Documente Academic
Documente Profesional
Documente Cultură
Unesco - Eolss Sample Chapters: Lysine Biosynthesis in Bacteria - An Unchartered Pathway For Novel Antibiotic Design
Încărcat de
Sheerin SulthanaTitlu original
Drepturi de autor
Formate disponibile
Partajați acest document
Partajați sau inserați document
Vi se pare util acest document?
Este necorespunzător acest conținut?
Raportați acest documentDrepturi de autor:
Formate disponibile
Unesco - Eolss Sample Chapters: Lysine Biosynthesis in Bacteria - An Unchartered Pathway For Novel Antibiotic Design
Încărcat de
Sheerin SulthanaDrepturi de autor:
Formate disponibile
U
N
E
S
C
O
E
O
L
S
S
S
A
M
P
L
E
C
H
A
P
T
E
R
S
BIOTECHNOLOGY Vol. XI - Lysine Biosynthesis in Bacteria An Unchartered Pathway for Novel Antibiotic Design - Con
Dogovski, Sarah C. Atkinson, Sudhir R. Dommaraju, Renwick C. J . Dobson, Matthew A. Perugini, Lilian Hor, Craig A. Huton, and
J uliet A. Gerrard
Encyclopedia of Life Support Systems (EOLSS)
LYSINE BIOSYNTHESIS IN BACTERIA AN UNCHARTERED
PATHWAY FOR NOVEL ANTIBIOTIC DESIGN
Con Dogovski, Sarah C. Atkinson, Sudhir R. Dommaraju, Renwick C. J. Dobson,
and Matthew A. Perugini
Department of Biochemistry and Molecular Biology, Bio21 Molecular Science and
Biotechnology Institute, University of Melbourne, Parkville, Victoria 3010, Australia
Lilian Hor
Department of Biochemistry and Molecular Biology, Bio21 Molecular Science and
Biotechnology Institute, University of Melbourne, Parkville, Victoria 3010, Australia
School of Chemistry, Bio21 Molecular Science and Biotechnology Institute, University
of Melbourne, Parkville, Victoria 3010, Australia
Craig A. Huton
School of Chemistry, Bio21 Molecular Science and Biotechnology Institute, University
of Melbourne, Parkville, Victoria 3010, Australia
Juliet A. Gerrard
School of Biological Sciences, University of Canterbury, Christchurch, New Zealand
Keywords: bacteria, antimicrobial, antibiotic, antibiotic resistance, lysine, meso-
diaminopimelate, lysine biosynthesis.
Contents
1. Introduction
2. Lysine Biosynthesis Pathway in Bacteria
3. Identification and Validation of Targets
4. Dihydrodipicolinate Synthase [DHDPS]
4.1 Function of DHDPS
4.2 Structure of DHDPS
4.3 Inhibition of DHDPS
5. Dihydrodipicolinate Reductase [DHDPR]
5.1. Function of DHDPR
5.2 Structure of DHDPR
5.3 Inhibition of DHDPR
6. Diaminopimelate (DAP) Epimerase
6.1 Function DAP epimerase
6.2 Structure of DAP epimerase
6.3 Inhibition of DAP epimerase
7. Conclusions
Glossary
Bibliography
Biographical Sketches
U
N
E
S
C
O
E
O
L
S
S
S
A
M
P
L
E
C
H
A
P
T
E
R
S
BIOTECHNOLOGY Vol. XI - Lysine Biosynthesis in Bacteria An Unchartered Pathway for Novel Antibiotic Design - Con
Dogovski, Sarah C. Atkinson, Sudhir R. Dommaraju, Renwick C. J . Dobson, Matthew A. Perugini, Lilian Hor, Craig A. Huton, and
J uliet A. Gerrard
Encyclopedia of Life Support Systems (EOLSS)
Summary
Antibiotics act by killing or inhibiting the growth of disease causing microorganisms.
Their mechanism of action often targets important microbial cellular processes,
including protein and cell wall synthesis. However, the effectiveness of many frontline
antimicrobials has become compromised through the emergence of antibiotic resistant
strains. Accordingly, there is a pressing need to develop novel antibiotics that target
previously unaffected biosynthetic pathways. This review describes lysine biosynthesis
in bacteria, an unchartered pathway for novel antibiotic design.
1. Introduction
The discovery of penicillin in 1929 by Alexander Fleming and subsequent development
by Howard Florey was one of the great achievements of the 20
th
century. The
development of other classes of antibiotics during the 1950s to 1970s allowed for the
treatment of many previously fatal diseases. Much recent scientific and media attention
has focused on the emerging resistance of bacteria to even our most powerful
antibiotics-of-last-resort, such as vancomycin. Although vancomycin had been used
for about 40 years without bacterial resistance, the rapidly increasing reports of resistant
strains, such as vancomycin-resistant enterococci (VRE) and vancomycin-resistant
Staphylococcus aureus (VRSA) highlight the need for continual development of new
antibacterial agents, ideally with a novel mode of action. Despite this dilemma, only a
small number of new antibiotics, such as linezolid and daptomycin, have been approved
in recent years, following a period of over two decades in which no new antibiotics
were developed. Accordingly, there is an urgent need to develop novel antimicrobials
and an equally urgent need to discover new antibiotic targets. One target of antibacterial
agents that has yet to be exploited clinically is the biosynthesis of the amino acid lysine,
and its immediate precursor meso-diaminopimelate (meso-DAP). Several publications
over the past decade have covered our increasing understanding of the enzymes of the
lysine biosynthetic pathway as antibiotic targets, and this review discusses progress
since 2003. The lysine biosynthetic pathway in plants and bacteria yields the de novo
synthesis of lysine for utilisation in protein synthesis. More importantly, lysine and
meso-DAP are vital constituents of the bacterial peptidoglycan cell wall in Gram-
positive and Gram-negative bacteria, respectively. Hence, inhibitors of the lysine
biosynthetic pathway may provide a new class of antibacterial agents through inhibition
of cell wall and/or protein synthesis. Additionally, mammals lack the ability to
biosynthesise lysine and it is therefore one of the 9 essential amino acids that must be
provided through a dietary source. The occurrence of the lysine biosynthetic pathway in
microorganisms and plants, but not in mammals, suggests that specific inhibitors of this
biosynthetic pathway may display novel antibacterial activity with low mammalian
toxicity.
