Documente Academic
Documente Profesional
Documente Cultură
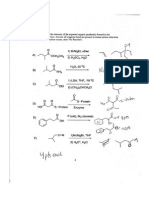
Miscellaneous Biochemistry Questions
Încărcat de
phoenixscarDrepturi de autor
Formate disponibile
Partajați acest document
Partajați sau inserați document
Vi se pare util acest document?
Este necorespunzător acest conținut?
Raportați acest documentDrepturi de autor:
Formate disponibile
Miscellaneous Biochemistry Questions
Încărcat de
phoenixscarDrepturi de autor:
Formate disponibile
Solutions for problems assigned with Lectures 1 & 2
Garrett & Grisham, pg. 27
3. Escherichia coli cells are about 2 m (microns) long and 0.8 m in diameter.
a. How many E. coli cells laid end to end would fit across the diameter of a pin head?
(Assume a pinhead diameter of 0.5 mm.)
Answer:
E. coli /pinhead di ameter =
0.5 mm/di a.
2 m/E. col i
=
0.5 10
3
m
2 10
-6
m
= 250 E. col i/pi nhead di ameter
b. What is the volume of an E. coli cell? (Assume it is a cylinder, with the volume of a
cylinder given by V=tr
2
h, where t = 3.14.)
Answer:
V = tr
2
h
= 3.14 (
0.8 m
2
)
2
2 m
= 3.14 (0.4 10
6
m)
2
(2 10
6
m)
= 1 18
18
m
3
But, 1 m
3
= (10
2
cm)
3
= 10
6
cm
3
= 10
6
ml = 10
3
L
V = 1 10
-18
m
3
= 1 10
-15
L
= 1 fL (femtoli ter)
c. What is the surface area of an E. coli cell? What is the surface-to-volume ratio of an
E. coli cell?
Note There is a mistake in this answer in the Study Guide.
Answer:
1 -
m
6
10 6 =
2
m
12
10 6
= SA/V
2
m
12
10 6 =
m)
6
10 m)(2
6
10 (0.8 3.14
2
m)
6
10 (0.4 3.14 2 =
dh
2
r 2 = SA
+
3
m
18
10 1
t t
d. Glucose, a major energy-yielding nutrient, is present in bacterial cells at a
concentration of about 1 mM. How many glucose molecules are contained in a typical E.
coli cell? (Recall that Avogadros number = 6.023 x 10
23
.)
Answer:
moles glucose = concentration volume
= 1 mM 1 10
-15
L (from b.)
= 1 10
-18
mol es
# mol ecul es = 1 10
-18
mol es 6.023 10
23
(mol ecule/mol)
= 6 10
5
mol ecul es
e. A number of regulatory proteins are present in E. coli at only one or two molecules per
cell. If we assume that an E. coli contains just one molecule of a particular protein, what
is the molar concentration of this protein in the cell?
Answer:
1 molecule
6.023 10
23
mol ecules/mol e
= 1.7 10
-24
mol e
Mol ar concentrati on =
mol es
volume(in l i ters)
=
1.7 10
-24
mol e
10
-15
L (from b.)
= 1.7 10
-9
M = 1.7 nM
f. An E. coli cell contains about 15,000 ribosomes, which carry out protein synthesis.
Assuming ribosomes are spherical and have a diameter of 20 nm (nanometers), what
fraction of the E. coli cell volume is occupied by ribosomes?
Answer:
volume of one ribosome =
4
3
tr
3
=
4
3
3.14 (10 10
9
m)
3
= 4.2 10
-24
m
3
volume of 15, 000 ribosomes = 4.2 10
-24
m
3
15, 000
= 6.3 10
20
m
3
fractional vol ume = vol ume ri bosome/vol ume E. col i
=
6.3 10
20
m
3
1 10
18
m
3
(from b.)
