Documente Academic
Documente Profesional
Documente Cultură
Metal Complexes or Coordination Compounds: Kfecn 4K Fe CN
Încărcat de
Pavan BoroTitlu original
Drepturi de autor
Formate disponibile
Partajați acest document
Partajați sau inserați document
Vi se pare util acest document?
Este necorespunzător acest conținut?
Raportați acest documentDrepturi de autor:
Formate disponibile
Metal Complexes or Coordination Compounds: Kfecn 4K Fe CN
Încărcat de
Pavan BoroDrepturi de autor:
Formate disponibile
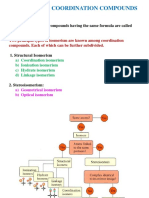
Metal Complexes or Coordination Compounds
In this chapter we shall study the following articles.
1. Introduction
2. Terminology of coordination compounds
3. IUPAC nomenclature of coordination compounds
4. Theories of bonding in coordination compounds
5. Isomerism in coordination compounds
6. Stability of coordination compounds
7. Organometallic compounds metal carbonyls.
1. Introduction:
In the study of inorganic chemistry so far, we have come across many
compounds like K
4
[Fe(CN)
6
], Na[Ag(CN)
2
], [Cu(NH
3
)
4
] SO
4
, K
2
[PtCl
6
] etc.
which if soluble in water will dissociate to give complex ion/s for
example
( ) ( )
+
( (
+
4
4
6 6
K Fe CN 4K Fe CN
(aq.) (aq.) (aq.)
Now in this solution we can test K
+
but not Fe
2+
or CN
, because the ion
[Fe(CN)
6
]
4
is a complex ion. Ofcourse a practical test eligible for this
complex ion will certainly prove its existence in the solution. Such
compounds are called complex compounds or since coordinate bonds
are formed within a complex ion between the metal and the electron
pair donor groups which we shall soon study as ligands, these
compounds are also known as coordination compounds. It must be
clearly understood that there is a clear distinction between coordination
compounds and double salts. For example we consider K
4
[Fe(CN)
6
] and
Mohr salt i.e. FeSO
4
.(NH
4
)
2
SO
4
.6H
2
O, then former is a complex but later
is a double salt because aqueous solution of Mohr salt will give positive
tests of all the ions present in its composition i.e. Fe
2+
, NH
4
+
and SO
4
2-
.
Potash alum K
2
SO
4
.Al
2
(SO
4
)
3
. 24H
2
O is another example of a double salt.
Well! we are concerned with coordination compounds in this chapter.
For the sake of convenience we can classify coordination compounds
into following four classes:
(i) A simple cation and complex anion e.g. K
4
[Fe(CN)
6
]
(ii) Complex cation and simple anion e.g. [Co(NH
3
)
6
]Cl
3
(iii) Complex cation and complex anion e.g.
[Co(NH
3
)
6
] [Cr(C
2
O
4
)
3
]
(iv) Non-ionic complex e.g.
[Co(NH
3
)
3
Cl
3
]
Now before we start actual study of metal complexes, it should be kept
in the mind that such compounds which have been prepared unlimited
in number and also exist in the nature play a vital role in our lives. For
example the green coloring matter of plants chlorophyll is a Magnesium
complex and the red content of the blood i.e. Haemoglobin is a complex
of iron.
2. Terminology of Coordination Compounds:
Consider the complex K
4
[Fe(CN)
6
] as below:
central metal atom/ion
K
4
[Fe(CN)
6
] Ligands
free cations complex anion
4 (K
+
) or coordination sphere
or the complex
Ligands
Co (NH
3
)
6
] Cl
3
free anions (3Cl
)
central metal atom/ion
complex catin
or
coordination sphere
If the complex contain cation and anion both in the complex state then there
shall be two coordination spheres.
Thus we can define a metal complex as a ion or neutral molecule in the
coordination sphere of which a metal atom or ion is bonded by a fixed
number of similar or different electron pair donors called Ligands.
These ligands are bonded t the central metal through coordinate bonds
hence these compounds are called coordination compounds. It is not
necessary that each coordination sphere must have only one metal atom
or ion, their number can be more than one also, such complexes are
called polynuclear complexes for example:
NH
2
(en)
2
Co Co (en)
2
(SO
4
)
2
OH
But our course is limited to mono nuclear complexes only.
Let us now discus in details the fundamental terms used in the study of a
complex.
(i) Ligand:
These are generally anions, or neutral molecules which are directly
attached to the metal atom or ion in the coordination sphere through
coordinate bonds. In some rare cases even a cation may behave like a
ligand provided it too is capable of donating lone pair/s some examples
of ligands are:
anions:
Cl
, CN
or [C = N]
etc.
neutral molecules:
NH
3
O CO etc.
H H
Since ligands are electron pair or pairs donors hence we can call them
Lewis bases also.
Types of Ligands:
This classification is based on the number of lone pairs a ligand can
donate to bind it self with the central metal atom or ion.
(a) Monodentate:
These ligands have one donor atom each such that only lone pair of
electrons is donated per ligand.
examples:
F
, Cl
, Br
, CN
, NO
2
, OH
NH
3
, H
2
O, CO, NO, C
5
H
5
N (pyridine) etc.
(b) Bidentate
Such a ligand has two donor atoms so that it can bind to the central
metal atom/ion at two locations by forming two coordinate bonds.
examples:
NH
2
CH
2
CH
2
NH
2
(ethylene diamine)
(2, 2' bipyridyl),
NH
2
CHCH
2
NH
2
|
CH
3
1, 2 propanediamine
O O H
|
CC CH
2
NH
O O, |
oxalate ion CO
(C
2
O
4
)
2
|| ()
O
glycine anion
(NH
2
CH
2
COO
)
OCO
||
O
Carbonate ion
(CO
3
2
)
etc.
(c) Tridentate: Ligands with three donor atoms:
example:
H
2
NCH
2
CH
2
NHCH
2
CH
2
NH
2
Diethylenetriamine
or
N(2-aminoethyl) 1, 2 ethane diamine
(d) Tetradentate: ligands with four donor atoms
example:
NH
2
CH
2
CH
2
NHCH
2
CH
2
NHCH
2
CH
2
NH
2
Triethylene tetramine
Similarly there are pentadentate, hexadentate or in general polydentate
ligands. One good example of a hexadentate ligand is the tetraanion of
EDTA i.e. ethylene diamine tetra acetic acid.
OOCCH
2
CH
2
COO
NCH
2
CH
2
--
OOCCH
2
CH
2
COO
Multi or polydentate ligands are also known as chelating agents if on
coordination with the metal a cyclic ring is formed. For example ethylene
diamine (en) forms a complex with Pt(II) as [Pt Cl
2
(en)].
It can be expanded as:
Cl NH
2
CH
2
Pt |
Cl NH
2
CH
2
So here ethylene diamine (en) is forming a five membered ring hence it is
behaving as a chelating agent.
Some ligands are known as Flexidentate because their denticity is variable. For
example EDTA may behave as hexa, penta or even tetradentate ligand
depending on the condition similarly SO
4
2
may behave as mono or
bidentate ligand. Some ligands are called Ambidentate which have
alternative donor atom for example cyanide ion [CN]
may bind itself to
the metal through carbon or through N depending on the conditions of
coordination. Similarly NO
2
may bind through N or through oxygen
atom.
(ii) Coordination Number:
It is the properly of the central metal. It is defined as the number of
atoms of the ligands that are directly bound to the central metal atom
or ion through coordinate bonds. For example in the complex ion
[Cu(NH
3
)
4
]
2+
; the coordination number of Cu(II) is four because it is
boned with 4NH
3
molecules (ligands) through four coordinate bonds.
NH
3
|
Cu
2+
H
3
N NH
3
|
NH
3
Similarly in complex ion [Co(NH
3
)
6
]
3+
, the coordination number of Co(III)
is six.
In the complex ion [Co(en)
3
]
3+
also the coordination number of Co(III) or
Co
3+
is six because 'en' i.e. ethylenediamine (NH
2
CH
2
CH
2
NH
2
) is bidentate
ligand. The most common coordination numbers usually exhibited by
metal atom/ions are 2, 4 and 6.
But many complexes of higher coordination numbers have been prepared. In the
following table, some common metal ions with their usual coordination
numbers have been shown.
Table
Metal ion Coordination Number
Cu
+
2, 4
Ag
+
2
Au
+
2, 4
Mg
2+
6
Fe
2+
6
Co
2+
6
Ni
2+
4, 6
Cu
2+
4, 6
Pt
2+
4
Al
3+
6
Fe
3+
6
Co
3+
6
Au
3+
6
Pt
4+
6
Pd
4+
6
3.
IUPAC Nomenclature of Coordination Compounds
Although some widely used complexes are still known by their trivial names for
example K
4
[Fe(CN)
6
] is commonly called potassium ferrocyanide but the
number of complexes is so high that it is not possible to have trivial
names of all of them. Hence IUPAC formulated certain rules to write a
systematic name of any complex molecule or ion. For this purpose IUPAC
has evolved names of ligands and metals too for writing the complete
name of a complex.
Let us first study the names of ligands:
Ligand IUPAC Name
H
hydrido
D
deuterido
Cl
Chlorido
Br
bromide
I
iodide
F
fluoride
CN
cyanide
CH
3
COO
acetate or
NO
2
nitrito N
ONO
nitrito O
OH
hydroxide
NH
2
amido
OCN
cyanatoN
NCO
cyanatoO
SCN
thiocyanatoN
NCS
thiocyanatoS
HSO
3
hydrogensulphito
HS
hydrogensulphido
or
mercapto
S
2
O
3
2
thiosulphato
O
2
oxido
2
2
O
peroxide
2
O
superoxido
3
O
ozonido
3
N
azido
2
2 4
C O
oxalate
2
3
CO
carbonato
2
3
SO
sulphito
2
4
SO
sujphato
S
2
sulphido
NH
2
imido
N
3
nitride
H
2
NCH
2
COO
glycenato
CH
3
CCH = CCH
3
|| |
O
acetonyl
acetonato
Thus in general if anion name ends in 'ide', 'ite' or 'ate' then the final 'e'
is replaced by 'O' giving ido, ito, and 'ato'.
Names of some neutral ligands
NH
3
ammine
CH
3
NH
2
methylamine
NH
2
CH
2
CH
2
NH
2
ethlyenediamine
H
2
O aquo or aqua
CO carbonyl
NO nitrosyl
C
5
H
5
N pyridine
CH
3
CONH
2
acetamide
PH
3
phosphane
CH
3
PH
2
methylphosphane
O
2
dioxygen
Names of some (rare) cationic ligands
NO
+
nitrosylium
NH
2
NH
3
+
hydrazinium
Some short notations very commonly used are:
Et = ethyl (CH
3
CH
2
)
Me = Methyl (CH
3
)
Ph = phenyl (C
6
H
5
)
Pr = propyl (CH
3
CH
2
CH
2
)
py = pyridine
en = ethylenediamine (NH
2
CH
2
CH
2
NH
2
)
dien = diethylenetriamine
(H
2
NCH
2
CH
2
NHCH
2
CH
2
NH
2
)
bpy = 2, 2' bipyridine
H
2
dmg = dimethyl glyoxime
NOH
CH
3
C
|
CH
3
C
NOH
Ur = Urea (NH
2
CONH
2
)
acac = acetylacetonato
CH
3
CCH = CCH
3
| |
OH O
etc.
