Documente Academic
Documente Profesional
Documente Cultură
Ep0194483a1 PDF
Încărcat de
SJ ChuaTitlu original
Drepturi de autor
Formate disponibile
Partajați acest document
Partajați sau inserați document
Vi se pare util acest document?
Este necorespunzător acest conținut?
Raportați acest documentDrepturi de autor:
Formate disponibile
Ep0194483a1 PDF
Încărcat de
SJ ChuaDrepturi de autor:
Formate disponibile
09}
(12
Europaisches
Pat ent amt
European
Patent Office
Publication number:
0 1 9 4 4 8 3
Office
europeen
des brevets A1
EUROPEAN PATENT APPLI CATI ON
Application number: 86102208.5
Int. CI.4: B 01 D 53/ 22
^
B 01 D 13/ 00
Date of filing: 20.02.86
Priority 01.03.85 US 707299 Applicant: AIRPRODUCTS ANDCHEMICALS, INC.
01.03.85 US 707298 P.O. Box 538
Allentown, Pennsylvania 18105(US)
Date of publication of application:
@ Inventor: Pez, Guido Peter
17.09.86 Bulletin 86/38 3705 Vale View Drive
Allentown, PA18103IUS)
Designated Contracting States:
BE DE FR GB NL Inventor: Carlin, Richard Trotter
Idylwood Apts, Apt No. 666 Slate Creek Drive
Cheektowaga New York 14227(US)
Representative: Kador 8t Partner
Corneliusstrasse 15
D-8000Miinchen5(DE)
(54) Method for
gas separation.
57 A
process
for separating a gas
from a mixture of
gases
comprises passing the
gas
mixture over a membrane,
selectively permeable by the
gas being separated, owing to
one or more reversible reactions between a continuous layer
of active molten material, immobilized in a thin, rigid,
porous, inert support therefor, and the
gas being separated.
Techni cal F i e l d
This i n v e n t i o n r e l a t e s to an i mproved method of
s e p a r a t i o n of a
g a s
from
a
mi xt ur e of
gases
and to i mproved membranes f or the s e p a r a t i o n
p r o c e s s .
Background A r t
Numerous
appr oaches to the s e p a r a t i o n of
a
gas
from a mi xt ur e o f
gases by d i f f e r e n t i a l
per meat i on have been
i n v e s t i g a t e d . Robb et a l . , i n
U.S. Pat ent 3, 335, 545, have pr oposed use of
a l i qui d, e nt r a ppe d in
a
porous or pe r me a bl e s uppor t , to s e p a r a t e
mi xt ur es of
gas es .
The
use of a
s o - c a l l e d " q u a s i - l i q u i d f i l m, " f or exampl e, di e t hyl e ne g l y c o l ,
in
a
s uppor t
has p e r mi t t e d s e p a r a t i o n of car bon di oxi de from n i t r o g e n ,
hydrogen or
oxygen,
the s e l e c t i v i t y bei ng somewhat hi gher t han would be
p r e d i c t e d on the bas i s of the mol ecul ar wei ght s
of the
gases as the s o l e
f a c t o r , i n f l u e n c i n g s e p a r a t i o n . Ward, I I I , has pr opos ed, in U.S. P a t e n t
3, 503, 186, a s i mi l a r p r o c e d u r e for s e p a r a t i n g s ul f ur di oxi de from o t h e r
g a s e s .
The
use
of f a c i l i t a t e d
t r a n s p o r t l i qui d membranes has been r e vi e we d
by Way et a l . , J. Membrane Sci ence, vol . 12 (1982),
pages
239- 259.
Anot her t y p i c a l d i s c l o s u r e of ga s s e p a r a t i o n usi ng a
f a c i l i t a t e d
t r a n s p o r t i mmobi l i zed membr ane is t ha t of Bas s et t et a l . , Bi ochi mi ca e t
Bi ophys i ca Act a, vol . 211 ( 1970) ,
pages
194-215. F a c i l i t a t e d
t r a n s p o r t
of
gases t hr ough l i q u i d membranes is al s o r e c i t e d , f or
exampl e,
in t h e
f ol l owi ng U.S. P a t e n t s :
Wa r d , I I I , et al . ' 510 r e c i t e us i ng an
i mmobi l i zed
l i q u i d
f i l m as a
per meabl e membrane, wher ei n t he l i q u i d f i l m c ont a i ns at l e a s t one
s o l u b l e , n o n - v o l a t i l e , di s s ol ve d c a r r i e r s pe c i e s , which is r e v e r s i b l y
c h e mi c a l l y r e a c t i v e wi t h
a
s e l e c t e d
component
of
a gaseous
mi x t u r e .
Ki mura, et al . (U.S. Pa t e nt 4, 318, 714) have r e c i t e d us i ng a n
i on- e xc ha nge
membrane to a c c o mg l i s h f a c i l i t a t e d s e p a r a t i o n of a gas
f r o m
a mi xt ur e of
g a s e s .
Yanamot o, et al . (U.S. Pat ent 3, 155, 467) have d i s c l o s e d s e p a r a t i o n
and p u r i f i c a t i o n of
hydr ogen, usi ng a pal l adi um a l l oy as a per meabl e wa l l
s t r u c t u r e .
Sol i d and mol t en s a l t e l e c t r o l y t e s have. been d i s c l o s e d , in t he f u e l
c e l l or e l e c t r o c h e mi c a l a r t s , in t he f ol l owi ng r e p r e s e n t a t i v e p a t e n t s :
Yo s h i s a t o , et a l . , in U.S. Pa t e nt 4, 330, 633, r e c i t e us i ng a s o l i d
e l e c t r o l y t e compr i s i ng a s i n t e r e d
body
of mixed i nor ga ni c met al oxi des a s
a membrane for the r e g e n e r a t i o n of
oxygen
at r e l a t i v e l y hi gh t e mp e r a t u r e s
( about 800 C) .
Oxygen is known to
per meat e s e l e c t i v e l y t hr ough me t a l l i c s i l v e r .
However,
oxygen
f l uxe s at r eas onabl e
t e mpe r a t ur e s ( about 400C)
are l ow,
as r e p o r t e d by Gr yaznov, et a l . , Rus s i an. J. Phys. Chem., vol . 47 ( 1973) ,
pages
1517- 1519. At hi gher t e mp e r a t u r e s , me t a l l i c membranes f or t h e
s e p a r a t i o n of
oxygen
from ot her
gases are u n s t a b l e , s e e Mul ha upt , U. S.
Pat ent 3 , 3 5 9 , 7 0 5 .
A l i mi t i n g f e a t ur e in
many
of t hese d i s c l o s u r e s is t ha t
a gas
s h o u l d
not c ompl e t e l y per meat e the e l e c t r o l y t e or e l e c t r o d e , s i nce c ompl e t e
p e n e t r a t i o n coul d s hor t c i r c u i t the d e v i c e .
Bat l gne et a l . , U.S. Pat ent 4, 396, 572, r e c i t e
usi ng a por ous
c e r a mi c
b a r r i e r , havi ng a p l u r a l i t y of super i mposed l a ye r s of
pa s t e s
of
v a r y i n g
c ompos i t i on, to
s e pa r a t e
urani um h e x a f l u o r i d e i s ot opes by u l t r a f i l t r a t i o n .
I t is
a ppa r e nt
t hat p r e s e n t l y
a v a i l a b l e membranes for
s e p a r a t i o n o f
gases by d i f f u s i o n , per meat i on or
u l t r a f i l t r a t i o n
are for the most
p a r t
r e l a t i v e l y u n s e l e c t i v e or complex in s t r u c t u r e .
I t is t h e r e f o r e
an obj e c t o f t h i s i nve nt i on to pr ovi de met hods a nd
a r t i c l e s of t he s e p a r a t i o n of
gases
from mi xt ur e s , which are much
mor e
hi ghl y
s e l e c t i v e and have hi gher f l uxes of per meat i ng
gas
t han
p r e s e n t l y
a v a i l a b l e met hods and to pr ovi de membranes which
are si mpl e to c o n s t r u c t
and to u s e .
Di s c l os ur e of I n v e n t i o n
In
one a s p e c t ,
t hi s i nve nt i on r e l a t e s to a pr ocess
f or
s e p a r a t i n g a t
l eas t
one gas
from
a mi xt ur e of
gas es ,
compr i s i ng pas s i ng the
gas
mi x t u r e
over a membrane s e l e c t i v e l y per meabl e to the
gas
bei ng s e p a r a t e d as a
r e s ul t of
one or more r e v e r s i b l e r e a c t i o n s , i nc l udi ng o x i d a t i o n - r e d u c t i o n
r e a c t i o n s , bet ween the
gas bei ng s e pa r a t e d
and
an
a c t i ve ma t e r i a l in t h e
membrane; wher ei n the membrane compr i ses a t hi n,
por ous,
i ne r t
s uppor t
i n
which is i mmo b i l i z e d t h e a c t i v e ma t e r i a l and the act i ve ma t e r i a l is
a
mol t en s a l t , p r e f e r a b l y capabl e of one or more
r e v e r s i b l e r e a c t i o n s wi t h
the
gas bei ng s e p a r a t e d .
In a not he r
a s pe c t ,
t h i s i nve nt i on r e l a t e s to a
pr oces s
f o r
s e p a r a t i n g a gas
from at l e a s t
one ot her
gas
in
a
mi xt ur e
by
the
s t eps
o f
i nt r oduc i ng the mi xt ur e i nt o a module havi ng at l eas t
a feed
gas
i n l e t
and a per meat e gas
out l e t and c ont a i ni ng a membrane s e l e c t i v e l y pe r me a bl e
to the
gas
bei ng s e p a r a t e d ,
which membrane compr i ses a t hi n,
porous
i n e r t
suppor t
in which an a c t i ve ma t e r i a l is i mmobi l i zed wi t hi n the
p o r e s ,
whi ch membrane has
a
i n l e t si de and a per meat e
si de and which a c t i v e
ma t e r i a l i s a
mol t en s a l t capabl e of under goi ng one or more r e v e r s i b l e
r e a c t i o n s , wi t h
t he gas bei ng s e p a r a t e d ; pas s i ng the mi xt ur e
t hr ough t h e
f eed i n l e t and i nt o c o n t a c t wi t h t he i n l e t si de of the membrane; r emovi ng
from t he i n l e t si de of t he membrane a
gas
st ream r e j e c t e d by the membrane
and
r emovi ng
from the
per meat e gas
o u t l e t the
gas pas s i ng by
r e a c t i o n
t hr ough the membr ane.
In s t i l l a not he r a s p e c t , t h i s i n v e n t i o n r e l a t e s to a me mb r a n e f o r
gas s e p a r a t i o n p r o c e s s e s ,
c ompr i s i ng a t hi n,
por ous,
i n e r t
s uppor t
i n
whi ch is i mmobi l i zed
a
mol t en a c t i v e s a l t , capabl e of
under goi ng one o r
more
r e v e r s i b l e r e a c t i o n s , ot he r t han o x i d a t i o n - r e d u c t i o n r e a c t i o n s , wi t h
t he
gas
bei ng s e p a r a t e d .
