Documente Academic
Documente Profesional
Documente Cultură
Diagram Ellingham
Încărcat de
cindycinpengDrepturi de autor
Formate disponibile
Partajați acest document
Partajați sau inserați document
Vi se pare util acest document?
Este necorespunzător acest conținut?
Raportați acest documentDrepturi de autor:
Formate disponibile
Diagram Ellingham
Încărcat de
cindycinpengDrepturi de autor:
Formate disponibile
Thermodynamics
Ellingham diagrams are a particular graphical form of the principle that the thermodynamic
feasibility of a reaction depends on the sign of G, the Gibbs free energy change, which is
equal to H TS, where H is the enthalpy change and S is the entropy change.
Simple Ellingham diagram for high temperature (0C 2500C) oxidation of several metals
and carbon
The Ellingham diagram plots the Gibbs free energy change (G) for each oxidation reaction
as a function of temperature. For comparison of different reactions, all values of G refer to
the reaction of the same quantity of oxygen, chosen as one mole O (
1
2
mol O
2) by some authors
[2]
and one mole O
2 by others.
[3]
The diagram at right refers to 1 mole O, so that for example the line marked Cr
2O
3 shows G for the reaction 2/3 Cr(s) +
1
2
O
2(g)
1
3
Cr
2O
3(s), which is
1
3
of the molar Gibbs energy of formation G
f
(Cr
2O
3, s).
In the temperature ranges commonly used, the metal and the oxide are in a condensed state
(liquid or solid), and oxygen is a gas with a much larger molar entropy. For the oxidation of
each metal, the dominant contribution to the entropy change (S) is the removal of
1
2
mol O
2, so that S is negative and roughly equal for all metals. The slope of the plots dG/dT =
S is therefore positive for all metals, with G always becoming more negative with lower
temperature, and the lines for all the metal oxides are approximately parallel. Since these
reactions are exothermic, they always become feasible at lower temperatures. At a
sufficiently high temperature, the sign of G may invert (becoming positive) and the oxide
can spontaneously reduce to the metal, as shown for Ag and Cu.
For oxidation of carbon, the red line is for the formation of CO: C(s) +
1
2
O
2(g) CO(g) with an increase in the number of moles of gas, leading to a positive S and a
negative slope. The blue line for the formation of CO
2 is approximately horizontal, since the reaction C(s) + O
2(g) CO
2(g) leaves the number of moles of gas unchanged so that S is small.
As with any chemical reaction prediction based on purely thermodynamic grounds, a
spontaneous reaction may be very slow if one or more stages in the reaction pathway have
very high activation energies E
A
.
If two metals are present, two equilibria have to be considered. The oxide with the more
negative G will be formed and the other oxide will be reduced.
Salient features
1. Curves in the Ellingham diagrams for the formation of metallic oxides are basically
straight lines with a positive slope. The slope is proportional to S, which is fairly
constant with temperature.
2. The lower the position of a metal's line in the Ellingham diagram, the greater is the
stability of its oxide. For example, the line for Al (oxidation of aluminium) is found to
be below that for Fe (formation of Fe
2O
3).
3. Stability of metallic oxides decreases with increase in temperature. Highly unstable
oxides like Ag
2O and HgO easily undergo thermal decomposition.
4. The formation free energy of carbon dioxide (CO
2) is almost independent of temperature, while that of carbon monoxide (CO) has
negative slope and crosses the CO
2 line near 700 C. According to the Boudouard reaction, carbon monoxide is the
dominant oxide of carbon at higher temperatures (above about 700 C), and the higher
the temperature (above 700 C) the more effective a reductant (reducing agent) carbon
is.
5. A reduced substance (such as a metal), whose Gibbs free energy of formation is lower
on the diagram at a given temperature, will reduce an oxide whose free energy of
formation is higher on the diagram. For example, metallic aluminium can reduce iron
oxide to metallic iron, the aluminium itself being oxidized to aluminium oxide. (This
reaction is employed in thermite.)
6. The greater the gap between any two lines, the greater the effectiveness of the
reducing agent corresponding to the lower line.
7. The intersection of two lines implies an oxidation-reduction equilibrium. Reduction
using a given reductant is possible at temperatures above the intersection point where
the G line of that reductant is lower on the diagram than that of the metallic oxide to
be reduced. At the point of intersection the free energy change for the reaction is zero,
below this temperature it is positive and the metallic oxide is stable in the presence of
the reductant, while above the point of intersection the Gibbs energy is negative and
the oxide can be reduced.