2. Lysine Biosynthesis Pathway in Bacteria.
The biosynthetic pathway leading to meso-DAP and lysine production in bacteria is
known as the DAP-pathway (Figure 1). The initial steps toward the DAP-pathway
involve the conversion of aspartate 1 to aspartyl phosphate 2, with subsequent reduction
to aspartate semialdehyde (ASA) 3. These steps are common to lysine, threonine,
U
N
E
S
C
O
E
O
L
S
S
S
A
M
P
L
E
C
H
A
P
T
E
R
S
BIOTECHNOLOGY Vol. XI - Lysine Biosynthesis in Bacteria An Unchartered Pathway for Novel Antibiotic Design - Con
Dogovski, Sarah C. Atkinson, Sudhir R. Dommaraju, Renwick C. J . Dobson, Matthew A. Perugini, Lilian Hor, Craig A. Huton, and
J uliet A. Gerrard
Encyclopedia of Life Support Systems (EOLSS)
isoleucine and methionine biosynthesis. The first committed step towards lysine
biosynthesis is the conversion of ASA and pyruvate to dihydrodipicolinate (DHDP) 5,
catalysed by DHDP synthase (DHDPS). DHDP is then reduced to
tetrahydrodipicolinate (THDP) 6 by DHDP reductase (DHDPR). The majority of
bacterial species convert THDP to N-succinyl-2-amino-6-ketopimelate 7a, a process
catalysed by an N-succinyltransferase (succinylase pathway). Some Bacillus species,
including B. subtilis, incorporate an N-acetyl group rather than an N-succinyl group
(acetylase pathway). Yet another small group of species (again including some Bacillus
species) possess the enzyme meso-DAP dehydrogenase, which catalyses the direct
conversion of THDP 6 to meso-DAP 10, thereby shortcutting the predominant route by
several steps (dehydrogenase pathway). Some bacterial species can use multiple
pathways to synthesise lysine. For example, Corynebacterium glutamicium can
synthesise lysine by either the dehydrogenase or succinylase pathway, whilst Bacillus
macerans can employ enzymes of the dehydrogenase or acetylase pathways. However,
most species, including Escherichia coli, only utilise the succinyl pathway: N-succinyl-
2-amino-6-ketopimelate 7a is converted to N-succinyl-L,L-DAP 8a by an
aminotransferase, with subsequent deacylation and epimerisation then providing meso-
DAP 10, which is converted to lysine 11 by DAP decarboxylase. Interestingly, while
plants had been assumed to follow the same biosynthetic pathway as the majority of
bacteria, only recently has it been discovered that both plants and bacteria of the
Chlamydiales order lack the genes encoding for the dapD, dapC and dapE enzymes,
which catalyze reactions central in the pathway. In fact, plants and Chlamydia possess
the enzyme diaminopimelate aminotransferase that directly converts THDP 6 to
diaminopimelate 9, and hence synthesise lysine via a variant of the DAP pathway.
Figure 1 - Diaminopimelate pathway in bacteria.
3. Identification and validation of targets
While enzymes of the lysine biosynthetic pathway have been extensively studied and
U
N
E
S
C
O
E
O
L
S
S
S
A
M
P
L
E
C
H
A
P
T
E
R
S
BIOTECHNOLOGY Vol. XI - Lysine Biosynthesis in Bacteria An Unchartered Pathway for Novel Antibiotic Design - Con
Dogovski, Sarah C. Atkinson, Sudhir R. Dommaraju, Renwick C. J . Dobson, Matthew A. Perugini, Lilian Hor, Craig A. Huton, and
J uliet A. Gerrard
Encyclopedia of Life Support Systems (EOLSS)
reviewed, only recently have rapid advances in genomics allowed for validation of this
pathway as a viable target for antibiotics. The complete sequencing of numerous
bacterial genomes has allowed the identification and validation of specific antibiotic
targets in these species. Most essential genes are present throughout a wide range of
bacteria, and analyses of essential genes from various strains show remarkable
similarity. In the Salmonella genome 219 out of ~2200 genes have been shown to be
essential, and in the Bacillus subtilis genome 271 of ~4100 genes have been shown or
predicted to be essential. In the E. coli and S. aureus genomes, 620 from 4291 genes and
658 from 2588 genes are predicted to be essential, respectively. Most enzymes of the
lysine biosynthetic pathway are amongst those shown to be essential or necessary for
growth under normal conditions. The asd gene, encoding for aspartate semialdehyde
dehydrogenase (ASA-DH) has been shown to be essential in all strains analyzed. The
dapA, dapB, dapD, and dapE genes have been shown indirectly to be essential, or
predicted to be so, in all strains. These genes code for DHDPS, DHDPR, THDP N-
succinyl transferase, and LL-DAP desuccinylase, respectively. Additionally, the dapF
gene, encoding for DAP-epimerase, is essential or predicted to be essential in most
bacteria.
The lysA gene, encoding for DAP decarboxylase, is also essential under certain
conditions, with auxotrophs exhibiting attenuated growth, but is not absolutely essential
as most growth media contain sufficient lysine for growth. Genes in the DAP pathway
shown to be non-essential include dapC, encoding for N-succinyl-L,L-diaminopimelate
aminotransferase, and dapG/lysC, encoding for aspartokinase. The nonessential nature
of the dapC gene is consistent with the recent finding that N-acetylornithine
aminotransferase (ArgD, NAcO-AT) possesses significant DAP-aminotransferase
activity, such that dapC auxotrophs are still viable. Additionally, many bacterial strains
produce multiple isozymes capable of catalyzing the production of aspartyl phosphate.
Finally, the asd, dapA and dapB genes have been found to be essential and preserved in
over 80% of a diverse range of bacterial genomes.
Recent investigations of enzymes of the lysine biosynthetic pathway and progress
towards the development of inhibitors of these enzymes will now be discussed further,
focusing on three well characterized enzymes that have been validated as antibiotics
targets, namely dihydrodipicolinate synthase (DHDPS), dihydrodipicolinate reductase
(DHDPR), and diaminopimelate (DAP) epimerase.
4. Dihydrodipicolinate Synthase (DHDPS)
4.1 Function of DHDPS
DHDPS was first purified in 1965 from E. coli extracts. The enzyme catalyzes the
condensation of pyruvate and aspartate semialdehyde (ASA) to form HTPA. It was first
suggested that the product released by DHDPS was DHDP, but studies using
13
C-
labelled pyruvate support the view that the product is the unstable heterocycle HTPA.
Rapid decomposition of the
13
C-NMR signals of HTPA following its formation indicate
that formation of DHDP occurs via a nonenzymatic step.
In all cases examined the DHDPS-catalyzed reaction proceeds via a ping-pong kinetic
U
N
E
S
C
O
E
O
L
S
S
S
A
M
P
L
E
C
H
A
P
T
E
R
S
BIOTECHNOLOGY Vol. XI - Lysine Biosynthesis in Bacteria An Unchartered Pathway for Novel Antibiotic Design - Con
Dogovski, Sarah C. Atkinson, Sudhir R. Dommaraju, Renwick C. J . Dobson, Matthew A. Perugini, Lilian Hor, Craig A. Huton, and
J uliet A. Gerrard
Encyclopedia of Life Support Systems (EOLSS)
mechanism in which pyruvate binds the active site, followed by the release of a
protonated water molecule. ASA then binds to the active site and the product, HTPA, is
released.
In the first step of the mechanism, the active site lysine, (Lys161 in E. coli DHDPS)
forms a Schiff base with pyruvate (Figure 2). Formation of the Schiff base proceeds
through a tetrahedral intermediate. It is proposed that a catalytic triad of 3 residues -
Tyr133, Thr44 and Tyr107 - act as a proton relay to transfer protons to and from the
active site via a water-filled channel leading to bulk solvent. The Schiff base (imine) is
converted to its enamine form, which then adds to the aldehyde group of ASA. In
aqueous solution, ASA is known to exist in the hydrated form rather than the aldehyde,
but the biologically relevant form of the substrate remains to be determined. HTPA is
then formed by nucleophilic attack of the amino group of ASA onto the intermediate
imine, leading to cyclisation and detachment of the product from the enzyme, with
release of the active site lysine residue.
