Documente Academic
Documente Profesional
Documente Cultură
Xelanj Tofacitinib Yale Alopecia Baldness Psoriasis Arthritis Cure Jid2014260a DrAlanBauman
Încărcat de
Alan J Bauman MD0 evaluări0% au considerat acest document util (0 voturi)
337 vizualizări11 paginiTreatment for Baldness due to Alopecia Totalis/Universalis using off-label Xeljanz - Tofacitinib; Report from Yale [Craiglow, King 2014 JID].
Tofacitinib/Xeljanz is a treatment for rheumatoid arthritis that has been shown in a report from Yale to reverse baldness in an Alopecia Universalis/Totalis & Psoriasis patient. Tofacitinib/Xeljanz for this type of alopecia is an off-label use.
Drepturi de autor
© © All Rights Reserved
Formate disponibile
PDF, TXT sau citiți online pe Scribd
Partajați acest document
Partajați sau inserați document
Vi se pare util acest document?
Este necorespunzător acest conținut?
Raportați acest documentTreatment for Baldness due to Alopecia Totalis/Universalis using off-label Xeljanz - Tofacitinib; Report from Yale [Craiglow, King 2014 JID].
Tofacitinib/Xeljanz is a treatment for rheumatoid arthritis that has been shown in a report from Yale to reverse baldness in an Alopecia Universalis/Totalis & Psoriasis patient. Tofacitinib/Xeljanz for this type of alopecia is an off-label use.
Drepturi de autor:
© All Rights Reserved
Formate disponibile
Descărcați ca PDF, TXT sau citiți online pe Scribd
0 evaluări0% au considerat acest document util (0 voturi)
337 vizualizări11 paginiXelanj Tofacitinib Yale Alopecia Baldness Psoriasis Arthritis Cure Jid2014260a DrAlanBauman
Încărcat de
Alan J Bauman MDTreatment for Baldness due to Alopecia Totalis/Universalis using off-label Xeljanz - Tofacitinib; Report from Yale [Craiglow, King 2014 JID].
Tofacitinib/Xeljanz is a treatment for rheumatoid arthritis that has been shown in a report from Yale to reverse baldness in an Alopecia Universalis/Totalis & Psoriasis patient. Tofacitinib/Xeljanz for this type of alopecia is an off-label use.
Drepturi de autor:
© All Rights Reserved
Formate disponibile
Descărcați ca PDF, TXT sau citiți online pe Scribd
Sunteți pe pagina 1din 11
Accepted Article Preview: Published ahead of advance online publication
Killing Two Birds with One Stone: Oral Tofacitinib Reverses
Alopecia Universalis in a Patient with Plaque Psoriasis
Brittany G Craiglow, Brett A King
Cite this article as: Brittany G Craiglow, Brett A King, Killing Two Birds with
One Stone: Oral Tofacitinib Reverses Alopecia Universalis in a Patient with
Plaque Psoriasis, Journal of Investigative Dermatology accepted article preview 18
June 2014; doi: 10.1038/jid.2014.260.
This is a PDF le of an unedited peer-reviewed manuscript that has been accepted
for publication. NPG are providing this early version of the manuscript as a service
to our customers. The manuscript will undergo copyediting, typesetting and a proof
review before it is published in its nal form. Please note that during the production
process errors may be discovered which could affect the content, and all legal
disclaimers apply.
