Documente Academic
Documente Profesional
Documente Cultură
Lam IV Udine
Încărcat de
MariusNeicuDrepturi de autor
Formate disponibile
Partajați acest document
Partajați sau inserați document
Vi se pare util acest document?
Este necorespunzător acest conținut?
Raportați acest documentDrepturi de autor:
Formate disponibile
Lam IV Udine
Încărcat de
MariusNeicuDrepturi de autor:
Formate disponibile
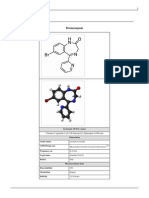
Lamivudine
1
Lamivudine
"3TC" redirects here. For the three-coach British electric trains sometimes known as 3TC, see British Rail Class 438.
Lamivudine
Systematic (IUPAC) name
4-amino-1-[(2R,5S)-2-(hydroxymethyl)-1,3-oxathiolan-5-yl]-1,2-dihydropyrimidin-2-one
Clinical data
AHFS/Drugs.com
monograph
[1]
MedlinePlus
a696011
[2]
Pregnancy cat. B3 (AU) C (US)
Legal status POM (UK) -only (US)
Routes Oral
Pharmacokinetic data
Bioavailability 86%
Protein binding Less than 36%
Half-life 5 to 7 hours
Excretion Renal (circa 70%)
Identifiers
CAS number
134678-17-4
[3]
ATC code
J05AF05
[4]
PubChem
CID 73339
[5]
DrugBank
DB00709
[6]
ChemSpider
66068
[7]
UNII
2T8Q726O95
[8]
KEGG
D00353
[9]
Lamivudine
2
ChEMBL
CHEMBL141
[10]
NIAID ChemDB
000388
[11]
Synonyms L-2,3-dideoxy-3-thiacytidine
PDB ligand ID
3TC (PDBe
[12]
, RCSB PDB
[13]
)
Chemical data
Formula C
8
H
11
N
3
O
3
S
Mol. mass 229.26 g/mol
(what is this?) (verify)
[14]
Lamivudine (2,3-dideoxy-3-thiacytidine, commonly called 3TC) is a potent nucleoside analog reverse
transcriptase inhibitor (nRTI). It is marketed by GlaxoSmithKline with the brand names Zeffix, Heptovir, Epivir,
and Epivir-HBV.
Lamivudine has been used for treatment of chronic hepatitis B at a lower dose than for treatment of HIV. It improves
the seroconversion of e-antigen positive hepatitis B and also improves histology staging of the liver. Long term use
of lamivudine unfortunately leads to emergence of a resistant hepatitis B virus (YMDD) mutant. Despite this,
lamivudine is still used widely as it is well tolerated.
It is on the World Health Organization's List of Essential Medicines, a list of the most important medication needed
in a basic health system.
Mechanism of action
Lamivudine is an analogue of cytidine. It can inhibit both types (1 and 2) of HIV reverse transcriptase and also the
reverse transcriptase of hepatitis B. It is phosphorylated to active metabolites that compete for incorporation into
viral DNA. They inhibit the HIV reverse transcriptase enzyme competitively and act as a chain terminator of DNA
synthesis. The lack of a 3'-OH group in the incorporated nucleoside analogue prevents the formation of the 5' to 3'
phosphodiester linkage essential for DNA chain elongation, and therefore, the viral DNA growth is terminated.
Lamivudine is administered orally, and it is rapidly absorbed with a bio-availability of over 80%. Some research
suggests that lamivudine can cross the bloodbrain barrier. Lamivudine is often given in combination with
zidovudine, with which it is highly synergistic. Lamivudine treatment has been shown to restore zidovudine
sensitivity of previously resistant HIV. Lamivudine showed no evidence of carcinogenicity or mutagenicity in in
vivo studies in mice and rats at doses from 10 to 58 times those used in humans.
