Documente Academic
Documente Profesional
Documente Cultură
AANA Journal Course: Update For Nurse Anesthetists Parkinson Disease
Încărcat de
Marianne Garcia0 evaluări0% au considerat acest document util (0 voturi)
22 vizualizări6 paginiJournal
Titlu original
p229-234
Drepturi de autor
© © All Rights Reserved
Formate disponibile
PDF, TXT sau citiți online pe Scribd
Partajați acest document
Partajați sau inserați document
Vi se pare util acest document?
Este necorespunzător acest conținut?
Raportați acest documentJournal
Drepturi de autor:
© All Rights Reserved
Formate disponibile
Descărcați ca PDF, TXT sau citiți online pe Scribd
0 evaluări0% au considerat acest document util (0 voturi)
22 vizualizări6 paginiAANA Journal Course: Update For Nurse Anesthetists Parkinson Disease
Încărcat de
Marianne GarciaJournal
Drepturi de autor:
© All Rights Reserved
Formate disponibile
Descărcați ca PDF, TXT sau citiți online pe Scribd
Sunteți pe pagina 1din 6
*6 CE Credits
AANA Journal Course
2
*AANA Journal Course No. 23: The American Association of Nurse Anesthetists is accredited as a provider of continuing education in nursing by the
American Nurses Credentialing Center Commission on Accreditation. The AANA Journal course will consist of 6 successive articles, each with objectives
for the reader and sources for additional reading. At the conclusion of the 6-part series, a final examination will be printed in the AANA Journal. Suc-
cessful completion will yield the participant 6 CE credits (6 contact hours), code number: 25468, expiration date: July 31, 2004.
Update for nurse anesthetists
Parkinson disease
Scott R. Doyle, RN, MSN
Michael J. Kremer, CRNA, DNSc
Chicago, Illinois
Objectives
At the completion of this course, the reader should be
able to:
1. Describe the clinical manifestations and clinical
course of Parkinson disease.
2. List the known etiologic factors related to Parkin-
son disease.
3. Discuss variables that modify the course of
Parkinson disease.
4. Review the pharmacological agents that are used
to treat this disease.
5. Describe anesthesia considerations for patients
with Parkinson disease.
Introduction
Parkinson disease (PD) is a degenerative neurologic dis-
order that affects the extrapyramidal system, leading to a
lack of coordinated motor control. The excitatory effects
of acetylcholine are left unchecked by a decrease in
dopamine levels, resulting in symptoms such as tremor,
muscular rigidity, hypokinesia, and postural instability.
1
One in 1,000 middle-aged adults and 50 in 1,000 older
adults are affected by PD.
2
Because PD has no known
cure, therapy is directed at ameliorating symptoms.
3
This
course reviews the pathophysiologic and etiologic factors
associated with PD and provides a review of the anes-
thetic assessment, intraoperative management, and post-
operative care of patients with PD. Contemporary issues
related to the medical and surgical management of PD
also are explored.
In this Journal course, the manifestations, etiologic and patho-
physiologic factors, and incidence of Parkinson disease are
reviewed along with current medical management. Medica-
tions and other factors that have an impact on the course of
Parkinson disease are discussed. Suggested preanesthetic,
intraoperative, and postoperative interventions are provided.
Key words: Anesthetic management, neuroanatomy, Parkinson
disease.
AANA Journal/June 2003/Vol. 71, No. 3 229
Pathophysiologic factors
Parkinson disease is a dopamine deficiency disorder that
involves the selective destruction of dopamine-producing
tissues in the midbrain called the substantia nigra.
Dopamine has an integral role as a neurotransmitter help-
ing to regulate motor coordination. This coordination
involves complex neuronal interplay among fibers in the
basal ganglia as part of the extrapyramidal system. Inputs
to the basal ganglia are directed from the cerebral cortex
to modulate motor control. The basal ganglia are 5 pri-
mary structures in the telencephalon that include the cau-
date and putamen (striatum), substantia nigra, globus
pallidus, and the subthalamic nucleus.
Two basic output pathways are defined through the
basal ganglia. In the direct output pathway, striatal D
1
receptors respond to dopamine by causing an excitatory
output to the medial globus pallidus. Conversely, stri-
atal D
2
receptors respond to dopamine by causing inhi-
bition of the indirect output pathway involving the sub-
thalamic nucleus and lateral globus pallidus. The net
result of inadequate dopamine production is inhibition
of the direct output pathway and increased activity of
the indirect output pathway.
4
Inhibitory output is medi-
ated to the thalamus and cerebral cortex, resulting in
classic PD symptoms (Table).
5
Etiologic factors
Nigral cell death is the defining pathology that dis-
tinguishes PD. Cell death in the substantia nigra results
in the presence of Lewy bodies with a loss of pigment
in this central nervous system (CNS) structure.
5
In
1913, Otto Lewy discovered a round, pink staining
bodythe Lewy bodyin the cytoplasm of PD nerve
cells. On autopsy, the presence of Lewy bodies in the
substantia nigra and the noradrenalin cells of the locus
ceruleus are considered essential for diagnosing PD.
Until the advent of positron emission tomography,
however, the clinical diagnosis of PD was based strictly
on symptomatology. The etiologic factors for selective
destruction of dopamine-producing fibers in the sub-
stantia nigra continue to be investigated.
Genetic links have been found (chromosome 4) in
some patients with PD, but most cases are not familial.
A popular hypothesis related to PD is that environmen-
tal toxins selectively kill dopamine neurons over time.
