Documente Academic
Documente Profesional
Documente Cultură
1 PB
Încărcat de
Juan Camilo HenaoTitlu original
Drepturi de autor
Formate disponibile
Partajați acest document
Partajați sau inserați document
Vi se pare util acest document?
Este necorespunzător acest conținut?
Raportați acest documentDrepturi de autor:
Formate disponibile
1 PB
Încărcat de
Juan Camilo HenaoDrepturi de autor:
Formate disponibile
Mechanical Engineering Research; Vol. 2, No.
2; 2012
ISSN 1927-0607
E-ISSN 1927-0615
Published by Canadian Center of Science and Education
Simulation and Parametric Analysis of Cryogenic Oxygen Plant for
Biomass Gasification
Vaijanath N. Raibhole1 & S. N. Sapali2
1
MESs college of Engineering, Pune, India
College of Engineering, Pune, India
Correspondence: Vaijanath N. Raibhole, MESs College of Engineering, Late principal V. K. Joag Path, Wadia
college campus, Pune, India. Tel: 91-800-793-7370. E-mail: vraibhole@gmail.com
Received: September 18, 2012
doi:10.5539/mer.v2n2p97
Accepted: October 18, 2012
Online Published: November 6, 2012
URL: http://dx.doi.org/10.5539/mer.v2n2p97
Abstract
Cryogenic air separation plants are used for production of oxygen, nitrogen and argon with required purity and
recovery.The first grade oxygen (purity over 99.99%) is required for welding, cutting, and medical applications.
These plants operate at low thermodynamic efficiency with specific power consumption in a range between
0.5-0.6 kw/scmh of O2. As air gasification produces poor quality syngas, oxygen is used as gasifying agent for
biomass gasification. Medium purity cryogenic air separation units (ASU) are chiefly required for gasification.
Biomass gasification with oxygen as gasifying agent has great potential in applications like integrated
gasification combined cycle (IGCC), chemical production and Fischer-Tropsch (F-T) products. In this work
simulation of medium purity oxygen cryogenic air separation plant integrated with biomass gasifier is carried out
by using Aspen plus. Such cryogenic air separation plants which produce oxygen in a range between 85-98% can
be used economically for gasification. The cryogenic oxygen plant produces oxygen with purity 96.2 % mole
basis with specific power consumption as 0.2435 kw/scmh of O2. The performance parameters like recovery,
purity, temperature and pressure and power consumption of cryogenic air separation unit are obtained. The
parameters like syngas composition and heating value also predicted in simulation of biomass gasifier. The effect
of parameters (parametric analysis), like vapour fraction of the feed on pure liquid (PL) flow and condenser duty,
effect of number of stages on PL and rich liquid (RL) flow and its purity and effect of oxygen flow and gasifier
temperature on syngas composition is discussed.
Keywords: ASPEN plus, biomass gasification, cryogenic ASU, IGCC, parametric analysis, syngas
1. Introduction
The development of cryogenic ASU has been taken place through various stages. As the process cycle
improvement is one of the areas for further development in cycle towards performance. So to understand
thermodynamics of the process cycle simulation of various unit operations in a cryogenic ASU is essential.
ASPEN plus (Advanced System for Process Engineering Plus) is one of the process simulator used for modeling
cryogenic air separation plant and biomass gasification. It is a steady state chemical process simulator, which
was developed at Massachusetts Institute of Technology (MIT) for the US DOE, to evaluate synthetic fuel
technologies. It uses unit operation blocks, which are models of specific process operations (reactors, heaters,
pumps etc.). The user places these blocks on a flow sheet, specifying material and energy streams.
Aspen plus is one of the most powerful and widely used process simulators in the process industry today. It has
several features that make it very intuitive and user friendly. Its graphic user interface and model manager make
an excellent guide for the user and allow for complete specifications and control at every stage of model
development (Aspen Tech, User Manual, 2011). Many large scale applications like gasification demands second
grade (medium purity) oxygen. The first grade oxygen production is too expensive which may not economical as
oxygen costs in such applications are critical. There are different methods available for production of second
grade oxygen as mentioned by (Schuftan, 1948) with merits and demerits. The continuous development of air
separation cycles has been taking place in terms of recovery, power consumption, purity, cost of manufacturing,
ease of maintenance etc. (Castle, 2000; Sapali, 2001; Agrawal et al., 1989; Zhu et al., 2006; Mandler, 2000;
Smith et al., 2001; Zhu et al., 2010). Gasification involves the production of gaseous fuel mainly consisting of
carbon monoxide (CO), carbon dioxide (CO2), hydrogen (H2), and with traces of methane (CH4); with useable
97
www.ccsenet.org/mer
Mechanical Engineering Research
Vol. 2, No. 2; 2012
heating value by partial combustion of solid fuel. Feedstocks of gasification like biomass and inferior quality
coal have great potential for energy production. Currently energy is recovered from these fuels through
combustion. The efficiency of these plants is very less. Thus coal/biomass gasification unit integrated with
combined power cycle offers higher efficiencies up to 60% (Doherty et al., 2008). As one of the attempt to study
performance of gasifier with air as gasifying agent, an equilibrium model based on minimization of Gibbs free
energy was developed for rubber wood and syngas composition obtained by (Paviet et al., 2009). Where as
mathematical model in Engineering Equation Solver (EES) was used to predict syngas composition in a small
downdraft gasifier (Chawdhury et al., 2011). However thermodynamic equilibrium composition prediction is the
important step in modeling the gasification process. An equilibrium model for biomass gasifier is developed and
equilibrium relations are solved by using MATLAB to get syngas composition and its properties (Khadse et al.,
2006). In addition exergy analysis performed on the basis of thermodynamic equilibrium for different feedstocks
by (Shrinivas et al., 2009). Gasification of rice husk was tested in fluidized bed gasifier experimentally for
studying various parameters like syngas composition, temperature and heating value of syngas obtained by
(Mansaray et al., 2010). Such clean syngas obtained from a gasifier can be used as fuel for a large combined
cycle system for electricity generation, where the gasified fuel is first burnt in a combustion turbinegenerator
unit, and then the hot exhaust gas from its gas turbine is used for generating steam to produce further power in a
steam turbinegenerator unit. The combination of a gasifier and a combined cycle is called the IGCC. Including
IGCC, other different applications where syngas can be used are highlighted in (Chen et al., 2010). The
multitude applications of syngas can be shown in Figure 1. In todays scenario biomass gasification with oxygen
as gasifying agent has great potential.
