Documente Academic
Documente Profesional
Documente Cultură
CN2116-HW7-Solution (XJP - 2011)
Încărcat de
Brian WatsonDescriere originală:
Titlu original
Drepturi de autor
Formate disponibile
Partajați acest document
Partajați sau inserați document
Vi se pare util acest document?
Este necorespunzător acest conținut?
Raportați acest documentDrepturi de autor:
Formate disponibile
CN2116-HW7-Solution (XJP - 2011)
Încărcat de
Brian WatsonDrepturi de autor:
Formate disponibile
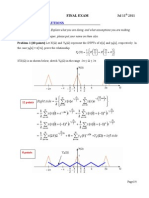
CN2116 Homework Set #7 (due 14-03-2011) CN2116-XJ P-2011-HW7
1
1. The F curve is shown below for a real reactor
What is the mean residence time?
Solution:
min 5 . 2 ) 4 )( 5 . 0 ( ) 2 )( 5 . 0 (
2
1
) (
) (
) ( ) (
1
0 0
0
= + = = =
=
=
tdF dt t tE t
dt t E dF
dt t E t F
m
t
2. The following data were obtained from a tracer test to a reactor.
t (s) 0 5 10 15 20 25 30 35
C(t)
(mg/dm
3
) 0 0 0 5 10 5 0 0
(a) Plot C(t), E(t) and F(t) curves.
(b) Evaluate the mean residence time and the variance.
Solution:
(a) Plot C(t)
Plot E(t)
CN2116 Homework Set #7 (due 14-03-2011) CN2116-XJ P-2011-HW7
2
100
) (
) (
) (
) (
100 ) 10 )( 20 30 (
2
1
) 10 )( 10 20 (
2
1
) ( Area
0
3
0
t C
dt t C
t C
t E
dm
s mg
dt t C
= =
= + = =
t(s) 0 5 10 15 20 25 30
C(t)(mg/dm
3
) 0 0 0 5 10 5 0
E(t) (s
1
) 0 0 0 0.05 0.1 0.05 0
0 ) ( , 30 For
) 30 ( 01 . 0 ) ( ) ( , 30 20 For
) 10 ( 01 . 0 ) ( ) ( , 20 10 For
0 ) ( , 10 For
2
1
=
= =
= =
=
t E t
t t E t E t
t t E t E t
t E t
Plot F(t)
5 . 3 3 . 0 005 . 0 ) 30 ( 01 . 0 5 . 0 ) ( 5 . 0 ) ( , 30 20 For
5 . 0 1 . 0 005 . 0 ) 10 ( 01 . 0 ) ( 0 ) ( , 20 10 For
) ( ) (
2
20 20
2 2
2
10 10
1 1
0
+ = + = + =
+ = = + =
=
t t dt t dt t E t F t
t t dt t dt t E t F t
dt t E t F
t t
t t
t
(b) s dt t t dt t t dt t tE dt t tE dt t tE t
m
20 ) 30 )( 01 . 0 ( ) 10 )( 01 . 0 ( ) ( ) ( ) (
30
20
20
10
30
20
2
20
10
1
0
= + = + = =
2 2
30
20
2
2
20
10
1
2 2
0
2
0
2 2
6 . 16 ) ( ) ( ) ( ) ( ) ( s t dt t E t dt t E t t dt t E t dt t E t t
m m m
= + = = =
CN2116 Homework Set #7 (due 14-03-2011) CN2116-XJ P-2011-HW7
3
3. A pulse input (N
0
=1 mol, v =4 liters/min) to a vessel gives the results shown in the
following figure.
(a) Check the material balance with the tracer curve to see whether the results are
consistent.
(b) If the result is consistent, determine the volume of the vessel V, the mean residence
time t
m
, and sketch the E(t) curve.