Figure 2 The catalytic mechanism of DHDPS.
4.1.1 Regulation of DHDPS activity
The activity of DHDPS is regulated by lysine in some organisms. Regulation involves
inhibition of the enzyme and has been investigated in several plant, Gram-negative and
Gram-positive bacterial species. Studies involving Triticum aestivium, Daucus carota
sativa, Spinacia aloeracea, Zea mays and Pivus sativum show that DHDPS from plant
species are generally strongly inhibited by lysine (IC
50
=0.01-0.05 mM). In contrast,
DHDPS from bacteria are significantly less sensitive to lysine inhibition than their plant
counterparts. DHDPS from Gram-negative bacteria such as E .coli, Bacillus sphaericus
U
N
E
S
C
O
E
O
L
S
S
S
A
M
P
L
E
C
H
A
P
T
E
R
S
BIOTECHNOLOGY Vol. XI - Lysine Biosynthesis in Bacteria An Unchartered Pathway for Novel Antibiotic Design - Con
Dogovski, Sarah C. Atkinson, Sudhir R. Dommaraju, Renwick C. J . Dobson, Matthew A. Perugini, Lilian Hor, Craig A. Huton, and
J uliet A. Gerrard
Encyclopedia of Life Support Systems (EOLSS)
and Methanobacterium thermoautotrophicum display IC
50
values that range from 0.25
mM to 1.0 mM. The enzyme from Gram-positive bacteria such as Bacillus
Lichenformis, Bacillus megaterium, Bacillus subtilis, Corynebacterium glutamicum,
Bacillus cereus, Lactobacillus plantarum and Staphylococcus aureus show little or no
inhibition by lysine.
The crystal structure of DHDPS in complex with lysine from E. coli shows that the
lysine binding site is situated in a crevice at the interface of the tight dimer, distal from
the active site, but connected to the active site by a water channel. Two inhibitory lysine
molecules are bound in close proximity in van der Waals contact to each other. Seven
residues located within the allosteric site bind lysine, namely Ala49, His53, His56,
Gly78, Asp80, Glu84, and Tyr106.
Studies show that lysine inhibition is cooperative; the second lysine molecule binds 10
5
times more tightly than the first. The mechanism by which lysine exerts regulatory
control over bacterial DHDPS is not well understood, although kinetic and structural
studies suggest that it is an allosteric inhibitor, causing partial inhibition (approximately
90%) at saturating concentrations. It has recently been suggested that lysine exerts some
effect on the first half reaction, affecting the proton-relay as well as the function of
Arg138, thought to be crucial for ASA binding. The crystal structure of the E. coli
DHDPS-lysine complex was solved in the absence of substrate; however,
thermodynamic studies have illustrated that the substrate pyruvate has a substantial
effect on the nature of enzyme-inhibitor association.
The mechanism of lysine inhibition has been investigated in DHDPS from wheat and
tobacco. Kinetic studies show that lysine is a non-competitive inhibitor relative to
pyruvate and a competitive inhibitor relative ASA. A structural study of DHDPS from
the plant species Nicotiana sylvestris shows that a significant change in conformation
occurs upon binding of lysine. Several residues involved in mediating inter-subunit
contact at the tight dimer interface are displaced when lysine is bound, thereby
dislocating the dimers in relation to each other. This translocation displaces the active
site residues Arg138 and Tyr133, which suggests inhibition may occur through
hindering coordination of the carboxyl group of ASA, thus preventing cyclisation of the
substrate. An equivalent conformational state is not observed in the lysine-bound
structure of DHDPS from E. coli. The role of ASA in the regulation of E. coli DHDPS
is not clearly defined. This is due to a number of conflicting studies reporting ASA
either does or does not mediate inhibition. Early reports of substrate inhibition in E. coli
DHDPS can be attributed to the quality of ASA preparations used in kinetic studies.
However, inhibition of DHDPS activity from Thermoanaerobacter tengcongensis,
Neisseria meningitidis, Bacillus anthracis and methicillin-resistant Staphylococcus
aureus (MRSA) has been observed with pure preparations of ASA.
4.2 Structure of DHDPS
4.2.1 Subunit and quaternary structure of DHDPS
DHDPS from E. coli, Mycobacterium tuberculosis, Thermotoga maritima,
Thermoanaerobacter tengcongensis and Bacillus anthracis and many other species is a
U
N
E
S
C
O
E
O
L
S
S
S
A
M
P
L
E
C
H
A
P
T
E
R
S
BIOTECHNOLOGY Vol. XI - Lysine Biosynthesis in Bacteria An Unchartered Pathway for Novel Antibiotic Design - Con
Dogovski, Sarah C. Atkinson, Sudhir R. Dommaraju, Renwick C. J . Dobson, Matthew A. Perugini, Lilian Hor, Craig A. Huton, and
J uliet A. Gerrard
Encyclopedia of Life Support Systems (EOLSS)
homotetramer in both crystal structure and solution (Figure 3). In E. coli, the monomer
is 292 amino acids in length and is composed of two domains. The N-terminal domain
is a (/)
8
TIM-barrel (E. coli DHDPS: residues 1-224) with the active site located
within the centre of the barrel (Figure 3). The C-terminal domain (residues 225-292)
consists of three -helices and contains several key residues that mediate contact at the
weak dimer interface.
Figure 3 - E. coli DHDPS structure. The active sites, allosteric sites, dimerization
interface (tight dimer interface) and tetramerization interface (weak dimer interface) are
shown.
The association of the four monomers leaves a large water-filled cavity in the centre of
the tetramer, such that each monomer has contacts with two neighbouring monomers
only. The tetramer is described as a dimer of dimers (monomers A and B and monomers
C and D, Figure 3) with strong interactions between the monomers A and B at the so-
called tight dimer interface and weaker interactions between the dimers at the weak
dimer interface (Figure 3).
4.2.2 Active site
The active site is located in cavities formed by the two monomers of the dimer. A long
solvent-accessible catalytic crevice with a depth of 10 is formed between -strands 4
and 5 of the barrel. Lys161, involved in Schiff-base formation is situated in the -barrel
near the catalytic triad of three residues, namely Tyr133, Thr44 and Tyr107, which act
as a proton shuttle transferring protons to and from the active site (Figure 4).
Thr44 is hydrogen bonded to both Tyr133 and Tyr107 and its position in the hydrogen-
bonding network may play a role in Schiff base formation and cyclisation. The dihedral
angles of Tyr107 fall in the disallowed region of the Ramachandran plot, suggesting an
important role in the enzymes function. It is believed to be involved in shuttling
protons between the active site and solvent. In contrast, Tyr133 plays an important role
in substrate binding, donating a proton to the Schiff base hydroxyl. It is also thought to
U
N
E
S
C
O
E
O
L
S
S
S
A
M
P
L
E
C
H
A
P
T
E
R
S
BIOTECHNOLOGY Vol. XI - Lysine Biosynthesis in Bacteria An Unchartered Pathway for Novel Antibiotic Design - Con
Dogovski, Sarah C. Atkinson, Sudhir R. Dommaraju, Renwick C. J . Dobson, Matthew A. Perugini, Lilian Hor, Craig A. Huton, and
J uliet A. Gerrard
Encyclopedia of Life Support Systems (EOLSS)
coordinate the attacking amino group of ASA, which requires the loss of a proton
subsequent to cyclisation. A marked reduction in activity is observed in single
substitution mutants, highlighting the importance of this catalytic triad.
