products and structural elements was investigated. The acid uptake constant
(K
H
) of TODGA was evaluated as 4.10.4, which was higher than CMPO (K
H
=2.0), DMDBTDMA (K
H
=0.32) and TBP (K
H
=0.20). The stoichiometry of
the extracted species of uranium, plutonium and americium conformed to
UO
2
(NO
3
)
2
3L, Pu(NO
3
)
4
3L and Am(NO
3
)
3
4L (where L =TODGA). The
stoichiometry of the extracted species changed with the nature of the diluent.
The conditional extraction constants (logK
ex
) for actinides varied in the order:
9.350.04 (Am(III)) >8.610.03 (Pu(IV)) >4.880.01 (U(VI)). The extraction
of actinides by TODGA was exothermic in nature. TODGA/n-dodecane forms
third phase at very low concentration of Nd (LOC at 3M HNO
3
by 0.1M
Summary and Conclusions
170
TODGA / n-dodecane =1.06g/L) and N,N-dihexyl octanamide (DHOA) was
found to be a promising phase modifier.
ii. The extraction behaviour of several metal ions, which are important from the
actinide partitioning point of view, was investigated from nitric acid medium
employing 0.1M TODGA +0.5M DHOA / n-dodecane. The extraction of
actinides and lanthanides increased gradually upto 2M HNO
3
and exhibited
very high distribution between 2-6M HNO
3
. The distribution values of
actinides (An) followed the order: An(III) ~An(IV) >An(VI). Though Sr(II)
was partially co-extracted along with actinides (D
Sr
at 3M HNO
3
=0.9), it can
be selectively stripped (scrubbed) with 6M HNO
3
. Negligible extraction of
Fe(III) and Cs(I) was observed as D
M
values for both the metal ions were <10
-2
in the acid range investigated. The D
M
values for actinides were lower under
Simulated High Level Waste (SHLW) conditions due to the co-extraction of
other metal ions present in SHLW; however, it was significantly higher for
their quantitative and efficient extraction. On the other hand, D
Sr
value
decreased significantly (~one tenth of the pure tracer value) from SHLW to an
acceptable level. Typical separation factor (SF) values for the metal ions
observed under SHLW condition were: 1375 (Pu/Sr), 1225 (Eu/Sr) and 775
(Am/Sr). TODGA is hydrolytically stable; however, it is susceptible to
radiolysis with the G-value of 8.50.6 molecules/ eV.
iii. Preliminary counter-current extraction studies carried out using a 16 stages
mixer-settler suggested that Nd(III) could be quantitatively extracted in five
stages from a feed solution containing 3.8g/L of Nd at 3.5M HNO
3
. Similarly,
the extracted Nd(III) could be stripped in three stages with 0.2M HNO
3
.
Quantitative stripping of Nd(III) was also possible in five stages at O/A =2 in
the stripping stages. Our findings suggested the possible application of TODGA
solvent system for the partitioning of actinides from HLW solution.
iv. The extraction chromatographic studies involving TODGA as the stationary
phase demonstrated its possible use for the concentration of lanthanides and
actinides from a large volume of dilute waste solutions of moderate acidity (3-
4M HNO
3
). The effect of macro concentrations of lanthanides, uranium and
Summary and Conclusions
171
iron suggested that the sorption of Am(III) on TODGA resin was mainly
affected by the presence of lanthanides. The column capacity with respect to
Am(III) was evaluated and it was observed that 200.30mg of Eu per gram of
resin could be loaded without any discharge of
241
Am activity at 4M HNO
3
.
v. A novel malonamide grafted resin was synthesis using N,N-dimethyl-N,N-
dibutyl malonamide (DMDBMA) as chelating ligand and Merrifield polymer as
the support backbone. The synthesized grafted resin exhibited superior binding
for hexavalent and tetravalent metal ions such as U(VI) and Pu(IV) over
trivalent metal ions, viz. Am(III) and Pu(III). The sorption of Am(III), Th(IV)
and U(VI) followed the first order Lagergren rate kinetics. The sorption of
uranium exhibited the Langmuir monolayer sorption phenomenon which was
confirmed by the theoretical model based on sorption kinetics. The sorption
capacities for uranium and thorium were found to be 18.781.53mg and
15.741.59mg/g resin, respectively. The resin could be used for pre-
concentration of U and Pu from environmental / waste effluents and could
continuously be used for a long period of time without any appreciable change
in the sorption properties towards the heavy metal ions.