Solution
(a) Check to see if the results are consistent
By material balance:
lit
mol
lit
mol
v
N
Area
min
25 . 0
min / 4
1
0
= = =
From the graph:
lit
mol
lit
mol
Area
min
25 . 0 min) 5 )( 05 . 0 (
= =
So the results are consistent.
(b)
1
0
min 2 . 0
25 . 0
05 . 0
25 . 0
) (
) (
) (
) (
= = = =
t C
dt t C
t C
t E
lit 10 min) / lit 4 min)( 5 . 2 (
min 5 . 2 ) 2 . 0 ( ) (
0
5
0
= = = = =
= = =
v t V
v
V
t
dt t dt t tE t
m m
m
CN2116 Homework Set #7 (due 14-03-2011) CN2116-XJ P-2011-HW7
4
4. The first-order reaction, B A , with
1
min 8 . 0
= k is carried out in a real reactor with the
following RTD function
For 2 0 t then
2 2
) ( ) ( = t t E min
-1
(hemi circle)
For 2 > t then 0 ) ( = t E
(a) What is the mean residence time?
(b) What is the variance?
(c) What is the conversion predicted by the segregation model?
(d) What is the conversion predicted by the maximum mixedness model?
Solution
(a) Mean residence time
By definition
=
0
1 ) ( dt t E . The area of the semicircle representing the E(t) is given by
1
2
2
= =
A and
2
= . For constant volumetric flow min 8 . 0
2
= = =
m
t .
(b) Variance
= =
2
0
2 2 2
0
2 2
) ( ) ( ) ( ) ( dt t t dt t E t
Using polymath:
159 . 0
2
=
Polymath results
POLYMATH Report
Ordinary Differential Equations 29-Nov-2010
Calculated values of DEQ variables
Variable Initial value Minimal value Maximal value Final value
1 E 0 0 0.7980869 0.0166534
2 E2 0 0 0.7980869 0.0166534
3 sigma 0 0 0.1593161 0.1593161
4 t 0 0 1.596 1.596
5 t1 1.596174 1.596174 1.596174 1.596174
6 tau 0.7980869 0.7980869 0.7980869 0.7980869
Differential equations
1 d(sigma)/d(t) = (t-tau)^2*E
CN2116 Homework Set #7 (due 14-03-2011) CN2116-XJ P-2011-HW7
5
Explicit equations
1 tau = (2/3.14)^0.5
2 t1 = 2*tau
3 E2 = (t*(2*tau-t))^(1/2)
4 E = if (t<t1) then (E2) else (0)
(c) Conversion predicted by the Segregation model
=
0
) ( ) ( dt t E t X X
kt
e t X
=1 ) (
( ) dt t e X
kt
2 / 1
2
0
2 2
) ( 1
=
Using Polymath
445 . 0 = X
Polymath Results
POLYMATH Report
Ordinary Differential Equations 29-Nov-2010
Calculated values of DEQ variables
Variable Initial value Minimal value Maximal value Final value
1 E 0 0 0.7980865 0.0166534
2 E2 0 0 0.7980865 0.0166534
3 k 0.8 0.8 0.8 0.8
4 t 0 0 1.596 1.596
5 t1 1.596174 1.596174 1.596174 1.596174
6 tau 0.7980869 0.7980869 0.7980869 0.7980869
7 X 0 0 0.7210716 0.7210716
CN2116 Homework Set #7 (due 14-03-2011) CN2116-XJ P-2011-HW7
6
8 Xbar 0 0 0.4447565 0.4447565
Differential equations
1 d(Xbar)/d(t) = X*E
Explicit equations
1 tau = (2/3.14)^0.5
2 t1 = 2*tau
3 E2 = (t*(2*tau-t))^(1/2)
4 E = if (t<t1) then (E2) else (0)
5 k = .8
6 X = 1-exp(-k*t)
(d) Conversion predicted by the Maximum Mixedness model
X
F
E
C
r
d
dX
A
A
) ( 1
) (
0
+ =
) 1 (
0
X kC kC r
A A A
= =
X
F
E
X k
d
dX
) ( 1
) (
) 1 (
+ =
= 596 . 1 ,
) ( 1
) (
) 1 ( z X
F
E
X k
dz
dX
(we need to change the variable such the integration proceeds forward)
Using Polymath
445 . 0 = X
X =0.445 as for the Segregation Model, but we knew this because for first order
reactions X
Seg
=X
MM
.