The aim of the present work is to simulate medium purity oxygen cryogenic plant by using Aspen plus for
gasification application and found out specific power consumption of cryogenic ASU. It is also an attempt to
develop a model of biomass/coal gasifier with air/oxygen and steam as oxidizing agent by using Aspen plus.
Simulation of biomass gasifier based on Gibbs free energy minimization is performed. The syngas is obtained
with various parameters like syngas composition, temperature, heating value for different feedstocks as rice husk,
wood pallets and Indian charcoal. The parametric analyses also discussed for cryogenic ASU and biomass
gasifier.
Biomass
Steam&
Power
Gasifier
Gas
Turbine
combined
cycle
ICEngine
Boiler
Ethanol
Power
Generatio
n
Refinery
Transport
ationfuel
Diesel/
Kerosene
Syngas
Fischer
Tropsch
Fuelcells
Wax
Gasoline
Methanol
Naphtha
Hydrogen
Fuelcells
Chemicals
Fertilizers
Figure 1. Multitude applications of syngas
98
www.ccsenet.org/mer
Mechanical Engineering Research
Vol. 2, No. 2; 2012
2. Cryogenic Air Separation Modeling
Distillation has long been used for air separation. The three major components of air (nitrogen, oxygen and
argon), do not form any azeotropes and can be separated by simple distillation. Cooling is achieved through the
Joule-Thomson effect, if higher pressure air gets expand; it cools down and can be partially liquefied. To
simulate this process the Peng Robinson EOS is used. The cryogenic air separation process is a tight integration
of heat exchangers and separation columns which is completely driven by the compression of the air at the inlet
of the unit. The air inlet stream is cooled to ambient temperatures with cooling water which is further cooled
against product and waste streams leaving the plant until partially liquefied. The liquefied fraction varies
between 25 to 35 mol% depending on cooling water temperature and compressor outlet pressure. This stream
enters the bottom of a high pressure (HP) column that produces pure liquid nitrogen (impurities 1ppm) at the top
of HP column. The HP column has roughly 40 equilibrium stages including condenser and operates at high
pressure. The cooling medium in the condenser of HP column is the oxygen, bottom product produced in a
second column. Thus, this second column must operate at a lower pressure to make the reboiler colder than the
condenser of the HP column (typically by 1 Kelvin only). Thus, the pressures of both columns are linked mainly
by the vapour pressures of nitrogen and oxygen and the temperature approach of the combined
condenser/reboiler. In order to exit oxygen from the plant, the bottom of the low pressure (LP) column is
typically around 1.2 bars which set a pressure of at least 5.7 bars for the HP column. Therefore compressor is
required to deliver air at slightly higher pressure. These two columns share the same column shell to minimize
the temperature difference between the condensing nitrogen and evaporating oxygen. The liquid bottom product
of the HP column is rich in oxygen called rich liquid. When this rich liquid is reduced in pressure, the
Joule-Thomson effect causes this rich liquid to cool further which is fed to the LP column. Additional
refrigeration is obtained by compressing air in a second stage compressor to a high pressure and expands in
expander. This air feeds directly to LP column through Joule -Thomson valve. Finally, the LP column is refluxed
with liquid nitrogen from the HP column, after having been flashed and cooled by the Joule-Thomson effect. The
liquid oxygen from the bottom of LP column is gasified and heated against the incoming air in the main heat
exchanger. The pure gaseous nitrogen of the top of the LP column is also heated to atmospheric conditions
against the incoming air. The products of the ASU are thus gaseous nitrogen (GN2) and oxygen (GO2).
3. Gaseous Oxygen Plant
There is large demand for the gaseous oxygen in the decarburization of steel, in an electric arc and open- hearth
furnaces and also in the bottom-blown Bessemer process of steel production in the steel industry. The steel
making process needs gaseous oxygen to accelerate the oxidation and conversion of iron to steel. The oxygen
purity required for above mentioned application is 99.99% (mole % basis). Gasification is one of the applications
which require huge quantity of medium pure gaseous oxygen with purity 94% to 96% (mole % basis). It is
because air gasification produces a poor quality gas with regard to the heating value, around 4-9 MJ/Nm3 higher
heating value while O2 and steam blown processes result in a syngas with a heating value in the range of 10-20
MJ/Nm3.