vi. The transport behaviour of long-lived radionuclides by TODGA impregnated
flat sheet liquid membrane (FSSLM) has been described with the help of
various diffusional parameters. Distilled water was used as the strippant for
actinides / fission products. The permeability coefficient (P) of Am(III)
increased gradually upto 2M HNO
3
and then there was a decline after 3M
HNO
3
due to co-transport of HNO
3
. The P value of Am(III) increased linearly
with ligand concentration from 3x10
-5
cm/s at 0.02M to 4x10
-4
cm/s at 0.15M,
which was typical of membrane diffusion controlled permeation process.
Similar to distribution behaviour, the P values of actinides and lanthanides
followed the order: An(III) ~Ln(III) >An(IV) >An(VI). The transport of
Zr(IV) followed Pu(IV) which was suppressed by addition of 0.4M oxalic acid.
The transport of Am(III) was not affected by the presence of macro
concentration of uranium and iron, but was significantly reduced by the
presence of lanthanides. Quantitative transport of Am(III) was observed in
Summary and Conclusions
172
20hrs from SHLW solution, which contained about 0.6g/L of lanthanides.
vii. Possible application of TODGA-based liquid membrane for the separation of
metal ions on large scale was demonstrated using hollow fibre membrane
modules. The transport of Nd(III) from nitric acid solution was studied in litre
scale with hollow fibre supported liquid membrane (HFSLM) system.
Interestingly, quantitative transport of Nd(III) was observed in 45min in
distilled water (used as receiver phase) from a feed solution containing 1g/L Nd
at 3.5M HNO
3
as compared to FSSLM where more than 25hrs were required
for quantitative transport. Similarly, quantitative transport of Am(III) was
achieved in 45min from a feed containing 1g/L Nd spiked with
241
Am tracer.
On the other hand, quantitative transport of Am(III) was observed in 30min
from SHLW which was ascribed to lower lanthanide concentration (~0.6g/L)
and salting out effect due to presence of large concentration of metal nitrate
salts. The stability of TODGA-HFSLM system was very good when evaluated
in ten successive cycles of operation. It appears that HFSLM offers a promising
alternate technique for actinide partitioning using TODGA as the carrier.
The results obtained during this work clearly demonstrate the potential of
TODGA in meeting the challenges encountered during the actinide partitioning. It
offers very high extraction for trivalent actinides and lanthanides over a large number
of fission products and structural elements. Preliminary mixer settler studies as well
as hollow fibre liquid membrane studies also confirm its potential application on
process scale. Possibility of complete incineration of amide based extractants has
additional advantage towards secondary waste management as compared to
phosphorous based extractants such as CMPO.
In conclusion, it can be said that TODGA appears to be very attractive
extractant for actinide partitioning as compared to other proposed extractants such
as CMPO, TRPO, DIDPA and DMDBTDMA etc. However, extensive mixer settler
runs and HFSLM runs need to be pursued to establish it as a process solvent.
-----------------------
173
STATEMENT UNDER ORDINANCE 771
The work described in the thesis was planned and carried out by me under the
supervision of Prof. V.K. Manchanda. A part of the work reported in this thesis has
been published in the following International Journals and National / International
Symposia.
Journals
1. N,N,N,N-tetraoctyl diglycolamide (TODGA): A promising extractant for
actinide-partitioning from high-level waste (HLW), S.A. Ansari, P.N. Pathak,
M. Husain, A.K. Prasad, V.S. Parmar and V.K. Manchanda, Solv. Extr. Ion
Exch., 23 (2005), 463-479.
2. Extraction chromatographic studies of metal ions using N,N,N,N-tetraoctyl
diglycolamide (TODGA) as the stationary phase, S.A. Ansari, P.N. Pathak, M.
Husain, A.K. Prasad, V.S. Parmar and V.K. Manchanda, Talanta, 68 (2006),
1273-1280.
3. Extraction of actinides using N,N,N,N-tetraoctyl diglycolamide (TODGA): A
thermodynamic study, S.A. Ansari, P.N. Pathak, M. Husain, A.K. Prasad, V.S.