" S a l t , " as used in t he s p e c i f i c a t i o n and cl ai ms , means a s u b s t a n c e
which i s compr i s ed of c a t i ons and ani ons and which is c h a r a c t e r i z e d
by
o v e r a l l e l e c t r i c a l n e u t r a l i t y . The c a t i ons and ani ons
may
be i n o r g a n i c ,
or ga ni c or a combi nat i on of bot h. I t wi l l be under s t ood t ha t s a l t s ha ve
a wide
range
of
mel t i ng poi nt s and t h a t , for the
pur poses
of t h i s
i n v e n t i o n , t he me l t i ng poi nt of t he s a l t used as a c t i ve ma t e r i a l is t h e
minimum
t e mp e r a t u r e , at which the membranes of t hi s i nve nt i on can be u s e d
f or s e p a r a t i o n of a
gas
from
a mi xt ur e of
g a s e s .
" I mmobi l i z e d, " as used in the s p e c i f i c a t i o n and cl ai ms , r e f e r s t o
mol t en a c t i ve ma t e r i a l , c a pt ur e d wi t hi n the
por es
or i n t e r s t i c e s of t h e
cer ami c or ot he r
s uppor t , as
wel l
as
any
ma t e r i a l in the form of
a t h i n
f i l m of s a l t mel t on t he s ur f a c e of t he s uppor t . Al t hough t he mo l t e n
s a l t need not n e c e s s a r i l y f i l l t he e n t i r e v o i d volume o f t h e
p o r o u s
s uppor t ,
i t is be l i e ve d
t ha t a t hi n cont i nuous l ayer
of mol t en s a l t in
o r
on t he
s uppor t i s n e c e s s a r y
f or the s u c c e s s f u l ope r a t i on of the membr anes.
" Re v e r s i b l e r e a c t i o n , " as used in the s p e c i f i c a t i o n and c l a i ms
e s s e n t i a l l y means a chemi cal r e a c t i o n which
can go
f or war d to the
r i g h t
or backwar ds to the l e f t , dependi ng upon
the r e l a t i v e c o n c e n t r a t i o n s o f
r e a c t a n t s and pr oduct s at a n y t i me . One
type
of r e v e r s i b l e r e a c t i o n
wi t hi n the meaning
of the i nve nt i on is f or mat i on of
a
c o o r d i n a t i o n
compl ex. A c o o r d i n a t i o n
complex or
c o o r d i n a t i o n compound c o n s i s t s of a
c e n t r a l atom or i on, s ur r ounde d by a s et of ot her at oms, i ons or s ma l l
mol e c ul e s . The at oms, ions or mol ecul es s ur r oundi ng t he c e n t r a l atom
a r e
c o n v e n t i o n a l l y c a l l e d l i ga nds . The r e s u l t i n g e n t i t y is
g e n e r a l l y a
complex and, s p e c i f i c a l l y ,
in the p r a c t i c e of t hi s i n v e n t i o n is a compl ex
i on. The
gas
bei ng s e p a r a t e d
from the mi xt ur e wi l l
a c c o r d i n g l y be a
l i ga nd in t hi s compl ex i o n .
Typi cal exampl es
of c o o r d i n a t i o n compounds
i nc l ude e n t i t i e s such a s
- +3 +3 ++
PF6. Cr(CO6), Cr(NH3)6+3, CO(NH3)6+3, CoCl4
and Zn(NH3)2++. The
compl exes
us ef ul in accor dance with t hi s i nve nt i on wi l l be t hose f or med
by an
ion of a s a l t , whet her a
cat i on or an a n i o n .
In a r e p r e s e n t a t i v e cas e,
the s e p a r a t i o n of ammonia from
ni t r oge n i n
c ont a c t wi t h a membrane compr i s i ng mol t en zinc c h l o r i d e , i t is p r o p o s e d
t hat the zi nc c h l o r i d e in t hi s ammoni a- r i ch envi r onment r e a c t s r e v e r s i b l y
to form
a
zi nc ammoni at e c o o r d i n a t i o n compound, Zn(NH3)++. The
i n i t i a l c o n d i t i o n o f a c r o s s - s e c t i o n of the membrane is
t hought to be
t hat de pi c t e d be l ow:
The zi nc ammoniate i ons t hus formed at the f eed si de wi l l d i f f u s e
t owa r d t he
per meat e s i de , where t her e is a lower c o n c e n t r a t i o n of s uc h
i ons. At the
per meat e
si de
membrane-gas i n t e r f a c e , where t her e is
a much
l ower p a r t i a l
p r e s s u r e
of ammonia, the zinc ammoniate i ons w i l l
r e v e r s i b l y d i s s o c i a t e back i nt o zi nc ions and f r ee ammonia, which i s
removed as the
per meat e s t r e a m.
The
s t eady s t a t e c o n d i t i o n of the membrane is shown in the di agr am
bel ow, wher ei n the zi nc i ons thus formed at the
per meat e
si de d i f f u s e
back to a r e g i o n o f l ower c o n c e n t r a t i o n at the feed s i de, t hus c o mp l e t i n g
the o v e r a l l cycl e, whi ch amounts to net t r a n s p o r t
of ammonia
t hr ough t he
membrane. This net t r a n s p o r t
of ammonia t hrough the membrane a r i s e s f r om
the r e v e r s i b l e r e a c t i v i t y of ammonia with zinc ions and the
mobi l i t y o f
t he z i n c i o n s and zi nc ammoni at e i ons in t he mol t en s a l t . Chl or i de a c t s ,
i n t h i s exampl e, to pr ovi de e l e c t r i c a l
n e u t r a l i t y .
The ot her f eed
gas, n i t r o g e n , does not r e a c t wi t h the mol t en s a l t .
Si nce t her e is
no mechani sm
by wh i c h n i t r o g e n can be
t r a n s p o r t e d a c t i v e l y
t h r o u g h t he membr ane, i t r emai ns l a r g e l y on t he f eed si de and a n
e x c e l l e n t o v e r a l l s e p a r a t i o n of ammonia n i t r o g e n is t hus a c h i e v e d .
Al t hough t he f o r e g o i n g gi ves a pr oposed t h e o r e t i c a l e x p l a n a t i o n o f
t he mechani sm
by
which t he
pr oc e s s
of t he p r e s e n t
i nve nt i on
o p e r a t e s , i t
wi l l b e unde r s t ood t ha t t he i n v e n t o r s do not . wi s h to be bound by s u c h
e x p l a n a t i o n
and
r e l y on t he appended
cl ai ms to def i ne the i n v e n t i o n .
In p r a c t i c e , p r e f e r r e d s e p a r a t i o n s empl oyi ng c o o r d i n a t i o n c ompl e xe s
ar e t hos e wh e r e i n :
(a) f us ed mol t en l i t h i u m n i t r a t e is used f or the s e p a r a t i o n
o f
ammonia from n i t r o g e n or hydr ogen or a mi xt ur e t h e r e o f , p r e f e r a b l y f r om
n i t r o g e n , b y t he r e v e r s i b l e f or ma t i on of
a l i t hi um ammoniate in t he me l t ;
(b) ammonia is
s e p a r a t e d
from
hydr ogen or n i t r o g e n usi ng mol t en z i n c
c h l o r i d e as
t h e a c t i v e ma t e r i a l in t he membrane and the complex
formed i n
t he mel t is
a mono or di ammoni at e; a n d
(c) wat er i s
s e p a r a t e d from
oxygen,
n i t r o g e n or ai r
by
f or ma t i on o f
one or more d i s s o l v e d
hydr a t e compl exes in a mel t of l i t hi um and s odi um
n i t r a t e s .
Anot her t ype of r e v e r s i b l e r e a c t i o n , a p p r o p r i a t e for t he p r a c t i c e o f
t h i s i n v e n t i o n is an a c i d - b a s e r e a c t i o n . This
t ype
of r e a c t i on i n c l u d e s
t h o s e of Ar r heni us a c i d s , Lowr y- Br ons t ed aci ds and Lewis aci ds a n d
b a s e s .
A
r e p r e s e n t a t i v e embodi ment of
a s e p a r a t i o n i nvol vi ng an
a c i d - b a s e
r e a c t i o n is the s e p a r a t i o n
of car bon di oxi de from
a
mi xt ur e of
g a s e s ,
usi ng as the a c t i ve membrane i n g r e d i e n t a mol t en
t e t r a a l k y l a mmo n i u m
c a r b o x y l a t e s a l t .
Al kyl can be the same or d i f f e r e n t
a l kyl
of 1 - 24
car bon atoms and is
p r e f e r a b l y of 1 - 10 car bon at oms. The c a r b o x y l a t e
can have
any
ani on r a d i c a l whi c h wi l l
give a
s a l t of t he mel t i ng p o i n t
r ange,
d e s i r e d f or o p e r a t i o n of the membrane. Tet r ahexyl ammoni um
benzoat e (THAB) is
p a r t i c u l a r l y p r e f e r r e d .
These
t e t r a a l kyl a mmoni um c a r b o x y l a t e s can
al s o be used for t h e
s e p a r a t i o n of
hydr ogen s u l f i d e from ot her
g a s e s .
A f u r t h e r r e v e r s i b l e r e a c t i o n , u t i l i z e d in the p r a c t i c e of t h i s
i n v e n t i o n , is f or ma t i on of
an or gani c chemi cal
compound, which is
c a p a b l e
of
s pont aneous f or ma t i on and d i s s o c i a t i o n at the
t e mp e r a t u r e
of
o p e r a t i o n
of the membrane. For exampl e, t et r aal kyl ammoni um s a l t s o f amino a c i d s
can r eact r e v e r s i b l y wi t h car bon di oxi de to form c a r ba ma t e s . Th i s
r e a c t i o n
can be used f or t he
s e p a r a t i o n
of carbon di oxi de from o t h e r
gases. Alkyl can be the
same or
d i f f e r e n t
al kyl of 1-24 car bon atoms. A
p r e f e r r e d embodiment is t ha t in which the amino aci d is gl yc i ne o r
s a r c os i ne and the al kyl i s of 1-10 car bon atoms. In t he
case
o f
s a r c o s i n e , the r e l e v a n t r e a c t i o n i s :
' I t is
pr oposed t hat s e p a r a t i o n
of ammonia, based on f or ma t i on o f
c o o r d i n a t i o n
compl exes wi t h zi nc c h l o r i d e ,
may
be
gover ned accor di ng t o
chemi cal
p r o p e r t i e s set f o r t h
by
Krasnov et a l . , "Tabl es and Diagrams o f
Thermodymanic Pr o p e r t i e s of Zinc Chl or i de Ammoniates in
Vapor and Li q u i d
Phas es , " Moskov.
Vsesoyuznyy Topl o- Te khni c he s ki y I n s t i t u t e . I n v e s t i y a ,
Moscow, n o . l l ( 1 9 3 5 ) ,
pages
44-49; St ar okadomskaya,
" S a t u r a t i o n P r e s s u r e
of Ammonia
During the Thermal Decompos i t i on of the Diammonium Sal t o f
Zinc Ch l o r i d e , " Zhur nal Pr i k l a d n o y Khi mi i , vol . 9 (1935 or 1936),
pa ge s
599-602; Zubakhi na, " I n v e s t i g a t i o n of Complexing in the
ZnCl2-NH3
Syst em, " Trudy Na u c h n o - l s s l e d o v a t e k ' s k o g o I n s t i t u t e Osnovnoi Khi mi i , v o l .
