Documente Academic
Documente Profesional
Documente Cultură
DSC: Differential Scanning Calorimetry
Încărcat de
Mouad ArradDescriere originală:
Titlu original
Drepturi de autor
Formate disponibile
Partajați acest document
Partajați sau inserați document
Vi se pare util acest document?
Este necorespunzător acest conținut?
Raportați acest documentDrepturi de autor:
Formate disponibile
DSC: Differential Scanning Calorimetry
Încărcat de
Mouad ArradDrepturi de autor:
Formate disponibile
1
DSC:
Differential Scanning Calorimetry
A bulk analytical technique
What Does a DSC Measure?
A DSC measures the difference in heat flow rate
(mW = mJ/sec) between a sample and inert
reference as a function of time and
temperature
-0 . 4
-0 . 3
-0 . 2
-0 . 1
0 . 0
0 . 1
H
e
a
t
F
l
o
w
(
W
/
g
)
0 2 5 5 0 7 5 1 0 0 1 2 5 1 5 0
Temperature (C)
Exo Up
Endothermic Heat Flow
Heat Flow
Endothermic: heat flows into the sample as
a result of either heat capacity (heating) or
some endothermic process (glass
transition, melting, evaporation, etc.)
-0.1
0.0
0.1
H
e
a
t
F
l
o
w
(
W
/
g
)
0 20 40 60 80 100 120 140 160
Temperature (C)
Exo Up
Exothermic Heat Flow
Heat Flow
Exothermic: heat flows out of the sample
as a result of either heat capacity
(cooling) or some exothermic process
(crystallization, cure, oxidation, etc.)
Temperature
What temperature is being measured and
displayed by the DSC?
Sensor Temp: used by most DSCs. It is
measured at the sample platform with a
thermocouple, thermopile or PRT.
Constantan Body
Chromel Wire
Chromel Area Detector
Constantan Wire
Chromel Wire
Base Surface
Thin Wall Tube
Sample Platform
Reference Platform
Temperature
What temperature is being measured and
displayed by the DSC?
Pan Temp: calculated by TA Q1000
based on pan material and shape
Uses weight of pan, resistance of pan, &
thermoconductivity of purge gas
What about sample temperature?
The actual temperature of the sample is
never measured by DSC
Temperature
What other temperatures are not typically
being displayed.
Program Temp: the set-point temperature
is usually not recorded. It is used to control
furnace temperature
Furnace Temp: usually not recorded. It
creates the temperature environment of
the sample and reference
Understanding DSC Signals
Heat Flow
Relative Heat Flow: measured by many
DSCs. The absolute value of the signal is
not relevant, only absolute changes are
used.
Absolute Heat Flow: used by TAs Q1000.
Dividing the signal by the measured heating
rate converts the heat flow signal into a heat
capacity signal
DSC Heat Flow
t) (T,
dt
dT
Cp
dt
dH
f + =
signal flow heat DSC
dt
dH
=
Weight Sample Heat x Specific Sample
Capacity Heat Sample Cp
=
=
Rate Heating
dt
dT
=
(kinetic) re temperatu absolute an at
time of function is that flow Heat t) (T, = f
Tzero Heat Flow Equation
R
s
R
r
q
s
q
r
C
s
C
r
T
r
T
0
T
s
Heat Flow
Sensor Model
( )
d
T d
C
d
dT
C C
R R
T
R
T
q
r
s
s r
r s r
=
1 1
0
How do we calculate these?
Besides the three
temperatures (T
s
, T
r
, T
0
);
what other values do we
need to calculate Heat
Flow?