Parmar and V.K. Manchanda, Radiochim. Acta, 94 (2006) 307-312.
4. Transport of americium(III) through a supported liquid membrane containing
N,N,N,N-tetraoctyl-3-oxapentane diamide (TODGA) in n-dodecane as the
carrier, S.A. Ansari, P.K. Mohapatra, D.R. Prabhu and V.K. Manchanda, J.
Membr. Sci., 282 (2006) 133-141.
5. Evaluation of N,N,N,N-tetraoctyl-3-oxapentane-diamide (TODGA) as a
mobile carrier in remediation of nuclear waste using supported liquid
membrane, S.A. Ansari, P.K. Mohapatra, D.R. Prabhu and V. K. Manchanda,
J. Membr. Sci., 298 (2007) 169-174.
6. Synthesis of N,N-dimethyl-N,N-dibutyl malonamide functionalized polymer
and its sorption affinities towards U(VI) and Th(IV) ions, S.A. Ansari, P.K.
Mohapatra and V. K. Manchanda, Talanta, 73 (2007) 878-885.
7. Transport of lanthanides and fission products through supported liquid
membranes containing N,N,NN-tetraoctyl diglycolamide (TODGA) as the
174
carrier, S.A. Ansari, P.K. Mohapatra, D.R. Prabhu and V. K. Manchanda,
Desalination, 232 (2008) 254-261.
8. A novel malonamide grafted polystyrene-divinyl benzene resin for solid phase
extraction, pre-concentration and separation of actinides, S.A. Ansari, P.K.
Mohapatra and V.K. Manchanda, J. Hazardous Mater., communicated.
9. Separation of actinides and lanthanides from high level waste using hollow fiber
supported liquid membrane containing TODGA as the carrier, S.A. Ansari,
D.R. Raut, P.K. Mohapatra and V. K. Manchanda, Sep. Purif. Technol.,
communicated.
10. Nuclear remediation: Counter-current extraction with N,N,NN-tetraoctyl
diglycolamide, D.R. Prabhu, S.A. Ansari, M.S. Murali, V.K. Manchanda, S.
Rajeswari, A. Suresh, R. Manivannan, M.P. Antony, T.G. Srinivasan, S.
Sundaramurthy and S.B. Koganti, Nucl. Technol., communicated.
Conferences / Symposia
1. N,N,N,N-tetraoctyl diglycolamide (TODGA): A promising extractant for
actinide-partitioning from high-level waste (HLW), S.A. Ansari, P.N. Pathak,
M. Husain, A.K. Prasad, V.S. Parmar and V.K. Manchanda, In Proceedings of
DAE-BRNS Theme Meeting on Emerging Trends in Separation Science and
Technology (SESTEC-2004), held at Bhabha Atomic Research Centre,
Mumbai, India, during July 22-23 2004. pp. 166-168.
2. Selective removal of uranium and evaluation of N,N,N,N-tetraoctyl
diglycolamide (TODGA) for actinide partitioning from high level waste
(HLW), J.M. Joshi, S.A. Ansari, P.N. Pathak and V.K. Manchanda, In
proceedings of the sixth international conference on nuclear and
radiochemistry (NRC-6), held at Aachen, Germany, during August 29 -
September 3, 2004. pp. 532-534.
3. Extraction chromatographic studies employing N,N,N,N-tetraoctyl
diglycolamide (TODGA) as the stationary phase, S.A. Ansari, P.N. Pathak, M.
Husain, A.K. Prasad, V.S. Parmar and V.K. Manchanda, In proceedings of
175
DAE-BRNS Symposium on Nuclear and Radiochemistry (NUCAR 2005),
held at Guru Nanak Dev University, Amritsar, India, during March 15-18,
2005. pp.145-146.
4. Thermodynamics of extraction of actinides using N,N,N,N-tetraoctyl
diglycolamide (TODGA), S.A. Ansari, P.N. Pathak, M. Husain, A.K. Prasad,
V.S. Parmar and V.K. Manchanda, In proceedings of DAE-BRNS Symposium
on Nuclear and Radiochemistry (NUCAR 2005), held at Guru Nanak Dev
University, Amritsar, India, during March 15-18, 2005. pp.275-276.
