Documente Academic
Documente Profesional
Documente Cultură
Încărcat de
Bằng SakerDescriere originală:
Titlu original
Drepturi de autor
Formate disponibile
Partajați acest document
Partajați sau inserați document
Vi se pare util acest document?
Este necorespunzător acest conținut?
Raportați acest documentDrepturi de autor:
Formate disponibile
Încărcat de
Bằng SakerDrepturi de autor:
Formate disponibile
* Corresponding author. Tel.
: +213 55 333 4904
E-mail addresses: zaizto@gmail.com (T. Zaiz), lanezhafnaoui@gmail.com (H.Lanez).
2013 IJ CPS. All rights reserved.
ASPEN HYSYS SIMULATION AND
COMPARISON BETWEEN ORGANIC
SOLVENTS (SULFOLANE AND DMSO) USED
FOR BENZENE EXTRACTION
*T. Zaiz, **H.Lanez, B.Kechida
Department of Process engineering, P.O. Box 789, 39000, El-Oued, Algeria
University of El-Oued., Algeria
ABSTRACT
Due to the high increase of the production of aromatic hydrocarbons: benzene, toluene and xylenes
BTX from oil because of the large activity of their big markets especially with the availability of
great quantities of these aromatic fractions in the oil. This study has two main parts the first
presents a general vision of the aromatic hydrocarbons, the second is going to focus on the liquid-
liquid extraction with the selected solvents as a separation method. The solvent selection depends
on many properties. In this second part there will be a simulation (conception and execution) of the
liquid-liquid extraction of aromatics by two different organic solvents Sulfolane and DMSO
followed by a comparison between the results obtained by the simulation. The simulator used will
be ASPEN HYSYS 7.2.
The results of the simulation showed that the use DMSO is better than Sulfolane because of the
separation efficiency the economic value and the regeneration rate although its use is more
dangerous (more toxic) than the Sulfolane
Keywords: Extraction, Aromatic, DMSO, Sulfolane, Simulation, HYSYS.
2013 IJ CPS. All rights reserved
1. INTRODUCTION
The petroleum industry has implemented several methods for separating
aromatic either cutting lubricant or light petroleum cuts, among these methods
there is liquid-liquid extraction. The liquid-liquid extraction is a separation
technique that takes advantage of the deference solubility of components of a
homogeneous liquid load in a suitable solvent [1, 4].
The addition to the load of a partially miscible solvent causes the appearance of a
second liquid phase which selectively transfers to the most soluble components.
Phase separation followed by decanting the solvent contained therein gives two
functions whose compositions depend on the parameters of the extraction [3,10].
It uses solvent extraction when distillation alone cannot provide a good separation
and good economic solution.
Tab
benz
Tolu
o-xy
m-xy
p-xy
T.Zaiz et
In orde
followi
- Solve
- Selec
be expe
2. OVE
2.1 Ch
Aromat
aromat
Benzen
the six
therefo
[1]:
It make
single d
For Xy
Orth
Met
Para
ble.1.Charac
form
zene C6H6
uene C7H8
ylene C8H1
ylene C8H1
ylene C8H1
t al. Internati
er to have
ing features
ent power.
tivity. (Solu
ensive, toxi
ERVIEW O
emical stru
tic are diff
ic cycles. A
ne has the fo
hydrogen i
ore be symm
es much of
derivative s
ylene three i
ho Xylene
a Xylene
a Xylene
teristics of a
mula MW
6 78.11
8 92.14
10 106.17
10 106.17
10 106.17
ional Journal
a good ext
:
ubility, sele
c, corrosive
OF AROMA
ucture
ferent than
And C/H rati
formula C
6
H
is a radical
metrical. Th
f the fact t
ubstituted w
isomers cou
romatic com
Boiling
Point (C)
80.1
110.6
7 144
7 139.1
7 138.4
l of Chemical
traction, it
ectivity, den
e, unstable .
ATIC HYD
other hydr
io is high.