This hypothesis is supported by the fact that PD is pri-
marily a disease of advanced age. The higher incidence
of PD demonstrated in persons working with pesticides
and certain metals or chemicals seems to support the
environmental toxin hypothesis.
6
Oxidative stress has an important role in the neu-
rodegenerative processes associated with PD. The sub-
stantia nigra has an intrinsically low ability to neutralize
oxygen free radicals. Deficiencies in superoxide dismu-
tase have been documented in some patients with PD.
Unfortunately, it is not known whether deficient oxygen
radical processing is a result or a cause of nigral destruc-
tion.
2
Iron processing is associated with oxidative
processes, and several studies demonstrate increased
iron levels in the substantia nigra of patients with PD. It
remains unclear whether elevated iron levels antedate
injury to dopamine-producing neurons in the CNS.
7
Lifestyle factors also may contribute to the incidence
of PD. Dietary intake of excess animal fats, certain herbal
teas, and a toxin in chick peas may be associated with an
increased incidence of PD.
6
Certain recreational drugs
are thought to contribute to the development of PD.
Methamphetamine, for example, is known to cause the
degeneration of dopamine-producing neurons. Another
popular club drug called Ecstasy (MDMA; 3,4-methyl-
enedioxymethamphetamine) is structurally similar to
methamphetamine. Ecstasy is known to cause a disrup-
tion in the transport of both dopamine and serotonin.
8
While these findings support the oxidative stress
hypothesis, the interaction among genetic factors and
environmental stress is confounding, making elucida-
tion of causes of PD difficult.
It is interesting, and somewhat counterintuitive, to
note that cigarette smoking is associated with a dimin-
ished incidence of PD. This relationship is well docu-
mented, but its explanation remains unclear. Proposed
explanations for the protective effects of cigarette
smoking include stimulation of dopamine release and
up-regulation of nicotinic receptors, carbon monoxide
inhibition of hydrogen peroxide, competitive inhibi-
tion of neurotoxins, and the inhibition of monoamine
oxidase.
9
Caffeine consumption also is associated with
a decreased incidence of PD.
10
Since caffeine is a
known CNS stimulant, it may have an important role in
neurotransmission and extrapyramidal modulation.
11
Literature review
To provide evidence-based perioperative care, the anes-
thetist must be aware of new ways to treat PD. While the
medications used to treat PD are not new, many investi-
gators now advocate using medications other than lev-
odopa during the initial stages of PD. Recent data sug-
gest that dopamine agonists have neuroprotective
effects, which delay CNS fiber destruction. Dopamine
receptor agonists such as bromocriptine meslyate and
pergolide mesylate are effective during the initial treat-
ment of PD. Monoamine oxidase inhibitors such as
selegiline hydrochloride are used to selectively increase
the level of dopamine in the basal ganglia. Anticholin-
ergic agents such as benztropine mesylate decrease the
transmission of acetylcholine and theoretically restore
the balance between excitatory and inhibitory neuro-
transmission. Amantadine hydrochloride, a nonspecific
antiviral agent, also may be useful during the initial
stages of PD. Entacapone and other catechol O-methyl-
transferase (also called COMT) inhibitors have been
shown to be effective adjuncts to levodopa therapy.
12,13
The inexorable progression of PD despite available
pharmacological therapy has led to a resurgence of inter-
est in surgical therapies. Surgical therapy for PD can be
classified broadly into 3 areas: ablative therapies, deep
Bradykinesia Slowness in movement, which
includes difficulty initiating movement
and incompleteness of movement
Tremor Tremor appears in the limbs during
rest.
Tremor most often involves the hand.
A tremor involving the thumb and
index finger is very typical and is
called a pill-rolling tremor.
Rigidity Rigidity sometimes is mistaken for
arthritis and is defined as increased
resistance to stretch.
Postural Defined as imbalance and the inabil-
instability ity to correct posture according to
environmental needs.
The shuffling gait associated with
Parkinson disease results from
postural instability and bradykinesia.
Table. Classic Parkinson disease symptoms
(Adapted from Liebermann.
5
)
230 AANA Journal/June 2003/Vol. 71, No. 3
AANA Journal/June 2003/Vol. 71, No. 3 231
brain stimulation (DBS), and transplantation proce-
dures.
14
Thalamotomy is an ablative procedure that tar-
gets the removal of the ventral intermediate nucleus of
the thalamus to reduce tremor. The procedure is not new,
but recent advances in neurologic imaging before surgery
have made the procedure safer.
15
These advances may
require anesthesia involvement during structural brain
imaging procedures done via computed tomography,
magnetic resonance imaging, or positron emission
tomography. Removal of neurologic circuits associated
with other thalamic structures such as the globus pallidus
is another ablative procedure that has been found to offer
improvement for some people with PD.
16
Subthalamic
nucleotomy is an intriguing ablative procedure that
remains experimental because of the deep neurologic
structures involved with this surgical intervention.
14
DBS offers a way of controlling PD symptoms with-
out permanently removing CNS structures. DBS
involves the application of high-frequency stimulation
to targeted brain structures via an implanted pacing
wire connected to a programmable pulse generator.
17
DBS offers the advantage of selectively blocking the
amount of neurologic outflow and is titratable without
permanently removing tissue. Compared with ablative
procedures, DBS offers similar neurologic outcomes for
suppression of severe tremor in PD.
18
Experimental transplantation procedures involving
embryonic neurons have increased the number of func-
tional dopaminergic fibers for patients with severe PD.
