Documente Academic
Documente Profesional
Documente Cultură
Calcium Carbonate: A Guide For GCSE Students
Încărcat de
Syed Muhammad AnasTitlu original
Drepturi de autor
Formate disponibile
Partajați acest document
Partajați sau inserați document
Vi se pare util acest document?
Este necorespunzător acest conținut?
Raportați acest documentDrepturi de autor:
Formate disponibile
Calcium Carbonate: A Guide For GCSE Students
Încărcat de
Syed Muhammad AnasDrepturi de autor:
Formate disponibile
CALCIUM
CARBONATE
A guide for GCSE students
KNOCKHARDY PUBLISHING
2010
SPECIFICATIONS
CALCIUM CARBONATE
INTRODUCTION
This Powerpoint show is one of several produced to help students
understand selected GCSE Chemistry topics. It is based on the requirements
of the AQA specification but is suitable for other examination boards.
Individual students may use the material at home for revision purposes and
it can also prove useful for classroom teaching with an interactive white
board.
Accompanying notes on this, and the full range of AS and A2 Chemistry
topics, are available from the KNOCKHARDY SCIENCE WEBSITE at...
www.knockhardy.org.uk/gcse.htm
All diagrams and animations in this Powerpoint are original and
created by Jonathan Hopton. Permission must be obtained for their
use in any commercial work.
LIMESTONE
Limestone is made of calcium carbonate CaCO
3
Origin Formed in the sea millions of years ago
from the remains of shells
Found in places like the Peak District of Derbyshire
Extraction Quarried in large amounts
e.g. 150 million tonnes each year in the UK
IT IS AN IMPORTANT RAW MATERIAL
Use building material and road chippings
neutralising excess acid in lakes and soil
making cement and concrete
added to bread
added to toothpaste
LIMESTONE PRODUCTS ARE VERY USEFUL
GLASS
CONCRETE
CEMENT / MORTAR
especially in building houses
CARBONATES
Formulae All carbonates contain the CO
3
unit
The compounds are ionic; the formula is found by
balancing the charges of the ions
Carbonate ions have a 2- charge CO
3
2-
CALCIUM CARBONATE
CaCO
3
SODIUM CARBONATE
Na
2
CO
3
CARBONATES
Formulae All carbonates contain the CO
3
unit
The compounds are ionic; the formula is found by
balancing the charges of the ions
Carbonate ions have a 2- charge CO
3
2-
CALCIUM CARBONATE
CaCO
3
SODIUM CARBONATE
Na
2
CO
3
CARBONATES
Formulae All carbonates contain the CO
3
unit
Chemical
formula
sodium carbonate Na
2
CO
3
calcium carbonate CaCO
3
magnesium carbonate MgCO
3
copper carbonate CuCO
3
CARBONATES
Formulae All carbonates contain the CO
3
unit
Chemical
formula
sodium carbonate Na
2
CO
3
calcium carbonate CaCO
3
magnesium carbonate MgCO
3
copper carbonate CuCO
3
PROCESSING LIMESTONE
Roasting Heating limestone very strongly makes it break up
The process is known as THERMAL DECOMPOSITION
calcium carbonate > calcium oxide + carbon dioxide
CaCO
3
> CaO + CO
2
Calcium oxide is also known as QUICKLIME
ACTION OF HEAT ON METAL CARBONATES
METHOD
Place a small amount of one of the
solid carbonates in a dry test tube.
Place about 2cm
3
of lime water in
another test tube. Heat the solid,
gently at first, then more strongly.
Test any gas with the limewater.
Appearance CO
2
produced
Calcium carbonate
CaCO
3
Copper carbonate
CuCO
3
Magnesium carbonate
MgCO
3
Zinc carbonate
ZnCO
3
Residue Conclusion
metal
carbonate
lime water
CO
2
ACTION OF HEAT ON METAL CARBONATES
WHITE
SOLID
Appearance CO
2
produced
Calcium
carbonate
CaCO
3
Copper
carbonate
CuCO
3
Magnesium
carbonate
MgCO
3
Sodium carbonate (Na
2
CO
3
) also decomposes on heating but it requires more heat
than an ordinary bunsen burner can supply.