Accepted article preview online 18 June 2014
2014 The Society for Investigative Dermatology
Killing Two Birds with One Stone: Oral Tofacitinib Reverses Alopecia Universalis in a
Patient with Plaque Psoriasis
Brittany G. Craiglow, MD and Brett A. King, MD, PhD
Department of Dermatology, Yale University School of Medicine, New Haven, CT
Address correspondence to Brett King, MD, PhD, Department of Dermatology, Yale
University School of Medicine, PO Box 208059, New Haven, CT 06520, or email
brett.king@yale.edu
Funding support: None reported
Financial Disclosure: None
Keywords: tofacitinib, alopecia areata, alopecia universalis, psoriasis, JAK inhibitor
Word count: 982; references: 14; figures: 2
To the Editor:
The patient is a 25-year-old male who presented for evaluation and management of
plaque psoriasis, which had begun five years earlier. Treatment with topical
corticosteroids had not been particularly helpful, and due to increasing body surface area
2014 The Society for Investigative Dermatology
involvement of psoriasis, systemic therapy with adalimumab had been initiated one year
before. While clearing of psoriasis was experienced early in the course of adalimumab,
the improvement faded. In addition to psoriasis, the patient reported a history of alopecia
areata (AA) beginning around age two years, which progressed to alopecia universalis
(AU) by age 18 years. Treatment of the alopecia with topical corticosteroids had not
been effective, and he had received no other therapy for it. He had no family history of
either psoriasis or alopecia areata, and his past medical history was otherwise
unremarkable.
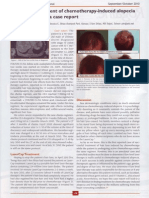
On examination, the patient demonstrated numerous well-marginated pink-red scaly
plaques on the scalp, torso, and elbows. He had no eyebrows, eyelashes (Figure 1a), or
facial hair and no hair on his arms, legs, torso, or in the axillae or groin. Notably, the
only prominent hair growth on the scalp was within areas of psoriasis (Figure 2a),
reminiscent of hair growth that occurs in areas of irritant contact dermatitis generated by
topical anthralin (Schmoeckel et al., 1979).
As treatment options for the patient were considered, we sought a therapeutic agent that
could potentially target both psoriasis and AU simultaneously. We identified tofacitinib
as a promising agent and began treatment.
Tofacitinib citrate (Xeljanz), formerly CP-690550 and tasocitinib, is a novel small
molecule selective Janus kinase 1/3 (JAK 1/3) inhibitor that was FDA-approved in late
2012 for the treatment of moderate to severe rheumatoid arthritis (RA). JAK inhibition
2014 The Society for Investigative Dermatology
has myriad effects on T-lymphocytes, and therefore it is not surprising that this
medication may be useful in the treatment of many inflammatory diseases (Pesu et al.,
2005). Indeed, both oral and topical formulations of tofacitinib have been demonstrated
to be safe and effective for the treatment of plaque psoriasis (Boy et al., 2009; Mamolo et
al., 2013; Papp et al., 2012; Ports et al., 2013; Strober et al., 2013), with additional
clinical trials for psoriasis and other inflammatory disorders now underway
(clinicaltrials.gov). In the case of psoriasis, increased interleukin-15 (IL-15) expression
is observed in lesional skin and may lead to epidermal hyperproliferation via keratinocyte
apoptotic resistance (Ruckert et al., 2000). Tofacitinib, which abrogates IL-15 signaling
(Johnston et al., 1995), might therefore be effective in psoriasis by normalizing
keratinocyte apoptosis (Ruckert et al., 2000).
In a murine model of AA, a Type I cytotoxic pathway has been demonstrated to be
responsible for the disease state, with NKG2D-expressing CD8+ cytolytic T-lymphocytes
identified as both necessary and sufficient for induction of disease. Upregulation of IL-
15 in the outer root sheath of the hair follicle activates cytolytic T-lymphocytes, which in
turn produce IFN, leading to activation of the hair follicle and upregulation of IL-15,
NKG2D ligands, and MHC molecules, all of which target the hair follicle for attack
(Jabbari et al., 2013). The same group has demonstrated that systemic treatment with the
JAK inhibitors tofacitinib and ruxolitinib (a JAK 1/2 inhibitor) prevents the onset of AA
in grafted AA mice (Jabbari et al., 2012, Dai et al., 2012) and that topical treatment
reverses AA in these mice (Jabbari et al., 2013). JAK 1/3 signaling mediates IL-15
activation of T-lymphocytes (Ghoreschi et al., 2011), explaining the success of these
2014 The Society for Investigative Dermatology
therapies.