Name of metals whenever the metal is present in the anionic complex ion. For
example: in the complex Na
2
[CuCl
4
], the metal ion Cu
2+
or Cu(II) is
present in complex anion hence its name will be written as Cuprate (II).
Similarly
Cr chromate; Pt palatinate;
Ag argentali Ni nickelate;
Zn zincate; Co Cobaltate
Fe ferrate; Hg mercurate;
W tungstate or wolframate;
Sc scandate; Pd palladate;
Ti titanate; V vanadate;
Cu cuprate; Au aurate etc.
But the name of the metal present in the cationic complex will be as usual
without any modification.
For writing the total number of one type of ligand whether in complex cation or
anion, the prefix to be used are:
Number of ligand prefix
1
2 di
3 tri
4 tetra
5 penta
6 hexa
7 hepta
8 octa
etc.
But in some cases alternative prefix is to be used in order to avoid any confusion.
For example of a complex has (CH
3
NH
2
)
2
as ligands then proceeding
ordinarily conc. may write it as
'dimethylamine'
But this creats a confusion on hearing that the ligand may be
CH
3
NH
CH
3
To avoid this confusion we must use alteration prefix which is bix for di.
Therefore the name of (CH
3
NH
2
)
2
will be written as
bis (methylamine)
Similarly (en)
2
will be written as
bis (ethylenediamine). The alternative prefix are:
bis for two
tris for three
tetrakis for four
pentakis for five
hexakis for six
and so on.
Now we are in a position that we can proceed for writing the name of
coordination compounds for that the procedure and rules to be adopted
are as follows:
(i) First of all calculate the oxidation number of the metal or metals
involved in coordination sphere.
(ii) Name of the cation whether simple or complex is always written first
then followed by the name of the anion.
(iii) Usually number (quantity) of free cations or free anions is not
mentioned. For example the name of K
4
[Fe(CN)
6
] will begin with
potassium but not tetrapotassium and the name of [Co(NH
3
)
6
]Cl
3
will
end with chloride not with trichloride. In the formula of a complex also
cation is always first and then the anion whether free or complex.
(iv) For writing he name of complex cation:
Write names of all the ligands present in the coordination sphere in
English alphabetical order with their quantities (not mono for one) as
prefixes followed by the name of metal with its oxidation number in
Roman within bracket ().
This will be one complete word. For example the name of
[Co(NH
3
)
6
]
3+
cation will be:
hexaammine colbalt (III) ion.
and the name of [CoCl(NH
3
)
4
(NO
2
)]
+
ioni will be:
tetraamminechloridonitritoNcobalt (III) ion.
Be careful NH
3
is written as ammine and not as amine, but CH
3
NH
2
or other
amines will be methylamine etc.
Also for deciding the alphabetical order of ligands, their IUPAC names must be
considered. The quantity of a particular ligand di, tri, tetra etc. are not to
be used for deciding the alphabetical order. Thispractice is to be retained
for writing the name of complex anion also.
(v) For writing the name of complex anion:
Follow the same order as for complex cation but now the name of the
metal will be modified as given earlier e.g. Cu will become cuprate.
Thusall anionic coordination entities will take the ending 'ate'.
For example name of
[Al(C
2
O
4
)
3
]
3
anion is:
trioxalatoaluminate (III) ion
[Fe(CN)
5
NO]
3
is pentacyanido nitrosyl ferrate (II) ion.
NOTE:
The name of a non-ionic complex will be written in the style of complex
cation e.g.
[NiCl
2
(PPh
3
)2] is dichloridobis (triphenylphosphine) nickel (II)
Now we can write the name of a complex molecule for example. There
must be a little gap between the names of cation and anion.
K
4
[Fe(CN)
6
] is: potassium hexacyanidoferrate (II)
+4 +2 6
?????????
[CO(NH
3
)
6
] Cl
3
is : hexaamminecolbalt (III) chloride
+3 + 0 + 3
[Pt Cl (NH
2
Me) (NH
3
)
2
] Cl is:
+2 1 + 0 + 0 1
diaminechlorido (methanamine) platinum (II) chloride
K
2
[OsCl
5
N] is: potassium pentachloridonitridoosmate (VI)
+ 2 + 6 5 3
[Co (en)
3
] Cl
3
is : tris (ethylenediamine) cobalt (III) chloride
+ 3 + 0 3
Names of bridge complexes:
Although the present course does not include bi or poly nuclear complexes yet
one or two examples of such complexes may be learnt for the sake of
curiosity.
In such complexes, all the rules which we have learnt so far are obyed. The
additional rule is that the names of bridging ligands have prefix, e.g.
name of
OH
[{Cr(NH
3
)
5
}
2
(OH)]Cl
5
or [(NH
3
)
5
Cr 1 Cr(NH
3
)
5
] Cl
5
O.NO. 0 + 3 + 3 0 5
is hydroxidobis (pentaamminechromium (III)) chloride
NH
2
[(en)
2
Co 1 Co (en)
2
] (SO
4
)
2
is:
O.NO. : 0 + 3 1 + 3 O 4
tetrakis (ethylenediamine)amidohydroxidodicobalt (II)
sulphate
or
it can also be written
bis (ethylenediamine) cobalt (III)amidohydroxide bis (ethylene
diamine) cobalt (III) sulphate.
To write the formula of the complex from the IUPAC name of the complex
(i) Cation is written first followed by anion whether complex or free.
(ii) In case of complex cation or anion or both within a square bracket the
order to be followed is metal, then ligands in the alphabetical order of
their formula with their quantities.
(iii) Finally with the help of oxidation number of metals given in the name of
the compounds, the charge of cation is balanced by charge of anion.
For example:
Write the formula of:
Q.(i) bromopentaamminecobalt (III) sulphate
Answer: [Co Br (NH
3
)
5
]
2+
O
4
2+
+ 3 1 0
Since cationic charge is balanced by anionic charge hence above formula
is correct. It can be finally written as
[Co Br (NH
3
)
5
] SO
4
Q.(ii) pentaamminenitritoNcolbalt (III) hexanitritoNcobalate
Answer: Since this name suggests that the compound has its cation and anion
both in complex state let us first prepare the formula of cation:
[Co (NH
3
)
5
NO
2
]
2+
or [Co (NH
3
)
5
NO
2
]
2+
+ 3 0 1
here NO
2
must not be ONO because then in the given name it would be
nitrito-O. The donor atom as suggested by name of such a ligand must
face the metal.
let us now write the formula of anion:
[Co (NO
2
)
6
]
3
or [Co (NO
2
)
6
]
3
+ 3 6
Now write cation formula first followed by anion
[Co (NH
3
)
5
NO
2
]
2+
[Co (NO
2
)
6
]
3
Finally to balance the charges we must multiply cation by 3 and anion by
2 so the formula of the compound becomes:
[Co (NH
3
)
5
NO
2
]
3
[Co(NO
2
)
6
]
2
4.
Theories of bonding in Coordination Compound
Since Werner (1893) who did the pioneering work in the field of complex many
theories have been developed to explain the properties and structures of
metal complexes. Following is the list of various theories:
(i) Wener's coordination theory
(ii) Sidgwic's effective atomic number rule
(iii) Valence bond theory (VBT)
(iv) Crystal field theory (CFT)
(v) Molecular Orbital Theory (Ligand filed theory)
We shall study all these theories except (V) the MOT or LFT which is beyond the
present syllabus.
(i) Werner's coordination theory:
Werner proposed the following postulate (a) Every metal or element
forming complex exhibits two types of valencies:
(i) Primary valence and (ii) Secondary valence.
In modern terminology, primary valence corresponds to oxidation
number of the metal involved in complexation and secondary valence
corresponds to its coordination number.
(b) The primary valence which is sometimes also known as ionic valence is
satisfied by negative ions.
(c) The secondary valence or coordination number is satisfied by neutral
molecules or anions which behave as ligands. Werner represented
secondary valence by thick lines and primary valence by dotted lines for
example CoCl
3
.6NH
3
complex can be represented as given below:
Cl
NH
3
NH
3
H
3
N Co NH
3
Cl Cl
NH
3
NH
3
Thus in this complex Co(III) [primary valence is 3] has its secondary
valence (CNo) equal to 6 which are satisfied by 6NH
3
molecules and
primary valence (3) is satisfied by three chloride ions.
(d) Every metal has a fixed value of its secondary valence as for example in
the above complex Co(III) needs 6NH
3
or 6 monomentate ligands to
stablise the complex.
(e) Every metal tends to satisfy both its primary and secondary valence. For
this purpose the anions present in the coordination sphere may play dual
role of satisfying secondary valence as well as primary if such a need
arises, for example consider the complex:
CoCl
3
.SNH
3
It must be written as
[Co Cl (NH
3
)
5
]
+2
Cl
2
2
+ 3 2 0
Thus the one Cl
in the coordination sphere is satisfying partly the
coordination number as well as primary valence Co
3+
.
Similarly
CoCl
3
.4NH
3
must be
[Co Cl
2
(NH
3
)
4
]
+1
Cl
1
+ 3 2 0
and CoCl
3
. 3NH
3
will be
[Co Cl
3
(NH
3
)
3
]
0
+ 3 3 0
But we cannot go beyond this for example CoCl
3
.2NH
3
cannot be
stabilized as there are insufficient number of ligands to satisfy the
secondary valence of Co(III).
(f) The lagands which satisfy the secondary valence are directed towards
fixed positions in the space. In other words, the geometry of complex
ion/molecule depends on its coordination number. Thus if coordination
is (6) then the geometry must be octahedral and if it is (4) then it can
be square planar or tetrahedral as shown below:
diagram here
Octahedral Square planar Tetrahedral
C.NO = 6 C.NO = 4 C.NO = 4
where M = metal atom/ion
L = Ligands (monodentate)
On the basis of directional character of secondary valencies, the cation of
complex [Co (NH
3
)
4
Cl
2
] Cl must be in two geometrical isomeric forms as
shown below:
diagram here
cis trans
Werner actually isolated these two geometrical isomers with different colors one
violet and the other green. Similarly the existence of two geometrical
isomers of the complex [Pt Cl
2
(NH
3
)
2
] proved that it must be a square
planar complex.
Werner's theory was purely based on his experiments, hence he proved the
postulates by many experimental evidences for example.
(a) reaction of Co(III), NH
3
and Cl
complexes with AgNO
3
solution.
( )
( ) ( )
3 3 3
6
3
6
Co NH Cl 3AgNO 3AgCl
Co NH NO
(
+
(
+
1 mole 2 moles 3 mole ppt.
Similarly one mole each of [Co (NH
3
)
5
Cl] Cl
2
and [Co (NH
3
)
4
Cl
2
] Cl will
react with 2 and one mole of AgNO
3
respectively. But the complex [Co
(NH
3
)
3
Cl
3
] does not react with AgNO
3
and moreover its solution is non-
conductor of electricity as it is a non-ionic complex. Similarly the complex
[Pt Cl
2
(NH
3
)
2
] cannot give any precipitate of AgCl with AgNO
3
.
(ii) Sidgwick's effective atomic number rule [EAN rule]
Sidgwick, on the basis of stability of electronic configurations of noble
gases gave the concept of effective atomic number (EAN)
EAN of the metal atom/ion involved in complixation
= number of electrons with that metal atom or ion
+ number of electrons gained from the donor atoms of the ligands.