= 0.063 or 6.3%
g. The E. coli chromosome is a single DNA molecule whose mass is about 3.0 x 10
9
Daltons. This macromolecule is actually a circular array of nucleotide pairs. The
average molecular weight of a nucleotide pair is 660 and each pair imparts 0.34 nm to
the length of the DNA molecule. What is the total length of the E. coli chromosome? How
does this length compare with the overall dimensions of an E. coli cell? How many
nucleotide pairs does this DNA contain? The average E. coli protein is a linear chain of
360 amino acids. If three nucleotide pairs in a gene encode one amino acid in a protein,
how many different proteins can the E. coli chromosome encode? (the answer to this
question is a reasonable approximation of the maximum number of different kinds of
proteins that can be expected in bacteria.)
Answer: The number of moles of base pairs in 3.1 x 10
9
D dsDNA is given by
775
m 2
m 1,550
coli E. length
DNA length
2mm = length
m 1,550 = mm 1.55 = m
3
10 1.55 =
(nm/bp) 0.34 bp/mole mole
6
10 4.55 = length
bp/mole mole
6
10 4.55 = dsDNA molecule 1
bp/mole mole
6
10 4.55
bp) e 660(gm/mol
dsDNA) (gm/mole
9
10 3.0
= =
coli E.
To calculate the number of different proteins:
360 aa/protein 3 bp/aa = 1080 bp/protei n
# di fferent protei ns =
4.55 10
6
bp
1080 bp/protei n
= 4, 213 proteins
10. Why does the central role of weak forces in biomolecular interactions restrict living
systems to a narrow range of environmental conditions?
Answer: The weak forces such as hydrogen bonds, ionic bonds, hydrophobic interactions, and
van der Waals interactions can be easily overcome by low amounts of energy. Slightly elevated
temperatures are sufficient to break hydrogen bonds. Changes in ionic strength, pH,
concentration of particular ions, etc., all potentially have profound effects on macromolecular
structures dependent on the weak forces.
11. Describe what is meant by the phrase "cells are steady-state systems".
Answer: Life is characterized as a system through which both energy and matter flow. The
consequence of energy flow in this case is order, the order of monomeric units in biopolymers,
which in turn produce macromolecular structures that function together as a living cell.
Garrett & Grisham, pg. 75
1. An enzymatic hydrolysis of fructose-1-P
Fructose1P +H
2
O fructose+P
i
was allowed to proceed to equilibrium at 25C. The original concentration of fructose-1-P
was 0.2 M, but when the system had reached equilibrium the concentration of fructose 1-
P was only 6.52 x 10
-5
M. Calculate the equilibrium constant for this reaction and the
standard free energy of hydrolysis of fructose 1-P.
Answer:
For fructose1P+H
2
O fructose+P
i
The equilibrium constant, Keq, is given by
K
eq
=
[f ructose]
eq
[P
i
]
eq
[ f ructose1 P]
eq
At 25C or 298
K (273 + 25), [fructose-1-P]eq = 6.52 x 10
-5
M. Initially [fructose-1-P] = 0.2 M. The
amount of the fructose produced is given by: 0.2 M - 6.52 x 10
-5
M.
And, since an equal amount of [Pi] is produced, Keq may be written as follows:
mol / kJ 9 . 15 G
ln613 K 298 K) J/mol 314 . 8 ( G
RTlnK G
M 613 K
10 52 . 6
) 10 52 . 6 2M . 0 )( 10 52 . 6 2M . 0 (
K
eq
eq
5
5 5
eq
= A
= A
= A
=
=
3. The standard state free energy of hydrolysis of acetyl phosphate is G = -42.3
kJ/mol.
AcetylP +H
2
O acetate+P
i
Calculate the free energy change for the acetyl phosphate hydrolysis in a solution of 2
mM acetate, 2 mM phosphate and 3 nM acetyl phosphate.
Answer:
kJ/mol -24.8 = G
) 10 (3
) 10 (2 ) 10 (2
ln K 20) + (273 K) l 8.314(J/mo + ol) 42,300(J/m = G
P] - [acetyl
] P [acetate][
ln K 20) + (273 K) l 8.314(J/mo + G = G
[Reactant]
[Product]
RTln + G = G
9 -
3 - 3 -
i
initial
initial
A
A
A A
A A
6. For the process A B, Keq(AB) is 0.02 at 37C. For the process B C, Keq(BC) is 1000
at 37C.
a. Determine Keq(AC), the equilibrium constant for the overall process
A C, from Keq(AB)
and Keq(BC).
b. Determine standard state free energy changes for all three processes, and use G(AC)
to determine Keq(AC). Make sure that this value agrees with that determined in part a, of
this problem.