Resistance
See also: resistance mutation
In HIV, high level resistance is associated with the M184V/I mutation in the reverse transcriptase gene as first
reported by Raymond Schinazi's group at Emory University. GlaxoSmithKline claimed that the M184V mutation
reduces "viral fitness", because of the finding that continued lamivudine treatment causes the HIV viral load to
rebound but at a much lower level, and that withdrawal of lamivudine results in a higher viral load rebound with
rapid loss of the M184V mutation; GSK therefore argued that there may be benefit in continuing lamivudine
treatment even in the presence of high level resistance, because the resistant virus is "less fit". The COLATE study
has suggested that there is no benefit to continuing lamivudine treatment in patients with lamivudine resistance. A
better explanation of the data is that lamivudine continues to have a partial anti-viral effect even in the presence of
the M184V mutation.
Lamivudine
3
In hepatitis B, lamivudine resistance was first described in the YMDD locus of the HBV reverse transcriptase gene.
The HBV reverse transcriptase gene is 344 amino acids long and occupies codons 349 to 692 on the viral genome.
The most commonly encountered resistance mutations are M204V/I/S.
[15]
The change in amino acid sequence from
YMDD to YIDD results in a 3.2 fold reduction in the error rate of the reverse transcriptase, which correlates with a
significant growth disadvantage of the virus. Other resistance mutations are L80V/I, V173L and L180M.
History
Racemic BCH-189 (the minus form is known as Lamivudine) was invented by Dr. Bernard Belleau while at work at
McGill University and Dr Paul Nguyen-Ba at the Montreal-based IAF BioChem International, Inc. laboratories in
1988 and the minus enantiomer isolated in 1989. Dr. Yung-Chi Cheng of Yale University in collaboration with R.F.
Schinazi and D.C. Liotta first reported the anti-hepatitis B virus (HBV) activity of Lamivudine in cell culture which
eventually led to the first oral antiviral agent for the treatment of HBV. Subsequently the group at Emory University
headed by Dr. Dennis C. Liotta Dr. Woo-Baeg Choi and Dr. Raymond F. Schinazi developed a synthesis for the
BCH-189 that gave exclusively the beta-enantiomers. They then went on to resolve the two enantiomers and
demonstrated that the antiviral activity at non-toxic concentrations resided in the (-)-enantiomer, now called
Lamivudine. The Emory patents to lamivudine were later invalidated by the original inventors. The drug's
effectiveness for treating HIV in combination with AZT was discovered accidentally when a patient took Zidovudine
secretly while in a clinical trial of Lamivudine monotherapy. The drug was later licensed to the British
pharmaceutical company Glaxo by Biochem Pharma (now Shire Pharmaceuticals) for a 14 percent royalty.
GlaxoSmithKline subsequently ceded the product to its ViiV Healthcare joint venture in 2009.
Lamivudine was approved by the Food and Drug Administration (FDA) on November 17, 1995 for use with
zidovudine (AZT) and again in 2002 as a once-a-day dosed medication. The fifth antiretroviral drug on the market, it
was the last NRTI for three years while the approval process switched to protease inhibitors. According to the
manufacturer's 2004 annual report, its patent will expire in the United States in 2010 and in Europe in 2011.
Presentation
Epivir 150mg or 300mg tablets (GlaxoSmithKline; US and UK) for the treatment of HIV;
Epivir-HBV 100mg tablets (GlaxoSmithKline; US only) for the treatment of hepatitis B;
Zeffix 100mg tablets (GlaxoSmithKline; UK only) for the treatment of hepatitis B.
