Documente Academic
Documente Profesional
Documente Cultură
Mixing Rules
Încărcat de
Cremorlab0 evaluări0% au considerat acest document util (0 voturi)
53 vizualizări15 paginichem eng
Drepturi de autor
© © All Rights Reserved
Formate disponibile
PDF, TXT sau citiți online pe Scribd
Partajați acest document
Partajați sau inserați document
Vi se pare util acest document?
Este necorespunzător acest conținut?
Raportați acest documentchem eng
Drepturi de autor:
© All Rights Reserved
Formate disponibile
Descărcați ca PDF, TXT sau citiți online pe Scribd
0 evaluări0% au considerat acest document util (0 voturi)
53 vizualizări15 paginiMixing Rules
Încărcat de
Cremorlabchem eng
Drepturi de autor:
© All Rights Reserved
Formate disponibile
Descărcați ca PDF, TXT sau citiți online pe Scribd
Sunteți pe pagina 1din 15
.
Fluid Phase Equilibria 152 1998 219233
Mixing rules for van-der-Waals type equation of state based on
thermodynamic perturbation theory
Navin C. Patel
)
, Vitaly Abovsky, Suphat Watanasiri
Aspen Technology, Ten Canal Park, Cambridge, MA 02141, USA
Received 15 August 1997; accepted 9 July 1998
Abstract
A concept based on the thermodynamic perturbation theory for a simple fluid has been applied to the
.
attractive term of a van-der-Waals type equation of state EOS to derive a simple mixing rule for the a
parameter. The new mixing rule is a small correction to the original one-fluid approximation to account for the
influence of particles of j-type on the correlation function of ii-type in a mixture consisting of particles of i and
j types. The importance of the correction has been shown by comparison of the calculated results for binary
.
mixtures of LennardJones fluids with the data obtained by numerical method Monte-Carlo simulation . The
new mixing rules can be considered as a flexible generalization of the conventional mixing rules and can be
reduced to the original v-d-W mixing rules by defaulting the extra binary parameters to zero. In this way the
binary parameters already available in the literature for many systems can be used without any additional
regression work. Extension of the new mixing rules to a multicomponent system do not suffer from
MichelsenKistenmacher syndrome and provide the correct limit for the composition dependence of second
virial coefficients. Their applicability has been illustrated by various examples of vaporliquid and liquidliquid
equilibria using a modified PatelTeja EOS. The new mixing rules can be applied to any EOS of van-der-Waals
type, i.e., EOS containing two terms which reflect the contributions of repulsive and attractive intermolecular
forces. q1998 Elsevier Science B.V. All rights reserved.
Keywords: Perturbation theory; Equation of state; Mixing rules; Vaporliquid equilibrium; Liquidliquid equilibrium
1. Introduction
.
Cubic equations of state EOS have become very popular in process design due to their simplicity
and ability to describe wide ranges of components and conditions. Most of these EOS are based on
the original van-der-Waals type EOS with two terms which accounts for contributions of repulsive
and attractive intermolecular forces. Recently, there has been considerable effort to extend the use of
)
Corresponding author
0378-3812r98r$ - see front matter q 1998 Elsevier Science B.V. All rights reserved.
. PII: S0378- 3812 98 00392- 6
( ) N.C. Patel et al.rFluid Phase Equilibria 152 1998 219233 220
cubic EOS to handle complex phase equilibrium problems containing polar and non-polar compo-
nents. In most cases, improvement in the representation of pure component properties is achieved by
using a flexible temperature dependence for the attractive parameter a, while the improvement in
.
representing the binary and multicomponent vaporliquid equilibrium VLE and liquidliquid
.
equilibrium LLE data is achieved by modifying the mixing rule for this parameter. A brief review of
various attempts to enhance conventional mixing rules for cubic EOS is presented by Mathias et al.
w x w x
1 . Many empirically designed flexible mixing rules 25 suffer from MichelsenKistenmacher
w x w x
syndrome 6 or the problem of invariance 1 . The main difficulty lies in the extension of binary
w x
results to calculate multicomponent systems. Mathias et al. 1 provided an empirical modification
which overcomes the problem of invariance but retains another problem which is due to the wrong
composition dependence of the second virial coefficient. This second problem is less significant in
w x
practice. Wong and Sandler 7 proposed a mixing rule which addresses the problem of composition
w x
dependence of the second virial coefficient but it is based on the Huron and Vidal 8 type approach
which duplicates the excess properties from the activity coefficient models and suffers from some
w x
thermodynamic inconsistency as shown by Michelsen and Heidemann 9 .
The main purpose of the present work is to extend the popular van-der-Waals one fluid model in
such a way as to avoid some of the deficiencies outlined above and to demonstrate its application to
systems which are difficult to correlate using currently available mixing rules, e.g., hydrocarbonal-
cohol and hydrocarbonwater. The present approach provides a theoretically-based generalization of
conventional mixing rules. The generalization is based on the first-order perturbation theory which
w x
can be identified with the van-der-Waals EOS under certain conditions 10,11 . The correction of
w x
conventional mixing rules arises in a natural way of enhancing the one-fluid approximation 12 for
w x
the first-order term of perturbation theory. The result was briefly mentioned by Abovsky 13,14 in
regards to the application of perturbation theory to strongly nonideal fluid mixtures. This result is now
extended to cubic EOS. It is shown that the way to improve conventional mixing rules is to consider a
correction to terms contributed by the interaction of similar particles. Work by Kabadi and Danner
w x w x
15 and Michel et al. 16 provides examples of empirical efforts using such a correction for fitting
liquidliquid equilibrium data.
2. Perturbation theory and one-fluid approximation for mixture
w x
Consideration 10 of a system of particles interacting with hard-core-with-attraction potential leads
to the following expression for Helmholz free energy;
AsA qA 1 .
r a
where A is the contribution of a reference system represented by particles with repulsive interaction
r
only and A is the first-order perturbation term. For simplicity we shall consider the A term for
a a
spherically symmetric intermolecular interaction, although results could be extended, without restric-
w x
tions, to system with angular-dependent interaction 14 :
`
3 2
A sy2pNs r g r ,, d f r r dr . .
H
a 0 a
. d T
2 .
`
3 2
sy2pNs r 1qh r ,, d f r r dr . .
H
0 a
. d T
( ) N.C. Patel et al.rFluid Phase Equilibria 152 1998 219233 221
where Nnumber of particles in the system; sgeometric parameter of the attractive part of the
.
pair potential function; energy parameter of the pair potential function; f r nondimensional
a
.
part of the pair potential function; -specific volume per particle; g r,,d radial distribution
0
function of a reference system; its dependence on temperature arises from the reduced diameter of
.
effective hard sphere dsD T rs ; the system of effective hard spheres is used to replace a
.
reference system with soft repulsive interaction; rreduced intermolecular distance; and h r,,d
0
correlation function of a reference system.