19 ( 1969) ,
pages
105- 110.
S o l u b i l i t y
be ha vi or of ammonia in mel t s of a l k a l i n i t r a t e a nd
p e r c h l o r a t e
is d i s c u s s e d b y Al l u l l i , J. Phys. Chem. , vol 73( 1969) ,
p a g e s
1084- 1087.
-
P r o p e r t i e s of c h l o r o a l u mi n a t e s , which
might be
germane
to the
use o f
mol t e n
cupr ous
c h l o r i d e c o o r d i n a t i o n compl exes, e . g . ,
(C2H5)3NH+ CuCl2-
or (C2H5)3 NH+CuCl 2- as
a c t i ve ma t e r i a l s f or the c o o r d i n a t i o n
of car bon monoxi de, ar e r e por t e d by Wal ker, "Sol i d and Li qui d Pha s e s
Co n t a i n i n g
CuAICI4, "
Symposium on
Advances in
Se pa r a t i on Te c hnol ogy,
p r e s e n t e d bef or e t he Di v i s i o n of Pet r ol eum Chemi s t r y, I nc . , S e a t t l e
Meet i ng, March, 1983,
pages
746-755; Kendal l , et a l . , "A St udy
of t h e
Fa c t o r s I n f l u e n c i n g Compound
For mat i on and S o l u b i l i t y
in Fused S a l t
Mi x t u r e s , " J. Am. Chem. Soc. , vol . 45 (1923)
pages
963, f f ; Hus s e y,
"Room
Te mpe r a t ur e
Mol t en Sal t Syst ems, Mamantov et a l . , ed. , Advances i n
Mol t en Sa l t Chemi s t r y 5, El s e v i e r , New York ( 1983) ,
pages
185-219;
H u s s e y
et a l . , "The El e c t r o c h e mi s t r y " o f Copper
in
a Room
Temper at ur e
Ac i d i c
Ch l o r o - a l u mi n a t e Me l t , " J. El ect r ochem. Soc. : So l i d - St a t e Sci ence a nd
Te c hnol ogy, v o l . 1 2 6 1979),
pages
1029-1034; and
Hussey, Mamantov e d . ,
Advances in Mo l t e n Sa l t Chemi s t r y 5,
pages
219- 223.
The r e a c t i o n of s i l v e r (1) compounds havi ng har d c o u n t e r i o n s ;
e . g . ,
AgBF4, AgC104,
wi t h o l e f i n s is r e c i t e d by Cot t on et a l . , "Advanced
I n o r g a n i c Chemi s t r y A Compr ehensi ve Text , " John Wiley & Sons, New Yor k
( 1980) ,
pages
9 7 1 - 9 7 2 . I t is
expect ed t hat the r e por t e d complex f o r ma t i o n
of monoval ent s i l v e r compounds
wi t h o l e f i n s coul d be e xpl oi t e d f o r
s e p a r a t i n g o l e f i n s from
a gas
mi xt ur e c o n t a i n i n g t hem.
Anot her r e v e r s i b l e r e a c t i o n is a " Re ve r s i bl e o x i d a t i o n - r e d u c t i o n
r e a c t i o n , " whi ch
as used in the
s p e c i f i c a t i o n
and cl ai ms
e s s e n t i a l l y
means an o x i d a t i o n - r e d u c t i o n r e a c t i o n which
can go
f or war d to the r i g h t
or
in
r e v e r s e to t he l e f t , dependi ng
upon
the r e l a t i v e c o n c e n t r a t i o n s o f
r e a c t a n t s a n d pr oduc t s at
any
t i me. Re p r e s e n t a t i v e r e a c t i o n s i n c l u d e
i n t e r c o n v e r s i o n bet ween n i t r a t e and n i t r i t e i o n s :
It is pr opos ed
t hat oxi des
are pr oduced by
r e a c t i ons such
a s :
and t hat the oxi des in the mel t , i nc l udi ng one or more of
0=, 02=
and
02r
c a t a l y z e the r e a c t i o n .
Anot her
t ype of o x i d a t i o n - r e d u c t i o n r e a c t i o n which can be e x p l o i t e d
in the
p r a c t i c e
of t hi s i nve nt i on i nc l ude s i n t e r c o n v e r s i o n o f
oxygen- oxi de s p e c i e s :
A
f u r t h e r e x e mp l a r y o x i d a t i o n - r e d u c t i o n r e a c t i o n is t hat i n v o l v i n g
o x i d a t i o n of
hydr ogen by hydr oxi de i o n s :
In a r e p r e s e n t a t i v e case, an a l k a l i met al n i t r a t e mol t en s a l t
membrane is f i r s t a c t i v a t e d by a l l owi ng some
of the n i t r a t e
(NO3)
ions to l ose
oxygen
and to f o r m n i t r i t e
(NO2)
i ons in the me l t
s p ont a n e o us l y, as
r e s u l t of h e a t i n g .
The worki ng mol t en s a l t membrane wi l l t hus cont ai n both an a l k a l i
met al n i t r a t e and an
a l k a l i metal n i t r i t e . The ope r a t i on
of the membrane
can be f u r t h e r v i s u a l i z e d as f o l l o w s :
At the feed si de of the membrane, ni t r oge n and
oxygen
are
in c o n t a c t
wi t h the s ur f ace of t he a l k a l i metal n i t r a t e / n i t r i t e mel t . The i n i t i a l
c o n d i t i o n i s as d e p i c t e d in t he f ol l owi ng c r o s s - s e c t i o n a l di agr am of t h e
membr ane:
The exces s
c o n c e n t r a t i o n of n i t r a t e ions at t he feed si de of t h e
membrane wi l l
promot e
t h e i r d i f f u s i o n t owar ds the
per meat e
s i de. At t h i s
i n t e r f a c e , t he r e i s
a much l ower p a r t i a l pr e s s ur e
of
oxygen,
t han at t h e
f eed i n t e r f a c e ; t h e r e f o r e , n i t r a t e i ons wi l l
r e v e r s i b l y d i s s o c i a t e i n t o
n i t r i t e i ons and l i b e r a t e
oxygen,
which is removed in the
p e r me a t e
s t r e a m. The s t e a dy s t a t e c o n d i t i o n of t he membrane is shown in t h e
di agr am bel ow, in which n i t r i t e i ons t hus formed at the
per meat e
s i d e
d i f f u s e back to a r egi on of l ower c o n c e n t r a t i o n at the feed si de t o
c o mp l e t e t h e o v e r a l l
c yc l e ,
which amount s t o n e t t r a n s p o r t
of
oxyge n
t hr ough
t he membrane. The net
t r a n s p o r t
of
oxygen
a r i s e s from r e v e r s i b l e
r e a c t i v i t y
of
oxygen
wi t h n i t r i t e i ons and mo b i l i t y of the n i t r i t e a n d
n i t r a t e i ons in t he s a l t .
The ot her feed
gas, ni t r oge n, does not r eact wi t h the mol t en s a l t .
Because t her e is
no
mechanism
by
which
n i t r o g e n can be a c t i v e l y
t r a n s p o r t e d t hr ough the mel t , i t wi l l be l a r g e l y r e j e c t e d at the f e e d
si de of the membrane., In t hi s
way,
e x c e l l e n t
s e p a r a t i o n
of n i t r o g e n and
oxygen
is a c h i e v e d .
Al t hough t he f or e goi ng gi ves an at t empt t o p r o v i d e a t h e o r e t i c a l
e x p l a n a t i o n
of the mechanism of the
pr oc e s s
of t he pr es ent i nve nt i on, i t
wi l l be under s t ood t hat the i nve nt or s do not wi sh to be bound by s u c h
e x p l a n a t i o n and r el y on the appended cl ai ms to def i ne the i n v e n t i o n .
It is
pr oposed t hat the
use
of
an
a l k a l i met al n i t r a t e mel t membrane
for s e p a r a t i o n of
oxygen
from ai r r e l i e s on the n i t r a t e - n i t r i t e
i n t e r c o n v e r s i o n . However, the r e a c t i on bet ween
oxygen
and oxi de s p e c i e s
may
al s o be o c c u r r i n g , as di s c us s e d more f u l l y by Zambonin, et a l . , J .
Am. Chem. Soc. , vol . 91 (1969),
pages
2225- 2228; Zamboni n,
E l e c t r o a n a l y t i c a l Chemi st r y and I n t e r f a c i a l El e c t r o c h e mi s t r y , vol . 45
( 1973) ,
pages
451-458 and Fl i nn et a l . , J. El e c t r o a n a l . Chem., vol 63
( 1975) ,
pages
39- 57.
It is al s o known t hat ot her mol t en s a l t syst ems
r eact r e v e r s i b l y
wi t h
oxygen
by
pr oc e s s e s i nvol vi ng e q u i l i b r i a wi t h reduced
oxygen
s p e c i e s , p a r t i c u l a r l y oxide ( O )
per oxi de
L02)
and
s u p e r o x i d e
(02).
For exampl e,
i t has been
r e por t e d
t hat
Na2O-Na2SO4
mel t s t ake
up oxygen r e v e r s i b l y at 920C, pr es umabl y by the r e a c t i o n s :
St er n et a l . , J. Phys. Chem. vol . 83 ( 1979) ,
pages
2848-2854. It is a l s o
t hought
t hat redox e q u i l i b r i a , i n v o l v i n g the decompos i t i on of s u l f a t e t o
s u l f i t e and
gaseous
s ul f ur di oxi de, are
i n v o l v e d .
It is
expect ed t hat
hydr oge n- hydr i de i n t e r c o n v e r s i o n in mo l t e n
a l k a l i met al hydr oxi de membranes of the i nve nt i on would
oper at e
i n
accor dance wi t h the behavi or r e por t e d by Bai kov, et a l . , " Ac t i va t i on o f
Mol ecul ar
Hydrogen by
Sol i d and Fused Hydr oxi des . I I I . Ki net i cs and
Mechanism of I s ot ope Exchange Between Hydrogen and Sol ut i ons of Water i n
Al kal i Mel t s" and "IV. The Role of Cat i ons in the
Ca t a l ys i s
o f
Homomol ecul ar Exchange of Hydrogen on Sol i d Hydr oxi de s , "
K i n e t i k a i
Ka t a l i z , vol . 23 ( 1982) ,
pages
573-577 and vol . 24 ( 1983) ,
p a g e s
5 0 2 - 5 0 5 .
Re v e r s i b l e
oxygen upt ake
from
Na 0-NaCl
mel t s is al s o known,
St e r n , et a l . , J. El ect r ochem. Soc. , vol . 124 ( 1977) ,
pages
641- 649.
Cupr ous
c h l o r i d e - a l k a l i me t a l h a l i d e mel t s ,
e . g . , CuCl - CuCl 2- KCl ,
are
a l s o known to r e a c t r e v e r s i b l e wi t h
oxygen, pr oba bl y by t he r e a c t i o n :
Font ana, et a l . , Ind. Eng. Chem., vol . 44 ( 1952) ,
pages
369- 378.