Situated at the entrance to the active site, Arg138 is essential for ASA binding. In the E.
coli DHDPS structure, a hydrogen bond is formed between Arg138 and Tyr107 and a
water mediated hydrogen bond is formed between Arg138 and Tyr133. Arg138 is thus
also important for stabilization of the catalytic triad, both of which are highly conserved
in all DHDPS enzymes.
Figure 4 E. coli DHDPS active site illustrating the catalytic triad Thr44, Tyr133 and
Tyr107 interdigitating from the opposing monomer
-
-
-
TO ACCESS ALL THE 21 PAGES OF THIS CHAPTER,
Visit: http://www.eolss.net/Eolss-sampleAllChapter.aspx
Bibliography
Blickling, S., Beisel, H-G., Bozic, D., Knaeblein, J ., Laber, B., and R. Huber. (1997). Structure of
dihydrodipicolinate synthase of Nicotiana sylvestris reveals novel quaternary structure. J. Mol. Biol., 274,
608-621. [The first reported structure of a plant DHDPS enzyme which shows a distinct three dimensional
architecture to that of bacterial DHDPS].
Blickling, S., Renner, C., Laber, B., Pohlenz, H. D., Holak, T. A., and R. Huber. (1997). Reaction
mechanism of Escherichia coli dihydrodipicolinate synthase investigated by X-ray crystallography and
U
N
E
S
C
O
E
O
L
S
S
S
A
M
P
L
E
C
H
A
P
T
E
R
S
BIOTECHNOLOGY Vol. XI - Lysine Biosynthesis in Bacteria An Unchartered Pathway for Novel Antibiotic Design - Con
Dogovski, Sarah C. Atkinson, Sudhir R. Dommaraju, Renwick C. J . Dobson, Matthew A. Perugini, Lilian Hor, Craig A. Huton, and
J uliet A. Gerrard
Encyclopedia of Life Support Systems (EOLSS)
NMR spectroscopy. Biochemistry, 36, 24-33. [This article describes the reaction mechanism of DHDPS].
Boughton, B. A., Dobson, R. C. J ., Gerrard J . A., and C. A. Hutton. (2008). Conformationally constrained
diketopimelic acid analogues as inhibitors of dihydrodipicolinate synthase. Bioorg. Med. Chem. Lett., 18,
460463. [An article describing the development of novel inhibitors of DHDPS].
Caplan, J . F., Zheng, R., Blanchard, J . S., and J . C. Vederas. (2000). Vinylogous amide analogues of
diaminopimelic acid (DAP) as inhibitors of enzymes involved in bacterial lysine biosynthesis. Org. Lett.,
2, 3857-60. [A study describing the most potent inhibitor of bacterial DHDPR described to date].
Hutton, C. A, Perugini, M. A., and J . A. Gerrard, (2007) Inhibition of lysine biosynthesis: an evolving
antibiotic strategy, Mol. BioSyst., 2007, 3, 458465. [A current review describing lysine biosynthesis].
Kobayashi, K., Ehrlich, S. D., Albertini, A., Amati, G., Andersen, K. K., Arnaud, M., Asai, K., Ashikaga,
S., Aymerich, S., Bessieres, P., Boland, F., Brignell, S. C., Bron, S., Bunai, K., Chapuis, J ., Christiansen,
L. C., Danchin, A., Debarbouille, M., Dervyn, E., Deuerling, E., Devine, K., Devine, S. K., Dreesen, O.,
Errington, J ., Fillinger, S., Foster, S. J ., Fujita, Y., Galizzi, A., Gardan, R., Eschevins, C., Fukushima, T.,
Haga, K., Harwood, C. R., Hecker, M., Hosoya, D., Hullo, M. F., Kakeshita, H., Karamata, D., Kasahara,
Y., Kawamura, F., Koga, K., Koski, P., Kuwana, R., Imamura, D., Ishimaru, M., Ishikawa, S., Ishio, I.,
Le Coq, D., Masson, A., Mauel, C., Meima, R., Mellado, R. P., Moir, A., Moriya, S., Nagakawa, E.,
Nanamiya, H., Nakai, S., Nygaard, P., Ogura, M., Ohanan, T., O'Reilly, M., O'Rourke, M., Pragai, Z.,
Pooley, H. M., Rapoport, G., Rawlins, J . P., Stragier, P., Studer, R., Takamatsu, H., Tanaka, T., Takeuchi,
M., Thomaides, H. B., Vagner, V., van Dijl, J . M., Watabe, K., Wipat, A., Yamamoto, H., Yamamoto,
M., Yamamoto, Y., Yamane, K., Yata, K., Yoshida, K., Yoshikawa, H., Zuber, U., and N. Ogasawara.
(2003). Essential Bacillus subtilis genes. Proc. Natl. Acad. Sci. USA. 100, 4678-4683. [A survey of the
Bacillus subtilis genome that shows a number of genes of the DAP pathway are essential].
Lloyd, A. J ., Huyton, T., Turkenburg, J . and D. I. Roper. (2004). Refinement of Haemophilus influenzae
diaminopimelic acid epimerase (DapF) at 1.75 A resolution suggests a mechanism for stereocontrol
during catalysis. Acta Crystallographica Section D 60, 397-400. [An article describing the structure and
function of DAP epimerase].
Mirwaldt, C., I. Korndorfer, and R. Huber. (1995). The crystal structure of dihydrodipicolinate synthase
from Escherichia coli at 2.5 A resolution. J. Mol. Biol., 246, 227-239. [First reported crystal structure of
DHDPS from E. coli].
Reddy, S. G., Sacchettini, J . C., and J . S. Blanchard. (1995). Expression, purification, and characterization
of Escherichia coli dihydrodipicolinate reductase. Biochemistry, 34, 3492-501. [An article which reports
the kinetic characteristics of DHDPR from E. coli].
Scapin, G., Blanchard, J . S., and J . C. Sacchettini. (1995). Three-dimensional structure of Escherichia coli
dihydrodipicolinate reductase. Biochemistry, 34, 3502-12. [This article reports the first crystal structure of
DHDPR].
Wiseman, J . S., and J . S. Nichols. (1984). Purification and properties of diaminopimelic acid epimerase
from Escherichia coli. J. Biol. Chem., 259, 8907-8914. [A article outlining the isolation and
characterization of DAP epimerase from E. coli].
Biographical Sketches
Dr Con Dogovski received his Ph.D. after completing his graduate studies in the Department of
Microbiology and Immunology, The University of Melbourne, Australia. During this period, his primary
research interests centered on studying the structure and function of bacterial integral membrane proteins.
Since then, he has worked within the Australian Department of Defence and private industry. Con is
currently located at the Department of Biochemistry and Molecular Biology, Bio21 Molecular Science
and Biotechnology Institute, The University of Melbourne. His research covers a broad range of areas
including, microbiology, molecular biology, protein chemistry, and biochemistry. More specifically, his
interests involve studying enzymes involved in the synthesis of meso DAP and lysine and the
development novel antimicrobials targeting several important human pathogens.