Introducing the following relations,
`
2
S T s f r r dr 3 . . .
H
0 a
. d T
and
`
2
S ,T s h r ,, d f r r dr 4 . . . .
H
h 0 a
. d T
results in
A sya T 1qs ,T r 5 . . .
a 0
.
3
. . .
where a T s2pNs S T and s ,T sS rS . Eq. 5 is obtained for a pure substance; the first
0 0 h 0
term on the right hand side is the contribution of the first order perturbation theory to the second virial
coefficient; the second term is the contribution of interactions of more than two particles.
.
Generalization of Eq. 5 for mixtures leads to
A sy 1r x x a T y 1r x x a T s x,,T 6 . . . . . .
am i j 0i j i j 0i j i j
i j i j
.
where the first term is the contribution of the mixture second virial coefficient. s x,,T is the ratio
i j
. .
of values defined by Eqs. 3 and 4 for mixtures and is generally a function of composition because
.
of the composition dependence of the correlation function h r ,x,,d . However, if we assume
0i j i j i j
.
s x,,T to be independent of composition, then it follows that:
i j
s ,T ss ,T 7 . . .
i j
. w x
Eq. 7 represents the one-fluid approximation 12 for the first-order term of a perturbation
expansion for mixtures. The structure of A within this approximation becomes identical to the
am
structure of A for a pure component:
a
A sya T 1qs ,T r 8 . . .
am 0m
where
a s x x a T 9 . .
0m i j 0i j
i j
which is the well known conventional one-fluid mixing rule for parameter a . s is different for each
0m
EOS as will be shown later.
( ) N.C. Patel et al.rFluid Phase Equilibria 152 1998 219233 222
w x
In general, s is composition-dependent and can be expressed as 14
i j
s x,,T ss ,T 1qc x,,T 10 . . . .
i j i j
.
where c is a correction to the one-fluid approximation and is related to h through Eq. 4 .
i j 0i j
w x .
Calculations by Lee and Levesque 17 have shown that h r ,x,,d can be substantially different
0i j i j i j
.
from h r,,d if diameters of hard spheres are significantly different which results in c being
0 i j
nonzero.
3. Correction to one-fluid approximation
w x
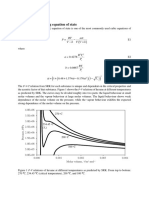
Figs. 1 and 2 illustrate applicability of perturbation theory described by Abovsky 14 for model
binary systems with a LennardJones potential function. Results of computational experiment by
w x ..
Monte-Carlo simulation 18 were used for comparison. While the one-fluid approximation Eq. 7
.
was sufficient for mixture of particles with equal size parameters Fig. 1 , one correction parameter
was required to fit adequately the simulation results for system with particles of substantially different
.
sizes Fig. 2 . Correction c for this system is equal to 0.2 x which confirms the assumption
21 1
c <1. These results were based on the assumption that c is independent of volume. Neglecting the
i j i j
Fig. 1. Reduced pressure ps
3
r vs. mole fraction x and vs. reduced density rrs
3
for binary system with
1 1 1
LennardJones interaction at reduced temperature k Tr s1.15. The ratios of parameters are: s rs s1.0, r s0.5.
b 1 2 1 1 2
w x The circles are results of MonteCarlo simulations 18 . The line represents one-fluid approximation of perturbation theory
w x 14 .
( ) N.C. Patel et al.rFluid Phase Equilibria 152 1998 219233 223
. Fig. 2. Same as in Fig. 1 except s rs s0.35, r s0.66. The dashline is obtained with k sy0.20, see Eqs. 10
2 1 1 2 c21
. and 11 .
volume dependence of c and expanding it in power series of compositions while keeping only the
i j
linear terms we obtain:
c x,T s x E c rE x s x k T 11 . . . .
i j 1 i j 1 1 ci j1 x (0
1
1 1
.
c should go to zero at the limit of a pure component according to Eq. 10 . To satisfy this condition;
i j
k s E c rE x s0 12 . .
x (0 ci i i i i i
i
. . .
Combining Eqs. 6 , 9 and 10 yields:
A sya x,T rya x,T 1qc s ,T r 13 . . . .
am 0m 0m i j
. .w x
Letting a x,T sa x,T 1qc , then
m 0m i j
A sya x,T rya x,T s ,T r 14 . . . .
am 0m m
where a is the mixture a parameter. Note that a can be considered as the sum of the one-fluid a ,
m m 0m
and a correction term Da, i.e.,
a sa qDa 15 .
m 0m
( ) N.C. Patel et al.rFluid Phase Equilibria 152 1998 219233 224
.
with Da defined subsequently in Eq. 28 . For a binary mixture:
a sa x
2
q2a x x qa x
2
16 .
0m 11 1 12 1 2 22 2
a sa x
2
1qx k q2a x x 1qx k qx k qa x
2
1qx k . . .
m 11 1 2 c112 12 1 2 1 c121 2 c122 22 2 1 c221
1r2
a s a a 1yk 17 . . .
12 1 2 12
By collecting terms and defining,
k sk q2k a ra 18 .
c12 c112 c121 12 11
k sk q2k a ra 19 .
c21 c221 c122 12 22
a sa x
2
1qx k q2a x x qa x
2
1qx k 20 . . .
m 11 1 2 c12 12 1 2 22 2 1 c21
.
Eq. 20 contains two new adjustable parameters k and k in addition to conventional parameter
c12 c21
.
k in Eq. 17 . The parameter k reflects the influence of component j on contribution of
12 ci j
ii-interaction to mixture free energy, and, similarly k reflects the influence of component i on
c ji
.
contribution of jj-interaction to mixture free energy. Eq. 20 can be generalized for a multicompo-
nent mixture as shown below:
a s x
2
a 1q k x q x x a 1q k x , 21 .
m i i i ci j j i j i j ci j1 1
/ /
i j/i i j/i 1/ij
where parameter k reflects the influence of component l on contribution of interaction ij. Although
ci jl
k is strictly a ternary parameter, it is more useful to evaluate this parameter from binary data. For
ci jl
this, a careful choice has to be made regarding the calculation of the cross term to avoid the
MichelsenKistenmacher syndrome. The geometric mean with retention of proper sign is necessary.
Thus, k can be calculated as follows:
ci jl
1r2
k s k k 22 . .
ci j1 ci1 c j1
where k and k are binary parameters which can be positive or negative. If both are positive, then
cil cjl
the positive sign is used for the square root; if both are negative, then the negative sign must be used;
if k and k have alternate signs, then it is appropriate to assume zero for k since comparable
cil c jl ci jl
values of opposite sign will result in zero interaction, and if one of the values is much less than the
other, the square root of the product will tend to be close to zero because binary parameters are
generally less than unity.