3TC 150mg tablets (GlaxoSmithKline; South Africa) for the treatment of HIV;
Lamivudine is also available in fixed combinations with other HIV drugs:
Combivir (with zidovudine);
Epzicom/Kivexa (with abacavir);
Trizivir (with zidovudine and abacavir)
Lamivudine
4
References
[1] http:/ / www. drugs. com/ monograph/ lamivudine.html
[2] http:/ / www. nlm. nih.gov/ medlineplus/ druginfo/ meds/ a696011. html
[3] http:/ / www. nlm. nih.gov/ cgi/ mesh/ 2009/ MB_cgi?term=134678-17-4& rn=1
[4] http:/ / www. whocc.no/ atc_ddd_index/ ?code=J05AF05
[5] http:/ / pubchem. ncbi. nlm.nih. gov/ summary/ summary. cgi?cid=73339
[6] http:/ / www. drugbank. ca/ drugs/ DB00709
[7] http:/ / www. chemspider.com/ Chemical-Structure.66068. html
[8] http:/ / fdasis.nlm. nih. gov/ srs/ srsdirect. jsp?regno=2T8Q726O95
[9] http:/ / www. kegg. jp/ entry/ D00353
[10] https:/ / www.ebi.ac. uk/ chembldb/ index.php/ compound/ inspect/ CHEMBL141
[11] http:/ / chemdb. niaid. nih.gov/ CompoundDetails. aspx?AIDSNO=000388
[12] http:/ / www.ebi. ac.uk/ pdbe-srv/ PDBeXplore/ ligand/ ?ligand=3TC
[13] http:/ / www.rcsb. org/ pdb/ search/ smartSubquery.do?smartSearchSubtype=ChemCompIdQuery& chemCompId=3TC&
polymericType=Any
[14] http:/ / en. wikipedia. org/ w/ index. php?title=Special:ComparePages& rev1=477168974& page2=Lamivudine
[15] http:/ / hivdb. stanford. edu/ index. html Stanford University Drug Resistance Database.
External links
Epivir (http:/ / www. viivhealthcare. com/ products/ epivir-3tc. aspx?sc_lang=en) (manufacturer's website)
Article Sources and Contributors
5
Article Sources and Contributors
Lamivudine Source: http://en.wikipedia.org/w/index.php?oldid=615872195 Contributors: A2-33, Alansohn, Aliwal2012, Amortias, Anaxial, Andries, Arcadian, Beetstra, Ben.c.roberts,
BruceSwanson, Carlo Banez, ChemNerd, DMacks, Daevatgl, Deli nk, Digitaldeepak, Einstoned, Fvasconcellos, Gak, Graham87, Gubernator, Hazard-SJ, Hoffmeier, Hopping, Identifier, JWBE,
Japanese Searobin, Jfdwolff, Jmh649, J, Kajasudhakarababu, Khoikhoi, Louisajb, MarinaVladivostok, MastCell, Meewam, Michael Hardy, Mort459, Mykhal, Naltang, Oneoffedit, Operative67,
OwenBlacker, P-kun80, Pashihiko, Piano non troppo, Redrose64, Rjwilmsi, Rodasmith, Rschinazi, Sedmic, Selket, Thickslab, Tony1, Trezatium, Vaccinationist, Volvariella, Wawawemn,
Wikipediatastic, , 62 anonymous edits
Image Sources, Licenses and Contributors
File:Lamivudine structure.svg Source: http://en.wikipedia.org/w/index.php?title=File:Lamivudine_structure.svg License: Public Domain Contributors: User:Vaccinationist
File:Lamivudine ball-and-stick.png Source: http://en.wikipedia.org/w/index.php?title=File:Lamivudine_ball-and-stick.png License: Creative Commons Zero Contributors: MarinaVladivostok
File:Yes check.svg Source: http://en.wikipedia.org/w/index.php?title=File:Yes_check.svg License: Public Domain Contributors: Anomie
File:X mark.svg Source: http://en.wikipedia.org/w/index.php?title=File:X_mark.svg License: Public Domain Contributors: User:Gmaxwell
License
Creative Commons Attribution-Share Alike 3.0
//creativecommons.org/licenses/by-sa/3.0/
S-ar putea să vă placă și
- Never Split the Difference: Negotiating As If Your Life Depended On ItDe la EverandNever Split the Difference: Negotiating As If Your Life Depended On ItEvaluare: 4.5 din 5 stele4.5/5 (838)
- Pharmaco KineticsDocument14 paginiPharmaco KineticsMariusNeicuÎncă nu există evaluări
- Shoe Dog: A Memoir by the Creator of NikeDe la EverandShoe Dog: A Memoir by the Creator of NikeEvaluare: 4.5 din 5 stele4.5/5 (537)
- AbacavirDocument5 paginiAbacavirMariusNeicuÎncă nu există evaluări