Freed and colleagues
19
reported clinical benefits to
younger, but not older, patients who received embryonic
transplant. Ethical considerations, the lack of available
embryonic tissue, and complications related to immuno-
suppression make transplantation of porcine cells and of
human retinal cells viable investigational options for the
treatment of advanced PD.
20
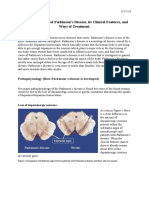
The Figure summarizes the
pathophysiology and contemporary medical or surgical
treatment options available for patients with PD.
The anesthetist should recognize that ablative, DBS,
and transplantation procedures are reserved for patients
with PD with severe functional disability. Neurosurgery
for PD is lengthy, making perioperative management,
Figure. Pathophysiology and treatment of Parkinson disease
Ach
1. Appropriate motor function is
dependent on a balance between
acetylcholine (Ach) and dopamine
in the central nervous system.
3. Medications used to treat Parkinson
disease include:
Dopamine precursors:
levodopa/carbidopa combinations
(Sinemet)
Dopamine releasers: amantadine
(Symadine, Symmetrel)
Dopamine receptor agonists:
bromocriptine (Parlodel), pergolide
(Permax)
Inhibitors of dopamine inactivation,
monoamine oxidase-B inhibitors:
selegiline (Eldepryl)
Anticholinergic and antihistamine
agents: benztropine (Cogentin),
biperiden (Akineton), diphenhydramine
(Benadryl), L-hyoscyamine (Levsin),
procyclidine (Kemadrin),
trihexyphenidyl (Artane)
These agents all have implications for the
anesthesia care plan.
Dopamine
4. Surgical treatment options for Parkinson disease include:
A. Ablation: destruction of the globus pallidus, the ventral
anterior and ventral lateral nuclei of the thalamus, or
(rarely) the subthalamic nuclei.
B. Electrical interruption (deep brain stimulation) of the
signals arising from the structures noted above.
C. Transplant of dopamine-producing cells (fetal, adrenal
medulla, retinal, porcine) to the striatum.
Subthalamic
nuclei
Substantia
nigra
Caudate
+
putamen
S
t
r
i
a
t
u
m
2. Parkinson disease causes the selective destruction of
neuronal fibers in the substantia nigra. The loss of
dopamine produced in these fibers allows the globus
pallidus to be disinhibited. Difficulty in movement and
coordination results.
Globus
pallidus
Thalamus
Basal ganglia
232 AANA Journal/June 2003/Vol. 71, No. 3
including positioning and close monitoring, even more
important. Understanding contemporary medical or sur-
gical treatment options helps the anesthetist anticipate
needs for the preoperative assessment, intraoperative
management, and postopertive care of patients with PD.
Preoperative assessment
The medication history provides clues about PD sever-
ity and can affect the intraoperative anesthesia plan.
The coadministration of levodopa, a metabolic precur-
sor to dopamine, and carbidopa, a peripheral decar-
boxylase inhibitor that prevents the breakdown of lev-
odopa outside the CNS, is the most effective medical
treatment for PD.
4
Levodopa administration has impli-
cations for the intraoperative anesthesia plan since it
can cause nausea, vomiting, orthostatic hypotension,
and cardiac irritability. Regardless of the pharmacologic
regimen used, the anesthetist should note that abrupt
withdrawal of any medication to treat PD may exacer-
bate PD symptoms. Patients should be instructed to
take PD-specific medications the day of surgery unless
otherwise indicated.
3
A focused assessment specific to neurologic func-
tion, respiratory function, and cardiac function is
appropriate for patients with PD. During the assess-
ment, unilateral tremor while signing the surgical con-
sent or during venipuncture are subtle clues that
should trigger additional investigation. Skeletal muscle
rigidity manifested in the proximal muscles of the neck
is an early sign of PD, alerting the anesthetist to the
potential for difficult airway management. Paresthesia
is not part of the normal PD process, but any move-
ment or sensation abnormalities should be docu-
mented. Other significant findings during the preoper-
ative assessment may include pupillary abnormalities,
diaphragmatic spasms, and oculogyric crises. PD does
not cause decreased cognitive function, but patients
may exhibit dementia or signs of depression in
advanced stages of the disease process.
21
PD is classified into 5 stages with functional ability
related to the degree of neurologic degeneration. Stage
I (onset) involves difficulty with unilateral movement.
The disease eventually progresses to stage V (severe
disease), which is characterized by immobilization of
the patient. In the absence of diagnosed PD, a surgical
patient with unexplained muscle stiffness or minor
tremor should be referred for postoperative neurologic
examination.
PD is not a primary cause of respiratory or cardiac
dysfunction. The dyskinesia associated with advanced
PD, however, often causes chest rigidity and is associ-
ated with chronic obstructive pulmonary disease. Care-
ful respiratory assessment, including documentation of
preanesthetic oxygen saturation, is essential. Compro-
mised respiratory function should be evaluated by arte-
rial blood gas analysis or chest radiograph. The major-
ity of medications used to treat PD cause increased car-
diac irritability and blunting of autonomic regulatory
responses. Close assessment of cardiac status and vol-
ume status is of particular importance.
3
Intraoperative management
The intraoperative anesthetic management of patients
with PD includes preparing for the induction of anes-
thesia, avoidance of specific anesthetic agents that
exacerbate PD symptoms, and preparing for emergence
from anesthesia. Before induction, aspiration prophy-
laxis may be considered due to alterations in muscular
tone and coordination that may predispose the patient
to pulmonary aspiration. A nonparticulate antacid such
as sodium citrate and an H
2
blocking agent such as
famotidine can be administered 30 to 60 minutes
before induction. The gastroprokinetic metoclo-
pramide should be avoided because it induces anti-
dopaminergic activity and may exacerbate extrapyami-
dal symptoms.