4. Equation of state of van-der-Waals type
w x
The v-d-W EOS can be derived from perturbation theory as shown by Smith and Alder 10 and
w x w x
Kac et al. 11 following the pioneering work by Zwanzig 19 on perturbation expansions based on a
.
hard sphere reference system. From Eq. 14 , we obtain the attractive term of any v-d-W type EOS:
2 2
p sya r y sy E srE a r 23 . .
am 0m m
( ) N.C. Patel et al.rFluid Phase Equilibria 152 1998 219233 225
. . . w .x
where a and a can be calculated using Eqs. 16 , 17 and 21 and the sy EsrE term is
0m m
.
different for each EOS. If we neglect the volume dependence of s, then Eq. 23 reduces to the v-d-W
w .x
attractive term. For other EOS sy EsrE can be obtained by matching the attractive term of the
.
EOS with Eq. 23 and by noting that a sa sa for pure components. For the RedlichKwong
m 0m
. w x
RK EOS 20 we obtain:
sy E srE sy br r 1qbr 24 . . . .
. w x
Similarly, for the three-parameter PatelTeja PT EOS 21 for which the pure component pressure
equation is
psRTr yb yar qb qc yb 25 . . . .
we obtain
sy E srE sy brqcr 1ybr r 1qbrqcr 1ybr 26 . . . .
. . .
For a mixture, a is different from a , therefore, if we substitute Eqs. 15 and 26 into Eq. 23 we
0m m
obtain the mixture form of the PT EOS:
2
psRTr yb ya r qb qc yb qDar 27 . . . .
m
.
where the mixing rule for a is given by Eq. 21 and
m
Das x
2
a k x q x x a k x 28 .
i ii ci j j i j i j ci j1 1
i j/i i j/i 1/ij
If all parameters k , k are equal to zero, then Das0 and a sa , which results in the
ci j ci jl m 0m
.
conventional one-fluid mixing rule. When Das0, the mixture form of the EOS, Eq. 27 will also
. .
reduce to the pure component form, Eq. 25 . The second virial coefficient from Eq. 27 is made up
.
of contributions from a and Da terms. The effect of the last term in Eq. 27 is to cancel out the
m
contribution of the Da term embedded in a , leaving only the contribution of a which is quadratic
m 0m
.
in composition. Therefore, Eq. 27 provides the correct composition dependence for the second virial
coefficient of the mixture. This term can be used with any EOS to satisfy this constraint on the
mixture second virial coefficient. The use of this term makes PengRobinson and PatelTeja EOS
quartic instead of cubic in volume which is less desirable if an analytical solution is required for the
density root, however, this is not a problem when a numerical solution is used to calculate the density.
In this study, we use a general three parameter PatelTeja EOS. This EOS when combined with a
flexible attractive term, allows accurate representations of vapor pressure, liquid density, and liquid
w x
heat capacity data 22 , the three most important pure component properties needed for a successful
.
process design. The EOS is given by Eq. 27 , with pure component parameters defined as follows,
R
2
T
2
RT RT
c c c
asV a T , bsV , csV 29 .
a b c
P P P
c c c
where, V , V , and V are functions of the predicted critical compressibility factor denoted by z .
a b c c
( ) N.C. Patel et al.rFluid Phase Equilibria 152 1998 219233 226
The following constraints at the critical points are applied to the EOS:
E P E
2
P P
c c
s0, s0, sz 30 .
c 2
/ /
E E RT
T
T c c
c
With the critical constraints, the following relationships are established between the three dimension-
less parameters V , V , and V with z .
a b c c
V s1y3z 31 .
c c
V
3
q 2y3z V
2
q3z
2
V yz
3
s0 32 . .
b c b c b c
V s3z
2
q3 1y2z V qV
2
q1y3z 33 . .
a c c b b c
.
V is obtained by solving for the smallest positive root of Eq. 32 . For the attractive term, the
b
following function of reduced temperature is used.
N
a T s1qc T y1 qc T y1 qc T y1 34 ( . . . / 1 r 2 r 3 r
Constants c , c , c , N and z were obtained by simultaneous regression of pure component
1 2 3 c
experimental data for vapor pressure, liquid density, and liquid heat capacity. Table 1 shows the
values of pure component parameters used in this work. Experimental data were taken from the
w x
DIPPR data compilation 23 .
For mixtures, the following mixing rules were used.
a sa qDa 35 .
m 0
. .
where Da is given by Eq. 28 ; however, it is more convenient to write Eq. 28 as follows:
1r2 1r2
Dasy x x x k a a , k s k k 36 . . .
i j 1 i j1 i j i j1 ci1 c j1
i j 1/ij
1r2
a s x x a a 1yk , k sk 37 . . .
0 i j i j i j i j ji
i j
3
1r3 1r3
b qb
i j
bs x x 1y1 , 1 s1 38 . .
i j i j i j ji
/
2
i j
c rb qc rb
i i j j
csb x x 1ym , m sm 39 . .
i j i j i j ji
/
2
i j
.
For a given binary system, we can have as many as five binary parameters k , k , k , l , m .
12 c12 c21 12 12
In most cases, however, we do not need all five parameters. Only one or two parameters are required
for most VLE calculations, and three parameters are required for complex VLE and LLE calculations.
To cover a wide range of temperatures, the parameters need to be temperature dependent. We have
used the following temperature dependence:
k sk1 qk2 rTqk3 ln T 40 . .
i j i j i j i j
( ) N.C. Patel et al.rFluid Phase Equilibria 152 1998 219233 227
Table 1
Pure-component parameters
. . Component T K P MPa c c c N z
c c 1 2 3 c
Propane 369.83 4.248 2.28968 y4.97686 y0.30851 2 0.3076
a
Butane 425.12 3.796 1.16274 y3.21657 y0.13352 2 0.3158
Pentane 469.70 3.370 1.65121 y3.99114 y0.21328 2 0.3121
Cyclohexane 553.58 4.073 2.43049 y4.79121 y0.38096 2 0.3142
Benzene 562.16 4.898 3.69025 y6.21024 y0.66095 2 0.3119
Methanol 512.64 8.097 y0.56959 y1.39897 0.05333 6 0.2753
2-Butanol 536.05 4.179 y5.34670 1.02041 2.02301 2 0.2988
Water 647.13 22.055 0.34350 y2.28139 0.05145 2 0.2724
a
c s1.08604 was used to match the pure component vapor pressure reported with the binary VLE data for the
1
w x w x butanemethanol system 30 . T and P were taken from DIPPR data compilation 23 .
c c
.
A temperature dependence similar to Eq. 40 is used for all parameters. In most cases, only the first
. .
two terms of Eq. 40 are needed. For some parameters l , m , the temperature dependence is
12 12
rarely required.
5. Results and discussions
Pure component parameters are listed in Table 1. These parameters give accurate representation of
pure component vapor pressure data which is important for VLE calculations. The binary parameters
are listed in Table 2.