After two months of tofacitinib 5 mg twice daily (the approved RA dose), there was some
improvement in psoriasis with partial hair regrowth on the scalp (Figure 2b) and face.
Given that higher doses of tofacitinib (up to 15 mg twice daily) have been shown to be
more effective in psoriasis (Papp et al., 2012), the dose was increased to 10 mg in the
morning and 5 mg at night. After three more months of therapy, there was complete
regrowth of the scalp hair (Figure 2c) along with significant regrowth of eyebrows,
eyelashes, and facial hair along with axillary and pubic hair. After 8 months of therapy,
there was full regrowth of hair at all body sites (Figures 1b and 2d), except for the arms
and legs, locations where the patient reports never having more than sparse hair growth
prior to the onset of AU. Improvement of the psoriasis has lagged behind reversal of the
alopecia, which is likely dose-related (Papp et al., 2012). While we considered
increasing the dose of tofacitinib, the patient is so pleased with the regrowth of his hair
(and is not particularly bothered by the remaining psoriasis) that he has chosen to
continue at the present dose. The patient has tolerated tofacitinib without subjective
complaints. Laboratory monitoring has revealed no abnormalities in serum creatinine,
electrolytes, glucose, complete blood count, hepatic function, or lipids.
To our knowledge, this is the first report of effective pathogenesis-based therapy for a
patient with alopecia universalis. While the results in this patient are provocative, a
clinical trial would more fully and systematically address the safety and efficacy of
tofacitinib and other JAK inhibitors in the treatment of AA and its variants. Aptly, an
2014 The Society for Investigative Dermatology
open label pilot study evaluating the efficacy of ruxolitinib in moderate to severe AA is
currently underway (Mackay-Wiggan, 2014). Given the potential for serious adverse
effects from oral JAK inhibitors, it would be particularly useful to explore the use of
topical formulations for these disorders.
The era of biological therapy has greatly enhanced the ability to make nuanced
therapeutic decisions. With every new agent, more thoughtful treatment algorithms
become possible, permitting better treatment of complex patients with multiple
comorbidities. As in the case presented herein, seemingly disparate diseases with
different pathomechanisms may be affected positively by a single agent. This case
highlights the interplay between advances in basic science and therapeutics and provides
a compelling example of the ways in which an increasingly complex understanding of
medicine and ingenuity in treatment benefit patients.
References:
Boy MG, Wang C, Wilkinson BE, et al. (2009) Double-blind, placebo-controlled, dose-
escalation study to evaluate the pharmacologic effect of CP-690,550 in patients with
psoriasis. J Invest Dermatol 129:2299-302.
2014 The Society for Investigative Dermatology
Dai Z, Xing L, Jabbari A, de Jong A, Christiano AM, Clynes R (2012) Treatment with
Ruxolitinib, an orally bioavailable JAK1/2 inhibitor, prevents the onset of alopecia areata
in C3H/HeJ mice. Immunology I: Adaptive Immunity Abstracts. J Invest Dermatol 132,
S97S107:S106.
Ghoreschi K, Jesson MI, Li X, et al. (2011) Modulation of innate and adaptive immune
responses by tofacitinib (CP-690,550). J Immunol 186:4234-43.
Jabbari A, Dai Z, Xing L, de Jong A, Christiano AM, Clynes R (2013) Reversal of
longstanding alopecia areata in C3H/HeJ mice using topical JAK inhibitors. 7th World
Congress for Hair Research Abstracts. J Invest Dermatol 133:1395.
Jabbari A, Dai Z, Xing L, de Jong A, Christiano AM, Clynes R (2012) Targeting of
JAK3 prevents onset of murine alopecia areata. Immunology I: Adaptive Immunity
Abstracts. J Invest Dermatol 132, S97S107:S104.