Zinc
carbonate
ZnCO
3
WHITE
SOLID
Calcium oxide
formed (needs very
strong heating)
BLACK
SOLID
Copper oxide
formed
WHITE
SOLID
Magnesium oxide
formed
YELLOW SOLID
WHICH TURNS
WHITE WHEN COOL
GREEN
SOLID
WHITE
SOLID
WHITE
SOLID
Residue Conclusion
Zinc oxide
formed
THERMAL DECOMPOSITION OF CARBONATES
All metal carbonates decompose when heated to form carbon dioxide and a
metal oxide. The process is known as THERMAL DECOMPOSITION
calcium carbonate > calcium oxide + carbon dioxide
copper carbonate > copper oxide + carbon dioxide
magnesium carbonate > magnesium oxide + carbon dioxide
sodium carbonate * > sodium oxide + carbon dioxide
zinc carbonate > zinc oxide + carbon dioxide
* THE THERMAL DECOMPOSITION OF GROUP I METAL CARBONATES
(SUCH AS SODIUM CARBONATE) REQUIRES A TREMENDOUS AMOUNT OF ENERGY
A BUNSEN BURNER IS NOT HOT ENOUGH
+
THERMAL DECOMPOSITION OF CARBONATES
All metal carbonates decompose when heated to form carbon dioxide and a
metal oxide. The process is known as THERMAL DECOMPOSITION
calcium carbonate > calcium oxide + carbon dioxide
copper carbonate > copper oxide + carbon dioxide
magnesium carbonate > magnesium oxide + carbon dioxide
sodium carbonate * > sodium oxide + carbon dioxide
zinc carbonate > zinc oxide + carbon dioxide
* THE THERMAL DECOMPOSITION OF GROUP I METAL CARBONATES
(SUCH AS SODIUM CARBONATE) REQUIRES A TREMENDOUS AMOUNT OF ENERGY
A BUNSEN BURNER IS NOT HOT ENOUGH
THERMAL DECOMPOSITION OF CARBONATES
CaCO
3
> CaO + CO
2
CuCO
3
> CuO + CO
2
MgCO
3
> MgO + CO
2
Na
2
CO
3
> Na
2
O + CO
2
ZnCO
3
> ZnO + CO
2
+
+
+
+
+
Write out the chemical equations
THERMAL DECOMPOSITION OF CARBONATES
CaCO
3
> CaO + CO
2
CuCO
3
> CuO + CO
2
MgCO
3
> MgO + CO
2
Na
2
CO
3
> Na
2
O + CO
2
ZnCO
3
> ZnO + CO
2
+
+
+
+
+
QUICKLIME
Calcium oxide CaO
Reacts with water to form SLAKED LIME (CALCIUM HYDROXIDE)
calcium oxide + water > calcium hydroxide + HEAT
CaO + H
2
O > Ca(OH)
2
Use added to soil to make it less acidic
added to lakes which have been polluted by acid rain
SLAKED LIME & LIME WATER
Lime water is a solution of calcium hydroxide in water
it is an alkali
it is used to test for the gas carbon dioxide
(limewater goes cloudy if CO
2
is present)
calcium hydroxide + carbon dioxide > calcium carbonate + water
Ca(OH)
2
+ CO
2
> CaCO
3
+ H
2
O
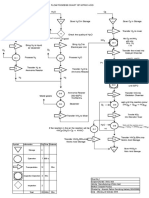
LIMESTONE CYCLE
AQUEOUS
CALCIUM
HYDROXIDE
(lime water)
SOLID CALCIUM HYDROXIDE (slaked lime)
CALCIUM CARBONATE (limestone)
CALCIUM
OXIDE
(quicklime)
CEMENT, CONCRETE & GLASS
All three rely on limestone for their manufacture
CEMENT powdered clay
powdered limestone
mix and roast them in a rotary kiln
CEMENT, CONCRETE & GLASS
All three rely on limestone for their manufacture
MORTAR cement
sand
water
mortar is a thin form of concrete used for bricklaying
CEMENT, CONCRETE & GLASS
All three rely on limestone for their manufacture
CONCRETE cement - bonds aggregate together
sand
powdered rock or chippings
water - makes it easier to work with
- causes a chemical reaction to harden the cement
allow the mixture to dry slowly (set)
you get a very hard solid
it is much stronger than simple cement
CEMENT, CONCRETE & GLASS
All three rely on limestone for their manufacture
CONCRETE cement - bonds aggregate together
sand
powdered rock or chippings
water - makes it easier to work with
- causes a chemical reaction to harden the cement
You choose the correct mixture for the job you are doing
foundations, driveways cement (1) large chippings (4)
paths cement (1) small chippings (3)
filling cracks, holes cement (1) coarse sand (3)
you must not add too much water otherwise it takes ages to dry
dont let it set too quickly otherwise it cracks
avoid laying concrete when it is frosty - it disintegrates
For plastering walls GYPSUM is used; it is a form of calcium sulphate
CEMENT, CONCRETE & GLASS
All three rely on limestone for their manufacture
GLASS made by heating a mixture of
limestone - calcium carbonate
sand - silica
soda - sodium carbonate
+ small amounts of other chemicals can be added to make special glass
REACTION OF ACIDS WITH CARBONATES
All carbonates react with acids to form a salt, carbon dioxide and water.