3.1 Simulation of Cryogenic ASU for Gaseous Oxygen Production
The cryogenic distillation of air is currently the only method available for the large oxygen production rates
required for future fossil fuel gasification and oxy-fuel combustion with CO2 capture. The current large scale
users are the chemical, steel and petroleum industries. O2 purities of up to 97% are economically favored for
gasification application as this requires the much easier separation of N2 from O2. Production of 99.99% O2
requires the more difficult O2/Ar separation with up to double the number of separation stages in a distillation
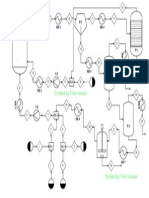
column. A process flow diagram of cryogenic air separation system is shown in Figure 2. Separation of over 95%
of the O2 in the air feed at 90% to 97% molar concentration is carried out in two distillation columns thermally
linked by a dual-function heat exchanger called as reboiler/condenser. This heat exchanger serves as a reboiler
for the HP column and as a condenser for LP column. The LP column operates at low pressure as 1.2 bars which
are close to ambient pressure to minimize energy use. The HP column operates at pressure 4 bar.
3.2 Simulation Considerations
The simulation considerations for cryogenic air separation unit are given as below.
1.
Air flow
: 1000 scmh
2.
Compressor discharge pressure : 4.2 bar
3.
LP Pressure
: 1.2 bar
4.
HP Pressure
: 4 bar
99
www.ccsenet.org/mer
Mechanical Engineering Research
5.
No. of stages in LP column
: 56
6.
No. of stages in HP column
: 40
7.
Heat leak
: 15 kw
Vol. 2, No. 2; 2012
12
15
B3
JT3
LP
2
20
6
JT1
M-COMPR
B14
5
26
13
1
B8
EXPANDER
25
JT2
GASN2
GASO2
HP
16
LM1
194
SUBCOL
MHX
14
FEED
22
17
Figure 2. Process flow diagram of cryogenic gaseous oxygen plant
Filtered air is first compressed to 4.2 bars and cooled to 45oC temperature by water cooled heat exchanger. The
compressed air then enters a main heat exchanger and is further cooled and partially liquefied by countercurrent
heat exchanger with cold nitrogen and oxygen streams from the columns. Partially liquefied air at 4.2 bars
pressure enters the HP column. The separated N2 gas condenses to provide reflux to the HP column and enters to
the LP column after sub cooling in the sub-cooler. Up to 30% of the air is further compressed to a pressure of 50
bars which gives a positive temperature difference. The refrigeration balance on the plant is provided by
expanding a portion of the high pressure air stream in a turbine. The discharge air stream from expander is feed
to LP column. The O2 rich liquid stream and a high pressure fraction of the air which has been liquefied are the
feed streams to the LP column. The distillation separates N2 gas stream from top of LP column and a liquid O2
stream from bottom of LP column are supplied to main heat exchanger and heated to ambient temperature, which
cools and partially liquefies the air feed streams.
4. Modeling of Biomass Gasifier
Gasification is a process that involves many complex reactions that depend on chemical kinetics, reactor
geometry, and many other parameters. As a result, the complexity of simulating gasification is simplified by
modeling the process based on thermodynamic and chemical equilibrium. Equilibrium is a good assumption
provided the residence time is long enough; however, in the case of most real world gasifiers this is not the case.
A reacting system is at its most stable composition at chemical equilibrium. This state is achieved when the
entropy of the system is maximized and its Gibbs free energy is minimized. Minimization of the Gibbs free
energy of the products is the method used to calculate equilibrium in this work. Aspen plus provides a unit
operation model called RGIBBS that calculates chemical and thermodynamic equilibrium based on minimizing
the Gibbs free energy of the system. Components in Aspen plus are classified as either conventional or
100
www.ccsenet.org/mer
Mechanical Engineering Research
Vol. 2, No. 2; 2012
nonconventional. Conventional components are ones with property data contained in the Aspen plus component
database. Nonconventional components are non-homogeneous substances that do not have a consistent
composition and are not contained in the Aspen plus component database. These components, which would
include coal and biomass, must be given physical attributes, such as those defined by the ultimate, proximate,
and sulfur analyses. Property methods must also be chosen to calculate the enthalpy and density of the substance.
For this work, the property methods HCOALGEN and DCOALIGT were respectively chosen to calculate the
enthalpy and density of biomass. These property methods use statistical correlations to calculate the specific heat,
enthalpy, and density of coal and coal-derived substances based on the ultimate, proximate, and sulfur analyses.
Biomass can be represented as a technical fuel through these analyses, these property methods were also used for
calculating the thermodynamic properties of biomass fuels. Furthermore, the property method HCOALGEN
offers different options for how the enthalpy of formation of the component is calculated. For this work, the
enthalpy of formation was calculated based on the higher heating value of the substance, which was specified
through ultimate analysis of biomass. The equilibrium reactor RGIBBS does not accept nonconventional
components as reactants. As a result, the fuel must be decomposed to conventional components which can be
used by the RGIBBS block. The conversion is accomplished with an RYIELD block, labeled DECOMP, which is
a reactor model that generates products based on known yields. The fuel feed stream enters DECOMP where it is
decomposed into its elemental constituents. A FORTRAN calculator script interacts with the RYIELD block such
that decomposition of the fuel is calculated based on the proximate and ultimate analyses of the nonconventional
component. The carbon content of the feed is converted to solid carbon graphite. The hydrogen, oxygen, nitrogen,
chlorine, and sulfur are converted to gaseous H2, O2, N2, Cl2, and S. Finally the moisture content is converted to
liquid H2O. These species are now contained in an intermediate stream called INGASFR, which then become the
reactants for the RGIBBS block. An O2/air and steam streams representing the gasifying oxidant also enters
RGIBBS reactor, and a products stream exits it. The heat stream QDECOMP connect the DECOMP and
RGIBBS reactor and represents the energy required to decompose the solid fuel. Although QDECOMP interacts
with RGIBBS reactor, the reactor is still considered to be adiabatic because DECOMP calculates the amount of
heat required for decomposition and draws it from RGIBS reactor.