Measuring the Cs & Rs
TzeroCalibration calculates the Cs & Rs
Calibration is a misnomer, THIS IS NOT A
CALIBRATION, but rather a measurement of
the Capacitance (C) and Resistance (R) of
each DSC cell
After determination of these values, they can be
used in the Four Term Heat Flow Equation
showed previously
Measuring the Cs & Rs
Preformed using TzeroCalibration Wizard
1. Run Empty Cell
2. Run Sapphire on both Sample & Reference
side
Measuring the Cs & Rs
Empty DSC constant heating rate
Assume:
0 =
r s
q q
Heat balance equations give sensor time constants
d
dT
T
R C
s
s s s
0
= =
d
T d
d
dT
T T
R C
s
r r r
= =
0
Measuring the Cs & Rs
Repeat first experiment with sapphire disks on sample
and reference (no pans)
Assume:
d
dT
c m q
s
sapph s s
=
d
dT
c m q
r
sapph r r
=
Use time constants to calculate heat capacities
1
0
=
s
s
sapph s
s
d
dT
T
c m
C
1
0
+
=
r
s
sapph r
r
d
T d
d
dT
T T
c m
C
Measuring the Cs & Rs
Use time constants and heat capacities to calculate
thermal resistances
s
s
s
C
R
=
r
r
r
C
R
=
A few words about the Cs and Rs
The curves should be smooth and continuous,
without evidence of noise or artifacts
Capacitance values should increase with
temperature (with a decreasing slope)
Resistance values should decrease with
temperature (also with a decreasing slope)
It is not unusual for there to be a difference
between the two sides, although often they are
very close to identical
Good TzeroCalibration Run
Can see that it is bad during
Tzerocal run
Bad TzeroCalibration Run
Before Running TzeroCalibration
System should be dry
Dry the cell and the cooler heat exchanger
using the cell/cooler conditioning template and
the default conditions (2 hrs at 75C) with the
cooler off
Preferably enable the secondary purge
Do not exceed 75C cell temperature with
the cooler off, although the time can be
extended indefinitely
Stabilization before Calibration
System must be stable before Tzero
Calibration
Stabilization is achieved by cycling the baseline
over the same temperature range and using the
same heating rate as will be used for the
subsequent calibration
Typical systems will stabilize after 3-4 cycles, 8
cycles recommended to ensure that the system
has stabilized
Example of Typical Results
40
50
60
R
e
f
e
r
e
n
c
e
R
e
s
i
s
t
a
n
c
e
(
C
/
W
a
t
t
)
0.02
0.03
0.04
R
e
f
e
r
e
n
c
e
C
a
p
a
c
i
t
a
n
c
e
(
J
o
u
l
e
/
C
)
0.01
0.02
0.03
0.04
0.05
S
a
m
p
l
e
C
a
p
a
c
i
t
a
n
c
e
(
J
o
u
l
e
/
C
)
30
40
50
60
70
S
a
m
p
l
e
R
e
s
i
s
t
a
n
c
e
(
C
/
W
a
t
t
)
-200 -100 0 100 200 300
Temperature (C)
Characteristics of the thermal resistances and heat capacities:
Both curves should be smooth, with no steps, spikes or inflection points.
Thermal resistances should always have negative slope that gradually decreases.
Heat capacities should always have positive slope that gradually decreases.
This cell is very well balanced. It is acceptable and usual
to have larger differences between sample and reference.
-0.4
-0.2
0.0
0.2
0.4
0.6
H
e
a
t
F
l
o
w
(
m
W
)
-100 0 100 200 300 400
Temperature (C)
Conventional Baseline
T zero Baseline
Tzerovs Conventional Baseline
Indium with Q Series Heat Flow Signals
Q1000
Q100
Q10
Keeping the DSC Cell Clean
One of the first steps to ensuring good data is
to keep the DSC cell clean
How do DSC cells get dirty?
Decomposing samples during DSC runs
Samples spilling out of the pan
Transfer from bottom of pan to sensor
How do we keep DSC cells clean?
DO NOT DECOMPOSE SAMPLES IN
THE DSC CELL!!!
Run TGA to determine the decomposition
temperature
Stay below that temperature!
Make sure bottom of pans stay clean
Use lids
Use hermetic pans if necessary
TGA Gives Decomposition Temperature
Cleaning Cell
If the cell gets dirty
Clean w/ brush
Brush gently both sensors and cell if
necessary
Be careful with the Tzerothermocouple
Blow out any remaining particles
Brushing the Sample Sensor
It Does Matter What Pan you use
Monohydrate
Pharmaceutical
sample
Sample Shape
Keep sample thin
Cover as much as the bottom of pan as
possible
Sample Shape
Cut sample to make thin, dont crush
If pellet, cut cross section
Sample Shape
Cut sample to make thin, dont crush
If pellet, cut cross section
If powder, spread evenly over the bottom of the
pan
Using Sample Press
When using crimped pans, dont over crimp
Bottom of pan should remain flat after crimping
When using Hermetic pans, a little more
pressure is needed
Hermetic pans are sealed by forming a cold
wield on the Aluminum pans
Crimped Pans
Hermetic Pans
Good Bad
Sealed
Not
Sealed
Sample Size
Larger samples will increase sensitivity
but.