It is an example of a NEUTRALISATION REACTION
CARBONATE + ACID > A SALT + CARBON DIOXIDE + WATER
Everyday examples
Bad - acid rain attacks limestone rocks in the countryside
- monuments and statues are attacked
Good - carbonates can neutralise excess acid in the stomach
REACTION OF ACIDS WITH CARBONATES
All carbonates react with acids to form a salt, carbon dioxide and water.
It is an example of a NEUTRALISATION REACTION
CARBONATE + ACID > A SALT + CARBON DIOXIDE + WATER
THE SALT THAT IS FORMED DEPENDS ON
THE METAL CARBONATE AND THE ACID USED
REACTION OF ACIDS WITH CARBONATES
All carbonates react with acids to form a salt, carbon dioxide and water.
It is an example of a NEUTRALISATION REACTION
CARBONATE + ACID > A SALT + CARBON DIOXIDE + WATER
THE SALT THAT IS FORMED DEPENDS ON
THE METAL CARBONATE AND THE ACID USED
HYDROCHLORIC ACID produces CHLORIDES
NITRIC ACID produces NITRATES
SULPHURIC ACID produces SULPHATES
REACTION OF ACIDS WITH CARBONATES
All carbonates react with acids to form a salt, carbon dioxide and water.
It is an example of a NEUTRALISATION REACTION
CARBONATE + ACID > A SALT + CARBON DIOXIDE + WATER
calcium + hydrochloric > calcium + carbon + water
carbonate acid chloride dioxide
sodium + sulphuric > sodium + carbon + water
carbonate acid sulphate dioxide
copper + nitric > copper + carbon + water
carbonate acid nitrate dioxide
HYDROCHLORIC ACID produces CHLORIDES
NITRIC ACID produces NITRATES
SULPHURIC ACID produces SULPHATES
REACTION OF ACIDS WITH CARBONATES
All carbonates react with acids to form a salt, carbon dioxide and water.
It is an example of a NEUTRALISATION REACTION
CARBONATE + ACID > A SALT + CARBON DIOXIDE + WATER
calcium + hydrochloric > calcium + carbon + water
carbonate acid chloride dioxide
sodium + sulphuric > sodium + carbon + water
carbonate acid sulphate dioxide
copper + nitric > copper + carbon + water
carbonate acid nitrate dioxide
HYDROCHLORIC ACID produces CHLORIDES
NITRIC ACID produces NITRATES
SULPHURIC ACID produces SULPHATES
REACTION OF ACIDS WITH CARBONATES
All carbonates react with acids to form a salt, carbon dioxide and water.
It is an example of a NEUTRALISATION REACTION
CARBONATE + ACID > A SALT + CARBON DIOXIDE + WATER
calcium + hydrochloric > calcium + carbon + water
carbonate acid chloride dioxide
sodium + sulphuric > sodium + carbon + water
carbonate acid sulphate dioxide
copper + nitric > copper + carbon + water
carbonate acid nitrate dioxide
HYDROCHLORIC ACID produces CHLORIDES
NITRIC ACID produces NITRATES
SULPHURIC ACID produces SULPHATES
REACTION OF ACIDS WITH CARBONATES
All carbonates react with acids to form a salt, carbon dioxide and water.