In Fig. 3, results for n-butanemethanol system are shown for 373.15 K. This system is very
difficult to correlate due to a flat region in the temperature vs. liquid composition curve. Many EOS
mixing rules will give false liquidliquid phase splitting that is often indicated by the maxima and
minima in the bubble point curve. As can be seen, the worst results are obtained when the size
Table 2
Binary interaction parameters
. . System Binary parameters see Eq. 38 for description of parameters
k k m I
i j ci j i j i j
a a a a
k1 k2 k3 k1 k2 k1 k2 m1 l1 l 2
12 12 12 c12 c12 c21 c21 12 12 12
. . Butane 1 Methanol 2 0.2553 0.4496 y1.4873
. . Pentane 1 Methanol 2 y0.1711 156.4 y0.1326 191.4 y1.3857
. . Propane 1 Water 2 0.2532 154.8 0.8445 y0.4188 828.0
. . Benzene 1 Water 2 0.3038 y4.458 0.3044 321.8
. . Cyclohexane 1 Water 2 0.2621 60.3 y0.6324 840.2
. . Cyclohexane 1 Benzene 2 0.0247
. . 2-Butanol 1 Water 2 8.9838 y118.2 y1.5057 y1.0909 y0.7422 325.9
a
Given in kelvin.
w x w x w x w x Data references: ButaneMethanol 30 , PentaneMethanol 31 , PropaneWater 25 , BenzeneWater 27,28 , Cyclohex-
w x w x w x aneWater 27,28 , CyclohexaneBenzene 32 , 2-ButanolWater 26 .
( ) N.C. Patel et al.rFluid Phase Equilibria 152 1998 219233 228
Fig. 3. Effect of adjusting different binary parameters for butanemethanol system at 373.15 K. Vaporliquid equilibrium
w x data from Ref. 30 .
.
parameters are not adjusted l and m equal to zero , and the best results are obtained by using the
12 12
new corrections k , k , and the size parameter m . It is interesting to note that there is no liquid
c12 c21 12
phase instability shown by the solid line and the results are identical to the gammaphi method using
w x
the best available activity coefficient model for this type of system, e.g., the Wilson model 24 . The
results for the Wilson-RK gammaphi method are not shown in Fig. 3 because they fall on the same
solid line shown for the EOS results.
In Fig. 4, results for the n-pentanemethanol system for two isotherms at 372.69 K and 422.59 K
are shown. In this case temperature-dependent parameters were used to improve the overall represen-
w x
tation of the data. This system was recently analyzed by Michelsen and Heidemann 9 . They also
w x Fig. 4. Vaporliquid equilibrium data for pentanemethanol system at 372.69 and 422.59 K. Data from Ref. 31 .
( ) N.C. Patel et al.rFluid Phase Equilibria 152 1998 219233 229
found that the Wilson model used to represent activity coefficients for this binary system could be
improved with the temperature-dependent parameters. Our results are slightly better than the MHV-2
model used by Michelsen and Heidemann; however, this is to be expected since we correlated the
data directly with the use of binary parameters while their results are based on calculations associated
with the implementation of an activity coefficient model into an EOS.
In Fig. 5, results of VLE and LLE data correlations are shown for the propanewater system. This
is a typical hydrocarbonwater system which exhibits VLE and LLE behavior. To evaluate binary
.
parameters, we used experimental data for VLE, LLE and GLE gas solubility covering the
temperature range from 313.15 to 413.15 K and the pressure range of 0.5 to 20.2 MPa. The binary
.
parameters are listed in Table 2. Two temperature-dependent parameters k and k were used with
i j c ji
.
one additional constant parameter k . The results are also compared with the Schwartzentruber et
ci j
w x
al. 5 mixing rule for the modified SRK equation of state. This is labeled SR-POLAR on the plots.
The SR-POLAR EOS needed a total of six binary parameters which includes the adjustable parameter
for the constant b in the EOS. As can be seen, the new mixing rules give better representation of the
solubility of propane in the water-rich phase for both VLE and LLE data. In Fig. 6, results for the
amount of water in the organic phase are shown; again the new mixing rules give a correct behavior
for the Px curve. Results for the amount of water in the vapor phase are in Fig. 7; the results are
within 5% for the data at 373.15 K. However, the vapor phase data reported by Kobayashi and Katz
w x
25 for 413.15 K gave deviations in the range of 1520% and the data appear to be inconsistent with
those at 373.15 K.
In Fig. 8, LLE data for 2-butanolwater system are shown as a function of temperature and
pressure. This system shows the closed-loop LLE behavior. Only data from 0.1 to 70.0 MPa reported
w x
by Moriyoshi et al. 26 were used. At higher pressures, we had some problems in convergence and
the data close to the hyper-critical solution point were not used in the regression. The new mixing
rules clearly show improved performance over the Schwartzentruber et al. mixing rules used in the
w x Fig. 5. Comparison of new mixing rules with the Schwartzentruber et al. 5 mixing rules. VLE and LLE data for
w x propanewater system 25 .
( ) N.C. Patel et al.rFluid Phase Equilibria 152 1998 219233 230
w x Fig. 6. Comparison of new mixing rules with the Schwartzentruber et al. 5 mixing rules, showing water content in organic
phase.
SR-POLAR EOS. The closed solubility loops in the isobaric Tx diagram are very well represented
by the new mixing rules. The pressure effect on the LLE data is normally small and is often neglected
in most modeling efforts. Fig. 8 shows that it is possible to model the effect of pressure on LLE data
using a simple cubic EOS. Most activity coefficient models give adequate representation for low
pressure data but fail at higher pressure due to the fact that activity coefficients are independent of
pressure.
Finally, Fig. 9 shows that our new mixing rules for the a parameter are free from the
w x
MichelsenKistenmacher syndrome 29 . There is no unusual maximum in the predicted water
w x Fig. 7. Comparison of new mixing rules with the Schwartzentruber et al. 5 mixing rules, showing water content in vapor
phase.
( ) N.C. Patel et al.rFluid Phase Equilibria 152 1998 219233 231
w x Fig. 8. Mutual solubility of 2-butanolwater system as a function of pressure. Data from Ref. 26 . Comparison of new
w x mixing rules with the Schwartzentruber et al. 5 mixing rules.
solubility in the benzenecyclohexane mixture. As shown, the SR-POLAR model based on the
Schwartzentruber et al. mixing rules does suffer from this syndrome. We have also checked the case
in which one of the binary components is split to give a ternary mixture. In this case, the same
numerical value was obtained for the parameter a and the composition derivative of a with respect to
mole number before and after the split indicating that the new mixing rules are free from this well
known deficiency. The equilibrium calculations were also identical before and after the split.
Fig. 9. Water solubility in benzenecyclohexane mixture.
( ) N.C. Patel et al.rFluid Phase Equilibria 152 1998 219233 232
6. Conclusion
.