5. Extraction chromatography of actinides, S.A. Ansari, P.N. Pathak, A.
Bhattacharyya, B.S. Shaibu, M.L.P. Reddy and V.K. Manchanda, In
proceedings of 13
th
International Conference on Nuclear Engineering
(ICONE 13), held at Beijing, China, during May 16-20, 2005. pp.329.
6. Uptake behaviour of actinides employing malonamide grafted polystyrene-
divinyl benzene resin, S.A. Ansari, P.K. Mohapatra and V.K. Manchanda, In
proceedings of International Conference on Application of Radiotracers in
Chemical, Environmental and Biological Sciences (ARCEBS 06), held at
Kolkata, India, during January 23-27, 2006. Volume II, pp. 88-89.
7. Facilitated transport of actinides, lanthanides and fission products through
supported liquid membrane containing N,N,NN-tetraoctyl diglycolamide
(TODGA) as the carrier, S.A. Ansari, P.K. Mohapatra, D.R. Prabhu and V.K.
Manchanda, In Proceedings of DAE-BRNS Theme Meeting on Emerging
Trends in Separation Science and Technology (SESTEC-2006), held at
Bhabha Atomic Research Centre, Mumbai, India, during September 29 -
October 1, 2006. pp. 208-209.
8. McCabe Thiele diagram for tetraoctyl diglycolamide (TODGA)NPHNd(III)
HNO
3
system, S.A. Ansari, D.R. Prabhu, R.B. Gujar, M.S. Murali and V.K.
Manchanda, In proceedings of DAE-BRNS Symposium on Nuclear and
Radiochemistry (NUCAR 2007), held at The Maharaja Sayajirao University of
Baroda, Vadodara, India, during February 14-17, 2007. pp.387-388.
9. Counter current extraction studies on Nd(III) using TODGA, D.R. Prabhu, S.A.
Ansari, M.S. Murali, V.K. Manchanda, S. Rajeswari, A. Suresh, R.
Manivannan, M.P. Antony, T.G. Srinivasan, S. Sundaramurthy and S.B.
176
Koganti, In proceedings of DAE-BRNS Symposium on Nuclear and
Radiochemistry (NUCAR 2007), held at The Maharaja Sayajirao University of
Baroda, Vadodara, India, during February 14-17, 2007. pp.281-282.
(Seraj Ahmad Ansari)
Candidate
I hereby, certify that the above statement is correct.
(Prof. V. K. Manchanda)
Research Guide
S-ar putea să vă placă și
- Ballast Water Treatment Using Electrochemical Disinfection TechnologyDocument233 paginiBallast Water Treatment Using Electrochemical Disinfection TechnologySupun KariyawasamÎncă nu există evaluări
- BioelectricityDocument99 paginiBioelectricitysharammaÎncă nu există evaluări
- Characterization Study and Utilization of Cameron Highlands Reservoir Sediments As Usable ProductsDocument28 paginiCharacterization Study and Utilization of Cameron Highlands Reservoir Sediments As Usable ProductsSima ChavoshiÎncă nu există evaluări
- Batch 05 FinalDocument64 paginiBatch 05 Final4047 MOHAMED RAJ SAFWAN.PÎncă nu există evaluări
- Efficiency Study of A Single Day Smoke Dryer: UbraryDocument10 paginiEfficiency Study of A Single Day Smoke Dryer: Ubraryrajatraj19pdfÎncă nu există evaluări
- Condition Montoring of TransformerDocument0 paginiCondition Montoring of TransformerDevansh SinghÎncă nu există evaluări
- ProjectthesisDocument66 paginiProjectthesisTatiana MendozaÎncă nu există evaluări
- PHD Thesis Mohammed AlqahtaniDocument138 paginiPHD Thesis Mohammed AlqahtaniagÎncă nu există evaluări
- Electroplating Thesis PDFDocument184 paginiElectroplating Thesis PDFmonu100% (1)
- Segun Final Project WorkDocument60 paginiSegun Final Project WorkSegun OlatujaÎncă nu există evaluări
- Final M.tech Project, Indranil, May 2019 UploadDocument62 paginiFinal M.tech Project, Indranil, May 2019 UploadIndranil HalderÎncă nu există evaluări