For example:
EAN of cobalt in [Co (NH
3
)
6
]
3+
Complex is :
(Atomic number (z) of Co = 27)
Co
3+
will have 24 electrons hence:
= 24 + 6 2 = 36
= Atomic number of Kr
EAN of iron in [Fe (CN)
6
]
4
is:
Atomic number of iron is 26 and here iron is Fe(II) hence electrons of
Fe
2+
= 26 2 = 24
and EAN = 24 + 6 2 = 36
= Atomic number of Kr.
On the basis of such simple calculations he proposed that EAN of a metal in a
complex is equal to the atomic number of next noble gas in the period of
that metal. Since noble gas has stable electronic configuration hence all
such complexes must also be stable.
But later on many exceptions were found for example:
K
3
[Fe(CN)
6
] or [Fe(CN)
6
]
3
+ 3 6
EAN = (26 3) + 6 2 = 35
[Cu (NH
3
)
4
] SO
4
or [Cu (NH
3
)
4
]
2+
+ 2 0
The EAN for copper (Z = 29) is (29 2) + 4 2 = 35
[Ag (NH
3
)
2
] Cl or [Ag (NH
3
)
2
]
+
The EAN for silver (Z = 47) is (47 1) + 2 2 = 50
None of these EAN is equal to atomic number of any noble or inert gas.
(iii) Valence Bond Theory (VBT) (Developed by Pauling)
We are already familiar with the VBT for simple covalent compounds
according to which a covalent bond is formed by partial overlapping of
two atomic orbitals of two similar or different atoms, each providing one
electron for boding.
But here since coordinate bond/s is/are formed between ligand and the metal
atom/ion hence metal atom or ion which is electron pair acceptor or
Lewis acid must provide the vacant orbital/s and ligand must provide
filled orbital/s for overlapping.
Thus according to Pauling in the formation of a metal complex, the metal atom or
ion must provide vacant orbitals which many undergo suitable
hybridization depending on the geometry of the complex. For
coordination number 6 this may be d
2
sp
3
or sp
3
d
2
and for coordination
number 4 it may be dsp
2
or sp
3
.
All this may involve rearrangement of electrons in the (n 1) d subshell of the
metal atom or ion. for this purpose we must have knowledge of the
magnetic moment of the complex or of its geometrical isomers etc.
Let us consider the following examples:
Coordination No. 4
Ex.1. Predict and explain the hybridization in the complex [Ni (CN)
4
]
2
. It is given
that this complex feels repulsion in the magnetic field. [Atomic Number
of Ni = 28]
Solution: Since the complex experiences repulsion in the applied magnetic field it
means it must be a diamagnetic complex.
Now the configuration of Ni (Z = 28) is
Ni = 3d
8
, 4s
2
|+ |+ |+ | | |+
Ni
2+
=
|+ |+ |+ | |
3d
8
4s
0
Further since in the given complex, ion, the C.No. of Ni
2+
is 4 hence this
complex can be either tetrahedral or square planar.
Now if we consider it to be tetrahedral so that Ni
2+
may use its vacant 4s
an 4p orbitals for sp
3
hybridization, then the two unpaired electrons in
3d
8
configuration will remain as such and then the complex must be
paramagnetic, but we are given an experimental fact which shows that it
is diamagnetic. Hence this complex ion cannot be tetrahedral.
Well! then it must be square planar but that requires dsp
2
hybridization
so one of the 3d orbitals must become vacant (it must be actually dx
2
y
2
). This is possible only when the two unpaired electrons are coupled in
one of the 3d orbitals. This rearrangement can be shown as below, with
the nameprehybridisation state.
Ni
2+
=
|+ |+ |+ |+
(pre-hybridization state) 3d 4s 4p
dsp
2
hybridization
It will provide four equivalent empty dsp
2
hybrid orbitals directed
towards the four corners of a square from its centre. Each dsp
2
hybrid
orbital will then overlape with a filled sp hybrid orbital of carbon of [CN]
so as to form a coordinate bond. Thus the structure of [Ni (CN)
4
]
2
becomes:
NC CN
Ni
2+
NC CN
Square planar
Ex.2. Predict and explain the geometry of tetrachloridonickelate (II) ion. Given
that its paramagnetic moment (spin only) is about 2.84 B.M.
Solution: The complex ion in the question is:
[NiCl
4
]
2
+ 2 4
and it is also given that it is paramagnetic with 2 unpaired electrons.
Now since Ni
2+
has the configuration:
Ni
2+
= (Z = 28) 3d
8
, 4s
0
|+ |+ |+ | |
3d 4s 4p
So this configuration does not require any rearrangement of electrons. There are
already two unpaired electrons as demanded by the question. Hence
Ni
2+
must use sp
3
hybridization and hence the complex must be
tetrahedral.
Cl
|
Ni
2+
Cl
Cl
Cl
Tetrahedral complex
The coordinate bond must be resulting from the overlapping of empty
sp
3
hybrid orbital of Ni
2+
and one of the filled '3d' orbital of Cl
.
Ex.3. Predict the hybridization state of Ni and geometry of the its complex [Ni
Cl
2
(PPh
3
)
2
]. Given that this complex does not show geometrical
isomerism.
Solution: In exercise (1) and (2) we were given the magnetic moments of the
complexes. But in this question that information is missing. However we
are given another equally important information, that this complex does
not show geometrical isomerism since the coordination of Ni(II) in this
complex is 4 hence its geometry can be either square planar or then it
must exist in two geometrical isomeric form cis and trans, which it does
not. Hence it must be tetrahedral i.e.
Cl
|
Ni
2+
Cl
PPh
3
|
PPh
3
Tetrahedral
Hence Ni(II) must be in sp
3
hybridisation state.
Ni (II) (Z = 28) 3d
8
, 4s
0
|+ |+ |+ | |
4s 4p
sp
3
hybridisation
And we may further predict that this complex must be paramagnetic with spin
only moment equal to nearly 2.83 B.M.
Coordination No. 6
The geometry of such complexes is bound to be octahedral. The problem is to
decide the hybridization of central metal atom/ion whether it is d
2
sp
3
(inner-d-complex) or sp
3
d
2
(outer-d-complex). Again it can be decided if
we have information regarding magnetic nature i.e. paramagnetic
moment of the complex under consideration, for example.
Ex.4. What is the state of hybridization of iron in hexacyanidoferrate (II) ion?
The complex ion is diamagnetic in nature.
Solution: Since the complex is diamagnetic hence it must not have any unpaired
electron.
Fe
2+
(Z = 26) = 3d
6
, 4s
0
p
0
|+ | | | |
3d 4s 4p
There must be rearrangement of electrons in '3d'.
Fe
2+
= |+ |+ |+
3d 4s 4p
(pre hybridization state)
d
2
sp
3
hybridisation
These six d
2
sp
3
empty orbitals will be directed towards
the six corners of
an octahedron from its centre. Each d
2
sp
3
hybrid empty orbital must be
overlapping with filled sp hybrid orbital of the (c) of CN
anion.
CN
--
NC CN
Fe
2+
--
NC CN
CN
Octahedral complex (d
2
sp
3
)
Hence it is a inner-d-complex.
Ex.5. Predict the hybridization of Fe(III) in [FeF
6
]
3
ion. Given that its
paramagnetic moment is about 5.9 B.M.
Solution: Paramagnetic moment =
( )
n n 2 5.9 + = means 'n' must be 5 i.e.
there must be 5 unpaired electron in each ion. Let us see how it is
possible?
Fe
3+
(Z = 26) = 3d
5
,
4s
0
p
0
d
0
| | | | |
3d 4s 4p
sp
3
d
2
hybridisation
Thus no electronic rearrangement is required here. Hence Fe
3+
must be
in sp
3
d
2
hybridsation state. Thus it is a outer-d-complex. Since it has high
number of unpaired electrons hence it is also known as high spin
complex.
Ex.6. Predict whether the complex ion [Zn(H
2
O)
6
]
2+
will be a inner -d or outer-d-
complex?
Solution: The coordination number is 6 hence geometry must be octahedral. But
to decide the hybridization state of Zn(II), we are not given any other
information like magnetic nature etc. Infact in some cases it is not
needed atall. The electronic configuration of the metal atom/ion itself
will decide the type of hybridization for example in this case:
Zn
2+
(Z = 30) = 3d
10
, 4s
0
d
0
|+ |+ |+ |+ |+
3d 4s 4p
sp
3
d
2
hybridisation
Since Zn
2+
has fully filled 3d subshell hence there came be any
rearrangement of electrons in it. Obviously it will undergo sp
3
d
2
hybridisation. Thus it is a outer-d-complex and diamagnetic in nature.
In the following table some more examples of various coordination numbers are
given with their hybridization and shape.
Table
Complex C.NO Magnetic
nature
Hybridisation
state
Shape
[Ag(NH
3
)
2
]
+
2 diamagnetic sp linear
[HgI
3
]
3 diamagnetic sp
2
trigonal
[ZnCl
2
]
2
4 diamagnetic sp
3
tetrahedral
[Ni(NH
3
)
4
]
2+
4 diamagnetic dsp
2
square planar
Fe(CO)
5
5 diamagnetic dsp
3
trigonal bi
pyramidal
[Ni(CN)
5
]
3
5 Paramagnetic
with 2
unpaired
electrons
sp
3
d square
pyramid (one
bond is longer
than the rest
four)
[Cr(NH
3
)
6
]
3+
6 Paramagnetic
with 3
unpaired
electrons
d
2
sp
3
octahedral
[FeF
6
]
3
6 Paramagnetic
with 5
unpaired
electrons
sp
3
d
2
octahedral
Limitations of Valence Bond Theory:
(i) Through VBT explains the geometry of the complexes with the concept
of hybridization of orbitals yet it does not do so in the absence of some
extra information like magnetic nature, geometrical isomers etc.
(ii) This theory does not explain the spectra of the complexes i.e. it provides
no explanation regarding colors of complexes.
(iii) In many cases electronic rearrangements in (n 1)d subshell has to be
done before applying the concept of hybridization, violating Hund's rule.
This theory does not provide a proper explanation for this
rearrangement.
(iv) According to this theory in square planar complex [Cu (NH
3
)
4
]
2+
, one
electron is excited from 3d subshell to 4p. Hence its unpaired electron is
present in he outershell which must be readily available for reducing
purpose. But practically this complex does not show reducing properties.
This theory does not provide any explanation for it.
(v) This theory does not explain why some complexes are labile while others
are inert Labile complexes are those in which substitution of one ligand
by other takes place speedly.
(iv) Crystal Field Theory:
This theory was proposed by H. Bethe and Van Vleck in (19291932) for
explaining the optical properties of ionic crystals, where a (+) ion is
surrounding symmetrically by some () ions depending on the
coordination numbers. They wanted to know the effect of presence of
anions on the energy of orbitals of cation and vice versa, hence the
name of the theory is crystal field theory.
Since the situation of ions in a ionic crystal can be compared with that in a
coordination sphere where a metal atom or ion is surrounded by mostly
negatively charged ligands, hence this theory was extended to metal
complexes with the view to study the energy changes in orbitals
(specially 'd' orbitals) of central metal atom or ion due to the presence of
several ligands surrounding it.
The important postulates of this theory are as follows:
(i) Ligands are treated as point charges hence it is assumed that attraction
between the metal atom/ion and the ligands is purely electrostatic.
(ii) There is no interaction between metal orbitals and the ligand orbitals.
(iii) The d-orbitals of the metal atom/ion are degenerate in he free state.
However, when a complex is formed then the surrounding ligands
destroy this degeneracy of 'd' orbitals.