Because of the compl exi t y of t he redox
pr oc e s s e s t hought to occur i n
s a l t me l t s , i t is unde r s t ood t hat t he
pr oc e s s e s
of t h i s i nve nt i on i n c l u d e
t r a n s f e r of
ga s e s , capabl e of under goi ng an o x i d a t i o n - r e d u c t i o n r e a c t i o n
wi t h a mol t en s a l t , i mmobi l i zed in
a s uppor t , r e g a r d l e s s of t he mechani sm
by
whi ch t he
pr oc e s s a c t u a l l y t akes p l a c e .
I t wi l l be unde r s t ood t ha t the r e v e r s i b l e r e a c t i o n s , a p p r o p r i a t e
f o r
use
in t he p r a c t i c e of t hi s i nve nt i on, are not l i mi t e d to t hose set f o r t h
a b o v e .
The membranes of the pr e s e nt
i n v e n t i o n are r e p r e s e n t e d
ma c r o s c o p i c a l l y in
Fi g. 1, f or the s e p a r a t i o n
of
a per meabl e
gas
(A) f r om
a
mi xt ur e of
gases
A and B. The
por ous
s ol i d s uppor t
is a r i gi d, o r
s l i g h t l y f l e x i b l e , i n e r t ma t e r i a l , s e l e c t e d from me t a l l i c , pol ymer i c o r
cer ami c s u b s t r a t e s . The
s uppor t
ma t e r i a l is f i l l e d
or coat ed wi t h a t
l e a s t a c ont i nuous
l ayer
of the s e l e c t e d mol t en s a l t , or mi xt ur e t h e r e o f .
The f eed
gas pas s es over t he feed si de of the membrane, as shown
by t h e
mi xt ur e A + B on the
upper
si de of Fi g. 1. The r e a c t i v e member of t h e
gas
mi xt ur e wi l l
undergo one or more r e v e r s i b l e r e a c t i o n s wi t h ions i n
t he mol t en s a l t , as
di s c us s e d above.
Eve nt ua l l y, at s t e a dy s t a t e
c o n d i t i o n s , the r e a c t i v e
gas
(A) per meat es the membrane. In a n
e xpe r i me nt a l c ont e xt , the
r e a c t i ve or per meat e gas
(A) is removed f r om
the syst em by an i ne r t
sweep gas
and a sample of the
sweep gas
st ream i s
t r a n s f e r r e d to a gas chr omat ogr aph
f or
a n a l y s i s . In a l ar ge
s c a l e
u t i l i z a t i o n of the i nve nt i on, i t wi l l be p r e f e r r e d to exhaus t
pe r me a t e
gas
from the
syst em
with
a compr es s or
and t r a n s f e r
r e l a t i v e l y pur e
per meat e gas
to a pr ocess
r e q u i r i n g
i t or to c o n t a i n e r s .
I t is
pr opos ed t hat the
gas pr e s s ur e ,
r e qui r e d to di s pl a c e l i q u i d
t hat is i mmobi l i zed by c a p i l l a r y
f or ces from the
pores
of a porous
we t t e d
membrane, conforms to the e qua t i on ( Por t e r , "Handbook of S e p a r a t i o n
Techni ques f or Chemi cal En g i n e e r s , " S c h we i t z e r , ed. , Mc Gr a w- Hi l l ( 1979) ,
Appendi x A,
page
2- 89) :
wher ei n Y is t he s ur f ace t e n s i o n of the g a s - l i q u i d i n t e r f a c e , 0 is t h e
c ont a c t angl e bet ween the l i q u i d and the
pore
wal l and d is the
pore
s i z e .
In q u a l i t a t i v e t erms, the s a l t or mi xt ur e of s a l t s s e l e c t e d f or t h e
membrane wi l l be f l u i d at the t e mpe r a t ur e
of
use,
but the melt wi l l n o t
have an
e xc e s s i ve r at e of flow
or t endency to dry out or mi gr at e
from t h e
i ne r t
s uppor t .
The s a l t wi l l be e s s e n t i a l l y n o n - v o l a t i l e under c o n d i t i o n s
of u s e .
Al t hough membranes, c ompr i s i ng a monomol ecul ar l ayer
of a c t i v e
mol t en s a l t in t he i ner t
s uppor t
wi l l f unc t i on in accor dance wi t h t h e
i nve nt i on, i t is p r e f e r r e d to use membranes, havi ng a cont i nuous f i l m o f
mol t en a c t i ve s a l t i mmobi l i zed in and l ayer ed on
the
s uppor t , or a
combi nat i on t h e r e o f . Membranes meet i ng t hi s c r i t e r i o n wi l l have a
r e l a t i v e l y low
p e r me a b i l i t y to a
n o n - r e a c t i v e
gas
at 760 t or r at t h e
t e mpe r a t ur e of
u s e , a s
measur ed a ga i ns t
the backgr ound cont ent of i n e r t ,
n o n - r e a c t i v e
sweep gas on the
per meat e
si de of the membrane. In a
p r e f e r r e d case,
the
p e r me a b i l i t y of ni t r oge n as the i ner t
gas
is s e l e c t e d
as a s t a nda r d and is below 20 Bar r er under t hes e c o n d i t i o n s .
The
s uppor t
is s e l e c t e d from cer ami c, g l a s s ,
me t a l l i c and
o r g a n i c
pol ymer i c ma t e r i a l s , s t a bl e at the t emper at ur e
of
use,
which
are e i t h e r
r i gi d or s l i g h t l y f l e x i b l e and which do not r e a c t wi t h the a c t i ve mo l t e n
s a l t or wi t h the
gas
mi xt ur e
bei ng t r e a t e d . A p r a c t i c a l lower l i mi t f o r
the t hi c kne s s of
s e l f - s u p p o r t i n g membranes is below 0.01 c e n t i me t e r s , b u t
some
t hi nne r ma t e r i a l s
may
l ack the r i g i d i t y r e qui r e d. However,
u l t r a f i n e
porous
cer ami c f i l ms , l ess than 20 mi cr omet er s in t hi c kne s s
a r e
known, Leemaars et a l . , J. Ma t e r i a l s Sci ence, vol . 19( 1984) ,
pa ge s
1077-1088. It wi l l be unde r s t ood t hat the
s uppor t can be coat ed o r
a d h e r e d t o a n o t h e r ma t e r i a l ,
u s u a l l y
of
g r e a t e r p o r o s i t y , which wi l l
pr ovi de t he s t r u c t u r a l r i g i d i t y r e q u i r e d .
For exampl e, a " c ompos i t e " or "asymmet r i c" membrane, as de s c r i be d i n
Sc h we i t z e r , "Handbook of Se p a r a t i o n Techni ques
f or Chemi cal
En g i n e e r s , "
McGraw Hi l l ( 1 9 7 9 ) , p a g e s 2-19 to 2- 26, c ont e mpl a t e d f or
use
in t h e
p r a c t i c e
of t h i s i n v e n t i o n woul d have a
t hi n f i n e l y
por ous l a y e r ,
c o n t a i n i n g t he a c t i v e mol t en s a l t , s uppor t ed
f u r t h e r on a ma c r opor ous
s uppor t c o mp r i s i n g pol yme r i c , cer ami c or
me t a l l i c ma t e r i a l .
I t i s f u r t h e r p r e f e r r e d t ha t t he s uppor t
have a por e
s i ze of 0 . 0 0 3 -
100 mi c r o me t e r s . It wi l l be a p p r e c i a t e d t hat l ar ge
e f f e c t i v e membrane
a r e a s c a n be a t t a i n e d by us i ng a p l u r a l i t y of s mal l er membr anes,
c onne c t e d
by a ma ni f ol d
or ot he r connect i ng means, or t ha t a l a r g e
membrane can be made me c h a n i c a l l y s t r ong by the use of s uppor t i ng means ,
wel l known to t hos e s k i l l e d in the a r t .
The membrane
may
be c o n t a i n e d wi t hi n a module in t he c o n f i g u r a t i o n
of hol l ow f i b e r s , a s p i r a l wi ndi ng or a
s e r i e s of f l a t
p l a t e s . A
d e s c r i p t i o n of hol l ow f i b e r and s pi r a l l y- wound
membrane c o n f i g u r a t i o n s i s
gi ven
in Sc h we i t z e r , i b i d . ,
pages
2-61 to 2-65 and 2- 21, r e s p e c t i v e l y .
In
any s e p a r a t i o n
p r o c e s s ,
c a r r i e d out under the p r i n c i p l e s of t h i s
i n v e n t i o n where the mol t en s a l t under goes
r e v e r s i b l e r e a c t i o n s wi t h t h e
gas
bei ng s e p a r a t e d , t he maximum a c hi e va bl e f l ux of
per meat e gas p e r
c r o s s - s e c t i o n a l
ar ea
of membrane is equal to the e f f e c t i v e d i f f u s i o n
c o e f f i c i e n t of t he c a r r i e r ( a c t i v e mol t en s a l t ) , mu l t i p l i e d by t h e
c o n c e n t r a t i o n g r a d i e n t of c a r r i e r ( dr i vi ng
f or ce) bet ween the t wo
i n t e r f a c e s of t he membrane. Thus ,
In g e n e r a l , the f l ux
t hr ough a
membrane may be e xpr e s s e d as t h e
pr oduc t
of
a s t a n d a r d p e r me a b i l i t y t i mes the c r o s s - s e c t i o n a l
ar ea
of t h e
membrane, t i me s (P/l. ), wher ei n l is the
pr e s s ur e
d i f f e r e n c e
a c r os s t he membrane and l is the t hi c kne s s of the membr ane.
As in
any f a c i l i t a t e d t r a n s p o r t syst em,
the a c t ua l f l ux wi l l a l s o
depend on t he k i n e t i c s of the r e v e r s i b l e r e a c t i ons of the per meat i ng
g a s
wi t h t he mol t e n s a l t .
Pe r me a b i l i t y (Po) is expr es s ed
in Bar r er u n i t s , t hat i s ,
3 2
1 Bar r er = ( c m . cm x
1 0 ) / ( s e c . c m . cmHg)
When pol ymer i c membranes are used to
s uppor t a melt and i t i s
de s i r e d to
e xpr e s s
the g a s f l u x t hr ough the membrane in
a manner whi c h
r e f l e c t s
onl y
the
s e p a r a t i o n per f or mance
of the mel t , a c o r r e c t i o n w i l l
be made for the
p o r o s i t y
and t o r t u o s i t y of t he membr ane, so t h a t :
Flux = Po . A. (P/l) ( p o r o s i t y / t o r t u o s i t y )
S e l e c t i v i t y ( S ) f or a mi xt ur e of
gas es ,
A and B, in which A is t h e
per meat e gas,
is expr es s ed by
the r a t i o of p e r me a b i l i t i e s :
Very hi gh s e l e c t i v i t i e s are obs er ved, usi ng t ypi c a l membranes o f
t hi s i nve nt i on. For exampl e,
s e l e c t i v i t i e s of 80-145 were measured f o r
s e p a r a t i o n of ammonia from ni t r oge n, usi ng a mol t en s al t membrane o f
l i t hi um c h l o r i d e . The
same s e p a r a t i o n , u t i l i z i n g molten zinc c h l o r i d e
a s
the a c t i ve mol t en s a l t in the membrane,
gave
s e l e c t i v i t i e s above 1000 : 1.