Reducing agents
In industrial processes, the reduction of metal oxides is often effected by a carbothermic
reaction, using carbon as a reducing agent. Carbon is available cheaply as coal, which can be
rendered to coke. Moreover, when carbon reacts with oxygen it forms the gaseous oxides
carbon monoxide and carbon dioxide, so the thermodynamics of its oxidation is different
from that for metals: its oxidation has a more negative G with higher temperatures (above
700 C). Carbon can thus serve as reducing agent. Using this property, reduction of metals
may be performed as a double redox reaction at relatively low temperature.
Use of Ellingham diagrams
The main application of Ellingham diagrams is in the extractive metallurgy industry, where it
helps to select the best reducing agent for various ores in the extraction process, purification
and grade setting for steel manufacturing. It also helps to guide the purification of metals,
especially the removal of trace elements. The direct reduction process for making iron rests
firmly on the guidance of Ellingham diagrams, which show that hydrogen can alone reduce
iron oxides to the metal.
Reducing agent for haematite
In iron ore smelting, haematite gets reduced at the top of the furnace, where temperature is in
the range 600 700 C. The Ellingham diagram indicates that in this range carbon monoxide
acts as a stronger reducing agent than carbon since the process
2 CO + O
2 2 CO
2
has a more-negative free energy change than the process:
2 C + O
2 2 CO.
In the upper part of the blast furnace, haematite is reduced by CO (produced by oxidation of
coke lower down, at higher temperatures) even in the presence of carbon though this is
mainly because the kinetics for gaseous CO reacting with the ore are better.
Reducing agent for chromic oxide-carbon cannot be used
The Ellingham curve for the reaction 2C(s) + O
2(g) 2CO(g) slopes down and falls below the curves for all the metals. Hence, carbon can
normally act as a reducing agent for all metal oxides at very high temperatures. But
chromium formed at these temperatures reacts with carbon to form its carbide, which gives
undesirable properties to the chromium metal obtained. Hence, for high temperature
reduction of chromic oxide, carbon cannot be used.
Alumino thermic process
Thermite reaction proceeding for a railway welding. Shortly after this, the liquid iron flows
into the mould around the rail gap
The Ellingham curve for aluminium lies below the curves of most metals such chromium,
iron, etc. This fact indicates that aluminium can be used as the reducing agent for oxides of
all these metals. This result is illustrated as follows:
The free energies of formation of chromium(III) oxide and aluminium oxide per mole of
oxygen consumed are -540kJ and -827kJ respectively. The processes are:
(1)
(2)
The second equation minus the first equation gives:
So aluminium oxide is more stable than chromium oxide (at least at normal temperatures, and
in fact all the way up to the decomposition temperatures of the oxides). Since the Gibbs free
energy change is negative, aluminium can reduce chromium oxide.
In pyrometallurgy, Al is used as a reducing agent in the alumino-thermic process or thermite
process to extract Cr and Mn by reduction of their oxides.
References
1. Ellingham, H. J. T. (1944), "Transactions and Communications", J. Soc. Chem. Ind.
(London) 63 (5): 125, doi:10.1002/jctb.5000630501.
2. Atkins, Peter; de Paula, Julio (2006), Physical Chemistry: Thermodynamics And
Kinetics (8th ed.), W.H. Freeman, p. 215, ISBN 0716785676. This reference plots the
diagram upside-down, with G decreasing upwards.