22
An anxiolytic such as midazolam is appropriate after
the anesthesia interview since emotional stress has
been found to trigger PD symptoms. Ensure that a ben-
zodiazepine is not contraindicated from a surgical per-
spective, eg, awake thalamotomy, pallidotomy, or DBS.
Diphenhydramine also is a valuable sedative for
patients with tremor.
23
Positioning on the operating
room table takes on added importance for patients who
have changes in physical mobility. Assuring proper
positioning and padding before anesthesia induction
and throughout surgery helps diminish injury risk for
patients with PD.
1
Relatively few contraindications exist regarding the
choice of induction agents and anesthesia techniques
for patients with PD. Hypotension related to hypo-
volemia, catecholamine depletion, and autonomic
instability often are side effects of medications used to
treat PD. For patients with severe PD, blood pressure
should be monitored invasively and the anesthetist
should be prepared to administer a direct-acting vaso-
pressor (phenylephrine or epinephrine) for profound
hypotension. Awake or rapid-sequence intubation is
indicated only in the most severe cases of PD in which
the disease has resulted in respiratory compromise or
esophageal spasms that increase aspiration risk.
24
There
is no reported contraindication to the use of succinyl-
choline that is specific to patients with PD.
Ketamine is the only commonly used induction agent
that should be avoided in patients with PD. Ketamine
produces sympathomimetic responses that may exacer-
bate PD symptoms. The choice of muscle relaxants is
not influenced by the presence of PD and should be
evaluated according to the type and duration of surgery,
the need for immediate airway access, and coexisting
disease. With the exception of halothane, PD does not
limit the choice of inhalation anesthetics. Halothane
sensitizes the myocardium to the effects of cate-
cholamines and could promote instability when com-
bined with pharmacologic agents used to treat PD.
24
Perioperative pain management may include the use
of systemic opioids. However, opioids decrease central
dopaminergic transmission resulting in dystonia, or a
woody chest. Alfentanil has been implicated in pro-
ducing acute dystonic reactions in patients with PD.
24
Judicious use of nonsteroidal anti-inflammatory drugs
and local anesthetic infiltration also can be considered
for perioperative analgesia.
Extubation can proceed normally as long as the
anesthetist recognizes that patients with PD are at
greater risk for developing postextubation respiratory
compromise due to hypokinesis of the chest cavity. In
its advanced stages, PD interferes with all muscles of
respiration, including intercostal, diaphragmatic, acces-
sory, and abdominal muscle groups, potentially leading
to the need for prolonged ventilatory support. An
anticholinesterase-anticholinergic combination may be
used if indicated to reverse neuromuscular blockade.
An additional dose of an anticholineric agent such as
glycopyrrolate, 0.2 mg intravenously, is indicated if the
anesthetist notes excessive secretions that often are part
of the PD disease process. Anticholinergic effects also
will diminish the excitatory effects of acetylcholine that
contribute to dystonia.
Postoperative management
Postoperative management of patients with PD should
include a plan for the resumption of preoperative med-
ications to avoid exacerbation of PD symptoms. Careful
assessment of neurologic function and movement
should be documented postoperatively. Postoperative
tremor can be treated with diphenhydramine, 25 mg
intravenously, which also is useful as a drying agent.
Problems with chest wall rigidity or respiratory compro-
mise should be assessed carefully and can be treated with
an anticholinergic such as glycopyrrolate or atropine to
decrease acetylcholine output. Scopolamine should be
avoided because it potentially causes changes in mental
status and may confound the neurologic examination of
patients with PD.
2
Opioids are acceptable for pain management, but
these drugs should be replaced with alternative agents
such as oral or intravenous nonsteroidal anti-inflam-
matory drugs as soon as possible. If it is not otherwise
contraindicated, ketorolac is a good choice for pain
management in patients with PD because it does not
interfere with respiratory function. Postoperative nau-
sea and vomiting are more likely for patients with PD.
Treatment plans must avoid phenothiazines, droperi-
dol, and other butyrophenones because they are
dopamine antagonists that potentially exacerbate PD
symptoms. Ondansetron and dolasetron are good
choices for the management of postoperative nausea
and vomiting since they do not interfere with the trans-
mission of dopamine.
24
Key points
Anesthesia care of patients with PD depends on knowl-
edge and skills of the anesthetist related to the follow-
ing key areas:
Classify the patient with respect to severity of PD
symptoms: mild (unilateral tremor), moderate, or
severe (very restricted movement).
Determine the medications used to control PD
symptoms and the duration of drug therapy.
Document a neurological assessment. Investigate
movement and strength, pupillary response, and
diaphragmatic movements. A stiff neck during air-
way evaluation may be indicative of PD.
Evaluate respiratory function. Anticipate chest
rigidity in patients with advanced PD.
During the intraoperative phase, the following ele-
ments are important:
Carefully position the patient and monitor pres-
sure points.
Anticipate and appropriately treat hypovolemia,
hypotension, and arrhythmias.
Avoid or consider avoiding the following agents:
metroclopramide, droperidol, haldoperidol, prometh-
azine, compazine, ketamine, alfentanil, meperidine,
and halothane.
Before extubation, assess spontaneous breathing
pattern, presence of gag reflex, ability of patient to
focus, and presence of 4 twitches or sustained tetanus.
During the postoperative phase, bear these consider-
ations in mind for patients with PD:
A plan to resume scheduled PD medications is a
priority.
Fully assess and document neurologic function.