H
6
, H are al
provided by
he first form
Fig.1.Benz
that all hyd
with a group
Fig.2.Tolue
uld exist
mpounds [2]
)
Melting
Point (C)
5.5
-93
-25.2
-47.8
13.3
and Petroleu
is necessar
nsity, viscos
.. etc...)[11]
DROCARB
rocarbons b
l identical s
y a single c
mula of ben
ene formula.
drogens are
p (Y)
ene formula.
)
density
(g/ml)
0.879
0.867
0.897
0.868
0.861
um Sciences, 2
ry to use so
sity, temper
].
BONS
by the fact
since the su
compound.
nzene by Ke
.
e identical,
.
Solubility i
water (g/100
0.18
0.05
Insoluble
Insoluble
Insoluble
2013, 2(1), 10
olvent cont
rature, it sh
that they
ubstitution o
The molecu
ekule was p
and there
in
0g)
Diele
Consta
2.2
2.38
e 2.5
e 2.3
e 2.2
0-19
11
tains the
hould not
have an
of one of
ule must
proposed
is not a
ectric
ant 3,4
fl
poin
28
(25)
57
37
27
flash
nt (C)
-11
4
32
27
27
T.Zaiz et al. International Journal of Chemical and Petroleum Sciences, 2013, 2(1), 10-19
12
2.2 Different methods of separation of aromatic
We can use several techniques to extract high purity aromatics species produced
either in the steam cracking, or catalytic reforming.
These treatments are mostly based on physico-chemical processes and are
sometimes Sir most specific economic, certain types of load or certain operating
conditions although they are, in principle, able to handle all aromatic types
essences. They are: Crystallization, adsorption, distillation, azeotropic distillation,
extractive distillation and solvent extraction [5, 7].
3. EXTRACTION BYSOLVENT
Liquid-liquid extraction (also called solvent extraction) was initially utilized in the
petroleum industry beginning in the 1930s. It has since been utilized in numerous
applications including petroleum, hydrometallurgical, pharmaceutical, and nuclear
industries. Liquid-liquid extraction describes a method for separating components
of a solution by utilizing an unequal distribution of the components between two
immiscible liquid phases. In most cases, this process is carried out by intimately
mixing the two immiscible phases, allowing for the selective transfer of solute(s)
from one phase to the other, then allowing the two phases to separate[8,9].
Typically, one phase will be an aqueous solution, usually containing the
components to be separated, and the other phase will be an organic solvent, which
has a high affinity for some specific components of the solution.
The process is reversible by contacting the solvent loaded with solute(s) with
another immiscible phase that has a higher affinity for the solute than the organic
phase. The transfer of solute from one phase into the solvent phase is referred to
as extraction and the transfer of the solute from the solvent back to the second
(aqueous) phase is referred to as back-extraction or stripping [4].
The two immiscible fluids must be capable of rapidly separating after being mixed
together, and this is primarily a function of the difference in densities between the
two phases. [3, 6]
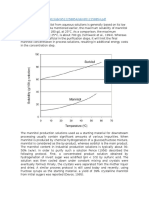
3.1 Different types of common solvents
The solvents used in industrial processes are either oxygenated sulfur compounds
such as tetra ethylene sulfone (sulfolane) or dimethyl derivative. They obviously
have the general properties of industrial solvents [7].
Fig.3.Sulfolane and DMSO structures.
T.Zaiz et al. International Journal of Chemical and Petroleum Sciences, 2013, 2(1), 10-19
13
3.2 Physico-chemical properties
Table.2.Physico-chemical properties of common solvents.
Solvent M(g/mol)
T
f
(C) T
b
(C)
p
Kg
m
3
2u (C)
p (mPo. s)
0 (C)
Pressure of
steam (kPa)
Sulfolane 120.2 27.6 287 1266 10.3/30
1.3310
-3
30C
DMSO 78.13 18.5 190.85 1100 1.99/25 0.084 25C
4. ENVIRONMENTAL PROPERTIES
4.1 Toxicological information
Table.3.Toxicological information of common solvents.