Sarah Atkinson is a 2
nd
year Ph.D. student at the University of Melbourne, with interests in molecular
biology, enzymology and structural biology. Her Ph.D. project focuses on understanding the structure-
U
N
E
S
C
O
E
O
L
S
S
S
A
M
P
L
E
C
H
A
P
T
E
R
S
BIOTECHNOLOGY Vol. XI - Lysine Biosynthesis in Bacteria An Unchartered Pathway for Novel Antibiotic Design - Con
Dogovski, Sarah C. Atkinson, Sudhir R. Dommaraju, Renwick C. J . Dobson, Matthew A. Perugini, Lilian Hor, Craig A. Huton, and
J uliet A. Gerrard
Encyclopedia of Life Support Systems (EOLSS)
function and inhibition of bacterial and plant DHDPS which is described in detail in this review. She also
plans to adopt an in silico drug design approach to her project with the potential of yielding lead
antimicrobial inhibitors targeting DHDPS. Sarah completed an undergraduate Bachelor of Science degree
at the University of Melbourne 2004-2006, before embarking on a successful biochemistry Honours (4th
year undergraduate) project in 2007. Her work focused on the DHDPS from the pathogen Clostridium
botulinum. Upon completing of her graduate studies, Sarah aspires to stay in biochemical research,
expanding her knowledge of structural biology.
Sudhir Dommaraju is a 3
rd
year Ph.D. student at the University of Melbourne, with interests in
bioinformatics, protein chemistry and microbiology. His Ph.D. project focuses on structural and
mechanistic characterization of bacterial DHDPR, a potential antimicrobial target.
Sudhir holds a Masters degree in medical microbiology from Manipal Academy of Higher Education,
India and a post graduate Diploma in Bioinformatics. Prior to the commencement of his Ph.D project in
2006, he worked as a scientist at the Institute of Bioinformatics, India and was involved in the
bioinformatic analysis and annotation of various human proteins, as part of a HUPO initiative project.
Upon completion of his Ph.D., Sudhir aspires to work on drug discovery projects that utilise his skill base
in protein chemistry and bioinformatics.
Lilian Hor completed her undergraduate Bachelor of Science (Honours) and Bachelor of Information
Systems degrees at the University of Melbourne. She is currently a 2
nd
year Ph.D. student at the
University of Melbourne with an interest in medicinal chemistry, enzymology and structural biology.
Lilians Ph.D. project focuses on understanding the structure-function relationships of bacterial DAP
epimerase, which is described in detail in this review. She also plans to adopt a rational drug design
approach to her project with the potential of yielding lead antimicrobial inhibitors targeting DAP
epimerase.
Dr Craig Hutton undertook his undergraduate and Ph.D. degrees at the University of Adelaide (Prof.
Christopher J . Easton, 1994) before completing postdoctoral studies at the University of California,
Berkeley (Prof. Paul A. Bartlett) and The University of Melbourne (Australian Postdoctoral Fellow). He
was appointed to his first independent academic position in 1999 in the School of Chemistry at The
University of Sydney, before returning to The University of Melbourne in 2003 as a Senior Lecturer. In
2005 his laboratory relocated to the newly-opened Bio21 Molecular Science and Biotechnology Institute
(Bio21 Institute), where he heads a group comprised of approximately a dozen research staff, graduate
and undergraduate students. His research interests include the design and synthesis of inhibitors of
enzymes in the lysine biosynthetic pathway, novel methods for the asymmetric synthesis of amino acids,
and the construction of cross-linked amyloid peptides associated with Alzheimers disease.
Dr Hutton also serves as an Associate Editor for the Australian J ournal of Chemistry, and on a number of
local committees including as secretary of the Royal Australian Chemical Institute (RACI) Victorian
branch (organic group) and the RACI/University of Melbourne Annual Synthesis Symposium organizing
committee.
Professor Juliet Gerrard trained at Oxford University, where she completed an Honours degree in
Chemistry and a DPhil in Biological Chemistry. In 1993, she was appointed as a research scientist at Crop
& Food Research Ltd, NZ, where her multidisciplinary research portfolio included a substantial element
of applied research in the food science area. She was appointed as a Lecturer in Biochemistry at the
University of Canterbury in 1998, promoted to Senior Lecturer in 2000, Assoc. Professor in 2004, and
Professor in 2006. J uliets research is interdisciplinary, cutting across biochemistry, chemistry, health,
agricultural and food science and biomaterial design. It also incorporates a full spectrum of applied and
fundamental research. At present, a major focus is the understanding of the quaternary structure of
proteins and the implications that this has for evolution of oligomeric proteins. This research has potential
application in the design of novel therapeutic agents and also in the assembly of novel materials. J uliet
has published over 90 papers in international refereed journals, several book chapters and three books,
two of which have been translated into other languages. She won a National Teaching Award for
Sustained Excellence in Tertiary Teaching in 2004 and is currently on the Editorial Board of Process
Biochemistry and an Academic Editor for PLoSONE. As the Principal Investigator on several major
research grants J uliet leads a productive research laboratory currently comprising 15 members; the team is
U
N
E
S
C
O
E
O
L
S
S
S
A
M
P
L
E
C
H
A
P
T
E
R
S
BIOTECHNOLOGY Vol. XI - Lysine Biosynthesis in Bacteria An Unchartered Pathway for Novel Antibiotic Design - Con
Dogovski, Sarah C. Atkinson, Sudhir R. Dommaraju, Renwick C. J . Dobson, Matthew A. Perugini, Lilian Hor, Craig A. Huton, and
J uliet A. Gerrard
Encyclopedia of Life Support Systems (EOLSS)
supplemented by national and international collaborators.
Dr Renwick Dobson is currently a C.R. Roper Senior Research Fellow in the Department of
Biochemistry and Molecular Biology at the University of Melbourne. He received in Ph.D. at the
University of Canterbury in 2004. His research is interdisciplinary, cutting across protein structural
biology, chemistry, enzymology and biochemistry. His current field of interest is the relationship between
protein structure and function, with a focus on the enzymology of lysine biosynthesis in bacteria. In
particular, he is interested in understanding the mechanisms that underpin function of the enzyme
DHDPS. This work is significant, since the enzyme is a potential drug target. Dr Dobsons goal is the
rational design of inhibitors of DHDPS, which is possible via a thorough knowledge of enzymes
structure and function.
An active member of the scientific community, Dr Dobson is on the organising committee of the biannual
Biomolecular Dynamics and Interactions 2009 (BDI2009) Symposium (Bio21, UOM). He is a current
member of the Royal Society of New Zealand, New Zealand Institute of Chemistry (NZIC), New Zealand
and Australian Societies of Biochemistry and Molecular Biology (NZSBMB & ASBMB), and the Society
of Crystallographers in Australia and New Zealand (SCANZ). Dr Dobson regularly referees manuscripts
for international journals, including Acta Crystallogr. D, Acta Crystallogr. F, J. Agric. Food Chem.,
Biochem, J., Biochemistry and ChemBioChem.
Dr Matt Perugini completed both his undergraduate BSc(Hons) degree in the 1990s and his Ph.D. at the
University of Melbourne in the Department of Biochemistry and Molecular Biology in 2002.