Larger samples will decrease resolution
Goal is to have heat flow of 0.1-10mW going
through a transition
Sample Size
Sample size depends on what you are
measuring
If running an extremely reactive sample (like
an explosive) run very small samples (<1mg)
Pure organic materials, pharmaceuticals
(1-5mg)
Polymers - ~10mg
Composites 15-20mg
Effect of Sample Size on Indium Melt
Size: 0.4900 mg
Size: 1.2100 mg
Size: 5.7010 mg
-25
-20
-15
-10
-5
0
H
e
a
t
F
l
o
w
(
m
W
)
150 152 154 156 158 160 162 164
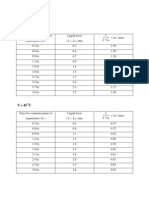
Temperature (C)
Weight Onset Peak Width
(mg) (C) (C) (C)
0.49 156.41 156.56 0.17
1.21 156.45 156.76 0.29
5.70 156.61 157.17 0.55
Purge Gas
Purge gas should always be used during DSC
experiments
Provides dry,inert atmosphere
Ensures even heating
Helps sweep away any off gases that might
be released
Nitrogen
Most common
Increases Sensitivity
Typical flow rate of 50ml/min
Purge Gas
Helium
Must be used with LNCS
High Thermo-conductivity
Increases Resolution
Upper temp limited to 350C
Typical flow rate of 25ml/min
Air or Oxygen
Used to view oxidative effects
Typical flow rate of 50ml/min
Sample Temperature Range
Rule of Thumb
Have 2-3 minutes of baseline before and
after transitions of interest - if possible
DO NOT DECOMPOSE SAMPLES
IN DSC CELL
Temperature range can affect choice of pans
Just because the instrument has a
temperature range of 90C to 550C (with
RCS) doesnt mean you need to heat every
sample to 550!
Start-up Hook
Do not attempt to interpret transitions
before Heating rate has stabilized
9.56mg PET @ 10C/min
0
2
4
6
8
10
12
D
e
r
i
v
.
T
e
m
p
e
r
a
t
u
r
e
(
C
/
m
i
n
)
-0.25
-0.15
-0.05
H
e
a
t
F
l
o
w
(
W
/
g
)
-5 5 15 25 35
Temperature (C)
Exo Up
Heating Rate
Faster heating rates increase sensitivity
but.
Faster heating rates decrease resolution
Good starting point is 10C/min
Effect of Heating Rate
PMMA
10.04mg
Thermal History
The thermal history of a sample can and will
affect the results
The cooling rate that the sample undergoes can
affect :
Crystallinity of semi-crystalline materials
Enthalpic recovery at the glass transition
Run Heat-Cool Heat experiments to see effect
of & eliminate thermal history
Heat at 10C/min
Cool at 10C/min
Heat at 10C/min
Heat-Cool-Heat of PET
Second Heat
First Heat
Cool
-1.5
-1.0
-0.5
0.0
0.5
1.0
1.5
H
e
a
t
F
l
o
w
(
W
/
g
)
20 60 100 140 180 220 260
Temperature (C)
S-ar putea să vă placă și
- Differential Scanning Calorimetry (DSC)Document96 paginiDifferential Scanning Calorimetry (DSC)Rajesh DwivediÎncă nu există evaluări
- DSC PresentationDocument16 paginiDSC PresentationVinoth MalaikaniÎncă nu există evaluări
- Denman’S Handbook for Auto Mechanics and TechniciansDe la EverandDenman’S Handbook for Auto Mechanics and TechniciansÎncă nu există evaluări
- Differential Scanning Calorimetry PresentationDocument16 paginiDifferential Scanning Calorimetry PresentationNebu MathewÎncă nu există evaluări
- PNEUMATICS AND AIR CIRCUITS UNDERSTANDING THE CASCADE VALVE AND PLC UNDERSTANDINGDe la EverandPNEUMATICS AND AIR CIRCUITS UNDERSTANDING THE CASCADE VALVE AND PLC UNDERSTANDINGÎncă nu există evaluări
- TemperatureDocument24 paginiTemperatureidayu111Încă nu există evaluări
- Handbook of Mechanical and Materials EngineeringDe la EverandHandbook of Mechanical and Materials EngineeringEvaluare: 5 din 5 stele5/5 (4)