Answer:
For A B and B C,
K
eq
(AB) =
[B]
eq
[A]
eq
, and K
eq
(BC) =
[C]
eq
[B]
eq
By solving for [B]
eq
in the above two equations we find:
[B]
eq
= K
eq
(AB) [ A]
eq
=
[C]
eq
K
eq
(BC)
This equation can be rearranged to give:
[C]
eq
[ A]
eq
= K
eq
(AC) =K
eq
(AB) K
eq
(BC) =0.021000 or,
K
eq
(AC) =20
The standard free energy change is calculated as follows:
mol / kJ 8 . 17 ) BC ( G
ln1000 K 310 ) molK / J ( 314 . 8 ) BC ( RTlnK ) BC ( G
mol / kJ 1 . 10 ) AB ( G
02 . ln0 K 310 ) K mol / J ( 314 . 8 ) AB ( RTlnK ) AB ( G
eq
eq
= A
= = A
= A
= = A
or
mol / 72kJ . 7 ) AC ( G
ln20 K 310 ) K mol / J ( 314 . 8 ) AC ( RTlnK ) AC ( G
eq
= A
= = A
l mo / 70kJ . 7 mol / 8kJ . 17 mol / 1kJ . 10 ) AC ( G
) BC ( G ) AB ( G ) AC ( G
= = A
A + A = A
8. Write the equilibrium constant, Keq, for the hydrolysis of creatine phosphate and
calculate a value for Keq at 25C from the value of G' in Table 3.3.
Answer: For the reaction:
7
eq
298 314 . 8
) 10 (-43.3
RT
G
eq
eq
eq 2 eq
eq i eq
eq
i 2
10 3.89 = K
e = e = K
: write can we , -RTlnK = G From
O] [H phosphate] [creatine
] [P [creatine]
= K
l -43.3kJ/mo = G , P + creatine O H + phosphate creatine
3
10. Calculate the free energy of hydrolysis of ATP in a rat liver cell in which the ATP,
ADP, and Pi concentrations are 3.4, 1.3, and 4.8 mM, respectively.
Answer: For [ATP] = 3.4 mM, [ADP] = 1.3 mM, and [P
i
] = 4.8 mM, calculate the G of hydrolysis
of ATP.
mol / kJ 7 . 46 G C, 37 At
mol / kJ 1 . 46 G
10 4 . 3
) 10 )(4.8 10 (1.3
ln K ) 5 2 273 ( 10 8.314 + 3.3) Table (From mol / 5kJ . 30 G
] ATP [
] P ][ ADP [
RTln G G
3 -
3 - 3 -
3 -
i
= A
= A
+ = A
+ A = A
11. Hexokinase catalyzes the phosphorylation of glucose from ATP, yielding glucose-6-P
and ADP. Using the values of Table 3.3, calculate the standard-state free energy change
and equilibrium constant for the hexokinase reaction. (The glucose to glucose-6-P
reaction is not in Table 3.3. Use G'= -13.9 kJ/mol for glucose-6-P hydrolysis.)
Answer:
Hexokinase catalyzes the following reaction:
glucose + ATP glucose -6-P + ADP
This reaction may be broken down into the following two reactions:
glucose +P
i
glucose -6-P + H
2
O (1) and,
ATP+ H
2
O ADP+P
i
(2)
From Table 3.3, we find that G'= -13.9 kJ/mol for glucose-6-P hydrolysis.
Thus, the reverse reaction, namely reaction (1), must have G' = +13.9 kJ/mol.
From Table 3.3, we also find that ATP hydrolysis has G' = - 30.5 kJ/mol.
The overall G' for phosphoryl transfer from ATP to glucose is:
AG' = +13.9 +( 30.5) = 16.6kJ/mol and,
K
eq
= e
AG'
RT
= e
(16.610
3
)
8.314310
= 626.9
Segel pg 205
1.