It is an example of a NEUTRALISATION REACTION
CARBONATE + ACID > A SALT + CARBON DIOXIDE + WATER
calcium + hydrochloric > calcium + carbon + water
carbonate acid chloride dioxide
sodium + sulphuric > sodium + carbon + water
carbonate acid sulphate dioxide
copper + nitric > copper + carbon + water
carbonate acid nitrate dioxide
HYDROCHLORIC ACID produces CHLORIDES
NITRIC ACID produces NITRATES
SULPHURIC ACID produces SULPHATES
REACTION OF ACIDS WITH CARBONATES
All carbonates react with acids to form a salt, carbon dioxide and water.
It is an example of a NEUTRALISATION REACTION
CARBONATE + ACID > A SALT + CARBON DIOXIDE + WATER
calcium + hydrochloric > calcium + carbon + water
carbonate acid chloride dioxide
sodium + sulphuric > sodium + carbon + water
carbonate acid sulphate dioxide
copper + nitric > copper + carbon + water
carbonate acid nitrate dioxide
HYDROCHLORIC ACID produces CHLORIDES
NITRIC ACID produces NITRATES
SULPHURIC ACID produces SULPHATES
REACTION OF ACIDS WITH CARBONATES
magnesium + nitric > magnesium + carbon + water
carbonate acid nitrate dioxide
zinc + hydrochloric > zinc + carbon + water
carbonate acid chloride dioxide
sodium + nitric > sodium + carbon + water
carbonate acid nitrate dioxide
zinc + sulphuric > zinc + carbon + water
carbonate acid sulphate dioxide
copper + sulphuric > copper + carbon + water
carbonate acid sulphate dioxide
potassium + hydrochloric > potassium + carbon + water
carbonate acid chloride dioxide
REACTION OF ACIDS WITH CARBONATES
magnesium + nitric > magnesium + carbon + water
carbonate acid nitrate dioxide
zinc + hydrochloric > zinc + carbon + water
carbonate acid chloride dioxide
sodium + nitric > sodium + carbon + water
carbonate acid nitrate dioxide
zinc + sulphuric > zinc + carbon + water
carbonate acid sulphate dioxide
copper + sulphuric > copper + carbon + water
carbonate acid sulphate dioxide
potassium + hydrochloric > potassium + carbon + water
carbonate acid chloride dioxide
AQA C1.2.1 Calcium carbonate - summary
a) Limestone, mainly composed of the compound calcium carbonate (CaCO
3
), is
quarried and can be used as a building material.
b) Calcium carbonate can be decomposed by heating (thermal decomposition) to
make calcium oxide and carbon dioxide.
c) The carbonates of magnesium, copper, zinc, calcium and sodium decompose on
heating in a similar way to give carbon dioxide and the metal oxide.
Not all carbonates of metals in Group 1 of the periodic table decompose at the
temperatures reached by a Bunsen burner.
d) Calcium oxide reacts with water to produce calcium hydroxide, which is an
alkali that can be used in the neutralisation of acids.
e) A solution of calcium hydroxide in water (limewater) reacts with carbon dioxide
to produce calcium carbonate. Limewater is used as a test for carbon dioxide.
Carbon dioxide turns limewater cloudy.
f) Carbonates (eg Mg, Cu, Zn, Ca, Na) react with acids to produce carbon dioxide,
a salt and water. Limestone is damaged by acid rain.
g) Limestone is heated with clay to make cement. Cement is mixed with sand to
make mortar and with sand and aggregate to make concrete.