- Segun's Project WorkDocument59 paginiSegun's Project WorkSegun OlatujaÎncă nu există evaluări
- Compressible Flow Analysis of Thrust Augmenting EjectorsDocument107 paginiCompressible Flow Analysis of Thrust Augmenting Ejectorscurtis.kaatzÎncă nu există evaluări
- PHI Book PDFDocument12 paginiPHI Book PDFSreerajÎncă nu există evaluări
- AreccoDocument220 paginiAreccorcarlos_810803Încă nu există evaluări
- I Love UtDocument386 paginiI Love UtJavon BakerÎncă nu există evaluări
- Synthesis and Applications of Silver NanoparticlesDocument7 paginiSynthesis and Applications of Silver NanoparticlesTigerÎncă nu există evaluări
- Fabrication and Characterization of Novel Iron Oxide/ Alumina Nanomaterials For Environmental ApplicationsDocument200 paginiFabrication and Characterization of Novel Iron Oxide/ Alumina Nanomaterials For Environmental Applicationsvlado2905100% (1)
- Vibrational Spectral Investigation, Molecular Structure and Electronic Interaction of 7-Diethylamino-4-Methyl CoumarinDocument16 paginiVibrational Spectral Investigation, Molecular Structure and Electronic Interaction of 7-Diethylamino-4-Methyl CoumarinramÎncă nu există evaluări
- Degradation of Acetaminophen by Sulfate RadicalDocument15 paginiDegradation of Acetaminophen by Sulfate RadicalWAQASÎncă nu există evaluări
- PolypyrroleDocument106 paginiPolypyrrolesurya rajÎncă nu există evaluări
- Comparative Study of Dye Removal by Adsorption Method and Nanocrystalline Tio Thin FilmsDocument11 paginiComparative Study of Dye Removal by Adsorption Method and Nanocrystalline Tio Thin FilmssumitÎncă nu există evaluări
- Omwoma Solomon MSC Thesis Maseno University KenyaDocument90 paginiOmwoma Solomon MSC Thesis Maseno University KenyaOmwoma SolomonÎncă nu există evaluări
- Stuart Modern SpectrosDocument5 paginiStuart Modern SpectrosDavor ŠestanÎncă nu există evaluări
- A Novel Chemical Approach to Fabricate ZnO NanostructuresDocument75 paginiA Novel Chemical Approach to Fabricate ZnO NanostructuresBharat chandra sahuÎncă nu există evaluări
- Full Thesis PDFDocument116 paginiFull Thesis PDFAdrian SelgasÎncă nu există evaluări
- Hydraulic Bearing Puller ReportDocument23 paginiHydraulic Bearing Puller ReportAman sharmaÎncă nu există evaluări
- Principles of Metal Surface Treatment and Protection: Pergamon International Library of Science, Technology, Engineering and Social Studies: International Series on Materials Science and TechnologyDe la EverandPrinciples of Metal Surface Treatment and Protection: Pergamon International Library of Science, Technology, Engineering and Social Studies: International Series on Materials Science and TechnologyÎncă nu există evaluări
- Experimental Study of Heating of Nanofluids in Microwave OvenDocument51 paginiExperimental Study of Heating of Nanofluids in Microwave OvenArun VermaÎncă nu există evaluări
- Thesis PDFDocument102 paginiThesis PDFEmayavaramban ManiÎncă nu există evaluări
- Project Report On Microbial Fuel CellDocument79 paginiProject Report On Microbial Fuel CellDhinesh Kumarv82% (11)
- A Project Report On: An Autonomous Institution Affiliated To Visvesvaraya Technological University, BelgaumDocument64 paginiA Project Report On: An Autonomous Institution Affiliated To Visvesvaraya Technological University, BelgaumDhananjay JahagirdarÎncă nu există evaluări
- Adriano Ambrosi 20120611164612Document231 paginiAdriano Ambrosi 20120611164612Omar EzzatÎncă nu există evaluări
- Ultrasonic Detection of Gas Porosity Defects in Aluminium Die CastingsDocument248 paginiUltrasonic Detection of Gas Porosity Defects in Aluminium Die CastingsGiovanni PucellaÎncă nu există evaluări