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeDe la EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeEvaluare: 4 din 5 stele4/5 (5794)
- Leonhard EulerDocument15 paginiLeonhard EulerMariusNeicuÎncă nu există evaluări
- The Yellow House: A Memoir (2019 National Book Award Winner)De la EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Evaluare: 4 din 5 stele4/5 (98)
- VenlafaxineDocument12 paginiVenlafaxineMariusNeicuÎncă nu există evaluări
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceDe la EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceEvaluare: 4 din 5 stele4/5 (894)
- BromazepamDocument6 paginiBromazepamMariusNeicuÎncă nu există evaluări
- The Little Book of Hygge: Danish Secrets to Happy LivingDe la EverandThe Little Book of Hygge: Danish Secrets to Happy LivingEvaluare: 3.5 din 5 stele3.5/5 (399)
- OlanzapineDocument12 paginiOlanzapineMariusNeicuÎncă nu există evaluări
- LorazepamDocument13 paginiLorazepamMariusNeicuÎncă nu există evaluări
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureDe la EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureEvaluare: 4.5 din 5 stele4.5/5 (474)
- Queti A PineDocument12 paginiQueti A PineMariusNeicuÎncă nu există evaluări
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryDe la EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryEvaluare: 3.5 din 5 stele3.5/5 (231)
- AmoxicillinDocument8 paginiAmoxicillinMariusNeicuÎncă nu există evaluări
- Grit: The Power of Passion and PerseveranceDe la EverandGrit: The Power of Passion and PerseveranceEvaluare: 4 din 5 stele4/5 (587)
- Levo Me PromazineDocument4 paginiLevo Me PromazineMariusNeicuÎncă nu există evaluări
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaDe la EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaEvaluare: 4.5 din 5 stele4.5/5 (265)
- AmpicillinDocument4 paginiAmpicillinMariusNeicuÎncă nu există evaluări
- Aspirin Paracetamol CaffeineDocument3 paginiAspirin Paracetamol CaffeineMariusNeicuÎncă nu există evaluări
- On Fire: The (Burning) Case for a Green New DealDe la EverandOn Fire: The (Burning) Case for a Green New DealEvaluare: 4 din 5 stele4/5 (73)
- HaloperidolDocument12 paginiHaloperidolMariusNeicuÎncă nu există evaluări
- The Emperor of All Maladies: A Biography of CancerDe la EverandThe Emperor of All Maladies: A Biography of CancerEvaluare: 4.5 din 5 stele4.5/5 (271)
- Malnutrition in Critical Illness and Beyond A Narrative Review PDFDocument9 paginiMalnutrition in Critical Illness and Beyond A Narrative Review PDFEsteban DavidÎncă nu există evaluări
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersDe la EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersEvaluare: 4.5 din 5 stele4.5/5 (344)
- Hodgkin LymphomaDocument44 paginiHodgkin LymphomaisnineÎncă nu există evaluări
- The Unwinding: An Inner History of the New AmericaDe la EverandThe Unwinding: An Inner History of the New AmericaEvaluare: 4 din 5 stele4/5 (45)
- NCP For SchizoDocument6 paginiNCP For SchizoGILIANNE MARIE JIMENEAÎncă nu există evaluări
- Team of Rivals: The Political Genius of Abraham LincolnDe la EverandTeam of Rivals: The Political Genius of Abraham LincolnEvaluare: 4.5 din 5 stele4.5/5 (234)
- AtherosclerosisDocument7 paginiAtherosclerosisFaris Mufid Madyaputra100% (1)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyDe la EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyEvaluare: 3.5 din 5 stele3.5/5 (2219)
- Endocrine System: Capillary Glucose MonitoringDocument34 paginiEndocrine System: Capillary Glucose Monitoringjoel david knda mj100% (1)