3. Ellingham diagram tutorial and interactive diagram (University of Cambridge)
S-ar putea să vă placă și
- Ellingham Diagram UsesDocument4 paginiEllingham Diagram Usesnanda rizkyÎncă nu există evaluări
- MINIMIZE CHROMIUM LOSSES IN EAFDocument3 paginiMINIMIZE CHROMIUM LOSSES IN EAFLegendaryÎncă nu există evaluări
- EllinghamDocument6 paginiEllinghamabhilashdbzÎncă nu există evaluări
- The Ellingham Diagram: Technical PresentationDocument22 paginiThe Ellingham Diagram: Technical PresentationaudahÎncă nu există evaluări
- Microscopy of Ceramics and Cements: Including Glasses, Slags, and Foundry SandsDe la EverandMicroscopy of Ceramics and Cements: Including Glasses, Slags, and Foundry SandsÎncă nu există evaluări
- Production Gas Carburising: The Pergamon Materials Engineering Practice SeriesDe la EverandProduction Gas Carburising: The Pergamon Materials Engineering Practice SeriesÎncă nu există evaluări
- Analysis of Organoaluminium and Organozinc Compounds: International Series of Monographs in Analytical ChemistryDe la EverandAnalysis of Organoaluminium and Organozinc Compounds: International Series of Monographs in Analytical ChemistryÎncă nu există evaluări
- Alumina to Zirconia: The History of the CSIRO Division of Mineral ChemistryDe la EverandAlumina to Zirconia: The History of the CSIRO Division of Mineral ChemistryEvaluare: 1 din 5 stele1/5 (1)
- The Working of Steel: Annealing, Heat Treating and Hardening of Carbon and Alloy SteelDe la EverandThe Working of Steel: Annealing, Heat Treating and Hardening of Carbon and Alloy SteelÎncă nu există evaluări
- Diamond Chemical Vapor Deposition: Nucleation and Early Growth StagesDe la EverandDiamond Chemical Vapor Deposition: Nucleation and Early Growth StagesÎncă nu există evaluări
- Alloys DensityDocument4 paginiAlloys DensityArnold Melissa CollettÎncă nu există evaluări
- Structure and Properties of Inorganic Solids: International Series of Monographs in Solid State PhysicsDe la EverandStructure and Properties of Inorganic Solids: International Series of Monographs in Solid State PhysicsÎncă nu există evaluări
- The Mechanical and Physical Properties of the British Standard EN Steels (B.S. 970 - 1955): EN 21 to EN 39De la EverandThe Mechanical and Physical Properties of the British Standard EN Steels (B.S. 970 - 1955): EN 21 to EN 39Evaluare: 5 din 5 stele5/5 (1)
- Continuous casting The Ultimate Step-By-Step GuideDe la EverandContinuous casting The Ultimate Step-By-Step GuideÎncă nu există evaluări
- Metallic Matrix Composites: Composite Materials, Vol. 4De la EverandMetallic Matrix Composites: Composite Materials, Vol. 4Kenneth G. KreiderÎncă nu există evaluări
- Advances in Research on the Strength and Fracture of Materials: An OverviewDe la EverandAdvances in Research on the Strength and Fracture of Materials: An OverviewD M R TaplinÎncă nu există evaluări
- Metallurgical Achievements: Selection of Papers Presented at the Birmingham Metallurgical Society's Diamond Jubilee Session, 1963-1964De la EverandMetallurgical Achievements: Selection of Papers Presented at the Birmingham Metallurgical Society's Diamond Jubilee Session, 1963-1964W. O. AlexanderÎncă nu există evaluări
- The Physical Metallurgy of Fracture: Fourth International Conference on Fracture, June 1977, University of Waterloo, CanadaDe la EverandThe Physical Metallurgy of Fracture: Fourth International Conference on Fracture, June 1977, University of Waterloo, CanadaD M R TaplinÎncă nu există evaluări
- Effect of Cooling Rate On Microstructure and Mechanical Properties of Gray Cast Iron - IsIDocument6 paginiEffect of Cooling Rate On Microstructure and Mechanical Properties of Gray Cast Iron - IsIgiokniessÎncă nu există evaluări
- Review of Magnesium Metal Matrix CompositesDocument19 paginiReview of Magnesium Metal Matrix CompositesGokulraju RangasamyÎncă nu există evaluări
- Cast Iron DampingDocument5 paginiCast Iron Dampinggabs88Încă nu există evaluări
- Reactions of Non-Metallic Inorganic CompoundsDe la EverandReactions of Non-Metallic Inorganic CompoundsÎncă nu există evaluări
- Mg Superplasticity TechniquesDocument23 paginiMg Superplasticity TechniquesashvaniÎncă nu există evaluări