CALCIUM
CARBONATE
THE END
2011 JONATHAN HOPTON & KNOCKHARDY PUBLISHING
S-ar putea să vă placă și
- Carbonates LimestoneDocument24 paginiCarbonates LimestoneVeronica HanyÎncă nu există evaluări
- Scientific American Supplement, No. 832, December 12, 1891De la EverandScientific American Supplement, No. 832, December 12, 1891Încă nu există evaluări
- Carbonates IGCSE NotesDocument4 paginiCarbonates IGCSE NotesMisbah KamranÎncă nu există evaluări
- 13.1 Carbonates: Calcium Oxide and Calcium CarbonateDocument3 pagini13.1 Carbonates: Calcium Oxide and Calcium CarbonateDavies MasumbaÎncă nu există evaluări
- Chapter#9 (Limestone) Ppt#1Document10 paginiChapter#9 (Limestone) Ppt#1X-Tremer The Phone GamerÎncă nu există evaluări
- 2 - Water N TreatmentDocument47 pagini2 - Water N TreatmentdarshanÎncă nu există evaluări
- 13.1 Carbonates: Calcium Oxide & Calcium CarbonateDocument11 pagini13.1 Carbonates: Calcium Oxide & Calcium CarbonateelizabethÎncă nu există evaluări
- Carbonation in Chemical Weathering by Zak FleschDocument1 paginăCarbonation in Chemical Weathering by Zak FleschlenanaÎncă nu există evaluări
- Soda Ash PresDocument32 paginiSoda Ash PresGaurav GuptaÎncă nu există evaluări
- Soda AshDocument21 paginiSoda Ashmlwbd2069Încă nu există evaluări
- WJEC LimestoneDocument30 paginiWJEC LimestoneGarethÎncă nu există evaluări
- Quimico DelatadDocument10 paginiQuimico DelatadKeiber GonzalezÎncă nu există evaluări
- CarbonatesDocument5 paginiCarbonatesUmar UsmanÎncă nu există evaluări
- Chapter Seven Water Softening AND Other MiscellaneousDocument22 paginiChapter Seven Water Softening AND Other Miscellaneoussami100% (1)
- Ocean Acidification:: The Other CO ProblemDocument24 paginiOcean Acidification:: The Other CO Problemnirbhay99Încă nu există evaluări
- Chapter 7Document21 paginiChapter 7Solomon DesalegnÎncă nu există evaluări
- Sodium Carbonate: NA CODocument44 paginiSodium Carbonate: NA COMg HÎncă nu există evaluări
- 12S-2023 Chemistry 9Document9 pagini12S-2023 Chemistry 9Alumbwe MubondaÎncă nu există evaluări
- Chem Mod1Document10 paginiChem Mod1baritone.exhaustÎncă nu există evaluări
- Extraction of MetalsDocument55 paginiExtraction of MetalsCatriona Chaikin100% (1)
- Name:Sourav Singh. Class:2Nd Year 3Rd Semester. Course: Civil Engineering. ROLLNO:CEN204040. Sub:Building MaterialsDocument36 paginiName:Sourav Singh. Class:2Nd Year 3Rd Semester. Course: Civil Engineering. ROLLNO:CEN204040. Sub:Building MaterialsSOURAAVÎncă nu există evaluări
- Softening FinalDocument23 paginiSoftening FinalSonali Jahagirdar100% (1)
- Questions LimestoneDocument1 paginăQuestions LimestonemillergraÎncă nu există evaluări
- Calcite Aragonite VateriteDocument2 paginiCalcite Aragonite VateriteManang JainÎncă nu există evaluări
- CarbonatesDocument4 paginiCarbonatesshehryar khanÎncă nu există evaluări
- Boiler Water ConditioningDocument3 paginiBoiler Water ConditioningShahin AfrozÎncă nu există evaluări
- Lime 2017Document2 paginiLime 2017Gaurav SinghÎncă nu există evaluări
- Carbon and Its Compounds NotesDocument20 paginiCarbon and Its Compounds NotesCORES TECHNOLOGIESÎncă nu există evaluări
- CE-121 Civil Engineering Materials Lecture 2Document74 paginiCE-121 Civil Engineering Materials Lecture 2Anonymous 0ClbS49QkuÎncă nu există evaluări
- Chapter 4 Lec1Document40 paginiChapter 4 Lec1Gemechis TolaÎncă nu există evaluări
- Limestone: Caco (S) Cao (S) + Co (G)Document1 paginăLimestone: Caco (S) Cao (S) + Co (G)Davies MasumbaÎncă nu există evaluări
- Lecture 6Document60 paginiLecture 6Ahmad NawazÎncă nu există evaluări
- Lecture #02 ConcreteDocument44 paginiLecture #02 ConcreteMuhammad UmairÎncă nu există evaluări