Solvent Toxicityague LD
50
Risk indication Values
carcinogenesis
(Oral) (skin)
Sulfolane 1941 4009 R22 0.37 Negative results
DMSO 28000 50000 R36, 37,38 50 Negative results
R36/37/38: Irritating to eyes, respiratory system and skin.
R22: Harmful if swallowed.
4.2 Ecological information
The organic solvents mentioned are all volatile organic compounds (VOCs).Their
vaporization in the atmosphere contributes to the production of ozone in the
troposphere by photo chemical reaction, thus increasing the risks, especially for
asthmatics or people with breathing difficulties.
The rejection of these solvents directly into the environment can contribute
significantly to the deterioration of the flora and fauna inhabiting the rivers and
streams.
5. THE SIMULATION STEPS
5.1 General methodology
This part is to simulate an aromatics extraction process by ordinary solvents
previously presented on an industrial scale by using simulation software well
known in the engineering field as ASPEN HYSYS 7.2.
5.2 Choosing a-separation system
The benzene extraction of benzene / heptane mixture by ordinary solvents was
chosen as a model for aromatic / aliphatic separation.
5.3 Choice of a thermodynamic model
Data interaction parameters for the system heptane +benzene +solvent were
estimated by choosing the thermodynamic model NRTL (Non Random Two
Liquid), because it is the most suitable model for the simulation of liquid-liquid
T.Zaiz et al. International Journal of Chemical and Petroleum Sciences, 2013, 2(1), 10-19
14
extraction processes.
5.4 The operating conditions
The average conditions of ordinary solvents studied in the extractor are
determined according to existing industrial processes.
Table.4.Operatingconditions of the extraction processes of aromatic industrial scale.
Table.5.Data of the extraction by organic solvents process.
Feed Solvent
Shell-UOP IFP Shell-UOP IFP
Temperature C 100 35 100 35
Pressure bar 1.013 1.013 1.013 1.013
Total flow total t/h 300 300 1600 1600
Benzene t/h 120 120 - -
Heptane t/h 180 180 - -
Solvent t/h - - 1600 1600
Composition by weight%
Benzene 40 40 - -
Heptane 60 60 - -
Solvent - - 100 100
6. PROCESS DESCRIPTION
The feed mixture is fed to the liquid-liquid extraction tower (T-100), where
aromatic hydrocarbons selectively dissolved with the solvent. The raffinate phase
rich non-aromatic hydrocarbons out the top of the column and sent to the tower
(T-102) for the purpose of recovering the small amount of solvent introduced into
the raffinate phase. The water-solvent mixture passed to the stripper for
separation. Aliphatic out the top of the splitter and sent to storage. The solvent-
rich extract phase exits the extraction tower with traces of aliphatic and aromatic
high quantity, they are sent to the stripper (101-T) to ensure good separation
solvent / aromatic solvent leaves the pure stripper (T-101) at the bottom and
recycled to the extraction tower and aromatics lot with high purity as distillate.
Process Solventused Operating
Conditions
Report
solvent/feedstock
Number
of stages
Sulfolane
(Shell-Uop)
Sulfolane 100C
2bar
4/1 12
IFP DMSO 35C
1bar
4/1 12
T.Zaiz et al. International Journal of Chemical and Petroleum Sciences, 2013, 2(1), 10-19
15
Fig.4.Technological scheme of aromatic extraction by organic solvent designed by
AspenHysys7.2.
T.Zaiz et al. International Journal of Chemical and Petroleum Sciences, 2013, 2(1), 10-19
16
Table.6.Results of the different processes studied.