Si mi l a r l y , the s e p a r a t i o n
of car bon di oxi de from ni t r oge n, u s i n g
mol t en
t et r aal kyl ammoni um benzoat e or s a r c o s i n a t e s a l t s in the membr anes ,
exceeded a s e l e c t i v i t y of 10. In the case
of the t e t r a l kyl a mmoni um
benzoat e membrane, i t
was s u r p r i s i n g l y f o u n d t h a t the
pr esence
of wa t e r
in the
gas
st r eam improved the s e p a r a t i o n , r a t h e r than a f f e c t i n g i t
d e l e t e r i o u s l y .
The s e l e c t i v i t y for
s e pa r a t i on
of wat er
vapor
from ni t r oge n, us i ng a
mol t en l i t hi um n i t r a t e / s o d i u m n i t r a t e membrane, was
about 200 : 1.
It is t h e r e f o r e
appar ent
t hat the membranes of the pr es ent i n v e n t i o n
give i mpr es s i ve s e l e c t i v i t i e s , compared to s e l e c t i v i t i e s of known
membranes, t y p i c a l exampl es of which
are set f o r t h in Table 1 be l ow.
For the
r e g e n e r a t i o n of
oxygen
from a i r , p r e f e r r e d
mel t s
are l i t h i u m
n i t r a t e
or sodium n i t r a t e , i mmobi l i zed in
a porous
me t a l l i c
or c e r a mi c
s uppor t . Pr e f e r r e d
ope r a t i ng t e mpe r a t ur e s are above 400C, more
p r e f e r a b l y above 450C. Mi xt ur es of n i t r a t e s , p a r t i c u l a r l y
of two
o r
more of sodium, l i t hi um and pot assi um n i t r a t e s , c ont a i ni ng a maximum o f
99%
by wei ght of
any
of the n i t r a t e s , are p a r t i c u l a r l y p r e f e r r e d .
S e l e c t i v i t i e s a ppr oa c hi ng
200 f or
oxygen
in a i r have been
a c h i e v e d ,
us i ng t he t e a c h i n g s of t hi s i n v e n t i o n . I t wi l l be a p p r e c i a t e d t h a t s uc h
s e l e c t i v i t i e s ar e much hi ghe r t han t hose obs er ved f or s e p a r a t i o n o f
o x y g e n - n i t r o g e n
mi xt ur e s usi ng c o n v e n t i o n a l
pol ymer membranes, as shown
in Tabl e 2 .
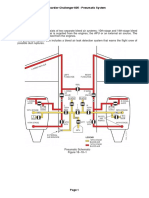
Br i e f De s c r i p t i o n of the Dr awi ngs
In Fi g.
1 i s shown a s i de vi ew of
a membrane, us e f ul f or t h e
p r a c t i c e
of t he i n v e n t i o n .
In F i g s . 2 , 3 and 4
ar e shown a t e s t
a ppa r a t us
f or
de mons t r a t i ng t h e
p r i n c i p l e s
of t h i s i n v e n t i o n , i n c l u d i n g a membrane, membrane hol der a nd
t e s t c e l l .
Best Mode for Car r yi ng Out the I n v e n t i o n
In a most p r e f e r r e d as pect
of t hi s i nve nt i on, the membrane c o mp r i s e s
an
i ne r t cerami c s uppor t
at l e a s t 0.02 cm
in t h i c k n e s s , and which has
a
pore
s i ze of 0 . 0 0 3 - 200 mi cr omet er s , is f i l l e d wi t h
a
cont i nuous f i l m o f
molten a c t i ve ma t e r i a l and has a p e r me a b i l i t y to
n i t r o g e n below 20
Bar r er s at 760 t o r r at the t e mpe r a t ur e
of
u s e .
Wi t hout f u r t h e r e l a b o r a t i o n , i t is be l i e ve d t hat
one
s k i l l e d in t h e
art can,
u s i n g t h e pr e c e di ng d e s c r i p t i o n , u t i l i z e t he
p r e s e n t
i n v e n t i o n
to i t s f u l l e s t e xt e nt . The f o l l o wi n g p r e f e r r e d s p e c i f i c embodi ment s
a r e ,
t h e r e f o r e , to be c ons t r ue d as merel y i l l u s t r a t i v e and not l i mi t a t i v e o f
the r emai nder o f t h e d i s c l o s u r e in
any way
what s oever . In the f o l l o wi n g
Exampl es, t e mpe r a t ur e s are set f or t h unc or r e c t e d in
degr ees Ce l s i u s .
Unless ot her wi s e i n d i c a t e d , al l par t s and pe r c e nt a ge s are by we i g h t .
For exper i ment s at hi gh t e mpe r a t ur e s , a membrane compr i s i ng a
t h i n
fi l m (about 0.2 mm) of
a
mol t en
s a l t s uppor t ed
in
a por ous
me t a l l i c o r
cerami c ma t e r i a l , was used. In a t y p i c a l case,
the membrane
s uppor t was
a c i r c u l a r pi ece
of #304 s t a i n l e s s s t e e l woven
wi re mesh (about 0.02 cm
in t h i c k n e s s ,
pore
si ze from 4-13 mi cr omet er s , Pal l
Cor p. , Cor t l a nd, NY),
s uppor t ed r i g i d l y
bet ween two I nconel 600 r i ngs about 10
cm
in i n n e r
di amet er . The me s h wa s l o a d e d wi t h a s al t
by me l t i ng the
dry
s a l t i n t o
i t s
por es ,
unt i l at l e a s t enough s a l t was used to pr ovi de a v i s i b l y
cont i nuous mol t en f i l m on one s ur f a c e of the wire mesh. The t h u s - c o a t e d
mesh and
suppor t assembl y was s eal ed i nt o a sample c e l l . In Fi gs. 2 and 3
are
shown a top view and a si de view, r e s p e c t i v e l y , of hol der a s s e mb l y 4
for
a wire mesh membrane s uppor t .
In Fig.
4 is shown the
sample c e l l 5,
i ncl udi ng the gol d 0- r i ng wi t h which the
suppor t as s embl y
is s e a l e d i n t o
the sample c e l l .
The sample
c e l l
or
module 5, as shown in
Fi g. 4, was pr ovi ded wi t h
means
f or i nt r oduc i ng f eed
g a s t h r o u g h
feed i n l e t 7 on the feed si de o f
the membrane 1, and for removal of a
gas
st ream r e j e c t e d by the membrane
t hr ough r e j e c t o u t l e t 8 wi t h per meat e sweep gas
i n l e t 10 on the o p p o s i t e
side of the membrane, f or pas s i ng
sweep gas
in c ont a c t wi t h the membrane
1 and
permeat e
o u t l e t means
11 for conveyi ng per meat e gases
and c a r r i e r
gas es
to an a n a l y z e r means. The a ppa r a t us was housed in
a t h e r mo s t a t t e d ,
t u b u l a r , h i g h - t e mp e r a t u r e he a t i ng mant l e and coul d
ope r a t e a t
t e mp e r a t u r e s up
to about 550C. The e xpe r i me nt a l assembl y al so i n c l u d e d
a gas
mani f ol d and was f u r t h e r pr ovi ded
wi t h e l e c t r o n i c
mass
f l ow
c o n t r o l l e r s t o per mi t p r e c i s e bl e ndi ng
of f eed
gas
mi xt ur e s . A
ga s
c hr oma t ogr a ph was used f or a n a l y s i s of the per meat e
gases
in the
sweep
s t r e a m.
A t y p i c a l l o we r - t e mp e r a t u r e membrane was pr e pa r e d by pl aci ng s e v e r a l
d r o p s o f a l ow- me l t i ng s a l t ( t et r ahexyl ammoni um be nz oa t e , Eastman Kodak
Co. ) on a c i r c u l a r pi ece
of 3501 ( Cel anese
Cor p. ) por ous pol ymer.
The
pol ymer was 27 mi c r ome t e r s t hi c k and had
pore
di mens i ons of 0.4
x
0 . 0 4
mi c r ome t e r s . The s a l t was smeared
over the s uppor t
wi t h a mi c r os c ope
s l i d e , which was
used t o remove excess
s a l t from the membrane. The
f i l l e d / c o a t e d membrane was l oaded i nt o a c e l l , ot he r wi s e as above, b u t
t h e r mo s t a t t e d i n a b a t h of e t hyl e ne g l y c o l .
In a not he r embodi ment of t he i nve nt i on
a s p e c t ,
t he method of t h i s
i n v e n t i o n wi l l be s e p a r a t i o n of
oxygen
from ot he r
gas es , usi ng mo l t e n
sodi um or l i t hi um n i t r a t e , i mmobi l i zed in
a por ous
met al s uppor t , at a
t e mp e r a t u r e above 400C.
Example 1
Se p a r a t i o n of Ammonia from Ni t r ogen us i ng a Li t hi um Ni t r a t e
I mmobi l i zed Mol t en Sa l t Membrane
S t a i n l e s s s t e e l woven
wi re mesh (# 304, about 0. 02 cm t hi ck, 4- 13
mi c r ome t e r s
pore
s i z e , ar ea about 0.8
cm2)
was coat ed wi t h f u s e d
l i t h i u m n i t r a t e
t o p r o v i d e a v i s i b l y c ont i nuous mol t en f i l m on one
s u r f a c e of t he wi re mesh, whi ch
was
mount ed i nt o the h i g h - t e mp e r a t u r e
t e s t i n g a p p a r a t u s .
The sampl e was heat ed to a f ur nace
t emper at ur e
o f
279C, us i ng a f eed fl ow of 30
cm3/min
f or f eed
gas
and a
sweep
flow on
t he
per meat e
si de of t he membrane of 30
cm3/min
of hel i um, both a t
about 1 at mos pher e p r e s s u r e
and 25C. The t o t a l
pr e s s ur e
in the c e l l
dur i ng the e xpe r i me nt s was about 760 t o r r . The c o n c e n t r a t i o n of
gases
i n
t he per meat e s t r eam was
de t e r mi ne d
by gas chr omat ogr aphy, usi ng a
305
cm
mo l e c u l a r s i eve column f or n i t r o g e n
and a
183
cm Chromosorb column f o r
ammonia, bot h ope r a t e d i s o t h e r ma l l y at 60C. Af t er the syst em ha d
s t a b i l i z e d at the o p e r a t i n g t e mp e r a t u r e ,
t her e was no l eak of
n i t r o g e n
acr os s
t he membrane, as
i n d i c a t e d by
very
low n i t r o g e n p e r me a b i l i t y .
The u t i l i t y
of the membrane in
s e p a r a t i n g
ammonia from ni t r oge n was
t e s t e d in t hr ee one- day runs. Duri ng each
run,
the c o n c e n t r a t i o n o f
ammonia in t h e a mmo n i a / n i t r o g e n
f eed
was var i ed from 0-100%. The
c o n c e n t r a t i o n s of ammonia and n i t r o g e n in the
per meat e were moni t or ed by
gas
c h r o ma t o g r a p h y .