In October 2004, he was awarded the Young Biophysicists Award (YBA) by the Australian Society for
Biophysics, largely for his contribution to the field of analytical ultracentrifugation; and then in 2005, Dr
Perugini was awarded an Australian Postdoctoral Fellowship (APD) by the Australian Research Council.
In the same year, he moved to the new Bio21 Molecular Science and Biotechnology Institute (Bio21
Institute) in Parkville, where he heads a laboratory comprised of more than a dozen research staff,
graduate students, and undergraduate (Honours) students. His research focuses on novel antibiotic design
targeting key enzymes of the lysine biosynthesis pathway of pathogenic bacteria and the more esoteric
aim of unravelling the molecular evolution in quaternary structure of these enzyme targets, including
DHDPS. As a result of his recent research activities, Dr Perugini was awarded the prestigious 2007
Applied Biosystems Edman Award by the Australian Society for Biochemistry and Molecular Biology.
Dr Perugini also serves on a number of local committees, including the Lorne Conference on Protein
Structure and Function and the Australasian Proteomics Society organization committees. In addition, he
has forged strong collaborations with local and international scientists focusing on the characterization of
protein structure and function, and the quantitation of protein-protein and protein-ligand interactions.
These collaborations are yielding exciting results that have provided insight into the structure and
function of several protein systems, including bacterial DHDPS, DHDPR and DAP epimerase, which are
described in detail in this EOLSS publication.
S-ar putea să vă placă și
- Reaction of ATCC 15697 To Oxidative Stress: Bifidobacterium Longum Subsp. Infantis StrainDocument15 paginiReaction of ATCC 15697 To Oxidative Stress: Bifidobacterium Longum Subsp. Infantis StrainMohammed SherifÎncă nu există evaluări
- GeLC-MS-based Proteomics of Chromobacterium Violaceum Comparison of Proteome Changes Elicited by Hydrogen PeroxidDocument12 paginiGeLC-MS-based Proteomics of Chromobacterium Violaceum Comparison of Proteome Changes Elicited by Hydrogen PeroxidLeidaiany SantosÎncă nu există evaluări
- 10 1016@j Ijbiomac 2020 05 135Document33 pagini10 1016@j Ijbiomac 2020 05 135MariÎncă nu există evaluări
- Zii 3122Document10 paginiZii 3122Muhammad Ricky RamadhianÎncă nu există evaluări
- LECTURA 1. Nicolaidis et al 2013. Resistance to antibiotics targeted to the bacterial cell wallDocument17 paginiLECTURA 1. Nicolaidis et al 2013. Resistance to antibiotics targeted to the bacterial cell wallAlmendra MosqueraÎncă nu există evaluări
- Study PlanDocument29 paginiStudy PlanMuhammad QasimÎncă nu există evaluări
- Colloids and Surfaces B: Biointerfaces: SciencedirectDocument9 paginiColloids and Surfaces B: Biointerfaces: SciencedirectMark AñazcoÎncă nu există evaluări
- Journal of Bacteriology-1983-Darveau-831.fullDocument8 paginiJournal of Bacteriology-1983-Darveau-831.fullanggi marlianaÎncă nu există evaluări
- Pharmaceuticals: Antimicrobial Peptides in 2014Document28 paginiPharmaceuticals: Antimicrobial Peptides in 2014Hermeson LimaÎncă nu există evaluări
- Taxonomy and Important Features of Probiotic Microorganisms in Food and NutritionDocument9 paginiTaxonomy and Important Features of Probiotic Microorganisms in Food and NutritionDhayu Mart Hindrasyah PandiaÎncă nu există evaluări
- EScholarship UC Item 372400z3Document24 paginiEScholarship UC Item 372400z3deslyati putri br sitepuÎncă nu există evaluări
- Mitochondrial Complex 1is Important For Plant Tolerance To Fungal Biotic StressDocument11 paginiMitochondrial Complex 1is Important For Plant Tolerance To Fungal Biotic StressSryahwa PublicationsÎncă nu există evaluări
- MC3 Group 5 Quiz #2Document6 paginiMC3 Group 5 Quiz #2Earl BenedictÎncă nu există evaluări
- Antioxidant Properties and Redox-Modulating Activity of ChitosanDocument9 paginiAntioxidant Properties and Redox-Modulating Activity of ChitosanGustavo RuizÎncă nu există evaluări
- Bioorganic & Medicinal Chemistry Letters: ArticleinfoDocument8 paginiBioorganic & Medicinal Chemistry Letters: ArticleinfoMehari AsratÎncă nu există evaluări
- Polya MineDocument10 paginiPolya MineLaili AlfinaÎncă nu există evaluări
- TMP 9 E27Document16 paginiTMP 9 E27FrontiersÎncă nu există evaluări
- CVBLIMDocument8 paginiCVBLIMfialophora0210Încă nu există evaluări
- A Culture-Independent Approach To Unravel Uncultured Bacteria and Functional Genes in A Complex Microbial CommunityDocument11 paginiA Culture-Independent Approach To Unravel Uncultured Bacteria and Functional Genes in A Complex Microbial CommunityBekele OljiraÎncă nu există evaluări
- Hylin DNA BBreportsDocument12 paginiHylin DNA BBreportsRamonquimicoÎncă nu există evaluări
- Metabolismo e MicrobiotaDocument14 paginiMetabolismo e MicrobiotaLívia Cristina Lima Dos SantosÎncă nu există evaluări
- Antibiotic Resistance Mechanisms in Bacteria: Biochemical and Genetic AspectsDocument11 paginiAntibiotic Resistance Mechanisms in Bacteria: Biochemical and Genetic AspectsD Wisam NajmÎncă nu există evaluări
- 2011 The LuxS-Dependent Quorum Sensing System Regulates Early Biofilm Formation by Streptococcus Pneumoniae Strain D39Document42 pagini2011 The LuxS-Dependent Quorum Sensing System Regulates Early Biofilm Formation by Streptococcus Pneumoniae Strain D39hazyaÎncă nu există evaluări
- Bioconversion of anthocyanins by probioticsDocument9 paginiBioconversion of anthocyanins by probioticsAnastasya AmandaÎncă nu există evaluări
- Activitty 1 LaboratoryDocument12 paginiActivitty 1 LaboratoryDarwin CauilanÎncă nu există evaluări
- Bioorganic & Medicinal ChemistryDocument6 paginiBioorganic & Medicinal ChemistryRuthaiwan KongcharoenÎncă nu există evaluări
- Planktocyclin, A Cyclooctapeptide Protease Inhibitor Produced by The Freshwater Cyanobacterium Planktothrix RubescensDocument5 paginiPlanktocyclin, A Cyclooctapeptide Protease Inhibitor Produced by The Freshwater Cyanobacterium Planktothrix RubescensJordana KalineÎncă nu există evaluări