Alimentation Solvent Bottoms Extracted Product
processes 1 2 1 2 1 2 1 2
Temperature C 100 35 100 33.36 129 36.16 98 -5.89
Pressurebar 1.013 1.103 1.103 1 3 1 3 1
Total flowt/h 300 300 1206 1520 1412 1737 93.45 82.94
Benzenet/h 180 180 7.71 - 253.86 162.08 2.93 -
Heptanet/h 120 120 - - 36.13 26.35 86.23 82.31
Solventt/h - - 1186.22 1289.67 1050.52 1013.86 4.19 0.60
H2O - - 12.06 230.32 71.49 234.70 0.08 0.02
Composition by weight %
Benzene 60 60 0.93 - 17.98 9.33 3.14 0
Heptane 40 40 - - 2.56 1.52 92.28 99.24
Solvent - - 92.77 84.85 74.4 75.64 4.49 0.73
H2O - - 6.29 15.15 5.06 13.51 0.09 0.03
Recovery rate%
Benzene - - - - 0.01 - - -
Heptane - - - - - - - -
Solvent - - - - 98.96 99.82 100 100
H2O - - - - 0.04 0.18 - -
. 1- Extraction by sulfolane 2- Extraction by DMSO
T.Zaiz et al. International Journal of Chemical and Petroleum Sciences, 2013, 2(1), 10-19
17
7. RESULTS AND COMPARISON
Following the processes carried out and which were designed to compare the rate
of solvent recovery without reducing the performance of processes it has been
found that there is a difference between these two.
On sulfolane, it was found that the extract contains 253 .86 t\h Benzene, while
DMSO contains only 162 .08 t\h (91.78 t\h more). The amount of benzene lost in
the raffinate is negligible (<3 t\h).
Pure benzene recovered was 184.19 t\h after extraction by sulfolane and 185.31
t\h by the DMSO.
Heptane for the amount in the extract was 36.1 t\h for sulfolane extraction and
26.35t\h for DMSO (difference 9.78 t\h) the major amount of heptane was in the
raffinate as in sulfolane extraction processes the amount of heptane was 86.23t\h
and 82.31 for DMSO (3.92 t\h difference).
The pure heptane to recover the end of the process is: 85.38 t \ h after extraction
sulfolane and 113.2 t \ h after extraction by DMSO.
According to that introduced 300 t\h mixing (180 t\h benzene and 120 t\h heptane)
in the extraction column was recovered 269.57 t\h in sulfolane extraction
(89.85%) and 298.51t\h by the method of DMSO (99.50%).
Fig.5.Products obtained at the end of the process.
70
75
80
85
90
95
100
sulfolane DMSO
benzne/heptane productivity %
T.Zaiz et al. International Journal of Chemical and Petroleum Sciences, 2013, 2(1), 10-19
18
7.1 The solvents
a- Regeneration rate
Sulfolane introduced into the extraction column is 1186.22 t\his recovered solvent
is 1179.84t\h (99.31% sulfolane was recovered).
DMSO introduced into the extraction column is 1289.67 t\h and recovered DMSO
is 1185.12 t\h (99.90% solvent was recovered).
b- Economic comparison
Proposed by the company of Liaoyang Guanghua Chemical Co.., Ltd. Standard
sulfolane price in the market is 3700 $ per ton. In the process we will use 1206 ton
of sulfolane that reach up to 4,462,200 $.
99.31% of sulfolane was recovered means that there is a lost value of 30,789.18$
per hour.
The reasonable price of DMSO is about 2100$ for ton (2.1$ / kg) so as proposed
by Hansen Zhuzhou Chemical Co., Ltd. 1520 ton will be used with the value of
3,192,000$.
99.90% DMSO was recovered means that the lost value of this solvent is 3.192$
per hour.
While DMSO is cheaper than sulfolane as the total difference is 1,270,200$ and
the difference of the lost (per hour) is 27,597.18$.
8. CONCLUSION
We have seen in this work the importance of separation processes and took the
liquid-liquid extraction as a separation method in industry and its economic
importance.
For benzene in the aromatic hydrocarbons, liquid-liquid extraction is probably the
best method of separation and the solvents used are generally organic.
The preparation and the choice of solvent is probably the main processes such as
extraction is chosen such as to form with the support a mixture of two immiscible
phases and must not only allow the separation of the products but also be easily
used in extractors and easily separable from the dissolved products and its use
should be as economical as possible.