K
eq
= 0.43
AG = -RTln K
eq
Using calories, not Joules,
RT = 1.987 cal/molK x 298 K = 592 cal/mol
AG = -592 cal/mol x ln(0.43)
AG = 500 cal/mol
2.
GTP + ATP ADPG + PP
i
; K
eq
= 1.0
a. AG = AG + RTln
] ][ [
] ][ [
ATP GTP
PPi ADPG
= -RTln(1) + (592 cal/mol)ln
] 10 ][ 10 [
] 10 ][ 10 [
3 4
5 5
= 0 + -4.09 kcal/mol = -4.09 kcal/mol
b. Spontaneously to the right.
10.
ATP cAMP + PP
i
; K
eq
= 0.065
ATP AMP + PP
i
; AG = -8 kcal/mol
So
ATP AMP + PP
i
; AG = -8 kcal/mol
cAMP + PP
i
ATP; AG = -RTlnK
eq
= -RTln )
065 . 0
1
( = -1618 cal/mol
______________
cAMP AMP ; AG = -8000 cal/mol + -1618 cal/mol = -9618 cal/mol
12.
G-6-P G-1-P ; K
eq
=
19
1
= 0.053 =
] 6 [
] 1 [
P G
P G
Initial: [G6P] = 1 M
Final: [G6P] = 1 X, [G1P] = X
0.053 =
X
X
1
X =
053 . 0 1
053 . 0
+
= 0.05
a. [G6P] = 1 0.05 = 0.95 M, [G1P] = 0.05 M;
] 1 [
] 6 [
P G
P G
= 19
b. [G6P] = 0.095 M, [G1P] = 0.005 M;
] 1 [
] 6 [
P G
P G
= 19
c. [G6P] = 0.0095 M, [G1P] = 0.0005 M;
] 1 [
] 6 [
P G
P G
= 19
etc.
S-ar putea să vă placă și
- RudrakshaDocument15 paginiRudrakshaT Sampath Kumaran100% (1)
- DBT BET JRF 2018 Solved Question Paper With Answer KeyDocument51 paginiDBT BET JRF 2018 Solved Question Paper With Answer KeyRana GhoshÎncă nu există evaluări
- Practical Guide StudentsDocument24 paginiPractical Guide StudentsTina Atlagić100% (1)
- Carter Solution Chapter 18Document13 paginiCarter Solution Chapter 18Maria SalazarÎncă nu există evaluări
- A History of Membrane Transport and Bioenergetics PDFDocument383 paginiA History of Membrane Transport and Bioenergetics PDFEdmilson RodriguesÎncă nu există evaluări
- Ch06 Homework ProblemsDocument6 paginiCh06 Homework Problemsheyyyale100% (1)
- Enzyme Kinetics ProblemsDocument5 paginiEnzyme Kinetics ProblemsBlessy Gabayno100% (1)
- Biochem Enzyme KineticsDocument53 paginiBiochem Enzyme KineticsJayvee Francisco67% (3)
- Balmes, Patricia R. - Enzyme KineticsDocument11 paginiBalmes, Patricia R. - Enzyme KineticsGlecie RasÎncă nu există evaluări
- Midterm Review SolutionsDocument16 paginiMidterm Review SolutionsKate SongÎncă nu există evaluări
- GenBio 1 Lesson 1 ATP and ADP CycleDocument2 paginiGenBio 1 Lesson 1 ATP and ADP CycleLlaban MunicaÎncă nu există evaluări
- Biochem Problem SolvingDocument53 paginiBiochem Problem SolvingNasser Gemina PantaoÎncă nu există evaluări
- ExChEL Group Study Session 13 - Day 1 ExaminationDocument15 paginiExChEL Group Study Session 13 - Day 1 ExaminationRochelle Louise SampagaÎncă nu există evaluări
- Sample Che Board Exam QuestionsDocument2 paginiSample Che Board Exam QuestionsGerry Lou Quiles93% (14)
- EXPERIMENT 9 Food ChemistryDocument7 paginiEXPERIMENT 9 Food ChemistryNurmazillazainal67% (3)
- Life Processes Class 10 Notes Biolog1Document8 paginiLife Processes Class 10 Notes Biolog1Eashurock KnigamÎncă nu există evaluări
- La Teoría de La Compuerta - AlDocument12 paginiLa Teoría de La Compuerta - AlAlterOsPegiamÎncă nu există evaluări
- Environmental Engineering Homework #1 SolutionDocument3 paginiEnvironmental Engineering Homework #1 Solutionjabenne2100% (2)