- Comparative Efficacy of Non-Sedating Antihistamine Updosing in Patients With Chronic UrticariaDocument6 paginiComparative Efficacy of Non-Sedating Antihistamine Updosing in Patients With Chronic UrticariadregleavÎncă nu există evaluări
- Cannabis-An IntroductionDocument5 paginiCannabis-An IntroductionSaleha TariqÎncă nu există evaluări
- Complications After CXLDocument3 paginiComplications After CXLDr. Jérôme C. VryghemÎncă nu există evaluări
- 328 IndexDocument29 pagini328 IndexDafi SanÎncă nu există evaluări
- A J B P R: Sian Ournal of Iochemical and Harmaceutical EsearchDocument5 paginiA J B P R: Sian Ournal of Iochemical and Harmaceutical EsearchNAVNEET BAGGAÎncă nu există evaluări
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreDe la EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreEvaluare: 4 din 5 stele4/5 (1090)
- BiopharmaceuticsDocument52 paginiBiopharmaceuticsDharma ShantiniÎncă nu există evaluări
- What Is ScabiesDocument7 paginiWhat Is ScabiesKenÎncă nu există evaluări
- Manage Ophthalmia NeonatorumDocument2 paginiManage Ophthalmia NeonatorumEjay BautistaÎncă nu există evaluări
- Assessment of The Intermediate Care Unit Triage SystemDocument6 paginiAssessment of The Intermediate Care Unit Triage SystemJHÎncă nu există evaluări
- Gender-Dysphoric-Incongruene Persons, Guidelines JCEM 2017Document35 paginiGender-Dysphoric-Incongruene Persons, Guidelines JCEM 2017Manel EMÎncă nu există evaluări
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)De la EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Evaluare: 4.5 din 5 stele4.5/5 (119)
- 2018 Conference AbstractsDocument155 pagini2018 Conference AbstractsBanin AbadiÎncă nu există evaluări
- German Gov't Bombshell - Alarming Number of Vaccinated Are Developing AIDS' - News PunchDocument8 paginiGerman Gov't Bombshell - Alarming Number of Vaccinated Are Developing AIDS' - News PunchKarla VegaÎncă nu există evaluări
- Psychiatric Assessment ToolDocument53 paginiPsychiatric Assessment ToolLori100% (4)
- SECOND YEAR PHARMD Syllabus PU PDFDocument14 paginiSECOND YEAR PHARMD Syllabus PU PDFRIYA ROYÎncă nu există evaluări
- Early Identification and Remediation For Infants With Poor SuckDocument10 paginiEarly Identification and Remediation For Infants With Poor SuckMara CadinoiuÎncă nu există evaluări
- Role of IV Meropenem in Current EraDocument38 paginiRole of IV Meropenem in Current EraImtiyaz Alam SahilÎncă nu există evaluări
- ThoracentesisDocument4 paginiThoracentesisCyntia Theresia Lumintang100% (1)
- History and P.E. of The Integumentary SystemDocument6 paginiHistory and P.E. of The Integumentary SystempazucenaÎncă nu există evaluări
- Kuisioner Nutrisi Mini Nutritional AssessmentDocument1 paginăKuisioner Nutrisi Mini Nutritional AssessmentNaufal AhmadÎncă nu există evaluări
- Jawaban UASDocument3 paginiJawaban UASJaclin Awuy SalembaÎncă nu există evaluări
- Case StudyDocument6 paginiCase StudyMattÎncă nu există evaluări
- Chapter 38 - Pediatric and Geriatric HematologyDocument3 paginiChapter 38 - Pediatric and Geriatric HematologyNathaniel Sim100% (2)
- Her Body and Other Parties: StoriesDe la EverandHer Body and Other Parties: StoriesEvaluare: 4 din 5 stele4/5 (821)
- Bench Marking of Operation Theatre ProcessDocument13 paginiBench Marking of Operation Theatre ProcessApollo Institute of Hospital AdministrationÎncă nu există evaluări
- Thesis On HPV VaccineDocument8 paginiThesis On HPV Vaccinegjftqhnp100% (2)
- ECG Localization of Culprit Artery in Acute Myocardial InfarctionDocument104 paginiECG Localization of Culprit Artery in Acute Myocardial Infarctionginaul100% (1)