- DSC PresentationDocument11 paginiDSC Presentationhareesh13h100% (1)
- Aim: Apparatus:: To Perform Thermo-Gravimetric Analysis of Calcium OxalateDocument10 paginiAim: Apparatus:: To Perform Thermo-Gravimetric Analysis of Calcium OxalateravideyÎncă nu există evaluări
- ThermalanalysisDocument88 paginiThermalanalysisbinteadamÎncă nu există evaluări
- Mechanical MeasurementsDocument8 paginiMechanical MeasurementsRam NiceÎncă nu există evaluări
- Heat 1Document21 paginiHeat 1Law TomÎncă nu există evaluări
- MCT Module 6Document23 paginiMCT Module 6AckshayaÎncă nu există evaluări
- Introduction To DSCDocument144 paginiIntroduction To DSCsecateÎncă nu există evaluări
- Specific Heat of CeramicDocument30 paginiSpecific Heat of CeramicAlbert Kaltenthaler VÎncă nu există evaluări
- Differential Scanning CalorimetryDocument38 paginiDifferential Scanning CalorimetrySukhwant SinghÎncă nu există evaluări
- 06082021024459am - S.1 Physics Heat IiDocument41 pagini06082021024459am - S.1 Physics Heat Iimarionmbambu9Încă nu există evaluări
- Brochure TGA DSC1Document14 paginiBrochure TGA DSC1Angela MoraÎncă nu există evaluări
- Gas DiffusionDocument6 paginiGas DiffusionAkashoujo AkariÎncă nu există evaluări
- How To Calibrate Your Autoclave PDFDocument3 paginiHow To Calibrate Your Autoclave PDFrafik1995Încă nu există evaluări
- TAM BasicTheory Applications 2019Document310 paginiTAM BasicTheory Applications 2019Ruchita PoilkarÎncă nu există evaluări
- Forced Convection Lab ManualDocument10 paginiForced Convection Lab Manualjohn paul.jaisonÎncă nu există evaluări
- Thermo Lab ReportDocument7 paginiThermo Lab ReportKartik BhararaÎncă nu există evaluări
- PRP OelDocument9 paginiPRP OelKashif hussainÎncă nu există evaluări
- AV300 High TempDocument5 paginiAV300 High TempAurelia BucurÎncă nu există evaluări
- Liquid-in-Glass Thermometer Calibration: Principle of Operation Traceability ChartDocument1 paginăLiquid-in-Glass Thermometer Calibration: Principle of Operation Traceability ChartbasdownloadÎncă nu există evaluări
- For Unmatched Performance: ThermogravimetryDocument14 paginiFor Unmatched Performance: ThermogravimetryAngela MoraÎncă nu există evaluări
- Gas ChromatographyDocument51 paginiGas ChromatographyDeepak SharmaÎncă nu există evaluări
- Differentialscanningcalorimetry 221101152123 464b39b3Document26 paginiDifferentialscanningcalorimetry 221101152123 464b39b3anujaÎncă nu există evaluări
- HMT Experiment 1Document18 paginiHMT Experiment 1Jr NtrÎncă nu există evaluări
- Isq Product SpecificationDocument2 paginiIsq Product SpecificationYan RiveraÎncă nu există evaluări
- IPC LAB - Experiment 1Document4 paginiIPC LAB - Experiment 1fayeenriquez100% (2)
- Notes To Study For Physics Paper 6Document7 paginiNotes To Study For Physics Paper 6Mohammad Nafai75% (4)
- ME3122E-Heat Transfer Lab A-Temperature MeasurementDocument9 paginiME3122E-Heat Transfer Lab A-Temperature MeasurementLinShaodun0% (1)
- How To Calibrate An RTD or PRTDocument36 paginiHow To Calibrate An RTD or PRTBAN ZANGHANAÎncă nu există evaluări
- Differential Scanning CalorimetryDocument7 paginiDifferential Scanning CalorimetryGintoki SakataÎncă nu există evaluări
- PCR April '00 V1.0 Part 1 NicDocument84 paginiPCR April '00 V1.0 Part 1 Nicvg04Încă nu există evaluări