Polymath Results
POLYMATH Report
CN2116 Homework Set #7 (due 14-03-2011) CN2116-XJ P-2011-HW7
7
Ordinary Differential Equations 29-Nov-2010
Calculated values of DEQ variables
Variable Initial value Minimal value Maximal value Final value
1 E 0.0166534 0 0.7980866 0
2 E1 0.0166534 0 0.7980866 0
3 F 1. -0.0005053 1. -0.0005053
4 k 0.8 0.8 0.8 0.8
5 lam 1.596 0 1.596 0
6 tau 0.7980869 0.7980869 0.7980869 0.7980869
7 x 0 0 0.4445289 0.4445289
8 z 0 0 1.596 1.596
Differential equations
1 d(x)/d(z) = -(-k*(1-x)+E/(1-F)*x)
2 d(F)/d(z) = -E
Explicit equations
1 k = .8
2 lam = 1.596-z
3 tau = (2/3.14)^0.5
4 E1 = (tau^2-(lam-tau)^2)^0.5
5 E = if (lam<=2*tau) then (E1) else (0)
CN2116 Homework Set #7 (due 14-03-2011) CN2116-XJ P-2011-HW7
8
[Discussion Topics]
1. A liquid macrofluid reacts according to R A as it flows through a vessel. Find the
conversion of A for the following flow patterns and kinetics.
Solution
(a) Using the segregation model, dt t E t X X
batch
) ( ) (
0
=
For a batch reactor we have
0 A
A
C
r
dt
dX
=
From the reaction kinetics, we have
zero order reaction, min mol/lit 3 =
A
r and mol/lit 6
0
=
A
C
Solving for X(t), we have
2min for t 1, X
min 2 for , 5 . 0 5 . 0 ) (
> =
= = t t X t t X
From the flow pattern (a), we have
3 t 0 for , min
3
1
) (
1
=
t E
Therefore, the mean conversion for a zero order reaction is
3
2
)
3
1
( ) 1 ( )
3
1
( ) 5 . 0 (
3
2
2
0
= + =
dt dt t X
(b) For a batch reactor we have
0 A
A
C
r
dt
dX
=
From the reaction kinetics, we have
zero order reaction, min mol/lit 03 . 0 =
A
r and mol/lit 1 . 0
0
=
A
C
Solving for X(t), we have:
min 33 . 3 for t 1, X
min 33 . 3 for , 3 . 0 3 . 0 ) (
> =
= = t t X t t X
CN2116 Homework Set #7 (due 14-03-2011) CN2116-XJ P-2011-HW7
9
From the flow pattern (b), we know nothing leaves the reactor before 4 min, so
everything has reacted. 1 = X
2. A step tracer input was used on a real reactor with the following results:
For 10 t min, then C
T
=0
For 30 10 t min, then C
T
=10 g/dm
3
For 30 > t min, then C
T
=40 g/dm
3
The second-order reaction B A with min /mol dm 1 . 0
3
= k is to be carried out in the real
reactor with an entering concentration of A of 1.25 mol/dm
3
at a volumetric flow rate of 10
dm
3
/min. Here k is given at 325 K.
(a) What is the mean residence time t
m
?
(b) What is the variance?
(c) What conversion do you expect from an ideal PFR, an ideal CSTR, and a real reactor
with the same t
m
?
(d) What is the conversion predicted by the Segregation model and the Maximum Mixedness
model?