Now in order to understand the destruction of degeneracy of 'd' orbitals, we
must first revise the shapes of the five types of d-orbitals. All the five 'd'
orbitals can be classified further into two categories. Three of them ic
dxy, dyz and dzx are called planar. They are also known as 't2g' which
means triply degenerate and 'g' stands for gerade which we have already
learnt in the chapter of chemical bonding while studying MOT. The other
two are called axial i.e.
2 2 2
x y z
d and d
because axes are passing
through their lobes. These are also called 'eg' means doubly degenerate
and gerade.
Five types of 'd' orbitals
diagram here
planar or t
2g
diagram here
axial or eg.
In a free state of the metal atom or ion all the five 'd' orbitals are of equal energy
i.e. degenerate. But ligands approach the metal atom or ion then the
electrons in these d-orbitals are repelled by the lone pairs of the ligands.
As a result of this interaction the degeneracy of 'd' orbitals is lost and
they split up into sets of orbitals known as t
2g
and eg. The energy gap
between these sets is called crystal field splitting (A). The magnitude of A
depends on the number of ligands and their positions around the central
metal atom or ion. Thus if six monodentate ligands are approaching the
metal so as to form an octahedral complex then A will be written as A
0
('0' stands for octahedral) and similarly for tetrahedral field it will
become A
t
.
Factors affecting A
0
and A
t
:
(i) Oxidation state of the metal:
usually more is the oxidation state more is the value of A.
(ii) Nature of metal atom/ion:
generally the order observed is 3d < 4d < 5d. Thus while going down the
group from Cr (Z = 24) to Mo (Z = 42) the value of A
0
under similar set of
ligands increases by 50%.
(iii) Geometry of coordination entity:
For the same metal and similar ligands it is observed that
t 0
4
9
A ~ A
(iv) Nature of ligands:
For this purpose ligands may be classified as Weak (WL) or strong (SL)
ligands. Obviously for SL the value of A will be more than for WL.
Infact ligands are arranged, on the basis of their power to cause crystal
field splitting, in a series which goes from WL to SL. This series is called
spectrochemical series. A series consisting of some important ligands is
given below:
I
< Br
< SCN
< Cl
< S
2
< NO
3
< N
3
< F
< OH
< C
2
O
4
2
< H
2
O < NCS
<
CH
3
CN < py < EDTA
4
< NH
3
< en < bipy < NO
2
< PPh
3
< CN
< CO
Thus in the above series I
is the weakest and CO is the strongest ligand. SCN
means bonding with the metal is through 'S' atom. Hence NCS
means it
is through 'N' atom of the ligand.
Let us now consider the case of an octahedral complex.
C.No = 6:
Suppose a metal atom/ion 'M' is situated at the centre of a cube and three axes
of geometry ic x, y and z are passing through the six face centres so that
origin concides with the centre of the cube where metal 'M' is present as
shown in the figure below:
diagram here
Now as we have seen in the figures of two sets of 'd' orbitals, that the axes pass
through the lobes of 'eg' orbitals i.e.
2 2 2
x y z
d and d .
So here we can
imagine that the total six lobes (four of
2 2 2
x y z
d and two of d )
are
along these axes pointing towards the six face centres of the above cube.
Further since axes make tangents with the lobes of the three t
2g
orbitals
hence we can imagine that the total 12 lobes of the thee t
2g
orbitals will
be pointing towards the 12 edge centres of this cube.
Now imagine that six monodentale ligands 'L' are approaching the metal 'M'
along the axes x, y and z so as to form an octahedron at the centre of
which M is situated. hen the lone pairs of ligands will repell the electrons
of e.g. orbitals much more than the electrons in t
2g
, because lobes of 'eg'
orbitals are just infront of ligands while lobes of t
2g
are at 45 angle. (The
angle extended by an edge centre and the nearest face centre on the
centre of the cube is 45). Due to these unequal repulsions the energy of
'eg' orbitals will become more than the level of energy of t
2g
orbitals.
This splitting is denoted by A
0
or some times by 10Dq. Such that energy
of two 'eg' orbitals will increase by
0
3
5
A and that of that of three 't
2g
'
will decrease by
0
2
.
5
A This is shown in the following figure. It must be
noted that as the ligands approach the metal in the beginning all the five
d-orbitals as a set of degenerate orbitals is elevated to some higher
energy level and then ligands very close to the metal then the splitting
takes place as shown below:
diagram here
Fig. 'd' orbital splitting or crystal field splitting in an octahedral complex.
Bary centre is represents the average energy level of the five 'd' orbitals from
bary centre the 'eg' orbitals have gone up by
0
3
5
A energy and 't
2g
' have
come down by
0
2
.
5
A
It means by putting a electron in a 't
2g
' orbital an energy equal to
0
2
5
A will be
released but on putting a electron in a 'eg' orbital an energy equal to
0
3
5
A will be absorbed.
Algebric sum of these two is known as Crystal field stabilization energy (CFSE)
for octahedral complex.
Thus we can write
'CFSE' = [ 0.4 (n) t
2g
+ 0.6 (n) eg] A
0
Where (n) t
2g
and (n) eg means the number of electrons present in t
2g
and eg orbitals respectively. Now before we deal with individual 'd'
orbital configurations, let us consider one specific example, say [Fe
(CN)
6
]
4
[Fe (CN)
6
]
4
i.e. hexacyanidoferrate (II) ion.
The oxidation number of iron in it is +2 and remember CN
are strong
ligands. Therefore its crystal field splitting will be as shown below:
diagram here
Since there are six electrons in the '3d' subshell of Fe
2+
and CN
are strong
ligands. Hence the value of A
0
will be large. Now these six electrons are
to be put in the splitted field. Naturally first of all the three degenerate
't
2g
' will be filled according to Hund's rule. So all the six electrons are
accommodated in 't
2g
' orbitals leaving behind 'eg' as empty orbitals.
Therefore the electronic configuration of Fe
2+
in [Fe (CN)
6
]
4-
complex ion
becomes.
Fe
2+
=
2, 2, 2 0, 0
2g
t , eg
Further since no unpaired electron is present there hence the complex [Fe
(CN)
6
]
4
must be a diamagnetic complex which it really is.
CSFE for [Fe (CN)
6
]
4
= [ 0.4 6 + 0.6 0] A
0
= 2.4 A
0
So that of we have numerical value of A
0
for this complex the value of CFSE can
be calculated.
Let us consider one more example. It is [FeF
6
]
3
. Here central metal ion in Fe
3+
whose configuration in free state in 3d
5
and do not forget that F
are
weak ligands.
It means the numerical value of A
0
is very small, so small that we can consider the
splitted 't
2g
' and 'eg' almost degenerate. Hence all the five electrons will
be put in these orbitals obeying Hund's rule right from t
2g
to eg. This is
shown in the following figure.
[FeF
6
]
3
diagram here
Thus the configuration of Fe
3+
in [FeF
6
]
3
becomes
1, 1, 1 1, 1
2g
t , eg with 5 unpaired
electrons.
CFSE = ( 0.4 3 + 0.6 2) A
0
= Zero.
[FeF
6
]
3
is highly paramagnetic complex ion and due to large number of unpaired
electrons it is called high spin complex. Similarly if we consider the
complex [Fe (CN)
6
]
3
then due to the presence of CN
which are strong
ligands, the configuration of Fe
3
will be =
2, 2, 1 0, 0
2g
t , eg
because now al the five 'd' electrons of Fe
3+
will first occupy 't
2g
'
according to Hund's rule. And its CFSE will be equal to :
CFSE = ( 0.4 5 + 0.6 0) A
0
= 2.0 A
0
Moreover, since this complex has only one unpaired electron hence it will known
as low spin complex.
So on the basis these examples we can formulate an important generalization for
C.No. 6 or octahedral complexes that :
Strong Ligands (SL) will prefer low spin complexes (more pairing and hence less
number of unpaired electrons) and Weak Ligands (WL) will prefer to
form high spin complexes (more unpaired electrons).
Let us now consider the metal atom/ion with 1, 2, 3 number of electrons one
by one and calculate their CFSE in teems of A
0
. As given in the following
table it can be concluded that for configurations d
1
,d
2
, d
3
,d
8
, d
9
and d
10
.
Whether the ligands are strong or weak the value of CFSE in terms of D
0
remains the same.
Table
For calculation of CFSE
for d
1
, d
2
, d
3
, d
8
, d
9
and d
10
. metal atom/ion.
whether ligands are strong or weak
d
n
with example configuration in
the ligand field
number of
unpaired
electrons
CFSE in teems of
A
0
Ti
3+
, d
1
1 0, 0
2g
t , eg
1 [0.4 + 0.6 0] A
0
= 0.4 A
0
V
3+
, d
2
1, 1 0, 0
2g
t , eg
2 0.8 A
0
Cr
3+
, d
3
1, 1, 1 0, 0
2g
t , eg
3 1.2 A
0
Ni
2+
, d
8
2, 2, 2 1, 1
2g
t , eg
2 1.2 A
0
Cu
2+
, d
9
2, 2, 2 2, 1
2g
t , eg
1 0.6 A
0
Zn
2+
, d
10
2, 2, 2 2, 2
2g
t , eg
Zero 0.0 A
0
Let us now discuss the metal atom/ion with configuration d
4
or d
5
or d
6
or d
7
.
Now the nature of ligands has an important role to play. But for ligands lying at
the extreme ends of the spectrochemical series we can say with
confidence that the ligands are weak or strong, what about those which
occupy intermediate positions in that series.
To deal with such a situation one more concept that is the concept of electron
pair energy (P) is to be taken in account. It is the amount of energy
needed to pair a electron with another electron i.e. to overcome the
repulsion between the two electrons.
The important thing is the comparison of A
0
and P.
(i) if A
0
> P, then the ligands are to be consider as strong (SL)
(ii) if A
0
< P, then the ligands are to be considered as weak (WL)
Let us try to understand it by considering the example of Cr
2+
with 3d
4
configuration.
Now whether ligands are strong or weak, the three electrons out of 4 of d
4
are
bound to occupy one orbital each o t
2g
i.e.
1, 1, 1
2g
t . The next problem is
where the 4th electron must go, to make a pair with one of the 3
electrons in t
2g
or to occupy higher eg orbital.
This can be decided as follows:
(i) if A
0
> P, means it is easier to pair the 4th electron in t
2g
than to lift it to
one of the eg orbital because A
0
is high. Hence this 4th electron will
prefer to be paired in t
2g
. So that the configuration of Cr
2+
in the field of
ligands shall become
2, 1, 1 0, 0
2g
t , eg . This is obviously the situation as it
ligands are strong.
(ii) However if we find that A
0
< P, means pairing of 4th electron in t
2g
requires more energy than to lift it to one of the eg orbitals, because
now A
0
is smaller. In such a case the configuration of Cr
2+
in the ligand
field will become
2, 1, 1 0, 0
2g
t , eg . Finally with the help of these
configurations, CFSE can be calculated as shown in the table given
below:
Calculation of CFSE for metal atom/ion with configuration d
4
, d
5
, d
6
or d
7
using
the concept of electron pair energy (P)
d
n
A
0
< P (WL)
or
A
0
< P (SL)
configuration in
ligand field
net CFSE in terms
of A
0
and P
d
4
A
0
< P (WL)
1, 1, 1 1, 0
2g
t , eg
(0.4 3 + 0.6
1) A
0
= 0.6 A
0
; No 'P' is
involved.