- Metallic Materials Sessional Microstructure StudyDocument39 paginiMetallic Materials Sessional Microstructure StudyMuhammedNayeemÎncă nu există evaluări
- Gating & RiserDocument8 paginiGating & Riserhegdemahesh1Încă nu există evaluări
- Alloy And Microstructural DesignDe la EverandAlloy And Microstructural DesignJohn TienÎncă nu există evaluări
- Resistance and Deformation of Solid Media: Pergamon Unified Engineering SeriesDe la EverandResistance and Deformation of Solid Media: Pergamon Unified Engineering SeriesÎncă nu există evaluări
- Overall Aspects of Non-Traditional Glasses: Synthesis, Properties and ApplicationsDe la EverandOverall Aspects of Non-Traditional Glasses: Synthesis, Properties and ApplicationsÎncă nu există evaluări
- Effect of Microstructure and Alloy Contents On The Luders Line Formation in Al-Mg AlloysDocument6 paginiEffect of Microstructure and Alloy Contents On The Luders Line Formation in Al-Mg AlloysJinsoo KimÎncă nu există evaluări
- Amorphous and Nano Alloys Electroless Depositions: Technology, Composition, Structure and TheoryDe la EverandAmorphous and Nano Alloys Electroless Depositions: Technology, Composition, Structure and TheoryÎncă nu există evaluări
- Chapter 6 Phase DiagramsDocument73 paginiChapter 6 Phase DiagramsSaiful AzrieÎncă nu există evaluări
- DME Assignment 1Document2 paginiDME Assignment 1sumikannuÎncă nu există evaluări
- Powder Metallurgy Process GuideDocument28 paginiPowder Metallurgy Process GuideAravindhan AnbalaganÎncă nu există evaluări
- Solidification and Crystallization Processing in Metals and AlloysDe la EverandSolidification and Crystallization Processing in Metals and AlloysÎncă nu există evaluări
- Controlling The Chemistry and The and The Section Size Is Very ImportantDocument2 paginiControlling The Chemistry and The and The Section Size Is Very ImportantHeuzerGomesÎncă nu există evaluări
- Transition Metal ToxicityDe la EverandTransition Metal ToxicityG. W. RichterÎncă nu există evaluări
- The effect of annealing on aluminum clad steel sheet propertiesDocument6 paginiThe effect of annealing on aluminum clad steel sheet propertiesRina OktapianiÎncă nu există evaluări
- Current Advances in Mechanical Design & Production IV: Proceedings of the Fourth Cairo University MDP Conference, Cairo, 27-29 December 1988De la EverandCurrent Advances in Mechanical Design & Production IV: Proceedings of the Fourth Cairo University MDP Conference, Cairo, 27-29 December 1988Y. H. KabilÎncă nu există evaluări
- Analytical Chemistry of Zirconium and Hafnium: International Series of Monographs in Analytical ChemistryDe la EverandAnalytical Chemistry of Zirconium and Hafnium: International Series of Monographs in Analytical ChemistryÎncă nu există evaluări
- Aluminium 2014 t6 2014 t651Document3 paginiAluminium 2014 t6 2014 t651Agung Sapto AjiÎncă nu există evaluări
- The Mechanical Behaviour of Engineering Materials: The Commonwealth and International Library: Structures and Solid Body Mechanics DivisionDe la EverandThe Mechanical Behaviour of Engineering Materials: The Commonwealth and International Library: Structures and Solid Body Mechanics DivisionÎncă nu există evaluări
- Equilibrium Crystallisation of Iron-Carbon Alloys LectureDocument25 paginiEquilibrium Crystallisation of Iron-Carbon Alloys LectureTisza_MÎncă nu există evaluări
- Si and Ni As Alloying Elements To Vary Carbon Equivalent of Austenitic Ductile Cast Iron - Microstructure and Mechanical Properties-2Document9 paginiSi and Ni As Alloying Elements To Vary Carbon Equivalent of Austenitic Ductile Cast Iron - Microstructure and Mechanical Properties-2Chun-Yi LinÎncă nu există evaluări
- Solidification of Metals (To Be Completed) : Prof. H. K. Khaira Professor, Deptt. of MSME M.A.N.I.T., BhopalDocument62 paginiSolidification of Metals (To Be Completed) : Prof. H. K. Khaira Professor, Deptt. of MSME M.A.N.I.T., BhopalIndranil Bhattacharyya100% (1)
- l4 The Clausius-UpadiDocument8 paginil4 The Clausius-UpadiMarcos Sánchez MartínezÎncă nu există evaluări
- Aluminium Alloy Applications PDFDocument3 paginiAluminium Alloy Applications PDFKhanHasibKaisarÎncă nu există evaluări