Solution
(a) The cumulative distribution function F(t) is given:
The real reactor can be modeled as two parallel PFRs:
The relative 30min min, 10 ), (
4
3
) (
4
1
) (
2 1 2 1
= = + = t t t E
Mean residence time
= + = =
1
0
min 25 ) 75 . 0 )( 20 ( ) 1 )( 10 ( tdF t
m
or
CN2116 Homework Set #7 (due 14-03-2011) CN2116-XJ P-2011-HW7
10
min 25
4
3
4
1
) (
4
3
) (
4
1
) (
2 1
0 0
2 1
= + =
+ = =
dt t t t dt t tE t
m
(b) Variance:
2 2 2 2
2
2
1
0 0
2 1
2 2 2
min 75 ) 25 30 (
4
3
) 25 10 (
4
1
) (
4
3
) (
4
1
) (
4
3
) (
4
1
) ( ) ( ) (
= + = + =
+ = =
m m
m m
t t
dt t t t t dt t E t t
(c) Conversion X from an ideal PFR, an ideal CSTR, and a real reactor.
For a PFR, second-order, liquid phase, irreversible reaction with min /mol dm 1 . 0
3
= k ,
min 25 = and
3
0
mol/dm 25 . 1 =
A
C
758 . 0
1
0
0
=
+
=
A
A
C k
C k
X
For a CSTR, second-order, liquid phase, irreversible reaction with min /mol dm 1 . 0
3
= k ,
min 25 = and
3
0
mol/dm 25 . 1 =
A
C
572 . 0
) 1 (
0
2
= =
X C k
X
X
A
For two parallel PFRs, min 10
1
= and min 30
2
= ,
0 01
4 / 1
A A
F F = and
0 02
4 / 3
A A
F F = ,
second-order, liquid phase, irreversible reaction with min /mol dm 1 . 0
3
= k , min 25 =
and
3
0
mol/dm 25 . 1 =
A
C
3
0 1
0 1
0 1
/ 556 . 0 )
1
1 ( dm mol
C k
C k
C C
A
A
A A
=
+
=
3
0 2
0 2
0 2
/ 263 . 0 )
1
1 ( dm mol
C k
C k
C C
A
A
A A
=
+
=
731 . 0
4
3
4
1
0 0
2 0 1 0 0 0
=
=
A
A A A
C v
C v C v C v
X
(d) Conversion predicted by the Segregation model.
731 . 0
1 4
3
1 4
1
) (
4
3
) (
4
1
1
) ( ) (
2 0
2 0
1 0
1 0
0
2 1
0
0
0
=
+
+
+
=
+
+
= =
A
A
A
A
A
A
kC
kC
kC
kC
dt t t
t kC
t kC
dt t E t X X
CN2116 Homework Set #7 (due 14-03-2011) CN2116-XJ P-2011-HW7
11
Conversion predicted by the Maximum Mixedness model
X
F
E
X kC
d
dX
X kC kC r
X
F
E
C
r
d
dX
A
A A A
A
A
) ( 1
) (
) 1 (
) 1 (
) ( 1
) (
2
0
2 2
0
2
0
+ =
= =
+ =
We need to change the variable such the integration proceeds forward:
X
z T F
z T E
X kC
dz
dX
A
) ( 1
) (
) 1 (
2
0
=
Solved by Polymath:
706 . 0 = X
POLYMATH Report POLYMATH Report
Ordinary Differential Equations 29-Nov-2010
Calculated values of DEQ variables
Variable Initial value Minimal value Maximal value Final value
1 ca 1.25 0.3593529 1.25 0.3672986
2 cao 1.25 1.25 1.25 1.25
3 E 0 0 1.25 0
4 E1 1.25 1.25 1.25 1.25
5 E2 1.25 1.25 1.25 1.25
6 E3 0 0 0 0
7 EF 0 0 2.427988 0
8 F 0.9999 -0.0001081 0.9999 -0.0001081
9 k 0.1 0.1 0.1 0.1
10 lam 40. 0 40. 0
11 ra -0.15625 -0.15625 -0.0129134 -0.0134908
12 t1 10. 10. 10. 10.