- Cell Kinetics and Fermenter Design 2Document17 paginiCell Kinetics and Fermenter Design 2rhia81% (16)
- Ian D. Young (Author) - Introduction To Risk Calculation in Genetic Counseling, Third Edition-Oxford University Press, USA (2006) PDFDocument252 paginiIan D. Young (Author) - Introduction To Risk Calculation in Genetic Counseling, Third Edition-Oxford University Press, USA (2006) PDFOmar AskanderÎncă nu există evaluări
- Membrane Prob.Document12 paginiMembrane Prob.Riki BiswasÎncă nu există evaluări
- Jawaban Soal BioprosDocument3 paginiJawaban Soal Bioproskuingin dicintaiÎncă nu există evaluări
- CH 01Document3 paginiCH 01mehÎncă nu există evaluări
- Homework Assignment 4: Due at 5 PM On FR Problems From The BookDocument7 paginiHomework Assignment 4: Due at 5 PM On FR Problems From The BookYi QinÎncă nu există evaluări
- MidSem KB3633 0810 BaruDocument6 paginiMidSem KB3633 0810 Baruellyn_zdeenÎncă nu există evaluări
- Assignment (Chemical Kinetics and Chemical Equilibrium)Document4 paginiAssignment (Chemical Kinetics and Chemical Equilibrium)Mapalo faith ChamaÎncă nu există evaluări
- Equations 1Document10 paginiEquations 1Deepak Singh Lourembam0% (1)
- Basics of ChemistryDocument7 paginiBasics of ChemistryManqabat WalayÎncă nu există evaluări
- Midsem CL-623Document4 paginiMidsem CL-623RajÎncă nu există evaluări
- Heat Transfer Sample ProblemsDocument5 paginiHeat Transfer Sample ProblemsdropgorgeousÎncă nu există evaluări
- Practical QuestionsDocument6 paginiPractical QuestionsLidiya MitikuÎncă nu există evaluări
- CHE 3122 Lecture Notes Energetics of Photosynthesis: 2018 Academic Year October 2019Document9 paginiCHE 3122 Lecture Notes Energetics of Photosynthesis: 2018 Academic Year October 2019Lucy ZuluÎncă nu există evaluări
- Hop AmDocument4 paginiHop AmAnonymous 5lZJ470Încă nu există evaluări
- # G /genome 3.1x10 1.66x10 G 5.1x10 G /genomeDocument2 pagini# G /genome 3.1x10 1.66x10 G 5.1x10 G /genomerickÎncă nu există evaluări
- Comments: General Questions and Numerical ProblemsDocument5 paginiComments: General Questions and Numerical ProblemsJiaming BiÎncă nu există evaluări
- PassGamsat Science Course Handout QuestionsDocument64 paginiPassGamsat Science Course Handout Questionsjoe lopasoÎncă nu există evaluări
- ch14 Allostery Problems 6-18-11Document9 paginich14 Allostery Problems 6-18-11Gary YuÎncă nu există evaluări
- Discussion 1Document10 paginiDiscussion 1Niomi ButtermilkÎncă nu există evaluări
- Bioprocess and Engeneering Revision QuestionDocument3 paginiBioprocess and Engeneering Revision QuestionGia BooÎncă nu există evaluări
- ATPaseDocument21 paginiATPaseDevin Maa100% (1)
- C Molecules Soln 07Document3 paginiC Molecules Soln 07Leah FeeneyÎncă nu există evaluări
- Practice Problems ThermodynamicsDocument5 paginiPractice Problems ThermodynamicsJana ChambersÎncă nu există evaluări
- CHEM311 191 Major2 SolvedDocument11 paginiCHEM311 191 Major2 SolvedhussainÎncă nu există evaluări
- Manual Biochemistry-10-2017Document65 paginiManual Biochemistry-10-2017Dental LecturesMMQÎncă nu există evaluări