Johnston JA, Bacon CM, Finbloom DS, et al. (1995) Tyrosine phosphorylation and
activation of STAT5, STAT3, and Janus kinases by interleukins 2 and 15. Proc Natl
Acad Sci U S A 92:8705-9.
Mackay-Wiggan J. Columbia University Medical Center. An Open-Label Pilot Study to
Evaluate the Efficacy of Ruxolitinib in Moderate to Severe Alopecia Areata. In:
ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). 2000-
[cited 2014 Mar 16]. Available from: http://www.clinicaltrials.gov/show/NCT01950780
NLM Identifier: NCT01950780.
Mamolo C, Harness J, Tan H, et al. (2013) Tofacitinib (CP-690,550), an oral Janus
kinase inhibitor, improves patient-reported outcomes in a phase 2b, randomized, double-
blind, placebo-controlled study in patients with moderate-to-severe psoriasis. J Eur Acad
Dermatol Venereol.
Papp KA, Menter A, Strober B, et al. (2012) Efficacy and safety of tofacitinib, an oral
Janus kinase inhibitor, in the treatment of psoriasis: a Phase 2b randomized placebo-
controlled dose-ranging study. Br J Dermatol 167:668-77.
Pesu M, Candotti F, Husa M, et al. (2005) Jak3, severe combined immunodeficiency, and
a new class of immunosuppressive drugs. Immunol Rev 203:127-42.
Ports WC, Khan S, Lan S, et al. (2013) A randomized phase 2a efficacy and safety trial
of the topical Janus kinase inhibitor tofacitinib in the treatment of chronic plaque
psoriasis. Br J Dermatol 169:137-45.
Ruckert R, Asadullah K, Seifert M, et al. (2000) Inhibition of keratinocyte apoptosis by
IL-15: a new parameter in the pathogenesis of psoriasis? J Immunol 165:2240-50.
2014 The Society for Investigative Dermatology
Schmoeckel C, Weissmann I, Plewig G, et al. (1979) Treatment of alopecia areata by
anthralin-induced dermatitis. Arch Dermatol 115:1254-5.
Strober B, Buonanno M, Clark JD, et al. (2013) Effect of tofacitinib, a Janus kinase
inhibitor, on haematological parameters during 12 weeks of psoriasis treatment. Br J
Dermatol 169:992-9.
Figure legends:
Figure 1. Eyebrows and eyelashes at baseline (a) and after 8 months of therapy (b).
2014 The Society for Investigative Dermatology
Figure 2. Representative photographs of patients scalp at intervals of therapy.
2a. Scalp of patient at baseline. The only prominent hair growth is seen within psoriatic
plaques.
2b. Scalp after 2 months of therapy. Prominent hair growth is now visible outside of
areas affected by psoriasis. Psoriasis is beginning to recede in areas.
2c. Scalp after 5 months of therapy. There is near complete regrowth of scalp hair.
Psoriasis continues to be present but is significantly improved compared to baseline.
2d. Scalp after 8 months of therapy. There is complete regrowth of scalp hair.