Re s ul t s for s p e c i f i c ammonia n i t r o g e n mi xt ur e s ,
r e p r e s e n t i n g a n
aver age
of the t hr ee one- days runs we r e :
These r e s u l t s show t hat good s e p a r a t i o n of ammonia and n i t r o g e n was
a c c ompl i s he d, usi ng the i mmobi l i zed l i t hi um n i t r a t e membrane. It is a l s o
a ppa r e nt t hat p e r me a b i l i t y is
a
f u n c t i o n of the p a r t i a l
p r e s s u r e s
at t h e
feed i n t e r f a c e of t he membr ane.
Example 2
(a) Se pa r a t i on of Ammonia from Ni t r ogen usi ng a Zinc Ch l o r i d e
I mmobi l i zed Mol t en Sal t Membrane
The membrane was pr epar ed by mel t i ng zinc c h l o r i d e i nt o the
pores
o f
s t a i n l e s s s t e e l mesh (about 0. 02 cm t hi c k, area 0.8
cm2).
The membrane
was pl a c e d i n t he hi gh t e mpe r a t ur e c e l l and checked f or
c o n t i n u i t y by
meas ur i ng p e r me a b i l i t y of
pure
n i t r o g e n at 310C.
The membrane was used f or
s e p a r a t i o n o f ammonia from
n i t r o g e n over a
21-day per i od
of cont i nuous o p e r a t i o n . Gas flow on
bot h the f eed and
per meat e
si de
was
20
cm3/min
at about 1 aim and 25C. P e r me a b i l i t i e s
were c a l c u l a t e d as
t he means
of 10-15 measurement s of ammonia a nd
n i t r o g e n f l uxe s over a t wo- hour pe r i od, a f t e r t he
syst em
had r e a c h e d
e q u i l i b r i u m under a gi ven set of c o n d i t i o n s . Re s ul t s
we r e :
Ni t r oge n
f l ux t hr ough the zi nc c h l o r i d e membrane was
very
low, c l o s e
to t he backgr ound
l evel of
n i t r o g e n
in the
sweep
s t r eam. The r e f or e , t h e
s e l e c t i v i t y c o u l d n o t be de t e r mi ne d q u a n t i t a t i v e l y , but wa s e s t i ma t e d t o
be at l e a s t 1000 : 1.
(b) Se p a r a t i o n of Ammonia from Hydr oge n
Expe r i me nt s were
done
as
in
Example 2( a) , us i ng as the f eed st r eam a
mi xt ur e of ammonia and hydr ogen. Argon was
used as t he
per meat e sweep
gas
to f a c i l i t a t e d e t e c t i o n of hydr ogen by the
gas c h r o ma t o g r a p h .
At 311C, us i ng f eed of
pure
hydr ogen (800 t o r r ) , t he s t a n d a r d
hydr ogen p e r me a b i l i t y was c a l c u l a t e d t o be 9 Ba r r e r . Under the same
c o n d i t i o n s , us i ng pure
ammonia as f eed, the p e r me a b i l i t y of ammonia
was
2.9 x
104
Ba r r e r s . Th e r e f o r e , t he ammoni a/ hydr ogen s e l e c t i v i t y was
about 3 2 0 0 : 1 .
Example 3
Se p a r a t i o n of Ammonia from Ni t r oge n usi ng Zi nc Chl or i de I mmobi l i z e d
Mol t en Sa l t Suppor t ed in
a Porous Cerami c Membrane
A c i r c u l a r
sampl e
of
woven zi r coni um di oxi de c l o t h (about 0.038 cm
in t h i c k n e s s , 87% p o r o s i t y , t ype ZYW-15, Zi r c a r Cer ami cs , Fl o r i d a , N. Y. )
was soaked in mol t en zi nc c h l o r i d e . The t h u s - i mp r e g n a t e d membrane
was
s andwi ched bet ween two a nnul a r I nconel r i ngs ( out er di amet er about 1
cm,
i nner di a me t e r about 0.6 cm). The r e s u l t i n g assembl y was s eal ed i nt o t h e
hi gh t e mp e r a t u r e t e s t c e l l us i ng gol d O- r i n g s .
Pe r me a b i l i t y
measurement s were
made
as in
Example
2 at
gas p r e s s u r e s
of about 1 atm, f eed r at es of a mmoni a / ni t r oge n
or hel i um
sweep gas
of 20
cm3/min
at about 1 ai m and 25C and an
i n t e r n a l membrane t e mp e r a t u r e o f
311C.
Owing to u n c e r t a i n t i e s about the t hi c kne s s of the membr ane,
p e r me a b i l i t i e s i n Ba r r e r ,
a s i n d i c a t e d below, are u n c e r t a i n . Membrane
pe r f or ma nc e ,
de f i ne d as
Po/l
( cm / cm .
sec. cmHg) was
obt a i ne d
by
di vi di ng
s t a n d a r d p e r me a b i l i t y by the assumed t h i c k n e s s of the membrane
(l = 0. 038 cm). The ni t r oge n f l ux was a c c o r d i n g l y v e r y low, compar ed
to the backgr ound
l evel of n i t r o g e n
in the hel i um
sweep.
The e s t i ma t e d
PoNH3/PoN2 was
at l eas t 1400: 1
Example 4
Se p a r a t i o n of Carbon Di oxi de from Ni t r ogen usi ng a
Tet r ahexyl ammoni um Benzoat e I mmobi l i zed Mol t en Sal t Membrane
The membrane was pr epar ed by pl a c i ng s e ve r a l drops
o f
t e t r a he xyl a mmoni um be nz oa t e (THAB, Eastman Kodak Corp. ) on a
c i r c u l a r
pi ece
of 3501
Cel gar d" ( Cel anese Co r p . , t h i c k n e s s 0.0027
cm, ar ea 2 . 5 6
cm2,
pore
di mens i ons 0.4 x 0 . 0 4 mi c r o me t e r s , t o r t u o s i t y 1. 25, p o r o s i t y
0. 5) . The s a l t
was smeared over the pol ymer f i l m and al l owed to ent er t h e
porous
pol ymer. Excess s a l t was removed. The membrane was l oaded i n t o
the l o we r - t e mp e r a t u r e c e l l , t h e r mo s t a t t e d wi t h an et hyl ene gl ycol b a t h .
The i n i t i a l f eed flow was 11.7
cm3/min
of car bon di oxi de and 16. 9
cm3/min
of n i t r o g e n
and the per meat e
flow
was 10
cm3/min
of h e l i u m,
bot h at 1 atm. The per meat e was a na l yz e d
f or car bon di oxi de and n i t r o g e n
by gas chr omat ogr aphy, us i ng an
183 cm Por opak P col umn, run i s o t h e r ma l l y
at 60 C.
Carbon di oxi de and n i t r o g e n p e r me a b i l i t i e s were measured as a
f u n c t i o n of
t e mpe r a t ur e , t a ki ng i nt o account t he t o r t u o s i t y and
p o r o s i t y
of t he
s u p p o r t .
Re s u l t s wer e:
These r e s u l t s show t ha t car bon di oxi de p e r me a b i l i t y i nc r e a s e s wi t h
i n c r e a s i n g t e mpe r a t ur e ,
but s e l e c t i v i t y d e c r e a s e s .
Example 5
Se p a r a t i o n of Carbon Di oxi de from Ni t r oge n us i ng a
Tet r ahexyl ammoni um Sa r c o s i n a t e (THAS) I mmobi l i zed Molten S a l t
Membrane
Tet r ahexyl ammoni um hydr oxi de was pr e pa r e d by s t i r r i n g t o g e t h e r
t et r ahexyl ammoni um bromi de (2. 2
g,
East man Kodak Cor p. ) and 6.5
g.
o f
s i l v e r (I) oxi de in 25 ml of 80% met hanol : 20% wat er by volume. The
hydr oxi de was
t i t r a t e d wi t h
aqueous
s a r c o s i n e s o l u t i o n . Removal o f
s o l v e n t , us i ng a r ot a r y e v a p o r a t o r , gi ve t et r ahexyl ammoni um s a r c o s i n a t e ,
N( n- C6H11) 4 + NH(CH3)-CH2COO,
in t he form of
a yel l ow l i qui d. The
s a r c o s i n a t e
was
used to
pr e pa r e a membrane, s uppor t e d in a porous p o l y me r
f i l m as
in
Example
4. The membrane
was 0. 0027
cm in t h i c k n e s s ,
2. 56
c m
in
ar ea,
and had
a t o r t u o s i t y
of 1. 25 and p o r o s i t y of 0 . 5 .
The
pe r me a t i on exper i ment s were done at
gas p r e s s u r e s
about 760 t o r r a t
f eed f l ows of 11.7
cm3/min
f or car bon di oxi de and 16.9
cm3/min
f o r
n i t r o g e n and
sweep gas
fl ow (hel i um) of 10
cm3/ mi n.
Res ul t s wer e:
The THAS membrane was ope r a t e d
f or about four days at or below 75C
wi t hout
any
obser ved a l t e r a t i o n in i t s
p r o p e r t i e s . Af t er the membrane
was
heat ed to 95C, the car bon d i o x i d e p e r me a b i l i t y at 60C
was 218
Ba r r e r s and
PoCO2/PoN2
was 1 2 . 8 .
Al t hough the THAS membrane had l ower carbon di oxi de
p e r me a b i l i t y
t ha t the THAB membrane
of
Exampl e 4, the THAS
membrane
had
h i g h e r
s e l e c t i v i t y at e l e va t e d t e mp e r a t u r e s t han the THAB
membr ane.
Example 6
Se pa r a t i on of Water Vapor from Ni t r oge n usi ng Li t hi um Ni t r a t e / S o d i u m
Ni t r a t e I mmo b i l i z e d Mol t en Sal t Membrane
A mol t en s a l t membrane of
a
mi xt ur e of l i t hi um n i t r a t e / s o d i u m
n i t r a t e ( 0. 75: 0. 25 mol %)
was
i mmobi l i zed in
a s t a i n l e s s s t e e l wire mesh
as
in
Example 1. The membrane was pl a c e d
in the high t e mpe r a t ur e c e l l
and used to s e pa r a t e wat er
vapor
from n i t r o g e n . The d e t e c t i o n
syst em was
s i mi l a r to t hat of Example 1, except
t ha t a set of
hygr omet er s was
a t t a c h e d to each of the f eed and
per meat e
st r eams to measure dew
or f r o s t
poi nt (wat er c o n c e n t r a t i o n ) . The n i t r o g e n / wa t e r
feed mi xt ur e s
wer e
pr e pa r e d by pas s i ng n i t r o g e n t hr ough a bubbl e r , immersed in
a
wa t e r / e t h y l e n e gl ycol c ons t a nt t e mpe r a t ur e bat h. Feed mi xt ur e s of 1-3%
(by volume) of wat er were t hus o b t a i n e d .