- Building A Living CellDocument12 paginiBuilding A Living Cellgre.mayÎncă nu există evaluări
- Essential Chemical Concepts Session IIDocument26 paginiEssential Chemical Concepts Session IIHamza QureshiÎncă nu există evaluări
- 200115spring 2001Document85 pagini200115spring 2001combatps1Încă nu există evaluări
- Enzym ESDocument21 paginiEnzym ESpoopnoodlemanÎncă nu există evaluări
- How Big Is A Protein MoleculeDocument6 paginiHow Big Is A Protein MoleculeAdamÎncă nu există evaluări
- CHEM 341 Physical Chemistry Final Exam: Do Not Open This Exam Until Told To Do SoDocument10 paginiCHEM 341 Physical Chemistry Final Exam: Do Not Open This Exam Until Told To Do SoOmarÎncă nu există evaluări
- F19 Midterm BlankDocument7 paginiF19 Midterm BlankAhmed OsmanÎncă nu există evaluări
- Tutorial 3 - Basic Calculations in Chem EnggDocument3 paginiTutorial 3 - Basic Calculations in Chem Engglifeworld2401Încă nu există evaluări
- Set8ans 10Document5 paginiSet8ans 10Agustina Evania DewiÎncă nu există evaluări
- CHEM-E2100 Polymer Synthesis Exercise 3: Chain Polymerization II: Chain Transfer ATRPDocument2 paginiCHEM-E2100 Polymer Synthesis Exercise 3: Chain Polymerization II: Chain Transfer ATRP王怀成Încă nu există evaluări
- Sans TitreDocument9 paginiSans TitrerollinpeguyÎncă nu există evaluări
- CSIR NET Dec 2015 Ls Answer KeyDocument32 paginiCSIR NET Dec 2015 Ls Answer KeyAbhay Kumar50% (2)
- 5 - Tutorial 1Document14 pagini5 - Tutorial 1039 Asma SanjumÎncă nu există evaluări
- Nuclear Chemistry WorksheetDocument4 paginiNuclear Chemistry WorksheetMigsÎncă nu există evaluări
- 2018 QuizDocument16 pagini2018 QuizrobsÎncă nu există evaluări
- Leskgenomics3e Exercises ch01Document3 paginiLeskgenomics3e Exercises ch01Reza SadeghiÎncă nu există evaluări
- BMSolutions PDFDocument48 paginiBMSolutions PDF曾hahaÎncă nu există evaluări
- Dec Exam AnswersDocument17 paginiDec Exam Answersvivivihohoho1Încă nu există evaluări
- Chapter 7Document8 paginiChapter 7api-201479236Încă nu există evaluări
- Chemical Principles 8th Edition Zumdahl Solutions ManualDocument35 paginiChemical Principles 8th Edition Zumdahl Solutions Manualdement.disturnlklpvp95% (22)
- CHEM102 062 Old-Exam First-Major UnsolvedDocument22 paginiCHEM102 062 Old-Exam First-Major UnsolvedAbdullah AltwirqiÎncă nu există evaluări
- Format of Lab Report Example 8609Document14 paginiFormat of Lab Report Example 8609herrk167% (3)
- 2016 Sample Paper 12 Chemistry 02 AnsDocument7 pagini2016 Sample Paper 12 Chemistry 02 AnsjrajaÎncă nu există evaluări
- Lab and Lect Aide Application W15Document2 paginiLab and Lect Aide Application W15phoenixscarÎncă nu există evaluări
- Organic Chemistry 118C Practice Exam + Key (Midterm 1)Document5 paginiOrganic Chemistry 118C Practice Exam + Key (Midterm 1)phoenixscarÎncă nu există evaluări
- Organic Chemistry 118C Practice Exam + Key (Midterm 2)Document7 paginiOrganic Chemistry 118C Practice Exam + Key (Midterm 2)phoenixscarÎncă nu există evaluări