A
0
< P (SL)
2, 1, 1 0, 0
2g
t , eg
(0.4 4 + 0.6
0) A
0
+ P
= 1.6 A
0
+ P
d
5
A
0
< P (WL)
1, 1, 1 1, 1
2g
t , eg
(0.4 3 + 0.6
2) A
0
= Zero
A
0
< P (SL)
2, 2, 1 0, 0
2g
t , eg
(0.4 5 + 0.6
0) A
0
+ 2P
(two pair)
= 2.0 A
0
+ 2P
d
6
A
0
< P (WL)
2, 1, 1 0, 1
2g
t , eg
(0.4 4 + 0.6
2) A
0
= 0.4 A
0
A
0
< P (SL)
2, 2, 2 0, 0
2g
t , eg
(0.4 6 + 0.6
0) A
0
+ 2P
2.4 A
0
+ 2P
d
7
A
0
< P (WL)
2, 2, 1 1,1
2g
t , eg
(0.4 5 + 0.6
2) A
0
= 0.8 A
0
A
0
< P (SL)
2, 2, 2 1, 0
2g
t , eg
(0.4 6 + 0.6
1) A
0
+ P
= 1.8 A
0
+ P
Practically the value of A
0
is determined spectrocopically by noting the
max
.
Which is required to raise a electron from t
2g
level to eg level in the given
complex.
Colour of Metal Complexes:
We have already learnt the origin of color in the study of transition metals.
Similarly if a coordination compound is capable of absorbing white light partly
then it will exhibit its complementary color. For example if a complex
absorbs wave length in the range 400 to 435 nm (violet color) then it will
appear yellow-green. Now what wave length a complex will absorb, it
depend on the difference of energy between lower-d- and higher -d-
orbitals, because a electron is to be excited from lower energy d-orbital
to a higher energy d-orbital. However if this difference of energy is
outside the 400-700 nm, the visible range, then the complex will appear
colorless.
For example let us solve the following question.
Q. For the complex [Ti(H
2
O)
6
]
3+
, A
0
= 240 KJ mol
1
. Predict the color of this
complex ion with the help of the table given in the study of properties
(color) of transition metals.
Solution: The complex given is [Ti (H
2
O)
6
]
3+
, the configuration of Ti
3+
in the crystal
field is
1, 0, 0 0, 0
2g
t , eg .
So this electron requires 240 KJ mol
1
of energy for exciting it to higher
level (eg). Let us calculate corresponding to this value.
1
0
240000 J mol
A =
=
23
240000
J per ion
6.02 10
= 3.99 10
19
J per ion
Now E =
hc
34 8
19
6.6 10 3 10
3.99 10
7
4.96 10 m
=
= 496 nm.
Now if we refer to that table we find that if absorbed l is in the range
490500 nm ie blue-green radiations, then it appears red or red-purple.
Infact this complex ion is purple in color.
Let solve some numerical questions based on
max.
, A
0
and CFSE.
Q.1. Calculate CFSE for [TiCl
6
]
3+
if
max.
for this is 770 nm.
Solution:
max.
hc
E =
=
34 8
9
6.6 10 3 10
J
770 10
= 2.571 10
22
kJ per complex ion
= 2.57 10
22
6.02 10
23
= 15.47 10
= 154.7 kJ mol
1
~ 155 kJ mol
1
This is equal to A
0
.
Now we can calculate CFSE for the given complex ion.
[TiCl
6
]
3+
,
3 1
Ti 3d
+
=
Hence the configuration of metal ion in the complex will be
1, 0, 0 0, 0
2g
t , eg ; Cl
are WL, otherwise also for d
1
it is immaterial whether ligands are strong
or weak.
Hence CFSE = 0.4 A
0
= 0.4 155
= 62 kJ mol
1
or numerically CFSE = 62 kJ mol
1
. Ans.
Q.2. Calculate CFSE for the complex ion [Cr (H
2
O)
6
]
2+
given that A
0
is 12600 cm
1
and P = 23500 cm
1
.
Solution: Since P > A
0
and Cr
2+
is 3d
4
.
Hence the configuration of Cr
2+
in the ligand field (WL) will be
1, 1, 1 1, 0
2g
t , eg
CFSE = 0.6 A
0
= 0.6 12600 cm
1
= 7560 cm
1
= 756000 m
1
Further since
E = hc v
or E =
34 8 23
1
6.6 10 3 10 756000 6.02 10
kJ mol
1000
= 90.11 kJ mol
1
Ans.
Q.3. Calculate the net stabilization energy for a d
6
complex having A
0
= 25000
cm
1
and P = 15000 cm
1
.
Solution:
0
P A > and metal is d
6
For SL field he configuration is
2, 2, 2 0, 0
2g
t , eg
and CFSE = (0.4 6 + 0.6 0) A
0
+ 2P
= 2.4 A
0
+ 2P
= 2.4 25000 2 15000
= 60000 + 30,000
= 30,000 cm
1
= 30,000 100 m
1
= 3 10
6
m
1
Now E hc = v
=
34 8 6 23
1
6.6 10 3 10 3 10 6.02 10
kJ /mol
1000
= 357.59 kJ mol
1
(numerical value)
Caution:
Some examples of octahedral complexes with their magnetic nature are given
below:
[Fe (CN)
6
]
4
Diamagnetic
[Fe (CN)
6
]
3
Paramagnetic (1 unpaired e) d
2
sp
3
[Fe (CN)
6
]
4
D
[Co (NH
3
)
6
]
3+
Diamagnetic
[Mn (CN)
6
]
4
Paramagnetic (1 unpaired e)
[Cr (NH
3
)
6
]
3+
Paramagnetic (3 unpaired e)
[Fe F
6
]
3
Paramagnetic (5 unpaired e)
[Mn F
6
]
3
Paramagnetic (4 unpaired e)
[Fe
I
(H
2
O)
5
NO
+
]
2+
Paramagnetic (3 unpaired e) sp
3
d
2
(brown ring for NO
3
)
[Ni (NH
3
)
6
]
2+
Paramagnetic (2 unpaired e)
[Co (H
2
O)
6
]
2+
Paramagnetic (3 unpaired e)
[Fe (H
2
O)
6
]
2+
Paramagnetic (4 unpaired e)
Coordination No. 4
Since such complexes can be either Tetrahedral or square planar hence let us
discuss them one by one.
Tetrahedral:
Let a metal atom or ion (M) be situated at the centre of a cube and four
monodentate ligands (L) approach it through four alternate corners of
this cube. We have already leant in the chapter of solid state that a
regular tetrahedron can be extracted from a cube by joining its alternate
corners. Thus as shown in the figure below:
diagram here
Fig. Tetrahedron in a cube.
M = metal atom/ion
L = monodentate ligands.
We have already learnt in the discussion of octahedral complexes that:
(i) Directions of x, y and z axes are towards the face centres.
(ii) The lobes of 'eg' orbitals are along these axes i.e. they are directed
towards face centres of the cube.
(iii) The lobes of 't
2g
' orbitals are directed towards theedge centres of the
cube.
Now as shown in the figure above the four monodentate ligands (L) are
approaching the central metal 'M' that they do not coincide exactly with
neither eg nor t
2g
lobes.
Hence it must be decided which of the two types of 'd' orbitals are nearer the
directions of approach of ligands.
This can be done in the following way:
The angle between LML is 109.5. Hence the angle between LM and a
lobe of eg orbital must be half of it. i.e.
109.5
54.75 .
2
=
But the angle between LM and a lobe of a t
2g
orbital must be 90 54.75 =
35.25.
Hence 't
2g
' orbitals are comparatively nearer the ligands. Therefore in a
tetrahedral field 't
2g
' orbitals experience more repulsions that the 'eg'
orbitals. Hence the crystal field splitting in a tetrahedral field must be of
opposite nature than in an octahedral field. This is shown in the figure
below:
diagram here
Fig. : Crystal field splitting in a tetrahedral field.
A
t
is smaller than
0 t 0
4
9
| |
A A ~ A
|
\ .
hence it is never energetically favourable to
pair electron and therefore all tetrahedral complexes are generally high
spin complexes.
It may be further noted that T-complexes are usually favoured where the ligands
are large and bulky theligands are weak and oxidation state of metal is
low. Even if the ligands are strong the CFSE is calculated by assuming
them to be weak ligands due to small value of A
t
. Thus
CFSE = [ 0.6 (n) eg + 0.4 (n) t
2g
] A
t
Hence for d
1
to d
10
configurations, the values of A
t
will be 1.2 A
t
, 0.8 A
t
, 0.4 A
t
,
0.0, 0.6 A
t
, 1.2 A
t
, 0.8 A
t
, 0.4 A
t
and 0.0 A
t
respectively.
Let us consider an example:
[MnCl
4
]
2
: Here Mn is in +2 state hence
Mn
2+
= 3d
5
, 4s
0
Its configuration in Tetrahedral field shall be eg
1, 1
,
1, 1, 1
2g
t , highly paramagnetic
and CFSE will be equal to
[ 0.6 2 + 0.4 3] = 0.0 A
t
Some other examples of tetrahedral complexes with sp
3
hybridisation of metal
are given below:
[Zn (NH
3
)
4
]
2+
Diamagnetic
[Zn (CN)
4
]
2
Diamagnetic
[Ni (CO)
4
] Diamagnetic
[Ni Cl
4
]
2
Paramagnetic with 2 unpaired electrons
[Ni Cl
2
(PPh
3
)
2
] Paramagnetic with 2 unpaired electrons
[Mn Cl
4
]
2
Paramagnetic with 5 unpaired electrons
[Fe Cl
4
]
2
Paramagnetic with 4 unpaired electrons
Hg [Co (SCN)
4
] Paramagnetic with 3 unpaired electrons
It is interesting to note that some times the cation attached to a complex anion
also affects the geometry of anion. For example,
Cs
2
[CuCl
4
] and (NH
4
)
2
[CuCl
4
].
The anion is tetrahedral in the former but square planar in the later.
Square Planar Complexes
In these complexes, the central metal atom/ion is dsp
2
hybridised and the 'd'
orbital involved is
2 2
dx y , because the four lobes of this orbital are
along the X- and Y axes, which can form a square.
Let us again imagine a metal atom/ion situated at the centre of a cube so that
lobes of 'eg' orbitals are pointing (along the axes) towards the six face
centres and those of t
2g
towards the 12 edge centres. Now in order to
understand the formation of a square planar complex we must first
imagine an octahedral complex (C.No = 6). Now further imagine that the
two ligands on the Z-axis (along dz
2
orbitals) are removed from the
complex. So as these ligands move away the energy of dz
2
orbital (one of
the two eg) will fall. As a secondary effect of this the energies of dyz and
dzx also fall but not much. The net result is this that dx
2
y
2
orbital
occupies the highest level and dyz and dzx the lowest. This total splitting
is much more than A
0
as shown in the figure below:
diagram here
The square planar complexes are rare except for d
8
metal ions for example:
Ni(II), Pd(II), Pt(II), Rh(I), Ir(I), Cu(III), Ag(III) and Au(III).
All the known square planar complexes of d
8
ions are diamagnetic. Because the
highest energy orbital (dx
2
y
2
) is greatly destabilised and pairing in 'dxy'
is more favourable than placing an unpaired electron in dx
2
y
2
orbital.