- 1 2 3 4 5 6 7 8 9 MergedDocument72 pagini1 2 3 4 5 6 7 8 9 Mergedtenguria samriddhÎncă nu există evaluări
- Temperature MeasurementDocument57 paginiTemperature MeasurementATUL ASWARÎncă nu există evaluări
- ThermometerCalibrationMethods PDFDocument5 paginiThermometerCalibrationMethods PDFDragoonbk89Încă nu există evaluări
- ME3122-1 Informal Lab ReportDocument5 paginiME3122-1 Informal Lab ReportLuqman MohdariÎncă nu există evaluări
- Determination of Vapor PressureDocument5 paginiDetermination of Vapor PressureAbhinav AnandÎncă nu există evaluări
- Minimum Number of SamplesDocument5 paginiMinimum Number of SamplesEdwin KohÎncă nu există evaluări
- Nicolet Nexus 670Document12 paginiNicolet Nexus 670huan heÎncă nu există evaluări
- 6.1 Temperature Section6.1Document15 pagini6.1 Temperature Section6.1UtterLuckÎncă nu există evaluări
- Thermistor A1Document4 paginiThermistor A1Ahmed Abu SharbainÎncă nu există evaluări
- First Order Dynamics Bulb ThermometerDocument5 paginiFirst Order Dynamics Bulb ThermometerRakesh Kumar ChaudharyÎncă nu există evaluări
- Thermal Analysis TechniquesDocument34 paginiThermal Analysis TechniquesMujahid AmeenÎncă nu există evaluări
- Lec 4Document37 paginiLec 4Akram TaÎncă nu există evaluări
- Santillan LBYME3B Laboratory Report 01Document11 paginiSantillan LBYME3B Laboratory Report 01Nygel Gian SantillanÎncă nu există evaluări
- HT Lab Report File 19112043 Jayesh Kumar Verma Group One Exp-4 Newton's Law of CoolingDocument6 paginiHT Lab Report File 19112043 Jayesh Kumar Verma Group One Exp-4 Newton's Law of CoolingJayesh VermaÎncă nu există evaluări
- Heat Conduction ExperimentDocument6 paginiHeat Conduction Experimenttallgah60% (5)
- Integration Start 20 Graus PDFDocument7 paginiIntegration Start 20 Graus PDFÉrick LimaÎncă nu există evaluări
- 19CH30018 - Mass Transfer-Lab-1Document25 pagini19CH30018 - Mass Transfer-Lab-1herewego759Încă nu există evaluări
- Measuring Instruments TemperatureDocument52 paginiMeasuring Instruments TemperaturemohammedhanafyÎncă nu există evaluări
- 18 Feb 03Document47 pagini18 Feb 03Patrick Bernard BanzemwaboÎncă nu există evaluări
- Lab Ys: From ToDocument4 paginiLab Ys: From TogostokhelwiÎncă nu există evaluări
- Thermal Applications NoteDocument5 paginiThermal Applications NoteOlger Ferreira PachecoÎncă nu există evaluări
- Flyer ORC-PLUS EnglishDocument2 paginiFlyer ORC-PLUS EnglishMouad ArradÎncă nu există evaluări
- A 1Document1 paginăA 1Mouad ArradÎncă nu există evaluări
- Density: STP-4.3 Page 1 of 6 Revised 5/5/2006Document6 paginiDensity: STP-4.3 Page 1 of 6 Revised 5/5/2006Mouad ArradÎncă nu există evaluări
- Kopp Rules Heat Capacity Solid PDFDocument134 paginiKopp Rules Heat Capacity Solid PDFMouad ArradÎncă nu există evaluări
- Biotech Math Problems Part1 AnswersDocument5 paginiBiotech Math Problems Part1 AnswersMouad ArradÎncă nu există evaluări
- Density: STP-4.3 Page 1 of 6 Revised 5/5/2006Document6 paginiDensity: STP-4.3 Page 1 of 6 Revised 5/5/2006Mouad ArradÎncă nu există evaluări
- Je 900974 UDocument6 paginiJe 900974 UMouad ArradÎncă nu există evaluări
- Levenberg ExamplesDocument2 paginiLevenberg ExamplesMouad ArradÎncă nu există evaluări
- PigmentDocument10 paginiPigmentMouad ArradÎncă nu există evaluări
- Uv Absorbers X DCDocument6 paginiUv Absorbers X DCMouad ArradÎncă nu există evaluări