2014 The Society for Investigative Dermatology
A
E F
B A
a
b
2014 The Society for Investigative Dermatology
a b c d
2014 The Society for Investigative Dermatology
S-ar putea să vă placă și
- Shoe Dog: A Memoir by the Creator of NikeDe la EverandShoe Dog: A Memoir by the Creator of NikeEvaluare: 4.5 din 5 stele4.5/5 (537)
- ISHRS 21st Abstract Book 2013 SanFran DR Alan Bauman FinalDocument542 paginiISHRS 21st Abstract Book 2013 SanFran DR Alan Bauman FinalAlan J Bauman MDÎncă nu există evaluări
- The Yellow House: A Memoir (2019 National Book Award Winner)De la EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Evaluare: 4 din 5 stele4/5 (98)
- Vampire PRP Hair Growth - South Florida Health Wellness - DR Alan BaumanDocument2 paginiVampire PRP Hair Growth - South Florida Health Wellness - DR Alan BaumanAlan J Bauman MD100% (1)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeDe la EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeEvaluare: 4 din 5 stele4/5 (5794)
- PRP Platelet Rich Plasma Hair Growth - DrBauman - PrimeDocument7 paginiPRP Platelet Rich Plasma Hair Growth - DrBauman - PrimeAlan J Bauman MDÎncă nu există evaluări
- LaserCap Use During Chemotherapy To Prevent Hair Loss - September/October 2010 - TK ShiaoDocument1 paginăLaserCap Use During Chemotherapy To Prevent Hair Loss - September/October 2010 - TK ShiaoAlan J Bauman MDÎncă nu există evaluări
- The Little Book of Hygge: Danish Secrets to Happy LivingDe la EverandThe Little Book of Hygge: Danish Secrets to Happy LivingEvaluare: 3.5 din 5 stele3.5/5 (400)
- Biphasic Dose Response in Low Level Light Therapy HarvardDocument26 paginiBiphasic Dose Response in Low Level Light Therapy HarvardMarcos Vinicios Borges GaldinoÎncă nu există evaluări
- Grit: The Power of Passion and PerseveranceDe la EverandGrit: The Power of Passion and PerseveranceEvaluare: 4 din 5 stele4/5 (588)
- ISHRS 20th Abstract Book 2012 Bahamas FinalDocument418 paginiISHRS 20th Abstract Book 2012 Bahamas FinalAlan J Bauman MDÎncă nu există evaluări
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureDe la EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureEvaluare: 4.5 din 5 stele4.5/5 (474)
- CeftriaxoneDocument2 paginiCeftriaxoneFlora Angeli PastoresÎncă nu există evaluări
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryDe la EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryEvaluare: 3.5 din 5 stele3.5/5 (231)
- Neuroshield ReportDocument2 paginiNeuroshield Reportrahim pathanÎncă nu există evaluări
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceDe la EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceEvaluare: 4 din 5 stele4/5 (895)
- Ijmrhs Vol 2 Issue 3Document399 paginiIjmrhs Vol 2 Issue 3editorijmrhs100% (1)
- Team of Rivals: The Political Genius of Abraham LincolnDe la EverandTeam of Rivals: The Political Genius of Abraham LincolnEvaluare: 4.5 din 5 stele4.5/5 (234)
- Tryptophan Supplement For SleepDocument35 paginiTryptophan Supplement For SleepZie Dak BlurÎncă nu există evaluări
- Never Split the Difference: Negotiating As If Your Life Depended On ItDe la EverandNever Split the Difference: Negotiating As If Your Life Depended On ItEvaluare: 4.5 din 5 stele4.5/5 (838)
- Approved Moh Guidelines On Submission of Documentation For Registration of Human Pharmaceutical Products RwandaDocument436 paginiApproved Moh Guidelines On Submission of Documentation For Registration of Human Pharmaceutical Products RwandaAry Bima WinardoÎncă nu există evaluări
- The Emperor of All Maladies: A Biography of CancerDe la EverandThe Emperor of All Maladies: A Biography of CancerEvaluare: 4.5 din 5 stele4.5/5 (271)
- Silver Is The New BlackDocument30 paginiSilver Is The New BlackSeptriyani KaswindiartiÎncă nu există evaluări
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaDe la EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaEvaluare: 4.5 din 5 stele4.5/5 (266)