Consider diphenhydramine for tremor, but avoid
meperidine for shivering since it may exacerbate PD
symptoms.
Evaluate respiratory function.
Use intravenous ketorolac or other nonopioid
analgesics, if possible. for postoperative pain.
Ondansetron or dolasetron may be used for post-
operative nausea and vomiting.
REFERENCES
1. Byrd DL, Marks WJ Jr, Starr PA. Deep brain stimulation for advanced
Parkinsons disease. AORN J. 2000;72:387-390, 393-408, 409-414,
416-418.
AANA Journal/June 2003/Vol. 71, No. 3 233
16. Rettig GM, York MK, Lai EC, et al. Neuropsychological outcome
after unilateral pallidotomy for the treatment of Parkinsons disease.
J Neurol Neurosurg Psychiatry. 2000;69:326-336.
17. Limousin P, Speelman JD, Gielen F, Janssens M. Multicentre Euro-
pean study of thalamic stimulation in Parkinsonian and essential
tremor. J Neurol Neurosurg Psychiatry. 1999;66:289-296.
18. Schuurman PR, Bosch DA, Bossuyt PMM, et al. A comparison of
continuous thalamic stimulation and thalamotomy for suppression
of severe tremor. N Engl J Med. 2000;342:461-468.
19. Freed CR, Greene PE, Breeze RE, et al. Transplantation of embryonic
dopamine neurons for severe Parkinsons disease. N Engl J Med.
2001;344:710-719.
20. Zesiewicz TA, Hauser RA. Neurosurgery for Parkinsons disease.
Semin Neurol. 2001;21:91-101.
21. Bobele GB. The extrapyramidal system. In: Kaufman CE, McKee PA,
eds. Essentials of Pathophysiology. Boston, Mass: Little, Brown & Co;
1996:679-688.
22. Burks TF. Gastrointestinal drugs. In: Brody TM, Larner J, Minneman
KP, eds. Human Pharmacology. St Louis, Mo: Mosby; 1998:827-841.
23. Morgan GE, Mikhail MS, Murray MJ. Clinical Anesthesiology. Nor-
walk, Conn: Appleton & Lange; 2002:243-244.
24. Stoelting RK, Dierdorf SF. Anesthesia and Co-Existing Diseases. New
York, NY: Churchill-Livingstone; 1993:209-210.
AUTHORS
Scott R. Doyle, RN, MSN, is a nurse anesthesia student at Rush Univer-
sity College of Nursing, Nurse Anesthesia Program, Chicago, Ill.
Michael J. Kremer, CRNA, DNSc, is associate professor in Adult
Health Nursing and assistant director of the Nurse Anesthesia Program in
the Rush University College of Nursing, Chicago, Ill. He also is chair of
the AANA Foundation, an associate editor of the AANA Journal, and a
chair reviewer of the Council on Accreditation of Nurse Anesthesia Edu-
cational Programs.
2. Carvey PM. Drug Action in the Central Nervous System. New York,
NY: Oxford University Press; 1998:225.
3. Cullen DJ, Crosby G. Central nervous system disorders. In: Sweitzer
B, ed. Handbook of Preoperative Assessment and Management.
Philadelphia, Pa: Lippincott Williams & Wilkins; 2000:255-284.
4. Gudelsky GA. Drugs for the treatment of Parkinsons disease. In:
Brody TM, Larner J, Minneman KP, eds. Human Pharmacology. St
Louis, Mo: Mosby; 1998:385-393.
5. Lieberman A. Curing Parkinsons disease in our lifetime: Part 1.
Miami, Fla: National Parkinson Foundation; 1999. Available at:
http://www.parkinson.org/lifetime.htm; Accessed February 20, 2002.
6. Tanner CM, Aston DA. Epidemiology of Parkinsons disease and aki-
netic syndromes. Curr Opin Neurol. 2000;13:427-430.
7. Berg D, Gerlach M, Youdim MB, et al. Brain iron pathways and their
relevance to Parkinsons disease. J Neurochem. 2001;79:225-236.
8. Tilson H. Assessing neurotoxicity of drug abuse. Available at:
www.drugabuse.gov. Accessed November 1, 2002.
9. Hernan MA, Zhang SM, Rueda-deCastro AM, Colditz AM, Speizer
FE, Ascherio A. Cigarette smoking and the incidence of Parkinsons
disease in two prospective studies. Ann Neurol. 2001;50:780-785.
10. Ascherio A, Zhang SM, Hernan MA, et al. Prospective study of caf-
feine consumption and risk of Parkinsons disease in men and
women. Ann Neurol. 2001;50:56-62.
11. Ross GW, Abbott RD, Petrovich H, et al. Association of coffee and
caffeine intake with the risk of Parkinson disease. JAMA.
2000;283:2674-2679.
12. Ahlskog JE. Parkinsons disease: medical and surgical treatment.
Neurol Clin. 2001;19:579-605.
13. Bhatia K, Brooks DJ, Burn DJ, et al. Updated guidelines for the man-
agement of Parkinsons disease. Hosp Med. 2001;62:456-470.
14. Lang AE. Surgery for Parkinson disease: a critical evaluation of the
state of the art. Arch Neurol. 2000;57:1118-1125.
15. Dagher A. Functional imaging in Parkinsons disease. Semin Neurol.
2001;21:23-32.