1 A new mixing rule has been derived for the parameter a of the van-der-Waals type EOS using
the first-order perturbation theory which leads to a small correction to the nonlinear by density term in
the expression for free energy departure.
.
2 The new mixing rule gives extra flexibility by considering the contribution of intermolecular
interactions at infinite dilution. In particular, the influence of particles of j-type on the correlation
function of ii-type in a mixture consisting of i and j types has been accounted for by the new mixing
rule.
.
3 The new mixing rule is free from the MichelsenKistenmacher syndrome and provides the
correct composition dependence of the second virial coefficient.
.
4 The new mixing rule is able to describe difficult phase equilibrium data such as those found in
hydrocarbonalcohol and hydrocarbonwater systems, and it is also capable of describing closed-loop
LLE data such as those found in alcoholwater systems.
7. List of symbols
A Helmholz free energy
a Temperature-dependent parameter of a cubic EOS
b Covolume parameter of a cubic EOS
c Parameter of PatelTeja EOS
c Correction to one-fluid approximation
i j
d Reduced diameter of effective hard sphere
g Radial distribution function of a reference system
0
h Correlation function of a reference system
0
k Boltzmann constant
B
k Any parameter of mixing rules
subscript
N Avogadros number
p Pressure
r Reduced intermolecular distance
R Gas constant
.
S Integral defined by Eq. 3
0
.
S Integral defined by Eq. 4
h
s Ratio of S to S
h 0
T Temperature
Specific volume per particle
x Composition
Greek letters
a Temperature dependent function for the attractive term in EOS
Energy parameter of the attractive part of pair potential function
f Nondimensional part of the pair potential function
a
s Geometric parameter of the pair potential function
( ) N.C. Patel et al.rFluid Phase Equilibria 152 1998 219233 233
Summation
z Predicted critical compressibility from EOS
c
V Dimensionless coefficient in EOS
Subscripts
a Contribution of attractive forces
c Correction
i,j,l Components
m Mixture
r Contribution of repulsive forces
References
w x . 1 P.M. Mathias, H.C. Klotz, J.M. Prausnitz, Fluid Phase Equilibria 67 1991 31.
w x . 2 A.J. Panagiotopoulos, R.C. Reid, ACS Symp. Ser. 300 1986 571.
w x . 3 R. Stryjek, J.H. Vera, Can. J. Chem. Eng., 64 1986 323, 334, 820.
w x . 4 Y. Adachi, H. Sugie, Fluid Phase Equilibria 28 1986 103.
w x . 5 J. Schwartzentruber, H. Renon, S. Watanasiri, Fluid Phase Equilibria 52 1989 127.
w x . 6 M.L. Michelsen, H. Kistenmacher, Fluid Phase Equilibria 58 1990 229.
w x . 7 D.S.H. Wong, S.I. Sandler, AIChE J. 38 1992 671.
w x . 8 M.J. Huron, J. Vidal, Fluid Phase Equilibria 3 1979 255.
w x . 9 M.L. Michelsen, R.A. Heidemann, Ind. Eng. Chem. Res. 35 1996 278.
w x . 10 E.B. Smith, B.J. Alder, J. Chem. Phys. 30 1959 1190.
w x . 11 M. Kac, G.E. Uhlenbeck, P.C. Hemmer, J. Math. Phys. 4 1963 216.
w x . 12 J.S. Rowlinson, Rep. Progr. Phys. 28 1965 169.
w x . . 13 V. Abovsky, AIChE Symp. Ser. 298 90 1994 188.
w x . 14 V. Abovsky, Fluid Phase Equilibria 116 1996 170.
w x . 15 V.N. Kabadi, R. Danner, Ind. Eng. Chem. Process Des. Dev. 24 1985 537.
w x . 16 S. Michel, H.H. Hooper, J.M. Prausnitz, Fluid Phase Equilibria 45 1989 173.
w x . 17 L. Lee, D. Levesque, Mol. Phys. 26 1973 1351.
w x . 18 A.M. Georgoulaki, I.V. Ntouros, D.P. Tassios, A.Z. Panagiotopoulos, Fluid Phase Equilibria 100 1994 153.
w x . 19 R.W. Zwanzig, J. Chem. Phys. 22 1954 1420.
w x . 20 O. Redlich, J.N.S. Kwong, Chem. Rev. 44 1949 233.
w x . 21 N.C. Patel, A.S. Teja, Chem. Eng. Sci. 37 1982 463.
w x . . 22 N.C. Patel, Int. J. Thermophys. 17 3 1996 673.
w x 23 T.E. Daubert, R.P. Danner, DIPPR Project 801 Data Compilation, 1994.
w x . 24 G.M. Wilson, J. Am. Chem. Soc., 86 1964 127, 133.
w x . . 25 R. Kobayashi, D.L. Katz, Ind. Eng. Chem. 45 2 1953 440.
w x . 26 T. Moriyoshi, S. Kaneshina, K. Aihara, K. Yabumoto, J. Chem. Thermodyn. 7 1975 537.
w x 27 J.M. Srenson, W. Artl, Dechema Chemistry Data Series, Vol. V, Part 1, 1979.
w x . 28 C. Tsonopoulos, G.M. Wilson, AIChE J. 29 1983 990.
w x . 29 G. Arich, I. Kikic, P. Alessi, Chem. Eng. Sci. 30 1975 187.
w x . . 30 A.D. Leu, C.J. Chen, D.B. Robinson, AIChE Symp. Ser. 272 85 1989 11.
w x . 31 R.A. Wilsak, S.W. Campbell, G. Thodos, Fluid Phase Equilibria 33 1987 157.
w x . 32 R.A. Mentzer, R.A. Greenkorn, K.C. Chao, J. Chem. Thermodyn. 14 1982 817.