13 t2 30. 30. 30. 30.
14 x 0 0 0.7125177 0.7061611
15 z 0 0 40. 40.
Differential equations
1 d(x)/d(z) = -(ra/cao+E/(1-F)*x)
2 d(F)/d(z) = -E
CN2116 Homework Set #7 (due 14-03-2011) CN2116-XJ P-2011-HW7
12
Explicit equations
1 cao = 1.25
2 k = .1
3 lam = 40-z
4 ca = cao*(1-x)
5 t1 = 10
6 t2 = 30
7 E3 = 0
8 ra = -k*ca^2
9 E2 = 0.75/(t2*2*(1-0.99))
10 E1 = 0.25/(t1*2*(1-0.99))
11
E = if ((lam>=0.99*t1)and(lam<1.01*t1)) then (E1) else( if ((lam>=0.99*t2)and(lam<1.01*t2)) then (E2) else
(E3))
12 EF = E/(1-F)
Summary
X
CSTR
X
MM
X
Seg
X
PFR
X
Real
0.572 0.706 0.731 0.758 0.731
S-ar putea să vă placă și
- HW #8 - SolutionDocument2 paginiHW #8 - SolutionMatty JakeÎncă nu există evaluări
- Solutions CN2116 HW7Document3 paginiSolutions CN2116 HW7Jian HongÎncă nu există evaluări
- Discrete-Time Evaluation of The Time Response: AppendixDocument6 paginiDiscrete-Time Evaluation of The Time Response: AppendixAnonymous WkbmWCa8MÎncă nu există evaluări
- Exercise: Specific Heat of SolidDocument3 paginiExercise: Specific Heat of SolidpcandiasÎncă nu există evaluări
- Reactors 2 SolutionsDocument4 paginiReactors 2 Solutionsnripesh_pokhrel100% (2)
- Engr-2500u Midterm SolutionsDocument6 paginiEngr-2500u Midterm SolutionsAbdullah AlshihriÎncă nu există evaluări
- L03 LCCDE LaplaceTransform PDFDocument40 paginiL03 LCCDE LaplaceTransform PDFJoseph Angelo BuenafeÎncă nu există evaluări
- 1.1 Basic Concepts. Modeling: Engineering Problems Physical Problems Economic ProblemsDocument24 pagini1.1 Basic Concepts. Modeling: Engineering Problems Physical Problems Economic Problems박태영Încă nu există evaluări
- Tutorial 6 - CT Fourier Transform (Solutions) PDFDocument9 paginiTutorial 6 - CT Fourier Transform (Solutions) PDFAna Maria Muñoz GonzalezÎncă nu există evaluări
- 2843 Solutions Chap9Document10 pagini2843 Solutions Chap9Thomas LiuÎncă nu există evaluări
- Assignment 2 PDFDocument5 paginiAssignment 2 PDFSourav kumar MeenaÎncă nu există evaluări
- 2011 Exam - Financial MathematicsDocument6 pagini2011 Exam - Financial MathematicsSylvia HuynhÎncă nu există evaluări
- Workbook Workbook Workbook Workbook Workbook: Try Yourself QuestionsDocument14 paginiWorkbook Workbook Workbook Workbook Workbook: Try Yourself QuestionsShubham mishraÎncă nu există evaluări
- Engr 311 Final 06Document2 paginiEngr 311 Final 06Roberto HoffmanÎncă nu există evaluări
- ControlDocument13 paginiControlNiaz ManikÎncă nu există evaluări
- PHYS 434 - Assignment 4 - Coupled Harmonic OscillatorsDocument10 paginiPHYS 434 - Assignment 4 - Coupled Harmonic Oscillatorsdec292010Încă nu există evaluări
- Sadiku 5th Ed Chapter 15Document74 paginiSadiku 5th Ed Chapter 15Lucas PlentzÎncă nu există evaluări
- Problem 1. A conducting slab: I ikz−ωtDocument38 paginiProblem 1. A conducting slab: I ikz−ωtHaroonRashidÎncă nu există evaluări
- 1982 - Holographic Antenna Measurements Further Technical ConsiderationsDocument20 pagini1982 - Holographic Antenna Measurements Further Technical Considerationszan wang100% (1)