234 AANA Journal/June 2003/Vol. 71, No. 3
S-ar putea să vă placă și
- Chapter 2 - Neurobiologic Theories and PsychopharmacologyDocument11 paginiChapter 2 - Neurobiologic Theories and PsychopharmacologyCatia FernandesÎncă nu există evaluări
- ptj4008504 PDFDocument8 paginiptj4008504 PDFDiva VashtiÎncă nu există evaluări
- PARKINSONISMDocument5 paginiPARKINSONISMPrashanth RajuÎncă nu există evaluări
- What Is ParkinsonDocument23 paginiWhat Is Parkinsonlakshmi chowdamÎncă nu există evaluări
- Stem Cell-Based Therapies For Parkinson DiseaseDocument17 paginiStem Cell-Based Therapies For Parkinson DiseaseYunita Christiani BiyangÎncă nu există evaluări
- Phytotherapy in Treatment of Parkinson's Disease: A Review: Pharmaceutical BiologyDocument9 paginiPhytotherapy in Treatment of Parkinson's Disease: A Review: Pharmaceutical BiologyG. Araya MoraÎncă nu există evaluări
- Novel Pharmacological Targets For The Treatment of Parkinson's DiseaseDocument10 paginiNovel Pharmacological Targets For The Treatment of Parkinson's Disease2013_10_33_03_11_164Încă nu există evaluări
- Toneuj 10 42 PDFDocument17 paginiToneuj 10 42 PDFpratiwifatmasariÎncă nu există evaluări
- Review (1) - 2004 - Tabakmna Et AlDocument11 paginiReview (1) - 2004 - Tabakmna Et Alshimonl3892Încă nu există evaluări
- BAB I PaperDocument22 paginiBAB I Paperindry fitriÎncă nu există evaluări
- Parkinson's Disease A Review 2014 PDFDocument19 paginiParkinson's Disease A Review 2014 PDFjeanÎncă nu există evaluări
- TMP - 4306 300 596 1 SM (3) 1791561448Document5 paginiTMP - 4306 300 596 1 SM (3) 1791561448Lulu LuwiiÎncă nu există evaluări
- A Statistical Approach To Classification of Mechanistic Computational Models of Parkinson's DiseaseDocument65 paginiA Statistical Approach To Classification of Mechanistic Computational Models of Parkinson's DiseaseADIL KARIMÎncă nu există evaluări
- Seminar On Gait Rehab in PDDocument41 paginiSeminar On Gait Rehab in PDPriya KuberanÎncă nu există evaluări
- Drug-Induced Parkinsonism: Hae-Won Shin, Sun Ju ChungDocument7 paginiDrug-Induced Parkinsonism: Hae-Won Shin, Sun Ju ChungBaskarazrÎncă nu există evaluări
- Jha SK Et Al. Final Version - Mar 2015Document20 paginiJha SK Et Al. Final Version - Mar 2015Dylan JacksonÎncă nu există evaluări
- Beitz 2014Document10 paginiBeitz 2014Anna Beatriz Silva EspindolaÎncă nu există evaluări
- 186-Book Chapter-902-2-10-20210323Document24 pagini186-Book Chapter-902-2-10-20210323Yelaena AbrauÎncă nu există evaluări
- 2019 European Academy of Neurology Parkinson DiseaseDocument16 pagini2019 European Academy of Neurology Parkinson DiseaseJuan Diego Gutierrez ZevallosÎncă nu există evaluări
- Parkinsonism A General Motor Disability PDFDocument9 paginiParkinsonism A General Motor Disability PDFRishabh SinghÎncă nu există evaluări
- Parkinson's DiseaseDocument14 paginiParkinson's DiseaseRohan MistryÎncă nu există evaluări
- Jurnal NeuropsikiatriDocument10 paginiJurnal NeuropsikiatriPosterilmiah Majestynas2019Încă nu există evaluări
- Toxin-Induced Models of Parkinson's Disease: Jordi Bove, Delphine Prou, Ce Line Perier, and Serge PrzedborskiDocument11 paginiToxin-Induced Models of Parkinson's Disease: Jordi Bove, Delphine Prou, Ce Line Perier, and Serge PrzedborskiSomanshu BanerjeeÎncă nu există evaluări
- Parkinsons Thesis StatementDocument8 paginiParkinsons Thesis StatementMartha Brown100% (1)
- Parkinson ThesisDocument4 paginiParkinson Thesisandreajimenezomaha100% (1)
- Parkinson's DiseaseDocument9 paginiParkinson's Diseaselucia desantisÎncă nu există evaluări
- CORTEX PDreviewDocument8 paginiCORTEX PDreviewNadineÎncă nu există evaluări
- Altered Oxidative Stress Levels in Indian Parkinson's Disease Patients With PARK2 MutationsDocument5 paginiAltered Oxidative Stress Levels in Indian Parkinson's Disease Patients With PARK2 MutationssandykumalaÎncă nu există evaluări
- Neuroprotective Activities of Curcumin in Parkinson's DiseaseDocument20 paginiNeuroprotective Activities of Curcumin in Parkinson's Diseasemohamed mezaniÎncă nu există evaluări
- Parkinson Report Comprev For MR John ClassDocument2 paginiParkinson Report Comprev For MR John ClassshaffanaÎncă nu există evaluări
- F1000research 9 28292Document12 paginiF1000research 9 28292agostinolongoÎncă nu există evaluări
- Management in Parkinson DiseaseDocument30 paginiManagement in Parkinson Diseasezendah123Încă nu există evaluări
- Yarn All 2012Document7 paginiYarn All 2012Lycopersicum EsculentumÎncă nu există evaluări