5. Simulation of Biomass Gasifier with Air/Oxygen as Gasifying Agent
The simulation model of biomass gasification consists of two stages; biomass decomposition and gasification
reaction with steam and oxygen. In decomposition stage, biomass is decomposed into its elements like C, H, O,
S, N and H2O.The Aspen plus yield reactor RYIELD is used to simulate decomposition of the feed. The stream
biomass is specified as non-conventional stream and ultimate and proximate analyses are given as input along
with thermodynamic conditions and mass flow rate. The Aspen plus Gibbs reactor, RGIBBS is used for partial
combustion based on assumption that reactions of biomass elements with oxygen follow Gibbs equilibrium. The
products from RGIBBS reactor are fed to separator block. The top outlet stream which is called SYNGAS is
composed of all the gases. The Process flow diagram of biomass gasification with air/oxygen and steam is
shown in Figure 3.
SYN GAS
BIOM ASS
D EC OMP
R YIELD
STEAM
O2
IN GASF R
GASF R
PR OD U C TS
R GIB BS
SPLIT
SS PLIT
ASH
Figure 3. Process flow diagram of biomass gasification with air/oxygen as gasifying agent
5.1 Characteristics of Feedstocks and Simulation Parameters of Model
On the basis of process cycle design calculations, the main simulation parameters in cryogenic ASU integrated
with gasifier model are: Oxygen flow rate- 10 kg/hr, Steam flow rate- 8 kg/hr, Input biomass mass flow-33.6
kg/hr and gasification pressure-1.05 bar. The proximate and ultimate analyses of different feed stocks are given
in Table 1.
101
www.ccsenet.org/mer
Mechanical Engineering Research
Vol. 2, No. 2; 2012
Table 1. Proximate and ultimate analyses of different feedstocks
Proximate Analysis(% mole )
FC
VM
Moisture ASH
Ultimate Analysis (% mole )
C
H
O
N
CL
Indian coal
39.83
26.74
5.9
27.53
56.09
3.58
6.05
0.72
0.24
--
Rice Husk
14.8
84.8
11.7
0.4
36.42
4.91
35.88
0.59
--
Wood pellets
10
85
8.55
0.574
48
46
--
--
Feedstock
---
6. Results and Discussion
In this simulation, it is found that a total of 209.78 scmh of gaseous O2 product with 96.2% purity is produced
which is safely used for gasification application. Gaseous N2 byproduct with purity 99.9% is also produced at
volumetric flow rate of 734 scmh. Heat duties of the reboiler of the LP column and condenser of the HP column
are matched by calculator block in Aspen plus. It is observed that from simulation results, the main compressor
consumes 47.22 kW power and booster consumes 12.22 kW power, which is partially compensated by the power
of 9.32 kW generated in the expander. The specific power consumption is obtained is 0.2435 kw/scmh of O2.
The details simulation result of cryogenic ASU for oxygen production are shown in table 2.
Steady state simulation model of biomass gasifier in Aspen plus predicts the composition of syngas. Syngas
composition for different biomass with air and oxygen as gasifying agent are shown in table.3. It is found that
from table. 3, with oxygen as gasifying agent H2 and CO in syngas composition are increased as it is not diluted
with nitrogen. Heating value of syngas with air as gasifying agent is low as compared with oxygen as gasifying
agent. Syngas with low heating value is suitable for applications like boiler and engine applications where as
syngas with medium or high heating value suitable for F-T Process.
Table 2. Simulation results of gaseous oxygen plant
Parameters
FEED
GASN2
GASO2
LM1
Temperature(K)
95.5
300.1
302.8
283.1
Pressure (bar)
4.1
1.1
1.15
50
Vapor Fraction
0.9
N2
0.755
0.998
5 ppb
0.755
O2
0.232
18 ppm
0.953
0.232
Ar
0.013
0.002
0.047
0.013
N2
663.765
780.900
TRACE
114.792
O2
178.33
0.012
209.78
30.814
Ar
7.905
1.088
8.212
1.367
N2
0.781
0.999
6 ppb
0.781
O2
0.210
16 ppm
0.962
0.21
Ar
0.009
0.001
0.038
0.009
Mass Fraction
Mole Flow (scmh)
Mole Fraction
102
www.ccsenet.org/mer
Mechanical Engineering Research
Vol. 2, No. 2; 2012
Table 3. Syngas composition of different feedstocks
Feedstock
Indian coal
Rice Husk
Wood
pellets
Gasifying
Agent
Syngas composition( % mole)
H2
CO
CO2
H2O
CH4
N2
CV(MJ/kg)
Air
8.8
41.8
0.623
0.018
17.3
32
12.59
Oxygen
15.3
60.1
0.003
0.492
0.23
0.8
19.55
Air
22.9
18.4
13.0
8.3
0.8
36.6
5.49
Oxygen
36.5
21.8
20.2
19.9
0.6
0.4
9.14
Air
32.1
29.8
7.9
5.7
0.9
23.6
9.22
Oxygen
4.07
37.8
11.3
8.1
1.7
0.9
13.19
7. Sensitivity Analysis for Cryogenic ASU Integrated with Biomass Gasifier
The sensitivity analysis tool is used in this study to analyze and predict the behavior of the model to changes in
key operating and design variables. In this sensitivity analyisis, process variables like vapour fraction (VF),
temperature, oxygen flow and number stages etc. on desired output are studied. These studies are helpful in
predicting scenarios over a range of operating variables and provide solutions in a What-If analysis.