- Time Dep SCH EqnDocument33 paginiTime Dep SCH Eqnutkarsh khandelwalÎncă nu există evaluări
- QB PDC-1 PDFDocument21 paginiQB PDC-1 PDFBalu BalireddiÎncă nu există evaluări
- EE341 FinalDocument4 paginiEE341 FinalLe HuyÎncă nu există evaluări
- Assignment1 2019Document8 paginiAssignment1 2019হিমাংশু লোহানীÎncă nu există evaluări
- Balanced Homodyne DetectorDocument31 paginiBalanced Homodyne DetectorKonstantinos Ladovrechis100% (1)
- ITI1100 Midterm Solutions PDFDocument7 paginiITI1100 Midterm Solutions PDFAmro HasanÎncă nu există evaluări
- Vasicek Model SlidesDocument10 paginiVasicek Model SlidesnikhilkrsinhaÎncă nu există evaluări
- Eee311 TermDocument38 paginiEee311 Termসামিন জাওয়াদÎncă nu există evaluări
- Calculus Instructor S Manual ComponentsDocument168 paginiCalculus Instructor S Manual ComponentsBez SofÎncă nu există evaluări
- Code 0 p2 SolutionDocument38 paginiCode 0 p2 Solutionanon020202Încă nu există evaluări
- TMRCA EstimatesDocument9 paginiTMRCA Estimatesdavejphys100% (1)
- 42663-0201596121 SMDocument85 pagini42663-0201596121 SMWael BazziÎncă nu există evaluări
- Solution 2Document5 paginiSolution 2Paulina MarquezÎncă nu există evaluări
- Assignment 4 SolutionsDocument7 paginiAssignment 4 SolutionsEllie AustinÎncă nu există evaluări
- EE207 Problem Set 1Document2 paginiEE207 Problem Set 1Rishabh AgarwalÎncă nu există evaluări
- DLTS Comb Alessandro SolutionDocument4 paginiDLTS Comb Alessandro SolutionShivshankar GhugeÎncă nu există evaluări
- δ (n) = u (n) - u (n-3) = 1 ,n=0Document37 paginiδ (n) = u (n) - u (n-3) = 1 ,n=0roberttheivadasÎncă nu există evaluări
- Macroeconomics 1 PS3 Solution PDFDocument10 paginiMacroeconomics 1 PS3 Solution PDFTaib MuffakÎncă nu există evaluări
- Physics of A Massive Vector Boson (Problem 5.5 of Peskin and Schroeder)Document5 paginiPhysics of A Massive Vector Boson (Problem 5.5 of Peskin and Schroeder)Bradley Nartowt100% (1)
- Elec3505 Formula SheetDocument10 paginiElec3505 Formula Sheetkavita4123Încă nu există evaluări
- MT1Document3 paginiMT1Pawan_Singh_6974Încă nu există evaluări
- 1 Exy 1 SDocument11 pagini1 Exy 1 SLukas BoskoÎncă nu există evaluări
- Quantenoptik Vorlesung6 PDFDocument11 paginiQuantenoptik Vorlesung6 PDFErinSuttonÎncă nu există evaluări
- Green's Functions For The Stretched String Problem: D. R. Wilton ECE DeptDocument34 paginiGreen's Functions For The Stretched String Problem: D. R. Wilton ECE DeptSri Nivas ChandrasekaranÎncă nu există evaluări
- 3assignment SolDocument7 pagini3assignment SolDalghak KhanÎncă nu există evaluări
- A Simple Yet Efficient Two Step Fifth Order Weighted Newton Method For Nonlinear ModelsDocument23 paginiA Simple Yet Efficient Two Step Fifth Order Weighted Newton Method For Nonlinear ModelsAntmel Rodriguez CabralÎncă nu există evaluări