The same thing is interpreted in VBT by the logic that dx
2
y
2
must be available
vacant so as to involve in dsp
2
hybridisation. For example if we consider
the diamagnetic complex [Ni (CN)
4
]
2
then
Ni
2+
= 3d
8
, 4s
0
Hence the configuration of Ni
2+
in the square planar field of 4CN
is
( ) 2 2 2
2 2 2 2 0
yz zx xy
z x y
d d , d , d , d
Calculation of CFSE can be done in terms of A
0
with the help of the figure given
above, but the relative energy considerations are sometimes
controversial.
It must be more important for us to note that square planar complexes are
favoured by metals with 4d
8
and 5d
8
configurations irrespective of the
type of ligands i.e. strong or weak because A
0
for these metals is already
very large.
But for 3d
8
metals such as Ni(II) a square planar complex will be formed only
when the ligands are strong. Thats why *Ni (CN)
4
]
2
is diamagnetic and
square planar but [Ni Cl
4
]
2
is paramagnetic and tetrahedral.
Some other examples of square planar complexes with their magnetic nature are
given below:
[Ni (en)
2
]
2+
Diamagnetic
[Ni (dmg)
2
] Diamagnetic
[Pt Cl
4
]
2
Diamagnetic, note here that although Cl
are weak ligands but Pt(II) is 5d
8
. Thus
Pt(II), Pd(II), Ir(I) and Rh(I) will always
form square planar complex whenever
they bind with 4 ligands.
[Pt (NH
3
)
4
]
2+
Diamagnetic
[AgF4]
Diamagnetic
[Rh Cl (PPh
3
)
3
] Diamagnetic
[Cu (NH
3
)
4
]
2+
Paramagnetic with unpaired electron
promoted to 4p orbital.
It may be interesting to note that Ni
2+
forms a red precipitate with
dimethylglyoxime in alkaline medium (NH
4
OH). This is a practical test
also for Ni
2+
.
The reaction can be shown as below:
OH
2 CH
3
C = N
| + Ni
2+
4
NH OH
CH
3
C = N
OH
O
HO
CH
3
C = N
N = CCH
3
Ni
2+
+2
N = CCH
3
OH..O
Red ppt.
(involves intramolecular H-bonding)
Now we shall study a very important property of coordination compounds that is:
5. Isomerism in Coordination Compounds
those metal complexes which have the same molecular formula but different
structures are called isomers. Like organic compound these also exhibit
mainly two types of isomerism
(A) Structural and
(B) Stereo-isomerism.
Let us first discuss structural isomerism which is further classified as:
(i) Ionisation isomerism:
This type of isomers have same molecular formula but give different ions
in solution such a situation arises when the counter ion in a complex is
itself a potential ligand and can displace a ligand from the coordination
sphere. The displaced ion then becomes a counter ion. For example
[Co (NH
3
)
5
SO
4
] Br and
(Red color)
[Co (NH
3
)
5
Br] SO
4
(Violet color)
The read colored complex will give free Br
in he solution which can be
tested by adding AgNO
3
to the solution so as to form a light yellow
precipitate of AgBr.
On the other hand the violet colored complex will furnish
2
4
SO
in the
solution which will give a white precipitate of BaSO
4
with BaCl
2
.
The other examples of this category are:
[Pt (NH
3
)
4
Cl
2
] Br
2
and
[Pt (NH
3
)
4
Br
2
] Cl
2
[Co (NH
3
)
4
Cl
3
] NO
2
and
[Co (NH
3
)
4
Cl NO
2
] Cl
(ii) Hydrate or solvate isomerism:
this type of isomers have difference number of water (or in general
solvent) molecules present inside and outside the coordination sphere.
Thus it is similar to ionization isomerism.
The examples are provided the molecular formula CrCl
3
.6H
2
O
(a) [Cr (H
2
O)
6
] Cl
3
; violet
(b) [Cr (H
2
O)
5
Cl] Cl
2
.H
2
O; green
(c) [Cr (H
2
O)
4
Cl
2
] Cl.2H
2
O; green
The other examples are :
[Co (NH
3
)
4
H
2
O Cl] Br
2
and
[Co (NH
3
)
4
(Br)
2
] Cl.H
2
O
[Cr (en)
2
(H
2
O) Cl] Cl
2
and
[Cr (en)
2
Cl
2
] Cl.H
2
O
(iii) Coordination isomerism:
This type of isomerism is show by those complexes whose cation and
anion both are in complex form, so that an exchange of ligands or metals
may form another isomer. For example,
[Pt (NH
3
)
4
] [CuCl
2
] and
[Cu (NH
3
)
4
] [Pt Cl
4
]
[Cr (NH
3
)
6
] [Co (C
2
O
4
)
3
] and
[Co (NH
3
)
6
] [Cr (C
2
O
4
)
3
]
[Co (en)
3
] [Cr (CN)
6
] and
[Cr (en)
3
] [Co (CN)
6
]
II IV IV II
[Pt (NH
3
)
4
] [Pt Cl
6
] and
[Pt (NH
3
)
4
Cl
2
] [Pt Cl
4
]
In the last example we see that metal is same but in different oxidation states. So
an exchange of oxidation states required the rearrangement of ligands
also to give an isomer.
(iv) Linkage isomerism:
This type of isomerism arises in those complexes which posses ambidentate
ligands such as
2
NO ,
which may be bonded with metal through 'N' or
through oxygen as
| |
ONO .
We have already seen the examples of
ambidentate ligands such as [CN]
, [SCN]
etc.
The examples of isomers are:
[Co (NH
3
)
5
(ONO)] Cl
2
and
pentaamminenitrito-o-cobalt (III)
chloride (Red)
[Co (NH
3
)
5
NO
2
] Cl
2
pentaamminenitrito-N-cobalt (III)
chloride (Yellow)
Another example is:
[Pd (bipy) (SCN)
2
] and
[Pd (bipy) (NCS)
2
]
Stereo isomerism
As usual stereo-isomerism is further classified into two types:
(A) Geometrical isomerism
(B) Optical isomerism
Although optical isomerism in metal complexes is not in present course of study
yet we will have a brief discussion of that. But let us first discuss
Geometrical isomerism. We shall study this first for coordination number
4 and then for coordination number 6.
Geometrical Isomerism for C.No. = 4:
We know compounds with coordination number 4 can be either tetrahedral or
square planar. Since tetrahedral compounds cannot show geometrical
isomerism hence we are left with square planar complexes only.
Square planar complexes with metal atom/ion 'M' and four monodentate ligands
a, b, c and d. Thus the total possible complexes can be
Ma
4
, Ma
3
b, Ma
2
b
2
, Ma
2
bc, Mabcd
Out of them Ma
4
and Ma
3
b complexes cannot show geometrical isomerism,
hence now we are having Ma
2
b
2
, Ma
2
bc and Mabcd for discussion.
2 2
Ma b : Such complexes can exist in following two geometrical forms.
b a b a
M M
b a a b
cis trans
An example of such a complex is [Pt Cl
2
(NH
3
)
2
] whose cis-isomer known
as cis-platin is used as an anti-tumor medicin.
H
3
N Cl
Pt
H
3
N Cl
cisplatin
[cis-diamminedichloridoplatinum (II)]
2
Ma bc : This will also exist in cis and trans two two geometrical isomeric
form as shown below:
a b b a
M M
a c a c
cis trans
example: cis and trans [Pt (NH
3
)
2
Cl NO
2
]
Mabcd : But such types of compounds will exist in three geometrical
isomeric forms as shown below:
a c a b a b
M M M
d b d c c d
(i) (ii) (iii)
These can be represented as:
(i) b is trans to a or 'b' T 'a'
(ii) c is trans to a or 'c' T 'a'
(iii) d is trans to a or 'd' T 'a'
An example of such a formula is
[Pt (NH
3
) (NH
2
OH) (NO
2
) (py)] NO
amminehydroxylamine nitrito-N-pyridineplatinum (II) nitrite
Square planar complexes with bidentate and bidentate with monodentate
ligands:
If (AA) represents a bidentate ligand whose both the donor atoms are similar and
(AB) has different, a, b, c etc. are as usual monodentate ligands, M is the
central metal atom/ion. Then the following complexes will have only
one form i.e. cannot show geometrical isomerism.
M(AA)
2
, M(AA) (BB), M(AA) (CD), M(AA) a
2
, M(AB) a
2
, M(AA) bc
But :
M(AB)
2
, M(AB) (CD), and M(AB) bc such will exist in two forms for
example:
M(AB)
2
can be written as
A B A B
M M
B B B A
cis Trans
Similarly M(AB) (CD):
A C A D
M M
B D B C
'A' T 'D' 'A' T 'C'
And M(AB) be:
A b A c
M M
B c B b
'A' T 'c' 'A' T 'b'
An example of M(AB)
2
complex is:
[Pt (Gly)
2
]
i.e. bis (glycenato) platinum (II) complex.
CH
2
NH
2
H
2
NCH
2
+2
Pt
COO
OCO
cis-isomer
and
COO
H
2
NCH
2
2+
Pt
CH
2
NH
2
OCO
trans isomer
Geometrical Isomerism in Octahedral
Complexes (C.No. = 6)
(A) Complexes with monodentate ligands only:
Ma
6
and Ma
5
b will be only of one form hence cannot show geometrical
isomerism.
Rest following nine combinations are possible and they have more than
one form possible. Thus
Formula Number of geometrical
isomers possible
Ma
2
b
4
2
Ma
4
bc 2
Ma
3
b
3
2
Ma
3
b
2
c 3
Ma
3
bcd 4
Ma
2
b
2
c
2
5
Ma
2
b
2
cd 6
Ma
2
bcde 9
Mabcdef 15
The above figures represent the maximum geometric isomers possible
theoretically. But it does not mean that all such isomers are practically
available many of them are yet to be prepared.
Anyway, let us see the arrangement of ligands in some of the above
cases.
2 2
Ma b :
b b
b a b b
M M
a a b a
b b
cis Trans
example:
[Co (NH
3
)
4
Cl
2
] Cl
4
Ma bc :
b b
a c a a
M M
a a a a
a a
cis (b and c) Trans (b and c)
example:
[Co (NH
3
)
4
Cl NO
2
] Cl
3 3
Ma b : The two geometrical isomers in this case are usually not termed as
cis and trans but they are known as:
fac and mer
example:
[Co (NH
3
)
3
Cl
3
]
Thus,
b b
a b b a
M M
a b a a
a b
fac mer
'fac', means facial. Since an octahedron has eight triangular faces, hence
in case of fac, the two faces are such that all its three corners are
occupied by identical ligands.
'mer', means meridian (a line drown from north to south pole in a map).
So in this each trio of identical ligands forms a plane which bisects the
molecule. In other words none of the eight faces of octahedron has a trio
of identical ligands on its three corners.
Similarly diagrams for all the other formula can be drawn.
Octahedral complexes with bidentate ligands with or without monodentate
ligands:
The various possible formula and their corresponding isomers number is
given below:
Formula number of geometrical
isomers
(1)
( )
3
M AA : only one form is possible as shown
below:
A
A A
M
A A
A
(2)
( )
3
M AB : Two forms are possible
example:
[Cr (NH
2
CH
2
COO)
3
]
A A
A A A B
M M
B B B B
B A
cis Trans
The three 'A' and
three 'B' are facial
(3)
( )
2
2
M AA a : Two forms are possible by placing
monodentate ligands in cis and trans.
example:
[Co (en)
2
Cl
2
] Cl
a a
A a A A
M M
A A A A
A a
cis Trans
(4)
( )
2
M AA ab : Two geometrical isomers are possible
by placing the mono dentate ligands 'a'
and 'b' in cis and trans positions like
(3).