350.0
PL
300.0
PL flow (Scmh)
400.0
7.1 Effect of VF on Pure Liquid (PL) Flow (Distillate of HP Column)
As VF of feed increases, the quantity of vapour entering the column gets increased and consequently the net
vapour reaching the top stage increased. Therefore, at higher VF of the feed air and for constant purity of top
product, the PL flow out of the column increases as shown in Figure 4.
0.6 0.61 0.62 0.63 0.64 0.65 0.66 0.67 0.68 0.69 0.7 0.71 0.72 0.73 0.74 0.75 0.76 0.77 0.78 0.79 0.8 0.81 0.82 0.83 0.84 0.85 0.86 0.87 0.88 0.89 0.9
VF of feed
Figure 4. Effect of VF on PL flow
7.2 Effect of VF on Condenser Duty of HP Column
As VF of feed increases, the quantity of vapour at the top of distillation column will be more. These vapours are
condensed by condenser provided at the top of the distillation column. As a result, condenser duty increases with
increase in VF of the feed as shown in Figure 5.
103
Condenser duty (Watts)
Mechanical Engineering Research
Vol. 2, No. 2; 2012
-55000.0-50000.0 -45000.0-40000.0 -35000.0
www.ccsenet.org/mer
QCOND
0.6 0.61 0.62 0.63 0.64 0.65 0.66 0.67 0.68 0.69 0.7 0.71 0.72 0.73 0.74 0.75 0.76 0.77 0.78 0.79 0.8 0.81 0.82 0.83 0.84 0.85 0.86 0.87 0.88 0.89 0.9
VF of feed
Figure 5. Effect of VF on condenser duty
0.996
0.994
0.992
0.99
0.988
PURITY
0.986
PL Purity ( mole basis )
0.998
1.0
7.3 Effect of number of stages on PL Purity and RL (Rich Liquid-Bottoms of HP column) Purity
In distillation column the vapour flows upward in the column through the liquid, while the liquid flows across
the vapour stream towards downwards. At every stage evaporation of N2 and condensation of O2 carried out in
counter flow. So as the vapour bubble moves upward through the liquid, it becomes richer in N2 and the liquid
through which the bubble moves becomes richer in O2. Thus by using a large number of stages in distillation
column PL purity and RL purity are increases as shown in Figure 6 and Figure 7.
10.0
12.5
15.0
17.5
20.0
22.5
25.0
27.5
30.0
Number of stages
Figure 6. Effect of number of stages on PL purity
104
32.5
35.0
37.5
40.0
RL Purity ( mole basis )
Mechanical Engineering Research
Vol. 2, No. 2; 2012
0.317 0.318 0.319 0.32 0.321 0.322 0.323 0.324 0.325 0.326
www.ccsenet.org/mer
RL
10.0
12.5
15.0
17.5
20.0
22.5
25.0
27.5
30.0
32.5
35.0
37.5
40.0
Number of stages
Figure 7. Effect of number of stages on RL purity
1.0
7.4 Effect of VF on PL Purity
The purity of the top product PL depends on the L/V at the top stage of the HP column. For more VF of the feed
air, liquid formation at the top stage will increase due to increased vapour rising to the upper stage. Therefore as
VF increases L/V increases which increases the reflux to the HP column for fixed PL flow and PL purity
increases as shown in Figure 8.
0.97
0.95
0.96
PL Purity
0.98
0.99
PLPURE
0.6
0.625
0.65
0.675
0.7
0.725
0.75
0.775
0.8
0.825
0.85
0.875
0.9
VF
Figure 8. Effect of number of stages on PL purity
7.5 Effect of Gasification Temperature on Syngas Composition
It is found that as temperature of gasifier increases CO and H2 in syngas composition increases while CO2 and
CH4 in syngas composition decreases as shown in Figure 9. It is because at high temperature methane
decomposes into hydrogen and monoxide carbon as well as shift reaction equilibrium inclines in the production
of CO and H2O.
105
Mechanical Engineering Research
Vol. 2, No. 2; 2012
0.35
0.3
0.25
0.2
0.15
H2
0.1
CO
CO2
H2O
0.05
syngas composition ( mole basis )
0.4
www.ccsenet.org/mer
CH4
N2
600.0
650.0
700.0
750.0
800.0
850.0
900.0
950.0
1000.0
Gasifier temperature
Figure 9. Effect of Gasifier temperature on syngas composition
7.6 Effect of Oxygen Flow Rate on Syngas Composition
In gasification process, the air/fuel ratio controls the gasification temperature. The composition profile of
individual species in syngas with oxygen flow rate is shown in Figure 10. As oxygen flow rate increases H2
increases and reach maximum at 9 kg/hr and then decreases. Similarly as oxygen flow rate increases CO
increases and reach maximum at 12 kg/hr and then decreases. As oxygen flow rate increases CO2 decreases
reach minimum and then increases. In a similar way H2O decreases reach minimum and then increases.
H2
CO
H2O
0.4
CH4
0.1
0.15
0.2
0.25
0.3
0.35
N2
0.05
Syngas composition ( mole basis )
0.45
CO2
0.0
2.0
4.0
6.0
8.0
10.0
12.0
14.0
16.0
Oxygen flow rate ( kg/hr )
Figure 10. Effect of oxygen flow rate on syngas composition
8. Conclusion
The simulation model of cryogenic ASU in Aspen plus predicts production of oxygen with required purity and
recovery. Oxygen is obtained with purity 96.2% which is economically suitable for biomass/coal gasification for
getting syngas with a higher heating value. The specific power consumption of the plant obtained is 0.2435
kw/scmh of O2, which is lower as compared to conventional cryogenic air separation plants.