S-ar putea să vă placă și
- High Pressure Phase Behaviour of Multicomponent Fluid MixturesDe la EverandHigh Pressure Phase Behaviour of Multicomponent Fluid MixturesÎncă nu există evaluări
- A Quasi-Newton Algorithm For Solving Multi Phase Equilibrium Flash ProblemsDocument22 paginiA Quasi-Newton Algorithm For Solving Multi Phase Equilibrium Flash ProblemsDaniel MedeirosÎncă nu există evaluări
- Solubility of Solids in Sub - and Supercritical Fluids A ReviewDocument26 paginiSolubility of Solids in Sub - and Supercritical Fluids A ReviewJonatas LopesÎncă nu există evaluări
- SRK Usage.14508.1580095490.5035 PDFDocument5 paginiSRK Usage.14508.1580095490.5035 PDFnutnicha suwannangkoonÎncă nu există evaluări
- Modelling The Extraction of Essential Oils With Compressed Carbon DioxideDocument14 paginiModelling The Extraction of Essential Oils With Compressed Carbon DioxideYusiana DewiÎncă nu există evaluări
- Thermophysical Properties-Industrial DirectionsDocument15 paginiThermophysical Properties-Industrial DirectionsfabiopdnÎncă nu există evaluări
- A Method To Estimate The Patel-Teja Equation of State ConstantsDocument7 paginiA Method To Estimate The Patel-Teja Equation of State Constants1940LaSalleÎncă nu există evaluări
- Partition Coefficients and Their UsesDocument92 paginiPartition Coefficients and Their UsesquelenigÎncă nu există evaluări
- Boltzmann ConstantDocument6 paginiBoltzmann ConstantuyenphuongdangÎncă nu există evaluări
- Experimental Optimization and Mathematical Modeling of The Supercritical - SADocument12 paginiExperimental Optimization and Mathematical Modeling of The Supercritical - SAJonatas LopesÎncă nu există evaluări
- A Improving Predictions of Equation of State by Modifying Its Parameters For Super Critical Components of Hydrocarbon Reservoir FluidsDocument17 paginiA Improving Predictions of Equation of State by Modifying Its Parameters For Super Critical Components of Hydrocarbon Reservoir Fluids13670319Încă nu există evaluări
- Dynamical Systems With Applications Using Python (Information)Document37 paginiDynamical Systems With Applications Using Python (Information)Abenesh KumaresanÎncă nu există evaluări
- Correlation of Ideal Gas Enthalpy, Heat Capacity, and EntropyDocument4 paginiCorrelation of Ideal Gas Enthalpy, Heat Capacity, and EntropyJosé Luís Díaz VargasÎncă nu există evaluări
- Computation of Phase and Chemical Equilibrium IDocument9 paginiComputation of Phase and Chemical Equilibrium IThou KanshieÎncă nu există evaluări
- R7222301 Mass Transfer & SeparationDocument2 paginiR7222301 Mass Transfer & SeparationsivabharathamurthyÎncă nu există evaluări
- Supercritical Carbon Dioxide Extraction of Pigments From Bixa Orellana Seeds (Experiments and Modelling)Document8 paginiSupercritical Carbon Dioxide Extraction of Pigments From Bixa Orellana Seeds (Experiments and Modelling)Jacob SteeleÎncă nu există evaluări
- Thermo SyllabusDocument5 paginiThermo SyllabusErica BradleyÎncă nu există evaluări
- Rate Based Vs Equilibrium ModelDocument12 paginiRate Based Vs Equilibrium Modelzorro21072107Încă nu există evaluări
- Ti Ti: 11.6 Fugacity and Fugacity Coefficient: Species in SolutionDocument4 paginiTi Ti: 11.6 Fugacity and Fugacity Coefficient: Species in Solutionsigit1058Încă nu există evaluări
- Vapor-Liquid Equilibrium of Systems Containing Alcohols, Water, CarbonDocument8 paginiVapor-Liquid Equilibrium of Systems Containing Alcohols, Water, CarbonJessica CehÎncă nu există evaluări
- Fluid Phase Equilibria Volume 95 Issue None 1994 (Doi 10.1016/0378-3812 (94) 80070-7) Monika Johannsen Gerd Brunner - Solubilities of The Xanthines Caffeine, Theophylline and Theobromine in SupeDocument12 paginiFluid Phase Equilibria Volume 95 Issue None 1994 (Doi 10.1016/0378-3812 (94) 80070-7) Monika Johannsen Gerd Brunner - Solubilities of The Xanthines Caffeine, Theophylline and Theobromine in Supenurul latifahÎncă nu există evaluări
- Sample Problem - ThermodynamicsDocument30 paginiSample Problem - ThermodynamicscyhdzÎncă nu există evaluări
- Thermodynamics Project: TOPIC: Fugacity of Pure SubstancesDocument6 paginiThermodynamics Project: TOPIC: Fugacity of Pure SubstancesRaman K. BediÎncă nu există evaluări
- Marrero and Gani 2001Document26 paginiMarrero and Gani 2001Jillian PeteÎncă nu există evaluări
- Flash Calc 1flash Calc 185Document18 paginiFlash Calc 1flash Calc 185Mohamed MamdouhÎncă nu există evaluări
- Flash Calc 152Document8 paginiFlash Calc 152Mohamed MamdouhÎncă nu există evaluări
- 071 - 88 Ing.3Document18 pagini071 - 88 Ing.3Leonardo SaggiomoÎncă nu există evaluări
- A Mathematical Model For Equilibrium Solubility of Hydrogen Sulfide and Carbon Dioxide in Aqueous Alkanolamine SolutionsDocument8 paginiA Mathematical Model For Equilibrium Solubility of Hydrogen Sulfide and Carbon Dioxide in Aqueous Alkanolamine Solutionsiitgn007100% (1)
- Group Contribution Estimation of Activity Coefficient in Non Ideal MixsDocument14 paginiGroup Contribution Estimation of Activity Coefficient in Non Ideal MixsArun EbenezerÎncă nu există evaluări
- CET 2 - Chemical Reaction EquilibriumDocument46 paginiCET 2 - Chemical Reaction EquilibriumMihir ParekhÎncă nu există evaluări
- Liquid Liquid Column Drop: Spray Holdup, and Continuous Phase Mass TransferDocument6 paginiLiquid Liquid Column Drop: Spray Holdup, and Continuous Phase Mass TransferBastien DautricheÎncă nu există evaluări
- Accepted Manuscript: J. of Supercritical FluidsDocument40 paginiAccepted Manuscript: J. of Supercritical FluidsjacerÎncă nu există evaluări
- H12/P, Equilibrium CoefficientDocument24 paginiH12/P, Equilibrium CoefficientFareeha HasanÎncă nu există evaluări
- Oliveira Et Al 2012Document10 paginiOliveira Et Al 2012Daniela De Araujo SampaioÎncă nu există evaluări
- Thermodynamics and Its ApplicationsDocument30 paginiThermodynamics and Its ApplicationspoletoÎncă nu există evaluări
- 7 1. Vapor Liquid EquilibriumDocument9 pagini7 1. Vapor Liquid Equilibriumwaseemkhan49Încă nu există evaluări