In this work we used two organic solvent which are: tetramethylene sulfone
(sulfolane) and dimethyl sulfoxide (DMSO) and compared the results of these last
two in the industry by simulation (with HYSYS 7.2).
We used the thermodynamic model NRTL (Non Random Two Liquid), because it
is the most suitable for the simulation of liquid-liquid extraction processes model.
The procedures were similar for both solvents means that the same equipment was
used for the separation and recovery same conditions operating power but
operating conditions solvents were not the same after the nature of the two
different solvents.
T.Zaiz et al. International Journal of Chemical and Petroleum Sciences, 2013, 2(1), 10-19
19
From the results obtained it was found that DMSO was better than sulfolane for
different reasons as it has better selectivity capacity miscibility regeneration rate
and economic value although it is more toxic than sulfolane.
9. REFERENCES
[1] Extraction des aromatiques: Calcul de vrification de la colonne de
Benzne de l'unit 200/RA1/K Ould Mohamed Lemine Beyrouk
Reziouk Faris.
[2] Vogel's Textbook of Practical Organic Chemistry 5th Ed. (Furnis B.S. Et
All, Longman 1989).
[3] Liquid-Liquid Extraction Equipment J ack D. Law and Terry A. Todd
Idaho National Laboratory.
[4] Etude de lutilisation des liquides ioniques comme cosolvants pour
lextraction des composs aromatiques lchelle industrielle BENNOUH
Oussama.
[5] P. WUITHIER : Raffinage et gnie chimique, Tome I,2
eme
dition 1972
Paris.
[6] P. WUITHIER : Raffinage et gnie chimique, Tome II,2
eme
dition 1972
Paris.
[7] J .P.WAUQUIER : Procds de sparation, Editions TECHNIP 1998
Paris.
[8] Engineering PERRY HAND BOOK, Edition 1972.
[9] Engineering DATA BOOK NINTH, Edition 1972.
[10] Manuel opratoire de lunit GPL et celui de lunit de traitement de
RHOURDE-NOUSS.
[11] R. ABDOULLAEV, V. KOSSIAKOV : Thorie et calcul de la
rectification des mlanges complexes,Editions INHC 1977 Boumerdes.
S-ar putea să vă placă și
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeDe la EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeEvaluare: 4 din 5 stele4/5 (5794)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreDe la EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreEvaluare: 4 din 5 stele4/5 (1090)
- Never Split the Difference: Negotiating As If Your Life Depended On ItDe la EverandNever Split the Difference: Negotiating As If Your Life Depended On ItEvaluare: 4.5 din 5 stele4.5/5 (838)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceDe la EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceEvaluare: 4 din 5 stele4/5 (895)
- Grit: The Power of Passion and PerseveranceDe la EverandGrit: The Power of Passion and PerseveranceEvaluare: 4 din 5 stele4/5 (588)
- Shoe Dog: A Memoir by the Creator of NikeDe la EverandShoe Dog: A Memoir by the Creator of NikeEvaluare: 4.5 din 5 stele4.5/5 (537)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersDe la EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersEvaluare: 4.5 din 5 stele4.5/5 (344)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureDe la EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureEvaluare: 4.5 din 5 stele4.5/5 (474)
- Her Body and Other Parties: StoriesDe la EverandHer Body and Other Parties: StoriesEvaluare: 4 din 5 stele4/5 (821)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)De la EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Evaluare: 4.5 din 5 stele4.5/5 (121)