It is seen that air gasification produces a syngas with lower heating value, while O2 and steam blown processes
106
www.ccsenet.org/mer
Mechanical Engineering Research
Vol. 2, No. 2; 2012
result in a syngas with a higher heating value. However, the use of oxygen does have other advantages such as
operation at lower equivalence ratio, smaller equipment size of gasifier and downstream equipment, and possibly
savings in compression cost of produced gas. The parametric analysis of cryogenic ASU integrated with biomass
gasifier discussed. It illustrates the impact of various parameters on the desired output of cryogenic ASU
integrated with biomass gasifier.
Acknowledgements
The authors are grateful to Dr. L.G. Navale, Principal and Prof. A.J. Hake, Vice Principal, MES College of
Engineering, Pune for their active help and guidance. We are also thankful to Dr. Uday Kulkarni for giving
guidelines in the simulation of Cryogenic air separation plant.
References
Agrawal, R., Erickson, D. C., & Woodward, D. W. (1989). High efficiency processes for cryogenic air separation.
Air products and chemicals, 33-36.
Anil, Khadse, Prasad, Parulekar, Preeti, Aghalayam, & Anuradda, Ganesh. (2006). Equilibrium model for
biomass gasification. Advances in Energy Research, IIT Mumbai.
Aspen plus 11.1 (2011). User Manual guide, Aspen Technology.
Castle, W. F. (2000). Air separation and liquefaction: recent developments and prospects for the beginning of the
new
millennium.
International
Journal
of
Refrigeration,
25(1),
158-172
http://dx.doi.org/10.1016/S0140-7007(01)00003-2
Chawdhury, M. A., & Mahkamov, K. (2011). Development of small downdraft biomass gasifier for developing
countries. Journal of Scientific Research, 3(1), 51-64. http://dx.doi.org /10.3329/jsr.v3i1.5613
Chen, Po-Chuang, Chiu, Hsiu-Mei, Chyou, Yau-Pin, & Yu, Chiou-Shia. (2010). Processes simulation study of
coal to methanol based on gasification technology. World academy of science, engineering and technology, 65.
Mandler, Jorge A. (2000). Modeling for control analysis and designing complex industrial separation and
liquefaction
process,
Journal
of
process
control,
10(2-3),
167-175.
http://dx.doi.org/10.1016/S0959-1524(99)00021-9
Mansaray, K. G., Ghaly, A. E., Al-Taweel, A. M., Ugursal, V. I., & Hamdullahpur, F. (2010). Mathematical
modeling of a fluidized bed rice husk gasifier: Part III - Model verification. Energy sources Part A:
Recovery,
utilization
and
Environmental
effects,
22(3),
281-296.
http://dx.doi.org/10.1080/00908310050014063
Paviet, Chazarency, & Tazeroutz. (2009). Thermo chemical equilibrium modelling of a biomass gasifying
process usng ASPEN PLUS. International Journal of chemical reactor engineering, 7(1), 1542-6580.
http://dx.doi.org/10.2202/1542-6580.2089
Sapali, S. N. (2001). A Thesis on cryogenic air separation plants: Parametric evaluation and exergy analysis.
Ph.D.Thesis, IIT Kharagpur.
Schuftan, P. M. (1948). Modern gaseous oxygen production methods. Transactions Institute of chemical
Engineers, 26a, 30-37.
Shrinivas, A. V. S., Gupta, S. K. S., & Reddy, B. V. (2009). Thermodynamic equilibrium model and exergy
analysis of biomass gasifier. Journal of energy resources technology, ASME, 131(3), 1-7.
http://dx.doi.org/10.1115/1.3185354
Smith, A. R, & Klosek, J. (2001). A review of air separation technologies and their integration with energy
conversion
processes.
Fuel
processing
technology,
2(2),
115-134.
http://dx.doi.org/10.1016/S0378-3820(01)00131-X
Wayne, Doherty, Anthony, Reynolds, & David, Kennedy. (2008). Simulation of a circulating fluidised bed
biomass gasifier using ASPEN plus: a performance analysis. International conference on efficiency, cost,
optimiztion, simulation and environmental impact of energy systems Karkow, poland, 1241-1248.
Zhu, Y., Liu, X. G., & Zhou, Z. Y. (2006). Optimization of cryogenic air separation distillation columns.
Proceeding of the world congress on intelligent control and automation, 7702-7705.
Zhu, Yu, Legg, Sean, & Laird Carl, D. (2010). Optimal design of cryogenic air separation columns under
uncertainty.
Computers
and
chemical
Engineering,
34(9),
1377-1384.