(5)
( )
4
M AA a : Only one form is possible.
(6)
( )
3
M AA a b : Two geometrical isomers are possible
as shown below:
b a
A a A a
M M
A a A b
a a
There 'a' are fac No three 'a' are fac and
and b T a i.e. 'b' is a T a
trans to 'a'
(7)
( )
2 2
M AA a b : Three geometrical isomers are
possible as shown below:
a a b
A b A a A a
M M M
A b A b A a
a b b
a T a a T b b T b
(8)
( )
M AA abcd : Six geometrical isomers are possible
which can be draw like (7).
Similar figures can be drawn for other possible formula but in case of larger
isomers, all the isomers are not available yet.
Optical Isomerism
A compound which can rotate the plane of a plane polarized light is called
optically active an those compounds which have the same molecular
formula but differ in their abilities to rotate this plane are called optical
isomers. Like organic compounds many coordination compounds show
this type of isomerism. As we know from our study of organic
compounds that optical isomers are non-superimposable mirror images
of each other and the essential requirement for a substance to be
optically active is absence of plane of symmetric in its structure, this is
truly valid for coordination compounds also. Those isomers which
rotate the plan towards right are called dextro (d-form) and those which
turn towards left are called lacvo (l-form). The two optically active
isomers are collectively called Enantiomers.
Coordination Compounds with C.No. = 4
As we know such compounds can be either Tetrahedral or Square Planar, out of
these square planar cannot show optical isomerism. Hence only
tetrahedral type of complexes will be able to do so but that too not all.
Only compounds of Mabcd are able to show optical isomerism.
a a
| |
M M
b d
d b
c c
mirro images
Coordination compounds with C. No = 6
(Octahedral complexes)
(A) Compounds containing monodentate ligands only:
It is observed that in such compounds optical isomerism is possible only
when the molecular contains 3 or more types of ligands but ligands of
one type must not be more than 2.
Hence compounds with general formula:
[Ma
2
b
2
c
2
], [Mabcdef], [Ma
2
b
2
cd], [Ma
2
bcde]
are capable of showing this type of isomerism.
For example:
2 2 2
Ma b c : This formula itself represents 5
geometrical isomers, out of them if we
consider the following in which similar
ligands are in cis position then the two
optical isomers are possible as shown
below:
example:
[Pt (py)
2
(NH
3
)
2
Cl
2
]
2+
py 2+ py 2+
| |
Cl py py Cl
Pt Pt
Cl NH
3
H
3
N Cl
| |
NH
3
NH
3
mirror
cis-d-isomer cis-l-isomer
(B) Compounds containing bidentate ligands with or without some
monodentate ligands:
Some of the possible compounds with general formula are: [M(AA)
3
], cis-
[M(AA)
2
a
2
], cis-[M(AA)b
2
c
2
]
For example:
( )
3
M AA : example: [Cr (C
2
O
4
)
3
]
3
has the following two optical isomers
O O
C O C
O C O
C = O OC = O
O = CO O
Cr Cr
OC = O
O = CO O O
O CC
O O
O O
mirror
The other examples are:
cis-[M(AA)
2
b
2
], example: cis-[Pt Cl
2
(en)
2
]
2+
in which both the Cl atoms
are in cis potion.
and
cis-[M(AA) b
2
c
2
], example: cis-[Cr Cl
2
(en) (NH
3
)
2
]
+
where both Cl and both NH
3
are in cis positions.
Infact these compounds do not have any plane of symmetry and hence
they are optically active. But compounds like trans-[M(AA)
2
a
2
] cannot be
optically active due to presence of plane of symmetry as it clear from the
structure given below:
a
|
A A
M
A A
|
a
(not optically active)
But its cis- form will be optically active as shown below:
cis-[M(AA) a
2
], example [Pt Cl
2
(en)
2
]
2+
2+ 2+
en en
Cl Cl
Pt Pt
Cl Cl
en en
mirror
cis-d-isomer cis-l-isomer
6. Stability of Coordination Compounds
The complexation of a metal ion in aqueous solution, with ligands can be
shown overall as:
( ) | |
2 n 2
n
M H O nL ML H O
(
+ +
So that according to Law of mass action, ignoring [H
2
O] we can write.
K =
| |
( ) | |
n
n
2
n
ML
M H O L
(
(stability constant)
The above formation of complex may take place in several steps as:
Equilibrium
constant
( ) ( )
2 2 2
n n 1
M H O L ML H O H O
( (
+ +
K
1
( ) ( )
2 2 2 2
n 1 n 2
ML H O L ML H O H O
( (
+ +
K
2
( )
( ) | |
2 n 2 n 1
ML H O L ML H O
(
+ +
K
n
So that the overall stability constant K (or some times denoted by |
n
) is
given by
K= K
1
K
2
K
3
.. K
n
(7) Organo-metallic CompoundsMetal Carbonyls
Compounds involving at least one meta-carbon (organic group) bond are
called organometallic compounds. These compounds may be classified in
the following three categories.
(i) Sigma (o) bonded compounds:
Examples of such compounds are
Girgnard Reagents RMgX, R
2
Zn i.e. dialk zinc, and similar
compounds like (C
2
H
5
)
4
Pb (TEL), Al
2
(CH
3
)
6
etc. In all these compounds
there exists single bonding between carbon and metal i.e. no double or
triple bonding.
(ii) Z-bonded compounds:
In these compounds, the t-electron of the organic group bind the metal.
For example
diagram here
Zeise's salt Dibenzene Ferrocene
K
+
[Pt Cl
3
(q
2
C
2
H
4
)] Cr (q
6
C
6
H
6
)
2
Fe (q
5
C
5
H
5
)
2
Classification of metal carbonyls:
Metal carbonyls are may be monomeric means containing only ne metal
atom, bridged, in which two metal atoms are linked via some CO groups
and polynuclear which contain more than two metal atoms in a
molecule. Fe(CO)
5
, Co
2
(CO)
8
and Fe
3
(CO)
12
are examples of these three
classes respectively. However our course is limited to monomeric
carbonyl only hence we shall concentrate on them only.
Examples of monomeric carbonyls:
V(CO)
6
Vanadium hexacarbonyl
Black crystals
(Paramagnetic)
Cr(CO)
6
Chromium hexa carbonyl
colorless solid
(Diamagnetic)
Fe(CO)
5
Iron penta carbonyl
yellow liquid
(Diamagnetic)
Ni(CO)
Nickel tetra carbon
colorless liquid
(Diamagnetic)
The other examples are Ru(CO)
5
, Os(CO)
8
, Mo(CO)
6
, W(CO)
6
etc.
Generally transition metals of VIB, VIIB and VIII groups or metals in 6th
to 10th vertical column form carbonyls. Thus all the metals are not
capable of forming carbonyls.
All these carbonyls are diamagnetic substances except V(CO)
6
which is
paramagnetic with one unpaired electron in the molecule.
Hence we can draw the structure as shown below:
CO
|
NI
CO
OC
CO
Similarly in Fe(CO)
5
, the iron atom is dsp
3
hybridised so that molecule is
trigonal bipyramidal and diamagnetic in nature.
CO
CO
Fe
OC CO
CO
Trigonal bipyramidal molecule
of Fe (CO)
5
Cr (O)
6
and V(CO)
6
both show d
2
sp
3
hybridisation of Cr and V
respectively. But V(CO)
6
is diamagnetic. They both have octahedral
geometry as shown below:
CO CO
CO CO CO Co
Cr Cr
OC CO OC CO
CO CO
Octahedral molecule Octahedral molecule
of Cr (CO)
6
of V(CO)
6
The same is sometimes known as 18 electron rule according to that
valence elect5rons of the metal atom + number of electrons donated by
CO groups, if this sum is equal to 18, ten the carbonyl is stable. Thus:
In Ni (CO)
4
;
( )
8 2
Ni Z 28 3d , 4s ; =
for this purpose (n 1)d and ns both the electrons are considered as
valence electrons, hence
10 + 4 2 = 18
(4CO)
Similarly in Fe(CO)
5
and Cr(CO)
6
this rule is valid. But in V(CO)
6
where the
configuration of V (Z = 23) is 3d
3
, 4s
2
. Hence it has only 5 valence
electrons.
Therefore total electrons will be 5 + 6 2 = 17.
Infact V(CO)
6
is not a stable carbonyl like other carbonyls.