- Be First Year Engineering Semester 1 2019 December Engineering Mechanics Emrev 2019'c' SchemeDocument43 paginiBe First Year Engineering Semester 1 2019 December Engineering Mechanics Emrev 2019'c' Schemesinghsitturaj78Încă nu există evaluări
- Assignment 3 Questions and AnswersDocument5 paginiAssignment 3 Questions and AnswersEllie AustinÎncă nu există evaluări
- hw1 SolutionsDocument3 paginihw1 SolutionsFatih İnalÎncă nu există evaluări
- Laplace Transform ExamplesDocument19 paginiLaplace Transform Exampleshamza abdo mohamoudÎncă nu există evaluări
- Three Period OLGDocument12 paginiThree Period OLGDavidChum Lai100% (1)
- EMwavesLectureNotes 1 7Document149 paginiEMwavesLectureNotes 1 7Anonymous BWdaO1lÎncă nu există evaluări
- 2016 Financial Mathematics AssignmentDocument1 pagină2016 Financial Mathematics AssignmentDfcÎncă nu există evaluări
- Modelos Estoc Asticos 2: Christian OjedaDocument6 paginiModelos Estoc Asticos 2: Christian OjedaChristian OjedaÎncă nu există evaluări
- May 2018 With SolutionDocument34 paginiMay 2018 With SolutionSagar AhireÎncă nu există evaluări
- ch11 PDFDocument43 paginich11 PDFmri_leon100% (1)
- VHDL Code For FilterDocument2 paginiVHDL Code For FilterArkadip GhoshÎncă nu există evaluări
- Solutions To Exercises: Chapter 10: 10.1 Use The Chemical Potential of An Ideal Gas in (10.1.9) and Obtain The BarometricDocument6 paginiSolutions To Exercises: Chapter 10: 10.1 Use The Chemical Potential of An Ideal Gas in (10.1.9) and Obtain The BarometricSalomé TorresÎncă nu există evaluări
- Ejer Cici Os Control DigitalDocument6 paginiEjer Cici Os Control DigitalLuis Alejandro MaccleshÎncă nu există evaluări
- SOAL P13-5 (Elements of Chemical Reaction Engineering 4 Edition Foggler)Document31 paginiSOAL P13-5 (Elements of Chemical Reaction Engineering 4 Edition Foggler)Adilla PratiwiÎncă nu există evaluări
- Chapter 3 - MatlabDocument59 paginiChapter 3 - MatlabZe SaÎncă nu există evaluări
- Explorations An Introduction To Astronomy 7Th Edition Arny Test Bank Full Chapter PDFDocument31 paginiExplorations An Introduction To Astronomy 7Th Edition Arny Test Bank Full Chapter PDFcarl.davis982100% (11)
- ASTM D6866 For Biobased ProductsDocument21 paginiASTM D6866 For Biobased ProductsBeta Analytic100% (1)
- UniversityPhysicsVolume3 OPDocument616 paginiUniversityPhysicsVolume3 OPSamuel Ciorap100% (2)
- Chapter 3Document6 paginiChapter 3Katsuki HashimotoÎncă nu există evaluări
- SCH4U Equilibrium Questions With SolutionsDocument28 paginiSCH4U Equilibrium Questions With SolutionsS P100% (1)
- CH 5Document23 paginiCH 5Ahmed GadÎncă nu există evaluări
- Zisman Plot MethodDocument3 paginiZisman Plot MethodJerome TeañoÎncă nu există evaluări
- Performance Comparison of New and Conventional Water RepellentsDocument6 paginiPerformance Comparison of New and Conventional Water RepellentsQuyen Tran Thi AnhÎncă nu există evaluări