Gas flows
were
20
CM /min
at about 1 atm. The membrane t h i c k n e s s
( s t e e l mesh) was
0.02
cm and the membrane area 0.8 cm. When t h e
membrane was heat ed to 230C,
very
low ni t r oge n p e r me a b i l i t y was
obs er ved. This i n d i c a t e s t hat the mol t en s a l t film
was c ont i nuous . The
membrane was
t e s t e d
over
f our
days
wi t h n i t r o g e n / wa t e r mi xt ur e s . Re s u l t s
we r e :
Owing to t he low l e ve l of n i t r o g e n pas s i ng t he f i l m, of t he or der o f
t he backgr ound l e ve l of n i t r o g e n , onl y a lower l i mi t f or
h y d r o g e n /
n i t r o g e n s e l e c t i v i t y
coul d be e s t i ma t e d. The t h u s - e s t i ma t e d
PoH2O/PoN2
is about 200: 1. I t is t h e r e f o r e
a ppa r e nt
t ha t t h e
s o d i u m/ l i t h i u m n i t r a t e i mmobi l i zed s a l t
membr ane per mi t s e x c e l l e n t
s e p a r a t i o n
of
wat er
from n i t r o g e n at 200 - 300C.
E x a m p l e 7
Ef f e c t of Wat er on Se p a r a t i o n of Carbon Di oxi de Usi ng an I mmobi l i z e d
Sa l t Membrane of Tet r ahexyl ammoni um Be nz oa t e
A membrane of t et r ahexyl ammoni um benzoat e, under c o n d i t i o n s
o t h e r wi s e as
in
Example 4, was used to s t u d y t h e e f f e c t of added wat er on
t he e f f i c i e n c y of
s e p a r a t i n g
car bon di oxi de from
n i t r o g e n . The wet f e e d
was pr oduced by bubbl i ng f eed t hr ough wat er at ambi ent
t e mpe r a t ur e
b e f o r e
pas s age over t he membr ane.
The f ol l owi ng r e s u l t s were o b t a i n e d :
These r e s u l t s show t hat moi st f eed had
hi gher car bon d i o x i d e
p e r me a b i l i t y
and s e l e c t i v i t y at lower
t e mpe r a t ur e s t han dry f eed. As t h e
t e mpe r a t ur e was r a i s e d, the e f f e c t of added wat er de c r e a s e d. I n
exper i ment s at 90C and above, a ddi t i on of wat er to the feed had
no
meas ur abl e e f f e c t .
Example 8
Se p a r a t i o n of
Oxygen
from Air usi ng a Li t hi um Ni t r a t e I mmobi l i z e d
Molten Sa l t Membrane
S t a i n l e s s s t e e l woven wire mesh (#304, about 0. 02
cm t hi c k, 4- 13
mi cr omet er s
pore
s i z e , area about 0.8
cm2)
was coat ed wi t h mo l t e n
l i t hi um n i t r a t e b y g r i n d i n g dry
l i t hi um n i t r a t e and p l a c i n g i t on t h e
mesh under an at mospher e
of
dry argon.
The cel l was c l os e d, connect ed t o
a gas
mani f ol d and heat ed to 285C under a feed flow of 10
cm3/min
o f
n i t r o g e n and per meat e
flow of 10
cm3/min
of hel i um at 1 atm
p r e s s u r e
for about 20 hr. At the end of t hi s time the l i t hi um n i t r a t e had we t t e d
t he s t a i n l e s s s t e e l mesh and formed
a v i s i b l y c ont i nuous mol t en f i l m
o n
one
s ur f a c e of t he wi r e mesh, and t hus
s e p a r a t e d t he feed and
p e r me a t e
s i des of t he c e l l :
The c e l l was
t hen heat ed to a f ur nace t e mp e r a t u r e of 429C, usi ng a
f eed flow of 10
cm3/min
of
zero gr ade
a i r and a
sweep
flow on t h e
p e r me a t e s i d e
of the membrane of 10
cm3/min
of hel i um, bot h at a b o u t
1 at mos pher e p r e s s u r e
and 25C. The t o t a l
p r e s s u r e
in t he cel l d u r i n g
t he e xpe r i me nt s was
about 760 t o r r .
The c o n c e n t r a t i o n of
gases
in the
per meat e
s t r eam was det er mi ned
by
gas
c hr oma t ogr a phy, us i ng a 183 cm 5A mol e c ul a r s i e ve column f or n i t r o g e n
and
oxygen,
o p e r a t e d i s o t h e r ma l l y at 60C. Af t er
t h e s y s t e m ha d
s t a b i l i z e d at t he o p e r a t i n g t e mp e r a t u r e ,
t he r e
was no l eak of
n i t r o g e n
a c r os s
t he membrane, as i n d i c a t e d by very
low n i t r o g e n p e r me a b i l i t y .
I t was obs e r ve d t ha t
oxygen
p e r me a b i l i t y i n c r e a s e d gr a dua l l y over 42
hour s of h e a t i n g .
Thi s
was
a t t r i b u t e d to an i n c r e a s e in t h e
c o n c e n t r a t i o n of oxi des (O .
O2 O2)
in the mol t en s a l t . I t
is pr opos ed t h a t t he d i s s o l v e d oxi des in t he mol t en s a l t c a t a l yz e d t h e
r e a c t i o n :
Ni t r oge n p e r me a b i l i t y was c ons t a nt ( about 12 Ba r r e r s ) . The
f o l l o wi n g r e s u l t s were o b t a i n e d :
These r e s u l t s show t hat a l i t hi um n i t r a t e i mmobi l i zed s a l t membrane
f u n c t i o n e d wel l f or a r e a s ona bl e t i me, and had good p e r me a b i l i t y a nd
s e l e c t i v i t y f or
oxyge n.
Example 9
Se p a r a t i o n
of Oxygen from Air usi ng a Sodium Ni t r a t e I mmobi l i z e d
Mol t en Sal t Membrane
A mol t en s al t membrane of
a sodium n i t r a t e
was i mmobi l i zed in
a
s t a i n l e s s s t e e l wire mesh as
in
Example
1. The sodi um n i t r a t e
was l o a d e d
ont o the s t a i n l e s s s t e e l mesh under an argon
a t mos phe r e a nd the cel l
was
a t t a c h e d to the
gas
mani f ol d. The c e l l was heat ed to 323C under a f e e d
flow of 10
cm3/min
o f n i t r o g e n and a per meat e
flow of 10
cm3/min
o f
hel i um f or about 15 h at 760 Torr. The f eed
gas was t hen changed t o
10
cm3/
min of zero grade
ai r at 760 To r r .
The c e l l
was
heat ed g r a d u a l l y to 450C, but no
oxygen was d e t e c t e d
in the per meat e st r eam, usi ng the a n a l y t i c a l method of
Example 1. At
477C,
oxygen was
obser ved in the
per meat e.
An e s s e n t i a l l y c o n s t a n t
val ue was
r eached a f t e r 4 h. The c e l l was hel d
at 477C f or 18 h and
t hen t he t e mpe r a t ur e was
va r i e d bet ween 452C and S25C t o det er mi ne t h e
e f f e c t of t e mpe r a t ur e
on
oxygen p e r me a b i l i t y . Re s ul t s
we r e :
Ni t r oge n p e r me a b i l i t y remai ned r e l a t i v e l y c o n s t a n t , at 12- 15
Ba r r e r . Thi s example shows t hat sodium n i t r a t e i mmobi l i zed mol t en s a l t
can
be used f or the s e p a r a t i o n of
oxygen
from a i r .
1. A
pr oc e s s
f or s e p a r a t i n g a
gas
from at l e a s t one ot her
gas
in
a
mi x t u r e , c ompr i s i ng pa s s i ng t he
gas
mi xt ur e
over a membrane s e l e c t i v e l y
pe r me a bl e to t he
gas bei ng s e p a r a t e d ,
which membrane compr i s es a t h i n ,
por ous
i n e r t
s uppor t
in which an a c t i v e ma t e r i a l is i mmo b i l i z e d wi t h i n
t he
por e s
and t he a c t i v e ma t e r i a l i s a mol t en s a l t .
2. The
pr oc e s s
of Cl ai m 1 wher ei n t he mol t en s a l t is
capabl e o f
u n d e r g o i n g one or more r e v e r s i b l e r e a c t i o n s , wi t h t he
gas bei ng s e p a r a t e d .
3. The
pr oces s
of Claim 2, wher ei n t he r e v e r s i b l e r e a c t i o n i s
f o r ma t i o n of a
c o o r d i n a t i o n
c ompl e x.
4. The
pr oc e s s
of Cl ai m 2, wher ei n t he r e v e r s i b l e r e a c t i o n is
a n
a c i d b a s e r e a c t i o n .
5. The
pr oc e s s
of Claim 2, wher ei n t he r e v e r s i b l e r e a c t i o n i s
f o r ma t i o n of an or ga ni c chemi cal compound.
6. The
pr oc e s s
of Claim 2 wher ei n t he r e v e r s i b l e r e a c t i o n is a n
o x i d a t i o n - r e d u c t i o n r e a c t i o n .
7. The
pr oc e s s
of Claim 1, wher ei n t he membrane i s c o n t a i n e d
wi t h i n
a module of hol l ow f i b e r s , a s p i r a l wi ndi ng or a
s e r i e s of f l a t
p l a t e s .
8. The
pr oc e s s
of Claim 1, wher ei n the r e v e r s i b l e
o x i d a t i o n - r e d u c t i o n r e a c t i o n i s n i t r a t e - n i t r i t e i n t e r c o n v e r s i o n ,
oxyge n
i s t he
gas
bei ng s e pa r a t e d and the a c t i ve ma t e r i a l in t he membrane i s
mol t e n a l k a l i met al n i t r a t e .
9. The
pr oces s
of Claim 1, wher ei n the
porous
membrane
c o n t a i n i n g
the mol t en s a l t i s s u p p o r t e d on a macr opor ous suppor t composed o f
cer ami c, g l a s s ,
me t a l l i c or pol yme r i c
ma t e r i a l .
10. The
pr oces s
of Claim 1, i nc l udi ng removing
gas pa s s i ng t h r o u g h
the membrane by. a st r eam of s weep
g a s .
11. The
pr oces s
of Cl ai m 1, i nc l udi ng removing gas pa s s i ng t h r o u g h
the membrane usi ng a c o mp r e s s o r .