- Full download book Recent Trends In Carbohydrate Chemistry Synthesis And Biomedical Applications Of Glycans And Glycoconjugates Pdf pdfDocument41 paginiFull download book Recent Trends In Carbohydrate Chemistry Synthesis And Biomedical Applications Of Glycans And Glycoconjugates Pdf pdfgeorge.berger158100% (18)
- Research Paper Pseudomonas AeruginosaDocument5 paginiResearch Paper Pseudomonas Aeruginosagatewivojez3100% (1)
- Production and Purification of Bacillus anthracis Protective AntigenDocument9 paginiProduction and Purification of Bacillus anthracis Protective AntigendyahTechÎncă nu există evaluări
- Caima2022 - Estabilidad y Actividad Biológica Mejoradas de Bacterioruberina en Nanovesículas MacrofagosDocument11 paginiCaima2022 - Estabilidad y Actividad Biológica Mejoradas de Bacterioruberina en Nanovesículas MacrofagosMark AñazcoÎncă nu există evaluări
- 1 s2.0 S0009308422000020 MainDocument10 pagini1 s2.0 S0009308422000020 MainHanan AqoubÎncă nu există evaluări
- 09campylobacter IDocument69 pagini09campylobacter Ianon_914901469Încă nu există evaluări
- Transcriptomic Analysis of The Under Oxidative Stress: Levilactobacillus Brevis 47f StrainDocument9 paginiTranscriptomic Analysis of The Under Oxidative Stress: Levilactobacillus Brevis 47f StrainMohammed SherifÎncă nu există evaluări
- Bacillus Subtillis PHDocument10 paginiBacillus Subtillis PHanon_816002920Încă nu există evaluări
- Bacterio Fag OsDocument11 paginiBacterio Fag OsTatiana CarrilloÎncă nu există evaluări
- Novel Biodemulsifier of Bacillus Mojavensis XH1 - Oxalate Decarboxylase With The Potential For Demulsification of Oilfield EmulsionDocument59 paginiNovel Biodemulsifier of Bacillus Mojavensis XH1 - Oxalate Decarboxylase With The Potential For Demulsification of Oilfield EmulsionMohamed AlhayaniÎncă nu există evaluări
- Bacterial LipasesDocument35 paginiBacterial LipasesLaura-Mihaela ChisÎncă nu există evaluări
- 2015-Appl Environ MicrobiolDocument9 pagini2015-Appl Environ MicrobiolMiguel Fernandez NiñoÎncă nu există evaluări
- Structural and Biological Aspects of Carotenoid CleavageDocument13 paginiStructural and Biological Aspects of Carotenoid CleavageMinh Chau NguyenÎncă nu există evaluări
- Biofilm Rpos PeroxidoDocument8 paginiBiofilm Rpos PeroxidoOmar MoralesÎncă nu există evaluări
- The Antioxidant Response of The Liver of Male SwisDocument7 paginiThe Antioxidant Response of The Liver of Male SwisLara Ayu LestariÎncă nu există evaluări
- Anaerobic Bacteriology: Clinical and Laboratory PracticeDe la EverandAnaerobic Bacteriology: Clinical and Laboratory PracticeÎncă nu există evaluări
- Oxidative Metabolism CanovaDocument13 paginiOxidative Metabolism CanovaA Sreenivasa ReddyÎncă nu există evaluări
- Aurantimonadaceae-Phylogenomics and Signature Proteins For The Alpha ProteobacteriaDocument20 paginiAurantimonadaceae-Phylogenomics and Signature Proteins For The Alpha ProteobacteriaAndrea EscobarÎncă nu există evaluări
- Article 1 PDFDocument19 paginiArticle 1 PDFAnonymous 5bgTK7qzsÎncă nu există evaluări
- Artigo 3Document6 paginiArtigo 3Uiara MariaÎncă nu există evaluări
- Cmls 2001 58 1650 65Document17 paginiCmls 2001 58 1650 65Nadia khalizaÎncă nu există evaluări
- Review: From Natural Product To Marketed Drug: The Tiacumicin OdysseyDocument14 paginiReview: From Natural Product To Marketed Drug: The Tiacumicin OdysseyJosué VelázquezÎncă nu există evaluări
- PQ 0403001990Document6 paginiPQ 0403001990Yuwono WibowoÎncă nu există evaluări
- Du Preez Et Al., 2008Document12 paginiDu Preez Et Al., 2008Linda ZommereÎncă nu există evaluări
- Campylobacter Jejuni Biofilms Up-Regulated in The Absence of The Stringent Response Utilize A Calcofluor White-Reactive PolysaccharideDocument11 paginiCampylobacter Jejuni Biofilms Up-Regulated in The Absence of The Stringent Response Utilize A Calcofluor White-Reactive PolysaccharideMichaelÎncă nu există evaluări
- Periodontology 2000 - 2022 - Pussinen - Periodontitis and Cardiometabolic Disorders The Role of Lipopolysaccharide andDocument22 paginiPeriodontology 2000 - 2022 - Pussinen - Periodontitis and Cardiometabolic Disorders The Role of Lipopolysaccharide andDr.Reshma RajendranÎncă nu există evaluări
- Said AhmedDocument19 paginiSaid Ahmedahmed mahdiÎncă nu există evaluări
- Metabolism and Interactions: The Chemistry and Biology of Compounds Containing Amino SugarsDe la EverandMetabolism and Interactions: The Chemistry and Biology of Compounds Containing Amino SugarsEndre A. BalazsÎncă nu există evaluări
- Fluid Mechanics, Bernoulli's Principle and Equation of ContinuityDocument10 paginiFluid Mechanics, Bernoulli's Principle and Equation of ContinuitySheerin SulthanaÎncă nu există evaluări
- J. Gen. Appl. Microbiol., 15, 387-398 (1969)Document12 paginiJ. Gen. Appl. Microbiol., 15, 387-398 (1969)Sheerin SulthanaÎncă nu există evaluări
- Trade-Related Intellectual Property Rights (Trips)Document20 paginiTrade-Related Intellectual Property Rights (Trips)Sheerin SulthanaÎncă nu există evaluări
- 32721Document33 pagini32721prasadbheemÎncă nu există evaluări
- Appl. Microbiol. 1968 Kato 1200 6Document8 paginiAppl. Microbiol. 1968 Kato 1200 6Sheerin SulthanaÎncă nu există evaluări
- Bioethics Timeline and ControversiesDocument18 paginiBioethics Timeline and ControversiesSheerin SulthanaÎncă nu există evaluări
- Human Genome ProjectDocument57 paginiHuman Genome ProjectDhaval ParekhÎncă nu există evaluări
- BCH440 (8) 32-33Document20 paginiBCH440 (8) 32-33Sheerin SulthanaÎncă nu există evaluări
- A Brief Review of Plants Having Anti Cancer PropertyDocument8 paginiA Brief Review of Plants Having Anti Cancer PropertysharammaÎncă nu există evaluări
- Chapter 26 Phylogeny and The Tree of LifeDocument41 paginiChapter 26 Phylogeny and The Tree of LifeSheerin Sulthana100% (1)
- Trade-Related Intellectual Property Rights (Trips)Document20 paginiTrade-Related Intellectual Property Rights (Trips)Sheerin SulthanaÎncă nu există evaluări
- A Business-Oriented Overview of Intellectual Property For Law StudentsDocument16 paginiA Business-Oriented Overview of Intellectual Property For Law StudentsSheerin SulthanaÎncă nu există evaluări