- The Development of Parkinson's Disease, Its Clinical Features, and Ways of TreatmentDocument4 paginiThe Development of Parkinson's Disease, Its Clinical Features, and Ways of TreatmenthanÎncă nu există evaluări
- PARKINSONDocument59 paginiPARKINSONvannyÎncă nu există evaluări
- TitleDocument2 paginiTitleshaffanaÎncă nu există evaluări
- The Development of Treatment For Parkinson's Disease: Harishankar Prasad Yadav, Yun LiDocument20 paginiThe Development of Treatment For Parkinson's Disease: Harishankar Prasad Yadav, Yun LiTherezia Sonia Gabriella TanggulunganÎncă nu există evaluări
- Science:, 819 (2003) Ted M. Dawson and Valina L. DawsonDocument5 paginiScience:, 819 (2003) Ted M. Dawson and Valina L. DawsonSomanshu BanerjeeÎncă nu există evaluări
- Schapira - v7 - DY - FinalDocument46 paginiSchapira - v7 - DY - FinalTamimjdÎncă nu există evaluări
- Herbal Treatment of Parkinsonism A ReviewDocument7 paginiHerbal Treatment of Parkinsonism A ReviewKartika BorraÎncă nu există evaluări
- Parkinson's: A Syndrome Rather Than A Disease?: BackgroundDocument8 paginiParkinson's: A Syndrome Rather Than A Disease?: BackgroundEduardo Santana SuárezÎncă nu există evaluări
- Parkinson's Disease DMDocument19 paginiParkinson's Disease DMMOH ABDUL FRENGKIÎncă nu există evaluări
- Cognitive and Behavioral Disorders in Parkinson's Disease (2017)Document9 paginiCognitive and Behavioral Disorders in Parkinson's Disease (2017)LaureanoÎncă nu există evaluări
- Background Parkinson Disease Is Recognized As One of The Most Common Neurologic Disorders, AffectingDocument27 paginiBackground Parkinson Disease Is Recognized As One of The Most Common Neurologic Disorders, AffectingCharan Pal SinghÎncă nu există evaluări
- Learning Objectives For The Pharmacology of Parkinson Disease Drugs.Document5 paginiLearning Objectives For The Pharmacology of Parkinson Disease Drugs.bl9nkverseÎncă nu există evaluări
- TMP FFC1Document28 paginiTMP FFC1FrontiersÎncă nu există evaluări
- Parkinson's DiseaseDocument3 paginiParkinson's Diseasehady920Încă nu există evaluări
- Lysosomal Impairment in Parkinson's Disease: ReviewDocument8 paginiLysosomal Impairment in Parkinson's Disease: ReviewRara Aulia IIÎncă nu există evaluări
- What Is Parkinson's Disease?Document3 paginiWhat Is Parkinson's Disease?pingkan septianiÎncă nu există evaluări
- EBNfinalDocument4 paginiEBNfinalSophia LalagunaÎncă nu există evaluări
- Parkinson Disease Epidemiology, Pathology, Genetics, and PathophysiologyDocument12 paginiParkinson Disease Epidemiology, Pathology, Genetics, and PathophysiologyAna María Arenas DávilaÎncă nu există evaluări
- Involvement of Interferon-Dopaminergic Neurons: in Microglial-Mediated Loss ofDocument10 paginiInvolvement of Interferon-Dopaminergic Neurons: in Microglial-Mediated Loss ofshayley9Încă nu există evaluări
- Content ServerDocument11 paginiContent ServerHendi Imam RamadhanÎncă nu există evaluări
- Glutathione Parkinsons DiseaseDocument4 paginiGlutathione Parkinsons Diseaseimm100% (3)
- Efficacy and Safety of The 5-Hydroxytryptophan On Depression and Apathy inDocument18 paginiEfficacy and Safety of The 5-Hydroxytryptophan On Depression and Apathy inIrma DanielleÎncă nu există evaluări
- Role of Preclinical Data in PDDocument23 paginiRole of Preclinical Data in PDIrving ParraÎncă nu există evaluări
- Parkinson's Disease: EpidemiologyDocument6 paginiParkinson's Disease: EpidemiologyCarlos MichasÎncă nu există evaluări
- McCutcheon Et Al-2020-World PsychiatryDocument19 paginiMcCutcheon Et Al-2020-World PsychiatryRob McCutcheonÎncă nu există evaluări
- Fifty Years of Clinical Holistic Treatment for Parkinson’s: A Unique ApproachDe la EverandFifty Years of Clinical Holistic Treatment for Parkinson’s: A Unique ApproachEvaluare: 1 din 5 stele1/5 (1)
- EthicsDocument7 paginiEthicsMarianne GarciaÎncă nu există evaluări
- Trunk Line +63 (02) 871 06 39: College of Physical Therapy and Occupational TherapyDocument2 paginiTrunk Line +63 (02) 871 06 39: College of Physical Therapy and Occupational TherapyMarianne GarciaÎncă nu există evaluări
- DeliriumDocument6 paginiDeliriumMarianne Garcia100% (1)
- Garcia, Marianne Faye N. Assignment in FCL 402 Bsot-Ii Mrs. Gloria RoqueDocument1 paginăGarcia, Marianne Faye N. Assignment in FCL 402 Bsot-Ii Mrs. Gloria RoqueMarianne GarciaÎncă nu există evaluări