S-ar putea să vă placă și
- 1 s2.0 S1385894713007869 Main PDFDocument7 pagini1 s2.0 S1385894713007869 Main PDFSJ ChuaÎncă nu există evaluări
- Nitric Acid PlantsDocument6 paginiNitric Acid Plantsabekat13Încă nu există evaluări
- Measurements of Oil-Water Separation Dynamics in Primary Separation Systems Using Distributed Capacitance SensorsDocument15 paginiMeasurements of Oil-Water Separation Dynamics in Primary Separation Systems Using Distributed Capacitance SensorsSJ ChuaÎncă nu există evaluări
- 1 s2.0 S0263876204726271 MainDocument8 pagini1 s2.0 S0263876204726271 MainSJ ChuaÎncă nu există evaluări
- Nitric Acid Manufacturing PlantDocument27 paginiNitric Acid Manufacturing Plantrasyid93Încă nu există evaluări
- Lecture 2 - Intro To HYSYSDocument40 paginiLecture 2 - Intro To HYSYSSJ Chua0% (1)
- Operational Experience of A Commercial Scale Plant of Electron Beam Puri®cation of Ue GasDocument5 paginiOperational Experience of A Commercial Scale Plant of Electron Beam Puri®cation of Ue GasSJ ChuaÎncă nu există evaluări
- 1 s2.0 S0016236107004085 MainDocument7 pagini1 s2.0 S0016236107004085 MainSJ ChuaÎncă nu există evaluări
- Radiation Physics and Chemistry: Ioan Calinescu, Diana Martin, Andrezj Chmielewski, Daniel IghigeanuDocument9 paginiRadiation Physics and Chemistry: Ioan Calinescu, Diana Martin, Andrezj Chmielewski, Daniel IghigeanuSJ ChuaÎncă nu există evaluări
- A Modification of The Demonstration of The Ostwald Process: Journal, 15 ItDocument1 paginăA Modification of The Demonstration of The Ostwald Process: Journal, 15 ItSJ ChuaÎncă nu există evaluări
- 1 s2.0 S0969806X04000921 MainDocument4 pagini1 s2.0 S0969806X04000921 MainSJ ChuaÎncă nu există evaluări
- ESP Sample Spec SheetDocument2 paginiESP Sample Spec SheetSJ ChuaÎncă nu există evaluări
- 1 s2.0 S0969806X07000941 MainDocument5 pagini1 s2.0 S0969806X07000941 MainSJ ChuaÎncă nu există evaluări
- Lecture 1 - Intro To Process SimulationDocument48 paginiLecture 1 - Intro To Process SimulationSJ ChuaÎncă nu există evaluări
- 1142 2908 1 PB PDFDocument4 pagini1142 2908 1 PB PDFIbrahim LahmidÎncă nu există evaluări
- Separation of Ammonia & WaterDocument14 paginiSeparation of Ammonia & WaterSiti Hajar JamaluddinÎncă nu există evaluări
- Fifty Years of Electrostatic PrecipitationDocument13 paginiFifty Years of Electrostatic PrecipitationSJ ChuaÎncă nu există evaluări
- Discharge ElectrodeDocument21 paginiDischarge ElectrodeSJ Chua100% (1)
- Turner Et Al 02Document13 paginiTurner Et Al 02SJ ChuaÎncă nu există evaluări
- 21st Century ESP DesignDocument12 pagini21st Century ESP DesignSJ ChuaÎncă nu există evaluări
- Krupp Nitric Acid PlantDocument16 paginiKrupp Nitric Acid PlantSJ ChuaÎncă nu există evaluări
- Turner Et Al 01Document15 paginiTurner Et Al 01SJ ChuaÎncă nu există evaluări
- Application of ESP For Gas Cleaning in Cement Industry - With Reference To IndiaDocument24 paginiApplication of ESP For Gas Cleaning in Cement Industry - With Reference To IndiaSJ ChuaÎncă nu există evaluări
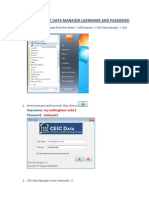
- CEIC Data Manager Username and PasswordDocument1 paginăCEIC Data Manager Username and PasswordIsmail Jamaluddin100% (1)
- DataStream Communications ConfigurationDocument1 paginăDataStream Communications ConfigurationIsmail JamaluddinÎncă nu există evaluări
- Physical Properties of Pure MethanolDocument1 paginăPhysical Properties of Pure MethanolSJ ChuaÎncă nu există evaluări
- Safety PrecautionDocument1 paginăSafety PrecautionSJ Chua100% (1)
- Shoe Dog: A Memoir by the Creator of NikeDe la EverandShoe Dog: A Memoir by the Creator of NikeEvaluare: 4.5 din 5 stele4.5/5 (537)
- Grit: The Power of Passion and PerseveranceDe la EverandGrit: The Power of Passion and PerseveranceEvaluare: 4 din 5 stele4/5 (587)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceDe la EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceEvaluare: 4 din 5 stele4/5 (890)
- The Yellow House: A Memoir (2019 National Book Award Winner)De la EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Evaluare: 4 din 5 stele4/5 (98)
- The Little Book of Hygge: Danish Secrets to Happy LivingDe la EverandThe Little Book of Hygge: Danish Secrets to Happy LivingEvaluare: 3.5 din 5 stele3.5/5 (399)
- On Fire: The (Burning) Case for a Green New DealDe la EverandOn Fire: The (Burning) Case for a Green New DealEvaluare: 4 din 5 stele4/5 (73)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeDe la EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeEvaluare: 4 din 5 stele4/5 (5794)
- Never Split the Difference: Negotiating As If Your Life Depended On ItDe la EverandNever Split the Difference: Negotiating As If Your Life Depended On ItEvaluare: 4.5 din 5 stele4.5/5 (838)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureDe la EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureEvaluare: 4.5 din 5 stele4.5/5 (474)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryDe la EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryEvaluare: 3.5 din 5 stele3.5/5 (231)
- The Emperor of All Maladies: A Biography of CancerDe la EverandThe Emperor of All Maladies: A Biography of CancerEvaluare: 4.5 din 5 stele4.5/5 (271)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreDe la EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreEvaluare: 4 din 5 stele4/5 (1090)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyDe la EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyEvaluare: 3.5 din 5 stele3.5/5 (2219)
- Team of Rivals: The Political Genius of Abraham LincolnDe la EverandTeam of Rivals: The Political Genius of Abraham LincolnEvaluare: 4.5 din 5 stele4.5/5 (234)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersDe la EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersEvaluare: 4.5 din 5 stele4.5/5 (344)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaDe la EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaEvaluare: 4.5 din 5 stele4.5/5 (265)
- The Unwinding: An Inner History of the New AmericaDe la EverandThe Unwinding: An Inner History of the New AmericaEvaluare: 4 din 5 stele4/5 (45)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)De la EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Evaluare: 4.5 din 5 stele4.5/5 (119)
- Her Body and Other Parties: StoriesDe la EverandHer Body and Other Parties: StoriesEvaluare: 4 din 5 stele4/5 (821)
- Sheet 2Document3 paginiSheet 2Abdalla Mohamed AbdallaÎncă nu există evaluări
- Norris SealDocument8 paginiNorris SealAbayÎncă nu există evaluări
- Gas burners one stage technical specifications and dimensionsDocument4 paginiGas burners one stage technical specifications and dimensionsMohamed FazilÎncă nu există evaluări
- Methanol ProDocument126 paginiMethanol Proinju rehaman100% (1)
- PBS & TBDocument3 paginiPBS & TBRajan BhandariÎncă nu există evaluări
- Introduction of Fired HeatersDocument14 paginiIntroduction of Fired HeatersLuis Torres50% (4)
- Cl605 PneumaticDocument12 paginiCl605 PneumaticMirko Novakovic100% (1)
- Economic Viability of UGS in NigeriaDocument87 paginiEconomic Viability of UGS in NigeriaNwachukwu Caleb Kelechi100% (3)
- Constant-Pressure Turbocharger Matching by Michael M. W. de SilvaDocument32 paginiConstant-Pressure Turbocharger Matching by Michael M. W. de SilvaMichael M. W. de Silva100% (1)
- Non-Reactive Ideal Gas MixtureDocument18 paginiNon-Reactive Ideal Gas MixtureGODÎncă nu există evaluări
- 5510 0172 00ppr Using Methanol Fuel in The LowDocument16 pagini5510 0172 00ppr Using Methanol Fuel in The LowFuchsbauÎncă nu există evaluări
- Suction Accumulator CatalogDocument1 paginăSuction Accumulator CatalogFirman ArrasyidÎncă nu există evaluări
- Bubble DewDocument15 paginiBubble DewSata AjjamÎncă nu există evaluări
- USOO8568134B2 Coanda gas burner apparatusDocument29 paginiUSOO8568134B2 Coanda gas burner apparatusFabrizio FerrerioÎncă nu există evaluări
- Introduction and Basic Concepts: Mehmet KanogluDocument54 paginiIntroduction and Basic Concepts: Mehmet Kanogluizzet9696Încă nu există evaluări
- RusBBTC2015 HALDOR TOPSOE ALEXANDRA KARYAGINA Eng PDFDocument28 paginiRusBBTC2015 HALDOR TOPSOE ALEXANDRA KARYAGINA Eng PDFlaquetengoÎncă nu există evaluări
- 6 - Loss of Energy (Or Head) in PipesDocument21 pagini6 - Loss of Energy (Or Head) in PipesMustafa ZahidÎncă nu există evaluări
- JJ 615 Mechanical Components & MaintenanceDocument36 paginiJJ 615 Mechanical Components & Maintenancedaussz60% (5)
- Fuderer, A. and Rudelstorfer, E. Selective Adsorption Process. U.S. Patent 3,986,849Document27 paginiFuderer, A. and Rudelstorfer, E. Selective Adsorption Process. U.S. Patent 3,986,849kay LauÎncă nu există evaluări
- Standard Technical Features of BTG System for Supercritical 660/800 MW Thermal UnitsDocument1 paginăStandard Technical Features of BTG System for Supercritical 660/800 MW Thermal UnitsDhiraj SatyamÎncă nu există evaluări
- Divex - Extinguidor HiperbaricoDocument2 paginiDivex - Extinguidor HiperbaricoCarlos GuevaraÎncă nu există evaluări
- 18MW Co-Generation system with low emissionsDocument2 pagini18MW Co-Generation system with low emissionsfrdnÎncă nu există evaluări
- Techno-Economic Comparative Analysis of Biomass Integrated Gasification Combined Cycles With and Without CO2 CaptureDocument12 paginiTechno-Economic Comparative Analysis of Biomass Integrated Gasification Combined Cycles With and Without CO2 CaptureMugdho HossainÎncă nu există evaluări
- Booster System Basics:: Constant Speed SystemsDocument49 paginiBooster System Basics:: Constant Speed SystemsJesus TineoÎncă nu există evaluări
- Pneumatic Conveying Systems..Document61 paginiPneumatic Conveying Systems..efka808Încă nu există evaluări
- SA-980-P-11443 - API 685 Offloading Pump Landside PZ - Vert02Document5 paginiSA-980-P-11443 - API 685 Offloading Pump Landside PZ - Vert02altipatlarÎncă nu există evaluări
- Module 2 - Pumps: Engr. P.C. Ramos - Mechanical Engineering Department - 1Document6 paginiModule 2 - Pumps: Engr. P.C. Ramos - Mechanical Engineering Department - 1Цедіе РамосÎncă nu există evaluări
- Performance Curve - Service1 - Item 1Document1 paginăPerformance Curve - Service1 - Item 1Fernando SanchezÎncă nu există evaluări
- Fan & SystemDocument31 paginiFan & SystemAlberto FranciscoÎncă nu există evaluări
- How to Select the Best Pump in 10 StepsDocument11 paginiHow to Select the Best Pump in 10 StepsTamani MoyoÎncă nu există evaluări