- XLPE Polyethylene Lifetime Detection PDFDocument120 paginiXLPE Polyethylene Lifetime Detection PDFconqiuÎncă nu există evaluări
- 2014 BoscainophdDocument187 pagini2014 BoscainophdFaldy LeimenaÎncă nu există evaluări
- PAOX 2018 Jiang Wu InglesDocument162 paginiPAOX 2018 Jiang Wu InglesZULAY MORA RIVAS0% (1)
- Ta ReportDocument13 paginiTa Report22ch028Încă nu există evaluări
- Synthesis and Characterization of Zinc Oxide Nanoparticles by Sol-Gel ProcessDocument45 paginiSynthesis and Characterization of Zinc Oxide Nanoparticles by Sol-Gel ProcessVivek CHOWDARYÎncă nu există evaluări
- Effects of Heat Treatment and Alloying Elements On Characteristics of Austempered Ductile IronDocument119 paginiEffects of Heat Treatment and Alloying Elements On Characteristics of Austempered Ductile IronFellow BetelÎncă nu există evaluări
- Efficient Removal of CR (Vi) From Aqueous Solution With Ppy-Fe O NanocompositeDocument8 paginiEfficient Removal of CR (Vi) From Aqueous Solution With Ppy-Fe O NanocompositeAnthony MuliwaÎncă nu există evaluări
- 18MPH021 Shanmuga Priya ThesisDocument55 pagini18MPH021 Shanmuga Priya ThesisElangopsgÎncă nu există evaluări
- Cover Pages For BVPDocument8 paginiCover Pages For BVPAashish GauravÎncă nu există evaluări
- DissertationDocument207 paginiDissertationhadrienÎncă nu există evaluări
- Data StreamDocument445 paginiData StreamJuniors Dueñas López100% (1)
- Thermal Barrier CoatingDocument41 paginiThermal Barrier CoatingDineshÎncă nu există evaluări
- Report FullDocument71 paginiReport FullVeera HasanÎncă nu există evaluări
- NMR Techniques & Applications in Geochemistry & Soil ChemistryDe la EverandNMR Techniques & Applications in Geochemistry & Soil ChemistryÎncă nu există evaluări
- ThesisDocument104 paginiThesisKastur ChakrabortyÎncă nu există evaluări
- Py Rig HT: Design and Measurement of Losses in Ac Induction Motor With Different Rotor Bar MaterialDocument24 paginiPy Rig HT: Design and Measurement of Losses in Ac Induction Motor With Different Rotor Bar MaterialMareta DanarÎncă nu există evaluări
- Shakeel Final Copy ThesisDocument81 paginiShakeel Final Copy ThesisshakelÎncă nu există evaluări
- Manufacture of Alum Project ReportDocument72 paginiManufacture of Alum Project ReportsampathkumarÎncă nu există evaluări
- RESALTADO KavDocument245 paginiRESALTADO KavFIORELLA GIANINA CHIPANA OCHOAÎncă nu există evaluări
- Synthesis and Characterization of Cowo Nanoparticles Via Chemical Precipitation TechniqueDocument6 paginiSynthesis and Characterization of Cowo Nanoparticles Via Chemical Precipitation TechniqueGirish GuptaÎncă nu există evaluări
- Rajiv ReportDocument59 paginiRajiv ReportmalaÎncă nu există evaluări
- Molecular Nano Dynamics: Vol. I: Spectroscopic Methods and Nanostructures / Vol. II: Active Surfaces, Single Crystals and Single BiocellsDe la EverandMolecular Nano Dynamics: Vol. I: Spectroscopic Methods and Nanostructures / Vol. II: Active Surfaces, Single Crystals and Single BiocellsHiroshi FukumuraÎncă nu există evaluări
- Study of Slurry Rheology and Pressure Drop with AdditivesDocument103 paginiStudy of Slurry Rheology and Pressure Drop with AdditivesAnonPawnÎncă nu există evaluări
- Amira: ARD Test HandbookDocument42 paginiAmira: ARD Test Handbookqcmin2Încă nu există evaluări
- Tescom 44 1300 Series Regulator Data SheetDocument4 paginiTescom 44 1300 Series Regulator Data SheetmatrixianuÎncă nu există evaluări