- The Emperor of All Maladies: A Biography of CancerDe la EverandThe Emperor of All Maladies: A Biography of CancerEvaluare: 4.5 din 5 stele4.5/5 (271)
- The Little Book of Hygge: Danish Secrets to Happy LivingDe la EverandThe Little Book of Hygge: Danish Secrets to Happy LivingEvaluare: 3.5 din 5 stele3.5/5 (400)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyDe la EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyEvaluare: 3.5 din 5 stele3.5/5 (2259)
- The Yellow House: A Memoir (2019 National Book Award Winner)De la EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Evaluare: 4 din 5 stele4/5 (98)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaDe la EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaEvaluare: 4.5 din 5 stele4.5/5 (266)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryDe la EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryEvaluare: 3.5 din 5 stele3.5/5 (231)
- Team of Rivals: The Political Genius of Abraham LincolnDe la EverandTeam of Rivals: The Political Genius of Abraham LincolnEvaluare: 4.5 din 5 stele4.5/5 (234)
- On Fire: The (Burning) Case for a Green New DealDe la EverandOn Fire: The (Burning) Case for a Green New DealEvaluare: 4 din 5 stele4/5 (73)
- The Unwinding: An Inner History of the New AmericaDe la EverandThe Unwinding: An Inner History of the New AmericaEvaluare: 4 din 5 stele4/5 (45)
- Alkali Corrosion of Refractories in Cement KilnsDocument69 paginiAlkali Corrosion of Refractories in Cement KilnsplazanaÎncă nu există evaluări
- CrystallizationDocument2 paginiCrystallizationoskrSSÎncă nu există evaluări
- Essential Oil Report SheetDocument1 paginăEssential Oil Report SheetoskrSSÎncă nu există evaluări
- Extraction of Eugenol From ClovesDocument12 paginiExtraction of Eugenol From ClovesmarcelompassosÎncă nu există evaluări
- 122 Lab Sample Report Rev 2Document7 pagini122 Lab Sample Report Rev 2karenÎncă nu există evaluări
- Extraction of Eugenol From ClovesDocument12 paginiExtraction of Eugenol From ClovesmarcelompassosÎncă nu există evaluări
- Extraction of Eugenol From ClovesDocument12 paginiExtraction of Eugenol From ClovesmarcelompassosÎncă nu există evaluări
- FC 0614 EmersonPortal OBanionDocument6 paginiFC 0614 EmersonPortal OBanionoskrSSÎncă nu există evaluări
- Aspen-Hysys Simulation of Sulfuric Acid PlantDocument3 paginiAspen-Hysys Simulation of Sulfuric Acid PlantacckypenrynÎncă nu există evaluări
- Experiment On Packed Bed Column - Mass TransferDocument15 paginiExperiment On Packed Bed Column - Mass TransferAllyana Marie TiemsimÎncă nu există evaluări
- Function: %KJ/KG %KJ/KG K %KJ/KG K %KDocument2 paginiFunction: %KJ/KG %KJ/KG K %KJ/KG K %KoskrSSÎncă nu există evaluări
- Vol 26 (3) BDocument7 paginiVol 26 (3) BoskrSSÎncă nu există evaluări
- Propane de AsphaltingDocument12 paginiPropane de AsphaltingFatma RedaÎncă nu există evaluări
- Science-Solid, Liquid and Gas: SolidsDocument4 paginiScience-Solid, Liquid and Gas: Solidsproma100% (1)
- Tutorial: Heat and Mass Transfer With The Mixture Model: PurposeDocument9 paginiTutorial: Heat and Mass Transfer With The Mixture Model: PurposemkbÎncă nu există evaluări
- IB EXtended Essay-Modeling The Variation in Specific Heat of A 4'-Octyl-4-Biphenyl-Carbonitrile Liquid Crystal Due To Phase TransitionDocument43 paginiIB EXtended Essay-Modeling The Variation in Specific Heat of A 4'-Octyl-4-Biphenyl-Carbonitrile Liquid Crystal Due To Phase TransitionAndrewÎncă nu există evaluări