- Decrease in Paco2 With Prone Position Is Predictive of Improved Outcome in Acute Respiratory Distress SyndromeDocument7 paginiDecrease in Paco2 With Prone Position Is Predictive of Improved Outcome in Acute Respiratory Distress SyndromedarwigÎncă nu există evaluări
- On Fire: The (Burning) Case for a Green New DealDe la EverandOn Fire: The (Burning) Case for a Green New DealEvaluare: 4 din 5 stele4/5 (74)
- Nabh Entry LevelDocument64 paginiNabh Entry LevelRenuka MuruganÎncă nu există evaluări
- Pharmacology Midterm Study GuideDocument17 paginiPharmacology Midterm Study GuidebkearnestÎncă nu există evaluări
- The Unwinding: An Inner History of the New AmericaDe la EverandThe Unwinding: An Inner History of the New AmericaEvaluare: 4 din 5 stele4/5 (45)
- MCQs 1Document10 paginiMCQs 1Mostafa MahmoudÎncă nu există evaluări
- Peripartum Bladder ManagementDocument6 paginiPeripartum Bladder ManagementYwagar YwagarÎncă nu există evaluări
- Biology SPMDocument17 paginiBiology SPMbloptra18Încă nu există evaluări
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersDe la EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersEvaluare: 4.5 din 5 stele4.5/5 (345)
- Physics of MRIDocument83 paginiPhysics of MRImishs14100% (1)
- Refeeding SyndromeDocument10 paginiRefeeding SyndromePhysiology by Dr RaghuveerÎncă nu există evaluări
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyDe la EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyEvaluare: 3.5 din 5 stele3.5/5 (2259)
- Mri Report - Left Knee Joint: Name Patient ID Accession No Age/Gender Referred by DateDocument2 paginiMri Report - Left Knee Joint: Name Patient ID Accession No Age/Gender Referred by Datefaiyaz432Încă nu există evaluări
- Sebaceous CystDocument4 paginiSebaceous CystristaniatauhidÎncă nu există evaluări
- Mission Urinalysis Strips InsertDocument1 paginăMission Urinalysis Strips Insertquirmche70Încă nu există evaluări
- Celiac Disease Comprehensive Cascade Test AlgorithmDocument1 paginăCeliac Disease Comprehensive Cascade Test Algorithmayub7walkerÎncă nu există evaluări
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreDe la EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreEvaluare: 4 din 5 stele4/5 (1090)
- Resume Mckenna JonesDocument1 paginăResume Mckenna Jonesapi-427456327Încă nu există evaluări
- Dorset County Hospital NHS Foundation TrustDocument1 paginăDorset County Hospital NHS Foundation TrustRika FitriaÎncă nu există evaluări
- Cardiovascular PharmacologyDocument77 paginiCardiovascular PharmacologyDhruva PatelÎncă nu există evaluări
- German Marine Agencies, Inc., Et Al. Petitioners, vs. Teodolah R. Caro, in Behalf of Her Husband Eduardo v. Caro, Respondent.Document5 paginiGerman Marine Agencies, Inc., Et Al. Petitioners, vs. Teodolah R. Caro, in Behalf of Her Husband Eduardo v. Caro, Respondent.Francis Coronel Jr.Încă nu există evaluări
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)De la EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Evaluare: 4.5 din 5 stele4.5/5 (121)
- Funny StoriesDocument5 paginiFunny Storiesamin jamalÎncă nu există evaluări
- Ahuja, Motiani - 2004 - Current and Evolving Issues in Transfusion PracticeDocument8 paginiAhuja, Motiani - 2004 - Current and Evolving Issues in Transfusion Practicesushmakumari009Încă nu există evaluări
- Case Study 103Document8 paginiCase Study 103Jonah MaasinÎncă nu există evaluări
- Pulmonary and Critical Care MedicineDocument48 paginiPulmonary and Critical Care MedicineCarlos HernándezÎncă nu există evaluări
- Saccharothrixsp Abh26 PDFDocument7 paginiSaccharothrixsp Abh26 PDFAlia RahmaÎncă nu există evaluări
- Sickle Cell Disease, Epidemiology, Genetic History, Complication and ManagementDocument52 paginiSickle Cell Disease, Epidemiology, Genetic History, Complication and ManagementAnastasiafynnÎncă nu există evaluări
- CE Strengths Based Nursing.24Document9 paginiCE Strengths Based Nursing.24THOHAROHÎncă nu există evaluări
- Her Body and Other Parties: StoriesDe la EverandHer Body and Other Parties: StoriesEvaluare: 4 din 5 stele4/5 (821)