- Internet and NetworkingDocument42 paginiInternet and Networkingآصف رضاÎncă nu există evaluări
- Analysis On The Impact of Madrid Protocol For The Economies of Developing CountriesDocument62 paginiAnalysis On The Impact of Madrid Protocol For The Economies of Developing CountriesSheerin Sulthana0% (1)
- Radioimmunoassay: Advanced Research Techniques in Basic Medical SciencesDocument23 paginiRadioimmunoassay: Advanced Research Techniques in Basic Medical SciencesKiran FriendooÎncă nu există evaluări
- Biological Safety Levels: Endia Ford Lori Gladney Izabella OsakweDocument34 paginiBiological Safety Levels: Endia Ford Lori Gladney Izabella OsakweSheerin Sulthana100% (1)
- ATCCDocument3 paginiATCCSheerin SulthanaÎncă nu există evaluări
- Elements of Biosafety: Janet Peterson Jpeterso@accmail - Umd.edu 405-3975Document28 paginiElements of Biosafety: Janet Peterson Jpeterso@accmail - Umd.edu 405-3975Sheerin SulthanaÎncă nu există evaluări
- Dimensions of Product Quality ManagementDocument5 paginiDimensions of Product Quality ManagementSheerin SulthanaÎncă nu există evaluări
- Lecture 21 - Respiratory SystemDocument59 paginiLecture 21 - Respiratory SystemSheerin SulthanaÎncă nu există evaluări
- The Range of Quality: Quality of Design Quality of Materials Quality of ProductsDocument4 paginiThe Range of Quality: Quality of Design Quality of Materials Quality of ProductsKin SkyeÎncă nu există evaluări
- Foodborne IllnessDocument13 paginiFoodborne IllnessSheerin SulthanaÎncă nu există evaluări
- Biological DatabaseDocument19 paginiBiological DatabaseMahesh Yadav100% (8)
- Foods Poli AgeDocument23 paginiFoods Poli AgeSheerin SulthanaÎncă nu există evaluări
- Pricing ProblemsDocument2 paginiPricing ProblemsSheerin SulthanaÎncă nu există evaluări
- 06iso Allo Idio09Document18 pagini06iso Allo Idio09Sheerin SulthanaÎncă nu există evaluări
- Emerging: Brewing Is The Production ofDocument16 paginiEmerging: Brewing Is The Production ofSheerin SulthanaÎncă nu există evaluări
- 10 1371-Journal Pone 0065654 g003Document1 pagină10 1371-Journal Pone 0065654 g003Sheerin SulthanaÎncă nu există evaluări
- (WWW - Entrance Exam - Net) Animal BiotechDocument34 pagini(WWW - Entrance Exam - Net) Animal BiotechSheerin SulthanaÎncă nu există evaluări
- Antinfek 10HDocument20 paginiAntinfek 10Hmistytewest100% (1)
- 10th Standard Tamilnadu State Board Science (English Medium)Document283 pagini10th Standard Tamilnadu State Board Science (English Medium)Karthick NÎncă nu există evaluări
- 30395-Article Text-56938-1-10-20220916Document8 pagini30395-Article Text-56938-1-10-20220916Djim KARYOMÎncă nu există evaluări
- Chapter 2 Medical Terminology Verified AnswersDocument5 paginiChapter 2 Medical Terminology Verified AnswersGregg ProducerÎncă nu există evaluări
- Transport Phenomena in Nervous System PDFDocument538 paginiTransport Phenomena in Nervous System PDFUdayanidhi RÎncă nu există evaluări
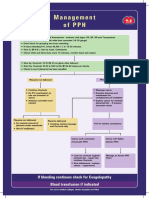
- Management of PPHDocument1 paginăManagement of PPH098 U.KARTHIK SARAVANA KANTHÎncă nu există evaluări
- 4 Factors Effecting Shelf Life and Storage Life of Fresh FishDocument26 pagini4 Factors Effecting Shelf Life and Storage Life of Fresh FishRafidah Zakaria100% (1)
- HistamineDocument16 paginiHistaminevg03ik2Încă nu există evaluări
- Cell Membrane: A Selective BarrierDocument23 paginiCell Membrane: A Selective BarrierHama JamalÎncă nu există evaluări
- Stress BreakerDocument7 paginiStress BreakerEshan Verma33% (3)
- Kingdom Protista: Diverse Eukaryotic MicroorganismsDocument79 paginiKingdom Protista: Diverse Eukaryotic MicroorganismsMeah PachecoÎncă nu există evaluări
- Adolescent Reproductive and Sexual HealthDocument42 paginiAdolescent Reproductive and Sexual HealthMuhammad Abbas WaliÎncă nu există evaluări
- 0610 IECR P3 Q1 v2 PDFDocument9 pagini0610 IECR P3 Q1 v2 PDFGAIA Educators - Home TuitionÎncă nu există evaluări
- Egg Quality Manual Web PDFDocument44 paginiEgg Quality Manual Web PDFVeterinario La EsperanzaÎncă nu există evaluări
- Classification and Characterization of Microsatellite Instability Across 18 Cancer TypesDocument13 paginiClassification and Characterization of Microsatellite Instability Across 18 Cancer TypeshadymatrixÎncă nu există evaluări
- AP Psychology Mnomonic DevicesDocument7 paginiAP Psychology Mnomonic DevicesBellony SandersÎncă nu există evaluări
- Introspect For DealersDocument13 paginiIntrospect For DealersOBERON-INTROSPECT-BIOSPECTÎncă nu există evaluări
- Molecular Cloning Technical GuideDocument40 paginiMolecular Cloning Technical GuideRÎncă nu există evaluări
- MR Spectroscopy: Technical Aspects and Clinical ApplicationsDocument5 paginiMR Spectroscopy: Technical Aspects and Clinical ApplicationsNotariana Kusuma AÎncă nu există evaluări
- Structure of Tooth m121Document13 paginiStructure of Tooth m121amirhossein vaeziÎncă nu există evaluări
- Biology Investigatory ProjectDocument17 paginiBiology Investigatory ProjectAnmol Dhungel100% (6)
- 4.1 - Cell Cycle Part 1Document5 pagini4.1 - Cell Cycle Part 1Deomar Joseph ParadoÎncă nu există evaluări
- Plant Transport - IGCSE Biology Notes (2020)Document6 paginiPlant Transport - IGCSE Biology Notes (2020)Zhi En LeeÎncă nu există evaluări
- Self Concept Inventory Hand OutDocument2 paginiSelf Concept Inventory Hand OutHarold LowryÎncă nu există evaluări
- Ewh Ix PDFDocument80 paginiEwh Ix PDFOR Premium FreeÎncă nu există evaluări
- Thesis Olof HerttingDocument57 paginiThesis Olof HerttingAjay IyerÎncă nu există evaluări
- Daftar PustakaDocument3 paginiDaftar PustakaAdnanÎncă nu există evaluări
- Noscapine - British PharmacopoeiaDocument3 paginiNoscapine - British PharmacopoeiaSocial Service (V)Încă nu există evaluări
- Theories of Tooth MovementDocument11 paginiTheories of Tooth MovementAhmedsy Ahmedsy AhmedsyÎncă nu există evaluări
- Short Periods of Incubation During Egg Storage - SPIDESDocument11 paginiShort Periods of Incubation During Egg Storage - SPIDESPravin SamyÎncă nu există evaluări