- Thermodynamic Property Predictions With The Trebble-Bishnoi EOSDocument18 paginiThermodynamic Property Predictions With The Trebble-Bishnoi EOSGeorge MissakÎncă nu există evaluări
- Hemp (Cannabis Sativa L.) Extract: Anti-Microbial Properties, Methods of Extraction, and Potential Oral DeliveryDocument22 paginiHemp (Cannabis Sativa L.) Extract: Anti-Microbial Properties, Methods of Extraction, and Potential Oral DeliveryCamilo Serna RestrepoÎncă nu există evaluări
- A New Generalized Alpha Function For A Cubic Equation of StateDocument11 paginiA New Generalized Alpha Function For A Cubic Equation of StateJenn QuintoÎncă nu există evaluări
- Isobaric Vapor-Liquid Equilibrium For Binary System of Methanol and AcetonitrileDocument4 paginiIsobaric Vapor-Liquid Equilibrium For Binary System of Methanol and AcetonitrileAlejandra Rodríguez MorenoÎncă nu există evaluări
- Phase EquilibriaDocument31 paginiPhase EquilibriaLogesh SelvamaniÎncă nu există evaluări
- Phase Equilibrium Properties of The Ternary Mixture Dibutylether + Toluene + Heptane at 313.15 KDocument5 paginiPhase Equilibrium Properties of The Ternary Mixture Dibutylether + Toluene + Heptane at 313.15 Kmurdanetap957Încă nu există evaluări
- A Comprehensive Comparison of Mixing Rules For Calculation of Phase Equilibria in Complex SystemsDocument8 paginiA Comprehensive Comparison of Mixing Rules For Calculation of Phase Equilibria in Complex Systemsmurdanetap957Încă nu există evaluări
- Elsevier Science Publishers B.V.. Amsterdam - Printed in The NetherlandsDocument16 paginiElsevier Science Publishers B.V.. Amsterdam - Printed in The NetherlandssonsdoedenÎncă nu există evaluări
- Improvement in Patel Teja Eqn of StatesDocument10 paginiImprovement in Patel Teja Eqn of StatesSumukh VermaÎncă nu există evaluări
- VLE Lactic Acid Ethyl Lactate Esterification PDFDocument7 paginiVLE Lactic Acid Ethyl Lactate Esterification PDFAseem Kashyap0% (1)
- Polyethylene Glycol-Salt PDFDocument14 paginiPolyethylene Glycol-Salt PDFlauraÎncă nu există evaluări
- 0378 3812 2889 2980369 3 PDFDocument12 pagini0378 3812 2889 2980369 3 PDFsaeedt538Încă nu există evaluări
- UNIFAC - Properties of Gases and LiquidsDocument37 paginiUNIFAC - Properties of Gases and LiquidsAngelicCamarisPereaÎncă nu există evaluări
- Vapor-Phase Chemical Equilibrium For The Hydrogenation of Benzene To Cyclohexane From Reaction-EnsembleDocument13 paginiVapor-Phase Chemical Equilibrium For The Hydrogenation of Benzene To Cyclohexane From Reaction-EnsembleEuse CalleÎncă nu există evaluări
- Super Critical CO2 Extraction of Red Pepper OleoresinDocument12 paginiSuper Critical CO2 Extraction of Red Pepper OleoresinsdhanupriyaÎncă nu există evaluări
- Phase Oil Equilibria of Oil-Water-Brine - Yaun Kun LiDocument11 paginiPhase Oil Equilibria of Oil-Water-Brine - Yaun Kun LiRonald NgueleÎncă nu există evaluări
- Flash Calculation Stability PDFDocument12 paginiFlash Calculation Stability PDFAndre BecÎncă nu există evaluări
- Supercritical CO2 Extraction of Helichrysum ItalicumDocument8 paginiSupercritical CO2 Extraction of Helichrysum ItalicumTaipe SollerÎncă nu există evaluări
- Fluid Flow Measurement and Analysis ExperimentDocument11 paginiFluid Flow Measurement and Analysis ExperimentJohn TomlinsonÎncă nu există evaluări
- Simulation of The Aromatic Recovery Process by Extractive DistillationDocument5 paginiSimulation of The Aromatic Recovery Process by Extractive DistillationaegosmithÎncă nu există evaluări
- Computation of Phase and Chemical Equilibrium IIDocument8 paginiComputation of Phase and Chemical Equilibrium IIThou KanshieÎncă nu există evaluări
- Can Bang Long HoiDocument370 paginiCan Bang Long HoiKhanh Nguyen100% (1)
- Flash Calc 3Document16 paginiFlash Calc 3Mohamed MamdouhÎncă nu există evaluări
- Solution Thermodynamics: Theory: Fundamental Property RelationDocument6 paginiSolution Thermodynamics: Theory: Fundamental Property RelationLim Ying PeiÎncă nu există evaluări
- KU To CentiPoiseDocument4 paginiKU To CentiPoiseUna Si Ndéso100% (1)
- Hdpe Pipe Design PDFDocument27 paginiHdpe Pipe Design PDFAshley Dillon TothÎncă nu există evaluări
- Well AbandonmentDocument30 paginiWell Abandonmentsmithyry2014Încă nu există evaluări
- 1st Periodical Test in English 2019-2020Document10 pagini1st Periodical Test in English 2019-2020ginadel timanÎncă nu există evaluări
- Visualising Magnetic FieldsDocument171 paginiVisualising Magnetic FieldshieudtqnÎncă nu există evaluări
- Seminar MehkaDocument23 paginiSeminar MehkaRudi HermanaÎncă nu există evaluări
- EFT GeorgiDocument47 paginiEFT GeorgiMaurits TepaskeÎncă nu există evaluări
- Analyses of Fat Oil and Grease in Wastewater or Receiving WaterDocument26 paginiAnalyses of Fat Oil and Grease in Wastewater or Receiving WaterFlorin Sorin Bolboaca100% (1)
- Lec5 PDFDocument15 paginiLec5 PDFRaviÎncă nu există evaluări
- The Mechanism of The Leuckart Reaction PDFDocument12 paginiThe Mechanism of The Leuckart Reaction PDFatomoscoÎncă nu există evaluări
- OPSS 1202 Nov08Document10 paginiOPSS 1202 Nov08Safa GhasemkhaniÎncă nu există evaluări
- A Strategy To Determine A Heating CurveDocument8 paginiA Strategy To Determine A Heating CurveHatef HajianÎncă nu există evaluări
- High Power CycloconverterDocument7 paginiHigh Power Cycloconverternapster26Încă nu există evaluări
- Comet Mystery Boxes: Educator GuideDocument4 paginiComet Mystery Boxes: Educator GuideABD00LÎncă nu există evaluări
- Calculation of Natural Frequency of Earth Dams by Means of AnalytDocument7 paginiCalculation of Natural Frequency of Earth Dams by Means of AnalytNathan VincentÎncă nu există evaluări
- Handwheel To Road Wheel 730039V001Document79 paginiHandwheel To Road Wheel 730039V001Dinesh Maheshwari100% (1)
- Structural Analysis NotesDocument166 paginiStructural Analysis NotesdunyÎncă nu există evaluări