- Organic Chemistry 118C Practice Exam + Key (Midterm 1)Document7 paginiOrganic Chemistry 118C Practice Exam + Key (Midterm 1)phoenixscarÎncă nu există evaluări
- Microbiology 1.03 Basic Concepts 1Document9 paginiMicrobiology 1.03 Basic Concepts 1Camila BarzagaÎncă nu există evaluări
- 172-1610826745-Case ReportDocument6 pagini172-1610826745-Case ReportBojan IlievÎncă nu există evaluări
- WV Nov 2023 First DraftDocument8 paginiWV Nov 2023 First DraftMandlakhe HadebeÎncă nu există evaluări
- 1st SEMESTER MIDTERM REVIEWER in MEDICAL SURGICAL NURSINGDocument4 pagini1st SEMESTER MIDTERM REVIEWER in MEDICAL SURGICAL NURSINGskoolrkiveÎncă nu există evaluări
- Acid Hydrolysis and Chemical Characterization of DNADocument28 paginiAcid Hydrolysis and Chemical Characterization of DNAai_ferminÎncă nu există evaluări
- Article: Contribution To The Knowledge of Ephemeroptera (Insecta) From Goiás State, BrazilDocument15 paginiArticle: Contribution To The Knowledge of Ephemeroptera (Insecta) From Goiás State, BrazilErikcsen Augusto RaimundiÎncă nu există evaluări
- Population Ecology: Aecc-I +3 1 YearDocument32 paginiPopulation Ecology: Aecc-I +3 1 YearAnita kumari SahuÎncă nu există evaluări
- Allegra 64R CentrifugeDocument64 paginiAllegra 64R Centrifugeluroguita-1Încă nu există evaluări
- Introduction History and Development of MicrobiologyDocument17 paginiIntroduction History and Development of Microbiologymoses samuelÎncă nu există evaluări
- Rasaoushadhis and Bhasmas in The Management of Arbuda: Dr. Santhosh B Dept. of RasashastraDocument69 paginiRasaoushadhis and Bhasmas in The Management of Arbuda: Dr. Santhosh B Dept. of Rasashastrasantosh S B100% (3)
- Overview of Muscle Tissues: Key ChoicesDocument36 paginiOverview of Muscle Tissues: Key ChoicesJustine Mae AbajoÎncă nu există evaluări
- Connect (4) New QuestionDocument18 paginiConnect (4) New QuestionGeorge ElsabaÎncă nu există evaluări
- Xerophilia - V 04 No - 4-15 Dec 2015Document110 paginiXerophilia - V 04 No - 4-15 Dec 2015golf2010100% (1)
- Sja 15 435Document6 paginiSja 15 435Nilamsari KurniasihÎncă nu există evaluări
- Reaka 2005 BiodiversityofCaribbeanCoralReefsDocument17 paginiReaka 2005 BiodiversityofCaribbeanCoralReefsNataly MendesÎncă nu există evaluări
- Uniit 9 399-408 DepEd-IPDocument10 paginiUniit 9 399-408 DepEd-IPpeucogcoÎncă nu există evaluări
- Goby Shirmp MutualismDocument20 paginiGoby Shirmp MutualismKhandoker Raisul AzadÎncă nu există evaluări
- Group Two'S Seminar Work: Topic: Enzyme Regulation Allosteric Regulation and Models OutlineDocument13 paginiGroup Two'S Seminar Work: Topic: Enzyme Regulation Allosteric Regulation and Models OutlineOluwasegun ModupeÎncă nu există evaluări
- Womersley Arterial Flow - SimpleDocument10 paginiWomersley Arterial Flow - SimplebhatsindhoorÎncă nu există evaluări
- Cerebrospinal FluidDocument3 paginiCerebrospinal FluidlecturioÎncă nu există evaluări
- Bionic EyeDocument1 paginăBionic EyesubhasishcÎncă nu există evaluări
- Pharmacokinetic Models NotesDocument32 paginiPharmacokinetic Models Notes슬기.Încă nu există evaluări