Methods of preparation of metal carbonyls:
Some examples are given below:
(i)
( )
80 C
4
Ni 4CO Ni CO
+
(ii)
( )
3 3
6
CrCl 6CO Al Cr CO AlCl + + +
(iii)
( ) ( )
2
5 9
2Fe CO Fe CO CO
A
+
(iv)
( ) ( )
2 2
8 4
Co CO H 2H Co CO
(
+
S-ar putea să vă placă și
- Aldehydes and Ketones - 1-MergedDocument94 paginiAldehydes and Ketones - 1-MergedseÎncă nu există evaluări
- Organic ChemistryDocument14 paginiOrganic ChemistryStuteeÎncă nu există evaluări
- Alcohols & Phenols:: GeneralizationsDocument27 paginiAlcohols & Phenols:: GeneralizationsdoudoudoudouÎncă nu există evaluări
- SAT Official GuideDocument774 paginiSAT Official GuidePavan Boro54% (13)
- Resonance and Inductive Effects PresentationDocument36 paginiResonance and Inductive Effects Presentationeagl33yeÎncă nu există evaluări
- MSCCH 604Document203 paginiMSCCH 604Gourav Biju100% (1)
- 01.coordination Chemistry Class Notes Part I-1 PDFDocument86 pagini01.coordination Chemistry Class Notes Part I-1 PDFShadrack Peter100% (1)
- Coaching Resource Pack Jade JoddleDocument12 paginiCoaching Resource Pack Jade JoddlePavan BoroÎncă nu există evaluări
- Chemistry of Reactive Intermediate FinalDocument38 paginiChemistry of Reactive Intermediate FinalTefera100% (1)
- NMR Solving StrategyDocument2 paginiNMR Solving Strategysorrow Lemon100% (1)
- Benzene and Aromatic Compounds-fazli-In ClassDocument57 paginiBenzene and Aromatic Compounds-fazli-In Classjokowi123Încă nu există evaluări
- Org Chem Sem 3 Paper 2Document15 paginiOrg Chem Sem 3 Paper 2Rohit DeshmukhÎncă nu există evaluări
- Geometry HandbookDocument82 paginiGeometry HandbookPavan Boro100% (1)
- CHM 101 Complete - LNDocument80 paginiCHM 101 Complete - LNSimon Adediran100% (1)
- C C, C N, C O CouplingDocument67 paginiC C, C N, C O CouplingAnonymous vRpzQ2BLÎncă nu există evaluări
- Inorg Reactionn MechanismsDocument38 paginiInorg Reactionn MechanismsThabang MolekoÎncă nu există evaluări
- Anthranilic Acid PDFDocument20 paginiAnthranilic Acid PDFGlibÎncă nu există evaluări
- An Introduction to Co-Ordination Chemistry: International Series of Monographs in Inorganic ChemistryDe la EverandAn Introduction to Co-Ordination Chemistry: International Series of Monographs in Inorganic ChemistryEvaluare: 3.5 din 5 stele3.5/5 (2)
- MSC. - Chemistry - 2013Document179 paginiMSC. - Chemistry - 2013Anonymous kT0ONWÎncă nu există evaluări
- Aromaticity 2019Document65 paginiAromaticity 2019Shreya PrakashÎncă nu există evaluări
- Practice Problems For Physical Chemistry 2Document1 paginăPractice Problems For Physical Chemistry 2Fatima CellonaÎncă nu există evaluări
- PolimerDocument22 paginiPolimerDhea Kana ZhafiraÎncă nu există evaluări
- Sigmatropic Rearrangement ReactionDocument18 paginiSigmatropic Rearrangement ReactionSuman ChauhanÎncă nu există evaluări
- Lab ManualDocument19 paginiLab Manualanon_467104036Încă nu există evaluări
- Bio Inorganic 1 PPT ChemistryDocument57 paginiBio Inorganic 1 PPT ChemistryShantanu MawaskarÎncă nu există evaluări
- Isolobal AnalogyDocument4 paginiIsolobal Analogyindu priyaÎncă nu există evaluări
- M.SC - Chemistry SEM 2 Syllabus 2018Document14 paginiM.SC - Chemistry SEM 2 Syllabus 2018Rukam Singh TomarÎncă nu există evaluări
- Csir Ugc JRF Net Chemistry Paper 1 (Part B) Series - 1Document22 paginiCsir Ugc JRF Net Chemistry Paper 1 (Part B) Series - 1polamrajuÎncă nu există evaluări
- Surface Chemistry MCQs - Questions - Paper 1Document7 paginiSurface Chemistry MCQs - Questions - Paper 1krishna prasad ghantaÎncă nu există evaluări
- SET-NET Pericyclic ReactionsDocument61 paginiSET-NET Pericyclic ReactionsBapu ThoratÎncă nu există evaluări
- Difficult Questions On Organic ChemistryDocument5 paginiDifficult Questions On Organic Chemistrytarunbirbanga100% (1)
- Classification of Organometallic CompoundsDocument28 paginiClassification of Organometallic CompoundsDingetegna GodanaÎncă nu există evaluări
- Problems On Named ReactionsDocument103 paginiProblems On Named ReactionsBapu ThoratÎncă nu există evaluări
- Organic Chemistry IIDocument7 paginiOrganic Chemistry IIRoberto SIlvaÎncă nu există evaluări
- NMR SpectrosDocument29 paginiNMR Spectroshareesh13h100% (1)
- Notes Chapter 8 Transition ChemistryDocument17 paginiNotes Chapter 8 Transition ChemistryGauravRajÎncă nu există evaluări
- Co-Ordination and Organometallic CompDocument85 paginiCo-Ordination and Organometallic CompDr. Dhondiba Vishwanath100% (1)
- Organometallics and Catalysis - IVDocument36 paginiOrganometallics and Catalysis - IVankit guptaÎncă nu există evaluări
- 1 IntroductoryDocument45 pagini1 IntroductoryTuhin Sahu100% (1)
- Books Suggested For M.sc. Semester 2Document1 paginăBooks Suggested For M.sc. Semester 2gsv988Încă nu există evaluări
- Acoplamiento ProtonicoDocument9 paginiAcoplamiento ProtonicoNICOLASÎncă nu există evaluări
- Organometallic CompoundsDocument40 paginiOrganometallic CompoundsHalida SophiaÎncă nu există evaluări
- Chalcone To Pyrimidine by Urea Indian PaperDocument7 paginiChalcone To Pyrimidine by Urea Indian PaperAnkit Kumar Singh100% (1)
- Atomic Structure and Spectra: Selection RulesDocument51 paginiAtomic Structure and Spectra: Selection RulesAdministracion OTIC IVICÎncă nu există evaluări
- The Transition Metals, The Lanthanides and The AntinidesDocument21 paginiThe Transition Metals, The Lanthanides and The AntinidesApril CruzÎncă nu există evaluări
- Organometallic ChemistryDocument24 paginiOrganometallic ChemistryFatma TaherÎncă nu există evaluări
- Experiments 3 Stage 2017-2018Document50 paginiExperiments 3 Stage 2017-2018Parawgay Danar100% (1)
- Electrochemistry: Introduction To Galvanic Cells and Nernst EquationDocument3 paginiElectrochemistry: Introduction To Galvanic Cells and Nernst EquationTinuviele EsguerraÎncă nu există evaluări
- Edexcel A2 Chemistry Paper 5Document386 paginiEdexcel A2 Chemistry Paper 5AbdulRahman Mustafa100% (1)
- Bioinorganic ChemistryDocument13 paginiBioinorganic ChemistryGiable AbilashÎncă nu există evaluări
- CSIR UGC NET Model Question Papers Chemical SciencesDocument32 paginiCSIR UGC NET Model Question Papers Chemical SciencesShiksha PortalÎncă nu există evaluări
- Aromatic CompoundsDocument25 paginiAromatic CompoundsElizabeth Vivar100% (1)
- Organic Chemistry IIDocument2 paginiOrganic Chemistry IIMahim MeenaÎncă nu există evaluări
- Chemistry of Natural Products PDFDocument21 paginiChemistry of Natural Products PDFhosseini_9864Încă nu există evaluări
- Lab Report Corrosion-1Document10 paginiLab Report Corrosion-1areniqwardiah_918730100% (1)
- 13C NMR SpectrosDocument16 pagini13C NMR Spectrosapi-3723327100% (4)
- Unit - 1 Lesson - 1Document271 paginiUnit - 1 Lesson - 1Rakesh SharmaÎncă nu există evaluări
- Complex SaltDocument29 paginiComplex SaltertaÎncă nu există evaluări
- Electron Transfer Reactions of Complex Ions in SolutionDe la EverandElectron Transfer Reactions of Complex Ions in SolutionÎncă nu există evaluări
- The Tech Fest 2016: Ticket Type QuantityDocument1 paginăThe Tech Fest 2016: Ticket Type QuantityPavan BoroÎncă nu există evaluări
- Physics ReviewDocument361 paginiPhysics ReviewPavan BoroÎncă nu există evaluări
- Hyperbola MathsDocument66 paginiHyperbola MathsPavan BoroÎncă nu există evaluări
- Geometry in PhysicsDocument79 paginiGeometry in PhysicsParag MahajaniÎncă nu există evaluări
- Differential Equation MathsDocument45 paginiDifferential Equation MathsPavan Boro100% (2)
- System of CirclesDocument25 paginiSystem of CirclesPavan Boro50% (2)
- Electric Current, Resistance and ResistivityDocument8 paginiElectric Current, Resistance and ResistivityPavan BoroÎncă nu există evaluări
- Alcohol, Phenol & Ether - ChemistryDocument156 paginiAlcohol, Phenol & Ether - ChemistryYoshitha Kuntumalla100% (1)
- Model Question Paper - ChemistrydocumentDocument7 paginiModel Question Paper - ChemistrydocumentHritik AgarwalÎncă nu există evaluări
- Coordination Compound Theory - EDocument34 paginiCoordination Compound Theory - Ethinkiit50% (2)
- Assignment On Co-Ordination CompoundsDocument2 paginiAssignment On Co-Ordination CompoundsMayank MundadaÎncă nu există evaluări
- CH2102 - VSEPR Theory and Coordination ChemistryDocument13 paginiCH2102 - VSEPR Theory and Coordination ChemistryJohn100% (1)
- Coordination Compounds Revision 2022Document2 paginiCoordination Compounds Revision 2022Dêêpák Sîñgh Ñîtwál100% (1)
- I. Results and Discussion: Will Be Zero and It Will Have No Effect To The Total Spin Quantum NumberDocument10 paginiI. Results and Discussion: Will Be Zero and It Will Have No Effect To The Total Spin Quantum NumberJohn Kenneth Ala SanguezaÎncă nu există evaluări
- Nandn: 1 Gate-Cy 2004 Question PaperDocument13 paginiNandn: 1 Gate-Cy 2004 Question PaperBABLI GUPTAÎncă nu există evaluări
- 9.coordination CompoundsDocument46 pagini9.coordination CompoundsSeenu MÎncă nu există evaluări
- SummerSch Intro Lewis 2015Document115 paginiSummerSch Intro Lewis 2015Sareh SabetÎncă nu există evaluări
- Ms Vandana Pandey PHD ThesisDocument263 paginiMs Vandana Pandey PHD ThesisashishkkrÎncă nu există evaluări
- CFT and Chelate Effect-IDocument65 paginiCFT and Chelate Effect-IHitesh vadherÎncă nu există evaluări
- (Fe (H2O) 6) 2 + and (Fe (CN) 6) 4 - Differ inDocument1 pagină(Fe (H2O) 6) 2 + and (Fe (CN) 6) 4 - Differ inSahil KaliaÎncă nu există evaluări
- DPP - 5 Coordination CompoundDocument3 paginiDPP - 5 Coordination CompoundTanisha SubudhiÎncă nu există evaluări
- 12 Chemistry Ncert Ch09 Coordination Compounds Part 01 QuesDocument43 pagini12 Chemistry Ncert Ch09 Coordination Compounds Part 01 Queshumayun khalidÎncă nu există evaluări
- CFT PDFDocument20 paginiCFT PDFRUFAS KANIKANTIÎncă nu există evaluări
- Coordination Chemistry Structures and IsomersDocument272 paginiCoordination Chemistry Structures and Isomersد.حاتممرقهÎncă nu există evaluări
- VBT - Theory and IsomerismDocument25 paginiVBT - Theory and Isomerismzuneid-adamoÎncă nu există evaluări
- Coordination Compound2Document63 paginiCoordination Compound2Lel LailiyÎncă nu există evaluări
- Chemistry Lecture Crystal Field TheoryDocument22 paginiChemistry Lecture Crystal Field TheoryMahendra Pratap SinghÎncă nu există evaluări
- CML 100: Inorganic Chemistry Contents: Bonding in Transition Metal ComplexesDocument19 paginiCML 100: Inorganic Chemistry Contents: Bonding in Transition Metal Complexestushar guptaÎncă nu există evaluări
- M.SC ChemistryDocument41 paginiM.SC ChemistryvishnuÎncă nu există evaluări
- Effective Ionic Radii in Oxides and Fluorides : ReferencesDocument22 paginiEffective Ionic Radii in Oxides and Fluorides : ReferencesMoad BarbariÎncă nu există evaluări
- Transition Metals and Coordination ComplexesDocument61 paginiTransition Metals and Coordination ComplexesGliezl ImperialÎncă nu există evaluări
- Valence Bond Theory For Complexes: Presentation OutlineDocument6 paginiValence Bond Theory For Complexes: Presentation OutlineNurul Izzah KaharÎncă nu există evaluări
- Lecture 9Document30 paginiLecture 9JetNoKunÎncă nu există evaluări
- Senior - 2020 - Class - 12 - Chemistry - Objective Questions - Coordination CompoundsDocument4 paginiSenior - 2020 - Class - 12 - Chemistry - Objective Questions - Coordination CompoundsJijendarÎncă nu există evaluări
- Co-Ordination CompoundsDocument11 paginiCo-Ordination CompoundsShashank AgarwalÎncă nu există evaluări
- 17 Doc 2844038 PDFDocument69 pagini17 Doc 2844038 PDFLewovÎncă nu există evaluări
- Crystal Field Theory IIDocument2 paginiCrystal Field Theory IIabhay j bavishiÎncă nu există evaluări
- General Chemistry IIDocument10 paginiGeneral Chemistry IIAravindan NatarajanÎncă nu există evaluări