- Soda Ash ProductionDocument10 paginiSoda Ash Productionimjaral75% (4)
- Water - OfficialDocument62 paginiWater - OfficialPushp BahukhandiÎncă nu există evaluări
- Laporan Anorganik Modul IIDocument19 paginiLaporan Anorganik Modul IITia Dwi Lestari NentoÎncă nu există evaluări
- Notes Chapter 6Document2 paginiNotes Chapter 6kelvin_890Încă nu există evaluări
- Manufacture of Soda Ash - LectDocument10 paginiManufacture of Soda Ash - LectIbrahim Al-MutazÎncă nu există evaluări
- LimestoneDocument19 paginiLimestoneKev WattsÎncă nu există evaluări
- FALLSEM2019-20 CHY1701 ETH VL2019201007061 Reference Material I 19-Jul-2019 FALLSEM2013-14 CP2530 31-Jul-2013 RM01 Presentation1Document66 paginiFALLSEM2019-20 CHY1701 ETH VL2019201007061 Reference Material I 19-Jul-2019 FALLSEM2013-14 CP2530 31-Jul-2013 RM01 Presentation1jaswanth chowdary lankaÎncă nu există evaluări
- Removal of ImpuritiesDocument27 paginiRemoval of ImpuritiesAbdullah ZaidÎncă nu există evaluări
- Production Technology of Cloro-Alkali IndustriesDocument71 paginiProduction Technology of Cloro-Alkali IndustriesBereket Tadesse100% (1)
- UNIT I - Water Treatment: (Soluble Sodium Stearate) (Insoluble Ca-Stearate) SoapDocument25 paginiUNIT I - Water Treatment: (Soluble Sodium Stearate) (Insoluble Ca-Stearate) Soap52 Shagun ChaudhariÎncă nu există evaluări
- Unit 1Document11 paginiUnit 1softsen10Încă nu există evaluări
- CementDocument55 paginiCementSajjad AhmadÎncă nu există evaluări
- UNIT V: Engineering Materials, Nanoscience & NanotechnologyDocument40 paginiUNIT V: Engineering Materials, Nanoscience & NanotechnologydileepÎncă nu există evaluări
- c1 Revision Notes - Set 1 OnlyDocument10 paginic1 Revision Notes - Set 1 Onlyapi-320022467Încă nu există evaluări
- Sulfates and CarbonatesDocument1 paginăSulfates and CarbonatesGirvinÎncă nu există evaluări
- Lime Kiln ProcessDocument9 paginiLime Kiln ProcessFelipe Santos100% (1)
- CP-XVII (Soda Ash & Caustic Soda)Document12 paginiCP-XVII (Soda Ash & Caustic Soda)Usman AliÎncă nu există evaluări
- Carbondioxide 1Document7 paginiCarbondioxide 1Willam SmithÎncă nu există evaluări
- Boiler WaterDocument70 paginiBoiler WaterDarius Dsouza100% (1)
- IndustrialchemistryDocument2 paginiIndustrialchemistryKimtuyen TranÎncă nu există evaluări
- Internship MpurDocument20 paginiInternship MpurKaranÎncă nu există evaluări
- Iwaste Hardness PDFDocument5 paginiIwaste Hardness PDFOnyx XynoÎncă nu există evaluări
- Sodiumcarbonate 180826152936Document31 paginiSodiumcarbonate 180826152936Aliha AzmatÎncă nu există evaluări
- Environmental Chemistry - Causes of Hardness in WaterDocument87 paginiEnvironmental Chemistry - Causes of Hardness in WaterVikas KabburiÎncă nu există evaluări
- 10ME TheoryDocument4 pagini10ME TheoryalfipincukÎncă nu există evaluări
- Water ChemistryDocument19 paginiWater ChemistryNupur ChoudharyÎncă nu există evaluări
- Phanerozoic Stratigraphy of Saudi Arabia - Part 1 PDFDocument74 paginiPhanerozoic Stratigraphy of Saudi Arabia - Part 1 PDFSyed Muhammad AnasÎncă nu există evaluări
- Astm D698Document7 paginiAstm D698Annilyn EncinasÎncă nu există evaluări
- Lithogeochemichal ExplorationDocument7 paginiLithogeochemichal ExplorationSyed Muhammad AnasÎncă nu există evaluări
- Theoretical GeomechanicsDocument4 paginiTheoretical GeomechanicsSyed Muhammad AnasÎncă nu există evaluări
- 4 TocDocument1 pagină4 TocSyed Muhammad AnasÎncă nu există evaluări
- Separating The Six Platinum MetalsDocument9 paginiSeparating The Six Platinum MetalsAFLAC ............100% (1)
- Us 4378342Document9 paginiUs 4378342هیمن مÎncă nu există evaluări