http://dx.doi.org/10.1016/j.compchemeng.2010.02.007
107
S-ar putea să vă placă și
- Nitrogen BlanketingDocument7 paginiNitrogen Blanketing3bandhuÎncă nu există evaluări
- Parker Tank Blanketing White Paper PDFDocument4 paginiParker Tank Blanketing White Paper PDFshashi kant kumarÎncă nu există evaluări
- Dow Filmtec BW30XFR 400 34iDocument2 paginiDow Filmtec BW30XFR 400 34iJuan Camilo HenaoÎncă nu există evaluări
- Uniteolstates Patent Office: "133.2535.lftii?iiii'tfiiiw 7 'Document2 paginiUniteolstates Patent Office: "133.2535.lftii?iiii'tfiiiw 7 'Juan Camilo HenaoÎncă nu există evaluări
- Purge With NitrogenDocument5 paginiPurge With Nitrogendeion29100% (1)
- When You Were A Child, What Job Did You Want To Do When You Grew Up?Document1 paginăWhen You Were A Child, What Job Did You Want To Do When You Grew Up?Juan Camilo HenaoÎncă nu există evaluări
- Batista 2013Document13 paginiBatista 2013Juan Camilo HenaoÎncă nu există evaluări
- Dow Filmtec BW30XFR 400 34iDocument2 paginiDow Filmtec BW30XFR 400 34iJuan Camilo HenaoÎncă nu există evaluări
- Papper Co2Document15 paginiPapper Co2Juan Camilo HenaoÎncă nu există evaluări
- Creative Science & Research - Screen Printing Press (2004)Document21 paginiCreative Science & Research - Screen Printing Press (2004)amrandconan100% (2)
- Algebra Angel Cap9Document66 paginiAlgebra Angel Cap9Ariel GomezÎncă nu există evaluări
- Learn English Podcasts Elementary 01 01 Support Pack TranscriptDocument22 paginiLearn English Podcasts Elementary 01 01 Support Pack Transcriptpramithbuddika100% (1)
- Created by Trial Version: HE-3 HE-4Document1 paginăCreated by Trial Version: HE-3 HE-4Juan Camilo HenaoÎncă nu există evaluări
- Ie00064a001 PDFDocument9 paginiIe00064a001 PDFJuan Camilo HenaoÎncă nu există evaluări
- Extreme Independence - All Combinations of 4 LimbsDocument3 paginiExtreme Independence - All Combinations of 4 LimbsJuan Camilo HenaoÎncă nu există evaluări
- CLASS 300 Welding Neck & Slip-On Flanges: ASTM A-105 & A181-CLASS 60Document3 paginiCLASS 300 Welding Neck & Slip-On Flanges: ASTM A-105 & A181-CLASS 60Juan Camilo HenaoÎncă nu există evaluări
- 02bfe50dacb1fe39e0000000 PDFDocument50 pagini02bfe50dacb1fe39e0000000 PDFJuan Camilo HenaoÎncă nu există evaluări
- (Doi 10.1021/ie50289a025) M. Souders G. G. Brown - Design of Fractionating Columns I. Entrainment and Capacity PDFDocument6 pagini(Doi 10.1021/ie50289a025) M. Souders G. G. Brown - Design of Fractionating Columns I. Entrainment and Capacity PDFJuan Camilo HenaoÎncă nu există evaluări
- IT H Unit IGDocument19 paginiIT H Unit IGJuan Camilo HenaoÎncă nu există evaluări
- Aspen HYSYS DynamicsDocument3 paginiAspen HYSYS DynamicsJuan Camilo HenaoÎncă nu există evaluări
- United States' Patent Office: Patented July 20, 1948Document5 paginiUnited States' Patent Office: Patented July 20, 1948Juan Camilo HenaoÎncă nu există evaluări
- Zirconia-Supported Vanadium Oxide Catalysts For Ammoxidation and Oxidation of Toluene: A Characterization and Activity StudyDocument22 paginiZirconia-Supported Vanadium Oxide Catalysts For Ammoxidation and Oxidation of Toluene: A Characterization and Activity StudyJuan Camilo HenaoÎncă nu există evaluări
- Benzoguanamine: Product Specification SheetDocument1 paginăBenzoguanamine: Product Specification SheetJuan Camilo HenaoÎncă nu există evaluări
- Dowtherm A PropertiesDocument2 paginiDowtherm A Propertiesflashiitm100% (1)
- Benzoguanamine: Product Specification SheetDocument1 paginăBenzoguanamine: Product Specification SheetJuan Camilo HenaoÎncă nu există evaluări
- Flash ConceptsDocument2 paginiFlash ConceptsJuan Camilo HenaoÎncă nu există evaluări
- Poços de CaldasDocument8 paginiPoços de CaldasAnninha FlavioÎncă nu există evaluări
- AbsorptionStripping PDFDocument25 paginiAbsorptionStripping PDFJuan Camilo HenaoÎncă nu există evaluări
- The Yellow House: A Memoir (2019 National Book Award Winner)De la EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Evaluare: 4 din 5 stele4/5 (98)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceDe la EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceEvaluare: 4 din 5 stele4/5 (895)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeDe la EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeEvaluare: 4 din 5 stele4/5 (5794)
- The Little Book of Hygge: Danish Secrets to Happy LivingDe la EverandThe Little Book of Hygge: Danish Secrets to Happy LivingEvaluare: 3.5 din 5 stele3.5/5 (399)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaDe la EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaEvaluare: 4.5 din 5 stele4.5/5 (266)
- Shoe Dog: A Memoir by the Creator of NikeDe la EverandShoe Dog: A Memoir by the Creator of NikeEvaluare: 4.5 din 5 stele4.5/5 (537)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureDe la EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureEvaluare: 4.5 din 5 stele4.5/5 (474)