- Thermodynamics - Theory T-V Diagram: Phase Change Process Under Constant Pressure (112 KB)Document3 paginiThermodynamics - Theory T-V Diagram: Phase Change Process Under Constant Pressure (112 KB)rajaraghuramvarmaÎncă nu există evaluări
- PHYSICAL CHEMISTRY-phase Diagram 1 Komponen (For Student)Document24 paginiPHYSICAL CHEMISTRY-phase Diagram 1 Komponen (For Student)Muhammad YanuarÎncă nu există evaluări
- Module 3 - Part 2 - Pure SubstanceDocument82 paginiModule 3 - Part 2 - Pure SubstanceRaja DuduluÎncă nu există evaluări
- Micro Emulsion TechnologyDocument3 paginiMicro Emulsion TechnologyRexona KhanomÎncă nu există evaluări
- Surface Tension (Liquid SolutionsDocument14 paginiSurface Tension (Liquid SolutionsHelenette Joy Vergara CaneÎncă nu există evaluări
- Lecture 15Document33 paginiLecture 15Anas Nasir officalÎncă nu există evaluări
- Quantity of HeatDocument40 paginiQuantity of HeatKiel JohnÎncă nu există evaluări
- ECE Catalogue PDFDocument26 paginiECE Catalogue PDFssn100% (1)
- Week 1-Intermolecular Forces and Liquids and SolidsDocument19 paginiWeek 1-Intermolecular Forces and Liquids and SolidsMark John Paul CablingÎncă nu există evaluări
- Jurnal CalorimeterDocument112 paginiJurnal CalorimeterAditya Pujasakti Yuswi100% (1)
- Use of Caustic in A Short Contact Time Approach To Selectively Scrub h2s From Co2 Contaminated Gas StreamsDocument15 paginiUse of Caustic in A Short Contact Time Approach To Selectively Scrub h2s From Co2 Contaminated Gas Streamsfika putriÎncă nu există evaluări
- Exam 1 AnswersDocument9 paginiExam 1 AnswersArvic Lacson0% (1)
- HPLC TroubleshootingDocument27 paginiHPLC TroubleshootingwingroupsÎncă nu există evaluări
- CHAPTER 3 Phase Diagram TTT HT - 1stDocument25 paginiCHAPTER 3 Phase Diagram TTT HT - 1stAriff AziziÎncă nu există evaluări
- Module 2Document15 paginiModule 2Ratay EvelynÎncă nu există evaluări
- Solubility of Liquids in LiquidsDocument12 paginiSolubility of Liquids in LiquidsYuppie RajÎncă nu există evaluări
- Muskat EvingerDocument14 paginiMuskat EvingerChristian PradaÎncă nu există evaluări
- 4th Grade - Lesson Overview-States of MatterDocument2 pagini4th Grade - Lesson Overview-States of MatterlchamblessÎncă nu există evaluări
- Seatwork No. 3 À Separation of Mixtures - Loreto Abigail B. Santos 6-TemperanceDocument2 paginiSeatwork No. 3 À Separation of Mixtures - Loreto Abigail B. Santos 6-TemperanceLoreto Abigail SantosÎncă nu există evaluări
- Vle Written Report 2Document19 paginiVle Written Report 2api-408316181Încă nu există evaluări
- (Progress in Soil Science) Viliam Novák (Auth.) - Evapotranspiration in The Soil-Plant-Atmosphere System-Springer Netherlands (2012)Document262 pagini(Progress in Soil Science) Viliam Novák (Auth.) - Evapotranspiration in The Soil-Plant-Atmosphere System-Springer Netherlands (2012)Nicolás Alberto Pereira LawrenceÎncă nu există evaluări
- 4.0 Preliminary Design of Equipment HeuristicsDocument10 pagini4.0 Preliminary Design of Equipment Heuristicssolehah misniÎncă nu există evaluări
- Lorna Parashin Portfolio 2009Document89 paginiLorna Parashin Portfolio 2009lornapaulÎncă nu există evaluări
- Technologies For The Design and Operation of Phosphate Fertilizer & Sulfuric Acid PlantsDocument12 paginiTechnologies For The Design and Operation of Phosphate Fertilizer & Sulfuric Acid PlantsJavier Alejandro RodriguezÎncă nu există evaluări
- Interactions Between Base Paper and Coating Color in Metered Size Press CoatingDocument99 paginiInteractions Between Base Paper and Coating Color in Metered Size Press CoatingHuy NguyenÎncă nu există evaluări