- OferDinamarca16feb - Graduate Talent Programme Novo NordiskDocument6 paginiOferDinamarca16feb - Graduate Talent Programme Novo NordiskClásico MovieÎncă nu există evaluări
- Material Hardness Conversion TableDocument3 paginiMaterial Hardness Conversion TableJessicalba LouÎncă nu există evaluări
- Human Skull 3D Papercraft: Paper ArtDocument8 paginiHuman Skull 3D Papercraft: Paper ArtReyes YessÎncă nu există evaluări
- Survey of Naval Surface Ship Propulsion and Auxiliary System DevelopmentsDocument90 paginiSurvey of Naval Surface Ship Propulsion and Auxiliary System DevelopmentsAn NgocSonÎncă nu există evaluări
- PDK 205481 KW26-S5-FSE-4Q enDocument83 paginiPDK 205481 KW26-S5-FSE-4Q enRoberto SacotoÎncă nu există evaluări
- SCX-8030ND 8040ND SMDocument450 paginiSCX-8030ND 8040ND SMMircea Stefan BogdanÎncă nu există evaluări
- Answers To Unit - Test Papers 2Document2 paginiAnswers To Unit - Test Papers 2geetaÎncă nu există evaluări
- Size Reduction PDFDocument53 paginiSize Reduction PDFnofriadyÎncă nu există evaluări
- 615-LS 42 ManualDocument48 pagini615-LS 42 ManualmelisaBÎncă nu există evaluări
- Soxhelet Extraction of Crude DrugsDocument38 paginiSoxhelet Extraction of Crude DrugsManjula Raghu0% (1)
- 07 Subsynchronous OscillationsDocument40 pagini07 Subsynchronous Oscillationssulemankhalid0% (1)
- Manual of Green Building MaterialsDocument153 paginiManual of Green Building MaterialsKawser HossainÎncă nu există evaluări
- SP Tools - MaytoJuly2013Document24 paginiSP Tools - MaytoJuly2013Riverland Welding and Tool SuppliesÎncă nu există evaluări
- Bachy TechnicalGuideSB2011Document106 paginiBachy TechnicalGuideSB2011Tân Nguyễn100% (2)
- Automotive Fuels and Engines Chemical PerspectiveDocument13 paginiAutomotive Fuels and Engines Chemical PerspectiveTeshome DengisoÎncă nu există evaluări
- Tempil Basic Guide To Ferrous Metallurgy PDFDocument1 paginăTempil Basic Guide To Ferrous Metallurgy PDFJose Alberto Gamiño Garcia100% (1)
- Assignment AASDocument2 paginiAssignment AASdean016026Încă nu există evaluări
- Paper, 49th Annual Syp, ADDocument12 paginiPaper, 49th Annual Syp, ADPhilÎncă nu există evaluări
- Cuptor Rotativ Morello ForniDocument26 paginiCuptor Rotativ Morello ForniscostelÎncă nu există evaluări
- Subsea Housing Design GuideDocument8 paginiSubsea Housing Design Guidemimi_chan_17100% (1)
- Anhydrous Ammonia Unloading Station & Storage/Vaporizer SystemDocument2 paginiAnhydrous Ammonia Unloading Station & Storage/Vaporizer SystemWalter Rigamonti100% (1)
- Triplex Pump ManualDocument11 paginiTriplex Pump ManualDustin WhiteÎncă nu există evaluări
- Alpha Wire CatalogDocument418 paginiAlpha Wire CatalogmycopteraviationÎncă nu există evaluări
- Cube Boom: Permenant DeploymentDocument1 paginăCube Boom: Permenant Deploymentapi-3703371Încă nu există evaluări
- Learn To Make A Quilt From Start To Finish: CraftsDocument3 paginiLearn To Make A Quilt From Start To Finish: CraftsStefaniaRosatiÎncă nu există evaluări
- Zephyr 20S 62-60519-01 PDFDocument42 paginiZephyr 20S 62-60519-01 PDFAnonymous K53TYtF0% (1)
- Contractors: Company - Address - PhoneDocument2 paginiContractors: Company - Address - PhoneMohammad Anwar HossainÎncă nu există evaluări
- Designation A516 A516M 10 PDFDocument4 paginiDesignation A516 A516M 10 PDFSiddharth GuptaÎncă nu există evaluări
- Koren - Reconfigurable Manufacturing SystemsDocument14 paginiKoren - Reconfigurable Manufacturing SystemsAhmed AtefÎncă nu există evaluări