- Applied Clay Science: Huaming Yang, Yuehua Deng, Chunfang Du, Shengming JinDocument5 paginiApplied Clay Science: Huaming Yang, Yuehua Deng, Chunfang Du, Shengming JinEL Hassania EL HERRADIÎncă nu există evaluări
- CHEM 210 Karty Exam 2Document19 paginiCHEM 210 Karty Exam 2nm100% (1)
- AG Gauge Valves AEDocument51 paginiAG Gauge Valves AEbaladiroyaÎncă nu există evaluări
- Applied Sciences: Immobilized Tio - Polyethylene Glycol: Effects of Aeration and PH of Methylene Blue DyeDocument1 paginăApplied Sciences: Immobilized Tio - Polyethylene Glycol: Effects of Aeration and PH of Methylene Blue DyeKuna JackÎncă nu există evaluări
- Chemistry For Engineers Set ADocument5 paginiChemistry For Engineers Set AMark Jecel RapirÎncă nu există evaluări
- Group A-1Document4 paginiGroup A-1pacoto livingstoneÎncă nu există evaluări
- Paper and Paper Based Packaging MaterialsDocument25 paginiPaper and Paper Based Packaging MaterialsPremnathÎncă nu există evaluări
- Biology 10th Edition Solomon Solutions ManualDocument5 paginiBiology 10th Edition Solomon Solutions Manualspawnerminutiaxae7n100% (35)
- I. Development of The Virial TheoremDocument14 paginiI. Development of The Virial Theoremjohnsmith37758Încă nu există evaluări
- Attractive Haldane Bilayers For Trapping Non-Abelian AnyonsDocument15 paginiAttractive Haldane Bilayers For Trapping Non-Abelian Anyonsreevu.officialÎncă nu există evaluări
- EAMCETDocument9 paginiEAMCETharshitÎncă nu există evaluări
- Physics 1 Week 9Document16 paginiPhysics 1 Week 9Santos, Alexsandria PaulaÎncă nu există evaluări
- Physics I Problems PDFDocument1 paginăPhysics I Problems PDFBOSS BOSSÎncă nu există evaluări
- Bio EnergeticsDocument8 paginiBio EnergeticsSamuel RookieÎncă nu există evaluări
- Exercises On Fluid Flow in HC ReservoirsDocument3 paginiExercises On Fluid Flow in HC ReservoirsMohamed SahnounÎncă nu există evaluări
- Saumyakanti Khatua Et Al - Plasmonic Nanoparticles-Liquid Crystal CompositesDocument7 paginiSaumyakanti Khatua Et Al - Plasmonic Nanoparticles-Liquid Crystal CompositesYlpkasoÎncă nu există evaluări
- Crystallization of Fats and Oils: Cargill Inc. Minneapolis, Minnesota University of Wisconsin Madison, WisconsinDocument29 paginiCrystallization of Fats and Oils: Cargill Inc. Minneapolis, Minnesota University of Wisconsin Madison, WisconsinPlácidoÎncă nu există evaluări
- Chemical BondingDocument50 paginiChemical BondingLeila BonÎncă nu există evaluări
- Handling of Hazardous MaterialsDocument3 paginiHandling of Hazardous MaterialsKuenieBondocoyÎncă nu există evaluări
- B.T. Kumaon Institute of Technology, Dwarahat End Semester (Back) Examination, 2020-2021Document2 paginiB.T. Kumaon Institute of Technology, Dwarahat End Semester (Back) Examination, 2020-2021verma.ashok031Încă nu există evaluări
- Sanjeevani NEETDocument44 paginiSanjeevani NEETemolicmuneeb768Încă nu există evaluări
- Agitan DF 6120 Tds enDocument1 paginăAgitan DF 6120 Tds enViktor Ragozin (Nakilon)Încă nu există evaluări