- Nitric OxideDocument11 paginiNitric OxideDr. M. Prasad NaiduÎncă nu există evaluări
- HKCEE Part 4 Acids and BasesDocument64 paginiHKCEE Part 4 Acids and BasesTiana LamÎncă nu există evaluări
- D3223.22337 - Standard Test Method For Total Mercury in WaterDocument8 paginiD3223.22337 - Standard Test Method For Total Mercury in WaterIngrid Mora100% (1)
- Msds Hidrogen PeroxideDocument6 paginiMsds Hidrogen PeroxideIntan Yeti SeptiyaniÎncă nu există evaluări
- June 2020 (9-1) (v1) QP - Paper 6 CIE Chemistry IGCSEDocument8 paginiJune 2020 (9-1) (v1) QP - Paper 6 CIE Chemistry IGCSENatalie RossetteÎncă nu există evaluări
- I-1 I-2 Throw If Not Good Throw If Not Good: Save HNO3 in Nitric Acid StorageDocument2 paginiI-1 I-2 Throw If Not Good Throw If Not Good: Save HNO3 in Nitric Acid StorageAmanda MelialaÎncă nu există evaluări
- 3.5 Sulphur and Its CompoundsDocument27 pagini3.5 Sulphur and Its CompoundsCharles OtienoÎncă nu există evaluări
- Laboratory Experiment 6 - Proteins (GROUP 4)Document14 paginiLaboratory Experiment 6 - Proteins (GROUP 4)Renee Dwi Permata Messakaraeng100% (1)
- The Canning Handbook 2005Document65 paginiThe Canning Handbook 2005Khomasan Jumpasri100% (3)
- P Block 15TH Group Elements NotesDocument11 paginiP Block 15TH Group Elements Notesiipud072.giridhar.k MESKKPUCÎncă nu există evaluări
- Chem Investigatory ProjectDocument26 paginiChem Investigatory ProjectDevanand Siva SankaradasÎncă nu există evaluări
- Chemistry Project On Food AnalyasisDocument16 paginiChemistry Project On Food Analyasisvikram100% (1)
- 5.4.1 Arenes635464Document6 pagini5.4.1 Arenes635464ArchitÎncă nu există evaluări
- Safe Practices For The Production of Nitrous Oxide From Ammonium NitrateDocument23 paginiSafe Practices For The Production of Nitrous Oxide From Ammonium NitrateWrig PrajapatiÎncă nu există evaluări
- The P-Block Elements - Short Notes - Lakshya JEE 2024Document6 paginiThe P-Block Elements - Short Notes - Lakshya JEE 2024krishiv vyas :- 1022Încă nu există evaluări
- Copper Leaching From Electronic Waste For The Improvement of Gold RecyclingDocument9 paginiCopper Leaching From Electronic Waste For The Improvement of Gold RecyclingOvijit DasÎncă nu există evaluări
- MSDS - AnilinDocument6 paginiMSDS - AnilinsaririskihasibuanÎncă nu există evaluări
- Chemical Methode ManualDocument211 paginiChemical Methode ManualThufeil Tufi Yunindika100% (1)
- A New Method For Nitration of Phenolic CompoundsDocument6 paginiA New Method For Nitration of Phenolic CompoundsOmar valdesÎncă nu există evaluări
- 1 s2.0 S2772582022000079 MainDocument18 pagini1 s2.0 S2772582022000079 MainfredyzÎncă nu există evaluări
- Archives of Animal NutritionDocument16 paginiArchives of Animal NutritiondedyÎncă nu există evaluări
- Kcse 2023 Chemistry Replica.Document150 paginiKcse 2023 Chemistry Replica.micah isabokeÎncă nu există evaluări
- FL53098 Chemical Analysis Brass Lesson PlanDocument6 paginiFL53098 Chemical Analysis Brass Lesson Planmbbk5783Încă nu există evaluări
- (BS 1016-9 - 1977) - Methods For Analysis and Testing of Coal and Coke. Phosphorus in Coal and CokeDocument10 pagini(BS 1016-9 - 1977) - Methods For Analysis and Testing of Coal and Coke. Phosphorus in Coal and CokeAdelÎncă nu există evaluări
- A. The Process of Making BiodieselDocument6 paginiA. The Process of Making BiodieselInayah VeraÎncă nu există evaluări
- 01calcium Ammonium NitrateDocument32 pagini01calcium Ammonium NitrateHear M100% (1)
- Electro HorticultureDocument64 paginiElectro Horticulturepetri_jv67% (6)
- Aoac 906.03 PDFDocument2 paginiAoac 906.03 PDFAngie Cerinza AcostaÎncă nu există evaluări