- Areas of Specialisation in GENCO and DISCO - PPDocument33 paginiAreas of Specialisation in GENCO and DISCO - PPchdiÎncă nu există evaluări
- Spectra II Data Sheet - Atg UV Technology - Rev 2 - 2018Document1 paginăSpectra II Data Sheet - Atg UV Technology - Rev 2 - 2018nattaponamornÎncă nu există evaluări
- Pelco Dome CCTV Camera ics090-CA8Document6 paginiPelco Dome CCTV Camera ics090-CA8Jamie DeltonÎncă nu există evaluări
- BZX55CV24Document4 paginiBZX55CV24kcraussÎncă nu există evaluări
- Release Notes For EQSP - 122 - E1 - AI - V2Document9 paginiRelease Notes For EQSP - 122 - E1 - AI - V29 9 9Încă nu există evaluări
- CT Requirement For MicomsDocument50 paginiCT Requirement For Micomsjaved shaikh chaandÎncă nu există evaluări
- Interfacing 8251 With 8086 PDFDocument10 paginiInterfacing 8251 With 8086 PDFMurthyÎncă nu există evaluări
- Manual Phenix BaseDocument34 paginiManual Phenix BaseEmerson De Andrade LimaÎncă nu există evaluări
- Org ChemDocument22 paginiOrg ChemRay-ann Dela FuenteÎncă nu există evaluări
- Physics II Problems PDFDocument1 paginăPhysics II Problems PDFBOSS BOSS0% (1)
- Pulse and Digital CircuitsDocument2 paginiPulse and Digital Circuitsshaik ahammad hussainÎncă nu există evaluări
- Firevac IVDocument4 paginiFirevac IVjanechuacruzÎncă nu există evaluări
- Mixed-Signal-Electronics: PD Dr.-Ing. Stephan HenzlerDocument33 paginiMixed-Signal-Electronics: PD Dr.-Ing. Stephan HenzlerAhmed HamoudaÎncă nu există evaluări
- MLX90614 SMBusDocument32 paginiMLX90614 SMBustime machineÎncă nu există evaluări
- MPD 810HDocument5 paginiMPD 810Hdan22yÎncă nu există evaluări
- 3W Ultra-Compact Power Module HLK-PM01 230V AC To 5V/3W DC: FeaturesDocument6 pagini3W Ultra-Compact Power Module HLK-PM01 230V AC To 5V/3W DC: FeaturesmachogamerÎncă nu există evaluări
- Business PlanDocument25 paginiBusiness PlanMahesan SinthujanÎncă nu există evaluări
- TC-2450 Equipment Case User's Manual ISI-4421-0061 Rev BDocument16 paginiTC-2450 Equipment Case User's Manual ISI-4421-0061 Rev BPeter BoongÎncă nu există evaluări
- Timing Consideration On A Safety PLCDocument11 paginiTiming Consideration On A Safety PLCerstendrainÎncă nu există evaluări
- Hammond AllDocument301 paginiHammond AllMohamed El AbasyÎncă nu există evaluări
- Philips HTS-3090 PDFDocument30 paginiPhilips HTS-3090 PDFmatias cavalottoÎncă nu există evaluări
- Job Log After Code ChangeDocument7 paginiJob Log After Code ChangetalupurumÎncă nu există evaluări
- AmpliTube 4.0.0 Ipad User ManualDocument152 paginiAmpliTube 4.0.0 Ipad User ManualValérie CharavelÎncă nu există evaluări
- A Comparison of Liquid-Filled and Dry-Type Transformers For Industrial ApplicationsDocument5 paginiA Comparison of Liquid-Filled and Dry-Type Transformers For Industrial Applicationsmilad rouhiniaÎncă nu există evaluări
- Electronic Music - Systems, Techniques and ControlsDocument281 paginiElectronic Music - Systems, Techniques and Controlskuatrovich100% (1)
- HVDC Fault Location PresentationDocument66 paginiHVDC Fault Location PresentationAmila Pathirana100% (1)
- BJC-8500 Service ManualDocument215 paginiBJC-8500 Service ManualLee Swee ChooÎncă nu există evaluări
- GOOLOO GE1200 - User ManualDocument1 paginăGOOLOO GE1200 - User ManualzoltanpolyakÎncă nu există evaluări
- List of Tools For AutomotiveDocument4 paginiList of Tools For AutomotiveGilbert MendozaÎncă nu există evaluări