- PTQ - Corrosion and Fouling Challanges and Solution PDFDocument10 paginiPTQ - Corrosion and Fouling Challanges and Solution PDFPhatchara Chuaykerd100% (1)
- Revision Checklist: SC8 Acids and AlkalisDocument3 paginiRevision Checklist: SC8 Acids and AlkalisturanlegendaryÎncă nu există evaluări
- CLS - ENG 22 23 XI - Che - Target 5 - Level 1 - Chapter 10 PDFDocument38 paginiCLS - ENG 22 23 XI - Che - Target 5 - Level 1 - Chapter 10 PDFSaksham Chamoli 10 G , 27Încă nu există evaluări
- Analisis Kandungan Arsenik (As) Dan Cianida (CN) Depot Air Minum Isi Ulang Di Kota BatamDocument5 paginiAnalisis Kandungan Arsenik (As) Dan Cianida (CN) Depot Air Minum Isi Ulang Di Kota BatamHairunnisa BrutuÎncă nu există evaluări
- Unit 1. Chemical Foundation of LifeDocument40 paginiUnit 1. Chemical Foundation of LifeAila Grace PeriodicoÎncă nu există evaluări
- Reactive Exhaust DyeingDocument12 paginiReactive Exhaust DyeingKhandaker Sakib Farhad67% (3)
- Typical List of Chemicals Used in Dyeing MillDocument6 paginiTypical List of Chemicals Used in Dyeing MillMohammed Atiqul Hoque ChowdhuryÎncă nu există evaluări
- Dianal Resin TypesDocument2 paginiDianal Resin TypesdamiendamÎncă nu există evaluări
- Lipidss (Autosaved)Document21 paginiLipidss (Autosaved)AÎncă nu există evaluări
- Chemical Company Brochure PDF Anmol Chemicals Pvt. Ltd. Company ProfileDocument21 paginiChemical Company Brochure PDF Anmol Chemicals Pvt. Ltd. Company ProfileAnmol ChemicalsÎncă nu există evaluări
- List of Solvents and Their Evaporation Vacuum PressuresDocument1 paginăList of Solvents and Their Evaporation Vacuum PressuresAntares1973Încă nu există evaluări
- Fundamentals of Chemistry and MineralogyDocument11 paginiFundamentals of Chemistry and MineralogyEvi Intan SariÎncă nu există evaluări
- Hydrofluoric Acid Scrubber SystemsDocument12 paginiHydrofluoric Acid Scrubber Systemsravichem823Încă nu există evaluări
- NaBH4 Reduction of CyclohaxanoneDocument5 paginiNaBH4 Reduction of Cyclohaxanonenurul1110Încă nu există evaluări
- 1 s2.0 S001085450600138X MainDocument29 pagini1 s2.0 S001085450600138X MainRoman RusnacÎncă nu există evaluări
- Chapter 1 Organic CompoundsDocument7 paginiChapter 1 Organic CompoundsMark DiezÎncă nu există evaluări
- DyStar Product Selection Guidance HM Chemical Restrictions May 082014Document57 paginiDyStar Product Selection Guidance HM Chemical Restrictions May 082014rajasajjad100% (1)
- 1986Document3 pagini1986bobothebioguyÎncă nu există evaluări
- Dyefix-CF - Chlorine Fastness ImproverFor Cellulosic FabricsDocument10 paginiDyefix-CF - Chlorine Fastness ImproverFor Cellulosic FabricsL.N.CHEMICAL INDUSTRYÎncă nu există evaluări
- MLS 5a - BSMLS2-E - Module4 - Group9Document8 paginiMLS 5a - BSMLS2-E - Module4 - Group9Lexi Evonne NacionalesÎncă nu există evaluări
- Coordination PolymerizationDocument14 paginiCoordination PolymerizationVijaypal Singh Rathore100% (1)
- SCILOGEX DispensMate Chemical CompatibilityDocument3 paginiSCILOGEX DispensMate Chemical CompatibilitynasmineÎncă nu există evaluări
- Stability of Metal Cyanide and Hydroxide Complexes Conference PaperDocument9 paginiStability of Metal Cyanide and Hydroxide Complexes Conference PaperYasirÎncă nu există evaluări
- French Fries - Chemistry Science Fair Projects Using - Robert GardnerDocument129 paginiFrench Fries - Chemistry Science Fair Projects Using - Robert GardnerCarlos VilachaÎncă nu există evaluări
- Vinblastine CodexDocument2 paginiVinblastine CodexRahma SantosoÎncă nu există evaluări
- Formula 1017 Face Moisturizer With Squalane and Watermelon ExtractDocument1 paginăFormula 1017 Face Moisturizer With Squalane and Watermelon ExtractKothari SaralÎncă nu există evaluări
- Oshareg2 PDFDocument548 paginiOshareg2 PDFGustavo FeisbukeroÎncă nu există evaluări
- Lab 2 Chemical Nomenclature and Formula Writing (Word)Document12 paginiLab 2 Chemical Nomenclature and Formula Writing (Word)Tinke WinkeÎncă nu există evaluări
- Required Labs Manual - MSJ 2016Document26 paginiRequired Labs Manual - MSJ 2016Semwezi EnockÎncă nu există evaluări
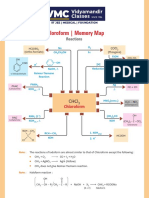
- Chloroform - Memory Map: ReactionsDocument1 paginăChloroform - Memory Map: ReactionsAryan GuptaÎncă nu există evaluări