- Pope'S Biography: Garcia, Marianne Faye N. FCL 602 The Perpetualite: A Minister of Life BSOT-3 Mr. John ValenzuelaDocument2 paginiPope'S Biography: Garcia, Marianne Faye N. FCL 602 The Perpetualite: A Minister of Life BSOT-3 Mr. John ValenzuelaMarianne GarciaÎncă nu există evaluări
- My Perpetualite VoiceDocument1 paginăMy Perpetualite VoiceMarianne GarciaÎncă nu există evaluări
- Ionic Packet For Lab Chem 2010 2011Document16 paginiIonic Packet For Lab Chem 2010 2011Marianne Garcia50% (2)
- Glassware: Brandy SnifterDocument2 paginiGlassware: Brandy SnifterMarianne GarciaÎncă nu există evaluări
- The Role of Nucleus Accumbency Dopamine in Motivated Behavior: A Unifying Interpretation With Special Reference To Reward-SeekingDocument15 paginiThe Role of Nucleus Accumbency Dopamine in Motivated Behavior: A Unifying Interpretation With Special Reference To Reward-SeekingMarianne GarciaÎncă nu există evaluări
- Divine Revelation Sacred ScriptureDocument14 paginiDivine Revelation Sacred ScriptureMarianne GarciaÎncă nu există evaluări
- Saah 2004 PDFDocument7 paginiSaah 2004 PDFIno MoxoÎncă nu există evaluări
- DOCUMENTARYDocument35 paginiDOCUMENTARYSuna SunaÎncă nu există evaluări
- Alcohol Withdrawal SyndromeDocument37 paginiAlcohol Withdrawal SyndromeSandra Diaz ArangoitiaÎncă nu există evaluări
- An Essay On The Shaking Palsy: Five Stages of Parkinsons DiseaseDocument16 paginiAn Essay On The Shaking Palsy: Five Stages of Parkinsons DiseaseBindu NairÎncă nu există evaluări
- DisulfiramDocument9 paginiDisulfiramManit ThaweehanÎncă nu există evaluări
- Antipsychotic Drugs: Referance Book .Essential of Pharmacology by Shah NawazDocument47 paginiAntipsychotic Drugs: Referance Book .Essential of Pharmacology by Shah NawazLaiba ShahÎncă nu există evaluări
- Jumpin The GapDocument16 paginiJumpin The GapUsamaÎncă nu există evaluări
- Patogenesis ParkinsonDocument10 paginiPatogenesis ParkinsonEric Yesaya TanÎncă nu există evaluări
- WC500020164 PDFDocument29 paginiWC500020164 PDFNeicu Marius-RăzvanÎncă nu există evaluări
- All India Open Mock Clat 07 (Clat 46) 5753815Document36 paginiAll India Open Mock Clat 07 (Clat 46) 5753815Likitha DashÎncă nu există evaluări
- Kaplan - Sadocks Synopsis of Psychiatry, 11th Edition-318-323Document6 paginiKaplan - Sadocks Synopsis of Psychiatry, 11th Edition-318-323nurmaulida568Încă nu există evaluări
- EcstasyDocument9 paginiEcstasyjasper_cannonÎncă nu există evaluări
- SPC Pramipexole 0.7 MGDocument14 paginiSPC Pramipexole 0.7 MGJehan SarahdiniÎncă nu există evaluări
- Summary Book Abnormal Psychology Barlow Durand PDFDocument58 paginiSummary Book Abnormal Psychology Barlow Durand PDFLee DonghyuckÎncă nu există evaluări
- TidometDocument1 paginăTidometkimtaehyung.kyv2830Încă nu există evaluări
- KEY ANSWERS.109 Questions and Rationale On Psychotic DisordersDocument21 paginiKEY ANSWERS.109 Questions and Rationale On Psychotic DisordersBecca Jhoy Emong100% (2)
- 10.1177 0269881113482532 PDFDocument18 pagini10.1177 0269881113482532 PDFjuraiddinÎncă nu există evaluări
- BullyingDocument13 paginiBullyingRon Virreld BustamanteÎncă nu există evaluări
- Effects of DrugsDocument5 paginiEffects of DrugsTom Rico DivinaflorÎncă nu există evaluări
- Topic 8 - Grey MatterDocument53 paginiTopic 8 - Grey MatterStudent 365Încă nu există evaluări
- Limbic SystemDocument49 paginiLimbic SystemBaishali DebÎncă nu există evaluări
- Smart DrugsDocument10 paginiSmart Drugsenlightearthhawaii514Încă nu există evaluări
- The Science of LoveDocument12 paginiThe Science of LoveJerico Kier YesyesÎncă nu există evaluări
- Aspartame Addiction H. J. Roberts, M.D., F.A.C.P., F.C.C.P.Document16 paginiAspartame Addiction H. J. Roberts, M.D., F.A.C.P., F.C.C.P.Amy Sasser100% (1)
- Up Clinical PharmacyDocument14 paginiUp Clinical Pharmacyyhaky.21Încă nu există evaluări
- Calc Drip Rates 2Document2 paginiCalc Drip Rates 2Charisse Nicole DiazÎncă nu există evaluări
- Parkinson's Disease - A Compl. Gde. For Patients, Families - W. Weiner, Et. Al., (JHU Press, 2001) WWDocument383 paginiParkinson's Disease - A Compl. Gde. For Patients, Families - W. Weiner, Et. Al., (JHU Press, 2001) WWAlexandra BalanÎncă nu există evaluări
- Le Aiom 7 Mock 46 Ques @CL - MockDocument36 paginiLe Aiom 7 Mock 46 Ques @CL - Mockjitendra kumarÎncă nu există evaluări
- Biochemistry and Cell Biology of Dopaminergic NeurotransmissionDocument18 paginiBiochemistry and Cell Biology of Dopaminergic NeurotransmissionOneng IfayaniÎncă nu există evaluări