- Never Split the Difference: Negotiating As If Your Life Depended On ItDe la EverandNever Split the Difference: Negotiating As If Your Life Depended On ItEvaluare: 4.5 din 5 stele4.5/5 (838)
- Grit: The Power of Passion and PerseveranceDe la EverandGrit: The Power of Passion and PerseveranceEvaluare: 4 din 5 stele4/5 (588)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryDe la EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryEvaluare: 3.5 din 5 stele3.5/5 (231)
- The Emperor of All Maladies: A Biography of CancerDe la EverandThe Emperor of All Maladies: A Biography of CancerEvaluare: 4.5 din 5 stele4.5/5 (271)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyDe la EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyEvaluare: 3.5 din 5 stele3.5/5 (2259)
- On Fire: The (Burning) Case for a Green New DealDe la EverandOn Fire: The (Burning) Case for a Green New DealEvaluare: 4 din 5 stele4/5 (73)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersDe la EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersEvaluare: 4.5 din 5 stele4.5/5 (344)
- Team of Rivals: The Political Genius of Abraham LincolnDe la EverandTeam of Rivals: The Political Genius of Abraham LincolnEvaluare: 4.5 din 5 stele4.5/5 (234)
- The Unwinding: An Inner History of the New AmericaDe la EverandThe Unwinding: An Inner History of the New AmericaEvaluare: 4 din 5 stele4/5 (45)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreDe la EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreEvaluare: 4 din 5 stele4/5 (1090)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)De la EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Evaluare: 4.5 din 5 stele4.5/5 (121)
- Her Body and Other Parties: StoriesDe la EverandHer Body and Other Parties: StoriesEvaluare: 4 din 5 stele4/5 (821)
- Generator Set Data Sheet: 250kVA Standby at 50HzDocument3 paginiGenerator Set Data Sheet: 250kVA Standby at 50Hzhonafa- R.O.N.OÎncă nu există evaluări
- DIMOIL SA: Business Plan 2012-2014Document47 paginiDIMOIL SA: Business Plan 2012-2014Dr. KIRIAKOS TOBRAS - ΤΟΜΠΡΑΣÎncă nu există evaluări
- Actual Sysem Setup Alpha LubricatorDocument7 paginiActual Sysem Setup Alpha LubricatorRanjan DiptanshuÎncă nu există evaluări
- O&M 883 IngDocument104 paginiO&M 883 IngRafael Antonio Calanche75% (4)
- Ecu - Injection System Magneti Marelli 6LPDocument17 paginiEcu - Injection System Magneti Marelli 6LPGrizono MassimoÎncă nu există evaluări
- IOCL MAthura PDFDocument52 paginiIOCL MAthura PDFKavisha AgrawalÎncă nu există evaluări
- S.M.Asghar (PVT) Limited Introduction For CMEC ComtransDocument152 paginiS.M.Asghar (PVT) Limited Introduction For CMEC ComtransSajidÎncă nu există evaluări
- Colorado Business Letter To Protect Arctic National Wildlife RefugeDocument9 paginiColorado Business Letter To Protect Arctic National Wildlife RefugeU.S. Senator Michael F. BennetÎncă nu există evaluări
- Petrol 1 Trial Bill Sample EmptyDocument1 paginăPetrol 1 Trial Bill Sample EmptyShubham gupta100% (1)
- W8L20 - Spare PartsDocument2 paginiW8L20 - Spare PartsTuan Linh VoÎncă nu există evaluări
- MM 540 DPDocument3 paginiMM 540 DPapi-27077834100% (1)
- Kubota Engine Parts List REV 200608 PDFDocument60 paginiKubota Engine Parts List REV 200608 PDFAndres HincapieÎncă nu există evaluări
- FSM (BPCL)Document15 paginiFSM (BPCL)Prabhat Kumar ChoubeyÎncă nu există evaluări
- C210 WML 208Document9 paginiC210 WML 208Efrén SantínÎncă nu există evaluări
- Hyd R3Document2 paginiHyd R3dddÎncă nu există evaluări
- Workover OperationsDocument19 paginiWorkover Operationslaaliauto100% (4)
- Engines EssayDocument5 paginiEngines EssayValiÎncă nu există evaluări
- Lab 6 - Gas TurbineDocument8 paginiLab 6 - Gas TurbinehaziqsajjadÎncă nu există evaluări
- Ingles TécnicoDocument5 paginiIngles TécnicoRigel OriónÎncă nu există evaluări
- Incadrare IMODocument8 paginiIncadrare IMOSamuel RobersonÎncă nu există evaluări
- Manual Yd25ddtiDocument299 paginiManual Yd25ddtiCato del Rio86% (44)
- General Knowledge Overhauling The CompressorDocument2 paginiGeneral Knowledge Overhauling The CompressorAfhnan TemiziÎncă nu există evaluări
- 194-323 Ford WSG1068 6.8L Industrial Engine Service Manual (Generac OE7083) (02-2002)Document358 pagini194-323 Ford WSG1068 6.8L Industrial Engine Service Manual (Generac OE7083) (02-2002)Miguel Sotelo100% (2)
- Scenario Based Assessment Method For Engine Room Simulator CoursesDocument8 paginiScenario Based Assessment Method For Engine Room Simulator Coursesrafif irfanÎncă nu există evaluări
- SPE 138798 Frac Con Sand Jetting PDFDocument8 paginiSPE 138798 Frac Con Sand Jetting PDFMauro Catano100% (1)
- PRYCEGAS LPG PERSONNEL (Supervisory)Document4 paginiPRYCEGAS LPG PERSONNEL (Supervisory)Марк Артём Лимот Апалла100% (2)
- 01 - Introduction To TrnsysDocument18 pagini01 - Introduction To TrnsysAnonymous 73gEYyEtLÎncă nu există evaluări
- JET 30 Multipurpose Single Pump UnitsDocument116 paginiJET 30 Multipurpose Single Pump UnitsAlberto VelásquezÎncă nu există evaluări
- July 08Document4 paginiJuly 08Mohamed HamedÎncă nu există evaluări