- Discussion Tray DryerDocument3 paginiDiscussion Tray DryerIskandar ZulkarnainÎncă nu există evaluări
- 10 Impedance TubeDocument17 pagini10 Impedance TubeMarco DanielÎncă nu există evaluări
- Self Healing ElectronicsDocument16 paginiSelf Healing ElectronicsVamsiKrishnaÎncă nu există evaluări
- XSteam Excel v2.6Document3 paginiXSteam Excel v2.6pchanycÎncă nu există evaluări
- A. Rothschild J Appl. Phys 2002Document9 paginiA. Rothschild J Appl. Phys 2002Johnny CrossÎncă nu există evaluări
- Equilibrium Stage Processes - Perpindahan MassaDocument30 paginiEquilibrium Stage Processes - Perpindahan Massaj0haNN3sÎncă nu există evaluări
- ASTM E824 Light Meter CalibrationDocument6 paginiASTM E824 Light Meter CalibrationreiazhÎncă nu există evaluări
- Validasi Spektrofotometer UV-VIS Pada Analisis ForDocument4 paginiValidasi Spektrofotometer UV-VIS Pada Analisis ForRizki Dyah RahmawatiÎncă nu există evaluări
- Guia Procedimiento Inspeccion MFL PDFDocument6 paginiGuia Procedimiento Inspeccion MFL PDFleonciomavarezÎncă nu există evaluări
- Muhammad Sahimi Heterogeneous Materials I LineaDocument793 paginiMuhammad Sahimi Heterogeneous Materials I LineadashatanliÎncă nu există evaluări
- The Settling ChamberDocument6 paginiThe Settling ChamberererdÎncă nu există evaluări
- Helmholtz Free Energy PDFDocument6 paginiHelmholtz Free Energy PDFDuà Zàhrä RìzvîÎncă nu există evaluări
- Foundation RockDocument8 paginiFoundation RockshashikantgaurÎncă nu există evaluări
- Knocking on Heaven's Door: How Physics and Scientific Thinking Illuminate the Universe and the Modern WorldDe la EverandKnocking on Heaven's Door: How Physics and Scientific Thinking Illuminate the Universe and the Modern WorldEvaluare: 3.5 din 5 stele3.5/5 (64)
- Hero Found: The Greatest POW Escape of the Vietnam WarDe la EverandHero Found: The Greatest POW Escape of the Vietnam WarEvaluare: 4 din 5 stele4/5 (19)
- Dark Matter and the Dinosaurs: The Astounding Interconnectedness of the UniverseDe la EverandDark Matter and the Dinosaurs: The Astounding Interconnectedness of the UniverseEvaluare: 3.5 din 5 stele3.5/5 (69)
- A Brief History of Time: From the Big Bang to Black HolesDe la EverandA Brief History of Time: From the Big Bang to Black HolesEvaluare: 4 din 5 stele4/5 (2193)
- Sully: The Untold Story Behind the Miracle on the HudsonDe la EverandSully: The Untold Story Behind the Miracle on the HudsonEvaluare: 4 din 5 stele4/5 (103)
- Summary and Interpretation of Reality TransurfingDe la EverandSummary and Interpretation of Reality TransurfingEvaluare: 5 din 5 stele5/5 (5)
- The End of Craving: Recovering the Lost Wisdom of Eating WellDe la EverandThe End of Craving: Recovering the Lost Wisdom of Eating WellEvaluare: 4.5 din 5 stele4.5/5 (82)
- The Future of Geography: How the Competition in Space Will Change Our WorldDe la EverandThe Future of Geography: How the Competition in Space Will Change Our WorldEvaluare: 4 din 5 stele4/5 (6)
- The Fabric of Civilization: How Textiles Made the WorldDe la EverandThe Fabric of Civilization: How Textiles Made the WorldEvaluare: 4.5 din 5 stele4.5/5 (58)
- A Beginner's Guide to Constructing the Universe: The Mathematical Archetypes of Nature, Art, and ScienceDe la EverandA Beginner's Guide to Constructing the Universe: The Mathematical Archetypes of Nature, Art, and ScienceEvaluare: 4 din 5 stele4/5 (51)
- Lost in Math: How Beauty Leads Physics AstrayDe la EverandLost in Math: How Beauty Leads Physics AstrayEvaluare: 4.5 din 5 stele4.5/5 (125)
- Quantum Spirituality: Science, Gnostic Mysticism, and Connecting with Source ConsciousnessDe la EverandQuantum Spirituality: Science, Gnostic Mysticism, and Connecting with Source ConsciousnessEvaluare: 4 din 5 stele4/5 (6)
- The Beekeeper's Lament: How One Man and Half a Billion Honey Bees Help Feed AmericaDe la EverandThe Beekeeper's Lament: How One Man and Half a Billion Honey Bees Help Feed AmericaÎncă nu există evaluări
- Dirt to Soil: One Family’s Journey into Regenerative AgricultureDe la EverandDirt to Soil: One Family’s Journey into Regenerative AgricultureEvaluare: 5 din 5 stele5/5 (125)
- Permaculture for the Rest of Us: Abundant Living on Less than an AcreDe la EverandPermaculture for the Rest of Us: Abundant Living on Less than an AcreEvaluare: 4.5 din 5 stele4.5/5 (33)
- When the Heavens Went on Sale: The Misfits and Geniuses Racing to Put Space Within ReachDe la EverandWhen the Heavens Went on Sale: The Misfits and Geniuses Racing to Put Space Within ReachEvaluare: 3.5 din 5 stele3.5/5 (6)
- System Error: Where Big Tech Went Wrong and How We Can RebootDe la EverandSystem Error: Where Big Tech Went Wrong and How We Can RebootÎncă nu există evaluări
- Four Battlegrounds: Power in the Age of Artificial IntelligenceDe la EverandFour Battlegrounds: Power in the Age of Artificial IntelligenceEvaluare: 5 din 5 stele5/5 (5)
- Midnight in Chernobyl: The Story of the World's Greatest Nuclear DisasterDe la EverandMidnight in Chernobyl: The Story of the World's Greatest Nuclear DisasterEvaluare: 4.5 din 5 stele4.5/5 (410)
- Fire on the Horizon: The Untold Story of the Gulf Oil DisasterDe la EverandFire on the Horizon: The Untold Story of the Gulf Oil DisasterÎncă nu există evaluări
- The End of Everything: (Astrophysically Speaking)De la EverandThe End of Everything: (Astrophysically Speaking)Evaluare: 4.5 din 5 stele4.5/5 (157)
- Mastering Logical Fallacies: The Definitive Guide to Flawless Rhetoric and Bulletproof LogicDe la EverandMastering Logical Fallacies: The Definitive Guide to Flawless Rhetoric and Bulletproof LogicEvaluare: 4 din 5 stele4/5 (91)
- Highest Duty: My Search for What Really MattersDe la EverandHighest Duty: My Search for What Really MattersÎncă nu există evaluări
- The Intel Trinity: How Robert Noyce, Gordon Moore, and Andy Grove Built the World's Most Important CompanyDe la EverandThe Intel Trinity: How Robert Noyce, Gordon Moore, and Andy Grove Built the World's Most Important CompanyÎncă nu există evaluări